Abstract
Background:
Comorbidities are common in patients with atrial fibrillation (AF) and affect prognosis, yet are often under-treated. However, contemporary rates of use of guideline-directed therapies (GDT) for non-AF comorbidities and their association with outcomes is not well described.
Methods:
We used the Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF) to test the association between GDT for non-AF comorbidities and major adverse cardiac or neurovascular events (MACNE- cardiovascular death, myocardial infarction, stroke/thromboembolism, or new onset heart failure), all-cause mortality, new-onset heart failure and AF progression. Adjustment was performed using Cox-proportional hazards models and logistic regression.
Results:
Only 6,782 (33%) of the 20,434 patients eligible for one or more GDT for non-AF comorbidities received all indicated therapies. Use of all comorbidity-specific GDT was highest for patients with hyperlipidemia (75.6%) and lowest for diabetes mellitus (43.1%). Use of “all eligible” GDT was associated with a non-significant trend towards lower rates of MACNE (HR 0.90 [0.79–1.02]) and all-cause mortality (HR 0.90 [0.80–1.01]). Use of GDT for heart failure was associated with a lower risk of all-cause mortality (HR 0.77 [0.67–0.89]) and treatment of obstructive sleep apnea was associated with a lower risk of AF progression (OR 0.75 [0.62–0.90]).
Conclusion:
In AF patients there is underuse of GDT for non-AF comorbidities. The association between GDT use and outcomes was strongest in heart failure and obstructive sleep apnea patients where use of GDT was associated with lower mortality and less AF progression.
Keywords: Atrial fibrillation, outcomes, guideline directed medical therapy
Introduction
Atrial fibrillation (AF) is an increasingly prevalent condition, which carries a substantial risk of stroke, heart failure, cognitive impairment, and mortality.1, 2 Comorbid conditions that carry additional cardiovascular risk are common among AF patients and are associated with higher rates of adverse outcomes.3 Many of these conditions have been associated with overall worsening of cardiac function,4 and progression of AF from paroxysmal to persistent forms.5, 6 Prior work has shown that the treatment of risk factors can improve outcomes in patients with AF and use of guideline directed therapies (GDT) for comorbid conditions can help reduce risk associated with these comorbidities.7–10 Yet, the majority of patients with AF do not receive all GDT for comorbid cardiovascular conditions for which they are eligible,11 and the impact of receiving all eligible GDT use on clinical outcomes in AF patients is not well studied. We hypothesized that the use of all eligible GDT for comorbidities is associated with lower rates of adverse events among AF patients, as well as less progression of AF (from paroxysmal to persistent/permanent).
Methods
Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Patients from the Outcomes for Better Informed Treatment of AF (ORBIT-AF and ORBIT-AF II) registries were considered for inclusion in this study. The details of these registries have been previously published.12, 13 Briefly, the ORBIT-AF and ORBIT-AF II registries enrolled ambulatory, adult patients with electrocardiographically documented AF from outpatient US practices and followed them semiannually tracking clinical outcomes and events for a minimum of 2 years. ORBIT-AF enrolled 10,137 patients from 176 sites from June 29, 2010 through August 9, 2011. ORBIT-AF II enrolled 13,394 patients from 244 US sites from February 20, 2013 through July 12, 2016. The study protocol was reviewed and approved by the Duke University Medical Center Institutional Review Board (IRB) and the IRB at each enrolling center. Primary funding was provided by Ortho-McNeil Janssen Scientific Affairs, LLC. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
For the purpose of this study, only patients with at least one of the following GDT eligible conditions (coronary artery disease [CAD], diabetes mellitus [DM], heart failure [HF], hyperlipidemia [HL], hypertension [HTN], peripheral vascular disease [PVD], or obstructive sleep apnea [OSA]) at baseline were included in this analysis. Additionally, patients were excluded if they did not have any available follow up data.
GDT Assessment
GDT eligibility was defined according to current professional guidelines published by the American College of Cardiology (ACC) and American Heart Association (AHA) and related professional societies for the management of CAD,14 DM,15, 16, HF,17 HL,16 HTN,15 PVD,18, and OSA.19 Specifically, GDT for CAD included use of a beta blocker, statin, angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) in the presence of DM or left ventricular ejection fraction (LVEF) ≤ 40%, and an antiplatelet agent if the patient had a myocardial infarction (MI), percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG) within the preceding 12 months. DM GDT included use of a statin and use of ACEi or ARB in the presence of CAD or LVEF ≤ 40%. While glycemic control agents (e.g. insulin, metformin) are an important component of DM GDT, their use was not captured in the ORBIT registry and thus not included in the assessment of GDT for DM in this study. GDT for HF included use of a beta blocker, ACEi/ARB in the setting of LVEF ≤ 40% or DM, aldosterone antagonist in the presence of New York Heart Association (NYHA) class II-IV symptoms and suitable creatinine level (≤ 2.5 mg/dL in men, ≤ 2.0 mg/dL in women), and implantable cardioverter-defibrillator (ICD) in the presence of LVEF ≤ 35% and NYHA class II-III symptoms. GDT for HL included use of a statin in the presence of CAD, DM, or PVD. GDT for HTN included use of an aldosterone antagonist, ACEi/ARB, calcium channel blocker, diuretic or beta blocker in the presence of previously diagnosed HTN or documented blood pressure ≥ 140/90 (≥ 130/80 in patients with DM or chronic kidney disease). GDT for PVD required use of a statin. While antiplatelet agents are also indicated in patients with PVD, given the high use of anticoagulants in the ORBIT-AF cohorts which may influence antiplatelet use, we did not require use of antiplatelet therapy in our assessment of GDT for PVD in this study. Patients with OSA were considered to be on GDT if they reported use of a continuous positive airway pressure (CPAP) machine.
Because the number of therapies included in the GDT estimate varied by disease state (and consequently between patients), GDT use was analyzed across several different levels. The primary analysis was performed at the individual level with GDT as a binary variable (“All eligible” vs “Not all eligible”). GDT was evaluated as a binary variable because there was variance in both the number of comorbidities each patient had as well as the number of therapies considered as GDT for each comorbidity in this study (four of the seven evaluated had only one therapy included as GDT). In addition, comorbidity-specific GDT use (also binary) was also assessed at an individual level to better understand how treatment of each comorbidity was associated with outcomes. Finally, GDT use was evaluated as a continuous variable (%GDT defined by [number of eligible therapies used] / [total number of eligible therapies]) at the site level to evaluate whether site-level treatment patterns had similar effects on outcomes as individual treatment effects.
Clinical Outcomes
The primary outcome of interest for this study was major adverse cardiac/neurovascular events (MACNE) including cardiovascular (CV) death, MI, embolic event (central nervous system or systemic) and new onset HF. Secondary outcomes included all-cause mortality, new onset HF, and AF progression from paroxysmal to persistent or from persistent to permanent (excluding patients with new-onset or permanent AF at baseline).
Statistical Analyses
Demographic and patient baseline characteristics were compared between patients who received “all eligible” GDT and those who received some or none of the GDT for which they were eligible (“not all eligible”). Variables following a Gaussian distribution were presented as mean ± standard deviation; variables following non-Gaussian distributions were presented as median (interquartile range). Variables were compared using chi-square tests for categorical variables and the Wilcoxon rank sum test for continuous variables.
The relationship between all eligible GDT use and clinical outcomes was assessed with adjusted and unadjusted Cox-proportional hazard models for clinical outcomes and pooled logistic regression for AF progression (with site included as a random effect). Covariates included in the adjusted model are listed in the Appendix (Supplemental material) with analyses stratified across 128 combinations of comorbidities to account for number and type of comorbidity. All continuous variables were tested for linearity and any non-linear associations were accounted for using linear splines. A non-linear association was noted between the site level %GDT use and outcomes so a linear spline with a knot at 60% was used for site level analyses. At the patient level, a robust covariance estimate was included in order to account for within site correlation. Missingness was handled by 5 fold multiple imputation.20 Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Study Population
Of the 23,531 patients included in the combined ORBIT-AF and ORBIT-AF II registries, 2,446 patients were excluded for not having any of the conditions eligible for the GDT evaluated in this study, and an additional 651 patients did not have follow-up data resulting in a final study cohort of 20,434 patients. Table I shows the distribution of demographics, past medical history, baseline laboratory data and enrolling provider specialty stratified by patients being on all eligible GDT (“all eligible”) vs some or none of the GDT for which they were eligible (“not all eligible”). The mean age was 72.3 ± 10.8 years, 41.7% were female, and 87.1% were white. A total of 6,782 (33.2%) patients were on all GDT for which they were eligible; whereas 13,652 (66.8%) were on some or none of their eligible GDT. Patients in the “not all eligible” group were more frequently black (5.8% vs 4.0%, p<0.001) and/or Hispanic (5.0% vs 4.5%, p<0.001) and were more commonly insured by Medicare or Medicaid (57.3% vs 53.4%, p<0.001). Of the cardiovascular comorbidities with treatments included in our assessment of GDT, patients in the “not all eligible” group more frequently had five of the seven studied comorbidities: CAD (38.8% vs 25.0%), DM (40.0% vs 12.3%), HF (33.4% vs 21.2%), PVD (13.3% vs 8.0%), and OSA (23.1% vs 13.2%) (p<0.001 for all). Evaluating total number of GDT-eligible comorbidities, patients in the “not all eligible” group had a median of 3 (2–4) GDT-eligible comorbidities compared to 2 (2–3) in patients on “all eligible” therapies (p<0.001). Patients in the “not all eligible” group also more commonly had a medical history of non-GDT related conditions including thyroid disease, COPD, anemia, prior stroke or transient ischemic attack, GI bleed, liver disease, and dialysis. More patients in the “not all eligible” group had frailty (defined as three or more of the following: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed or low physical activity) compared to the “all eligible” group (5.3% vs 3.4%, p<0.001). CHA2DS2VASC scores were higher in patients in the “not all eligible” group (4 [3–5] vs 3 [2–4], p<0.001) yet these patients had slightly lower rates of oral anticoagulation use (84.1% vs 85.5%, p=0.01).
Table I:
Patient characteristics by use of “all eligible” or “not all eligible” GDT.
| Overall | “Receiving all eligible” | “Not receiving all eligible” | P-Value | |
|---|---|---|---|---|
| N | 20,434 | 6,782 | 13,652 | |
| Age, yrs | 72.3 ± 10.8 | 71.8 ± 10.8 | 72.6 ± 10.7 | <0.001 |
| Sex (n, % female) | 8523 (41.7%) | 2782 (41.0%) | 5741 (42.0%) | 0.16 |
| Race | <0.001 | |||
| White | 17794 (87.1%) | 6022 (88.8%) | 11772 (86.3%) | |
| Black/African American | 1071 (5.3%) | 274 (4.0%) | 797 (5.8%) | |
| Asian | 256 (1.3%) | 80 (1.2%) | 176 (1.3%) | |
| American Indian/Alaska Native | 57 (0.3%) | 17 (0.2%) | 40 (0.3%) | |
| Hispanic | 987 (4.8%) | 308 (4.5%) | 679 (5.0%) | |
| Native Hawaiian/Pacific Islander | 20 (0.1%) | 10 (0.2%) | 10 (0.1%) | |
| Other | 234 (1.2%) | 69 (1.0%) | 165 (1.2%) | |
| Missing | 15 (0.1%) | 2 (<0.1%) | 13 (0.1%) | |
| Insurance | <0.001 | |||
| Medicare or Medicaid | 11454 (56.0%) | 3624 (53.4%) | 7830 (57.3%) | |
| Private | 7941 (38.9%) | 2804 (41.4%) | 5137 (37.6%) | |
| Cardiovascular comorbidities and risk factors | ||||
| Total number of GDT-eligible comorbidities | 3 (2–4) | 2 (2–3) | 3 (2–4) | <0.001 |
| Coronary artery disease | 6990 (34.2%) | 1692 (25.0%) | 5298 (38.8%) | <0.001 |
| Diabetes mellitus | 6294 (30.8%) | 835 (12.3%) | 5459 (40.0%) | <0.001 |
| Heart failure | 6001 (29.4%) | 1438 (21.2%) | 4563 (33.4%) | <0.001 |
| Hyperlipidemia | 14601 (74.4%) | 4863 (71.7%) | 9738 (71.3%) | 0.58 |
| Hypertension | 18474 (90.4%) | 6123 (90.3%) | 12351 (90.5%) | 0.67 |
| Peripheral vascular disease | 2358 (11.5%) | 543 (8.0%) | 1815 (13.3%) | <0.001 |
| Obstructive sleep apnea | 4045 (19.8%) | 894 (13.2%) | 3151 (23.1%) | <0.001 |
| Other medical history | ||||
| Cancer | 4437 (21.7%) | 1455 (21.4%) | 2982 (21.8%) | 0.52 |
| Thyroid disease | 4051 (19.8%) | 1233 (18.2%) | 2818 (20.6%) | <0.001 |
| COPD | 2930 (14.3% | 768 (11.3%) | 2162 (15.8%) | <0.001 |
| Anemia | 2921 (14.3%) | 788 (11.6%) | 2133 (15.6%) | <0.001 |
| Prior stroke or transient ischemic attack | 2697 (13.2%) | 771 (11.4%) | 1926 (14.1%) | <0.001 |
| Frailty | 949 (4.7%) | 229 (3.4%) | 720 (5.3%) | <0.001 |
| GI bleed | 1391 (6.8%) | 387 (5.7%) | 1004 (7.4%) | <0.001 |
| Cognitive impairment/Dementia | 468 (2.3%) | 146 (2.2%) | 322 (2.4%) | 0.35 |
| Liver disease | 442 (2.2%) | 123 (1.8%) | 319 (2.3%) | 0.02 |
| Dialysis | 237 (1.2%) | 46 (0.7%) | 191 (1.4%) | <0.001 |
| CHA2DS2VASC Score | 4 (3–5) | 3 (2–4) | 4 (3–5) | <0.001 |
| On oral anticoagulation | 17285 (84.6%) | 5799 (85.5%) | 11486 (84.1%) | 0.01 |
| Laboratory data, median (IQR) | ||||
| eGFR (ml/min/1.73 m2) | 69.7 (55.4–85.3) | 73.6 (61.6–87.0) | 67.1 (52.3–84.0) | <0.001 |
| Hemoglobin (g/dL) | 13.5 (12.2–14.7) | 13.7 (12.5–14.8) | 13.4 (12.1–14.6) | <0.001 |
| Provider specialty | <0.001 | |||
| Cardiology | 13929 (68.2%) | 4625 (68.2%) | 9304 (68.2%) | |
| Electrophysiology | 3775 (18.5%) | 1318 (19.4%) | 2457 (18.0%) | |
| Internal medicine/Family practice | 2646 (13.0%) | 802 (11.8%) | 1844 (13.5%) |
COPD = chronic obstructive lung disease; eGFR = estimated glomerular filtration rate; GDT = guideline directed medical therapy; IQR = interquartile range
Clinical Outcomes by Total GDT Use
Over the study period, there wsere 1,678 MACNE events (5.0 events per 100 patient years [event rate]), and 1,809 deaths (event rate = 5.2). A total of 419 patients developed new onset HF (event rate 1.8). Of the 11,238 patients with paroxysmal or persistent AF, 2,738 (24.4%) progressed to persistent or permanent AF over the course of follow up.
The association between use of “all eligible” GDT vs “not all eligible” GDT is shown in Figure 1. While there was an association between use of “all eligible” GDT and lower risk of MACNE (HR 0.84 [0.75–0.95]) in the unadjusted models, there was no association between “all GDT” use and these outcomes in the fully adjusted models. There were trends towards lower rates of MACNE and all-cause mortality (HR 0.90 [0.79–1.02], p=0.09 and HR 0.90 [0.80–1.01], p = 0.08 respectively), but these did not reach statistical significance.
Figure 1:
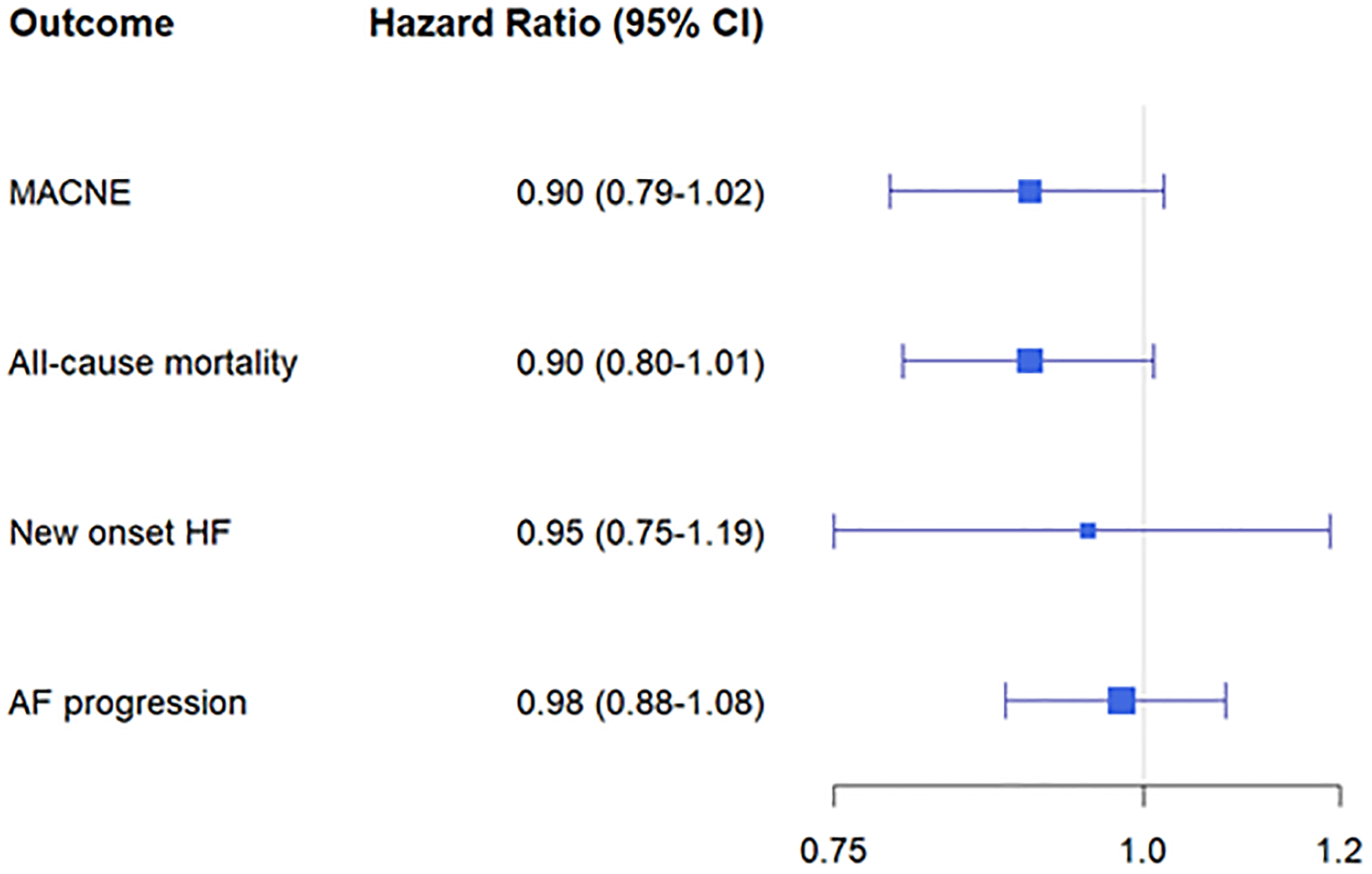
Association between use of “all eligible” GDT and clinical outcomes. Abbreviations AF = atrial fibrillation; HF = congestive heart failure; CV = cardiovascular; MACNE = major adverse cardiac/neurovascular events.
Comorbidity Specific GDT Use and Clinical Outcomes
In the study cohort, there were 6,990 patients with CAD, 6,294 patients with DM, 6,001 patients with HF, 14,601 with HL, 18,474 patients with HTN, 2,358 patients with PVD, and 4,045 patients with OSA. Use of each GDT by comorbidity is shown in Table II and Figure 2. Among CAD patients, usage was highest for antiplatelet agents (75.0%), beta blockers (73.5%), and statins (73.1%) and lowest for ACEi/ARB (63.7%). Use of all eligible CAD related GDT was substantially lower than any individual agent (45.0%). Similarly, among diabetic patients, use of ACEi/ARBs and statins were relatively high (60.7% and 65.9% respectively) compared to the number of patients on all eligible DM related GDT (43.1%). For patients with HF, there was high use of beta blockers (76.4%) and ACEi/ARBs (65.2%), lower for ICDs (45.3%) and lowest for aldosterone antagonists (16.4%). Use of all eligible HF related GDT was 47.0%. HL, HTN, PVD and OSA all had single therapies considered as GDT in this analysis. Most patients eligible for these therapies received them; 75.6% of hyperlipidemia patients and 67.3% of PVD were on a statin, 54.1% of hypertensive patients were on an antihypertensive, 57.7% of OSA patients used CPAP. Of patients eligible for beta blocker by any indication, 73.5% were on treatment. Similarly, 62.2% of patients eligible for ACEi/ARB and 67.0% of patients eligible for statins by any indication were on treatment.
Table II:
GDT use by comorbidity. Number and percent of patients receiving the components of GDT for each comorbidity.
| GDT use by comorbidity | Eligible | On Treatment | % On Treatment |
|---|---|---|---|
| CAD | |||
| Antiplatelet agent | 993 | 745 | 75.0% |
| Beta blocker | 6990 | 5137 | 73.5% |
| ACEi/ARB | 3447 | 2196 | 63.7% |
| Statin | 6990 | 5109 | 73.1% |
| Taking all eligible therapies | 6990 | 3145 | 45.0% |
| Diabetes mellitus | |||
| ACE/ARB | 6294 | 3818 | 60.7% |
| Statin | 6294 | 4150 | 65.9% |
| Taking all eligible therapies | 6294 | 2711 | 43.1% |
| Heart failure | |||
| Beta blocker | 6001 | 4584 | 76.4% |
| ACEi/ARB | 3509 | 2288 | 65.2% |
| Aldosterone antagonist | 1142 | 187 | 16.4% |
| ICD | 1119 | 507 | 45.3% |
| Taking all eligible therapies | 6001 | 2822 | 47.0% |
| Hyperlipidemia | |||
| Statin | 9282 | 7020 | 75.6% |
| Hypertension | |||
| Antihypertensive | 18474 | 9991 | 54.1% |
| PVD | |||
| Statin | 2358 | 1587 | 67.3% |
| Obstructive sleep apnea | |||
| CPAP | 4045 | 2333 | 57.7% |
ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta blocker; CAD = coronary artery disease; HF = heart failure; CPAP = continuous positive airway pressure; ICD = implantable cardioverter defibrillator; OSA = obstructive sleep apnea; PVD = peripheral vascular disease.
Figure 2:
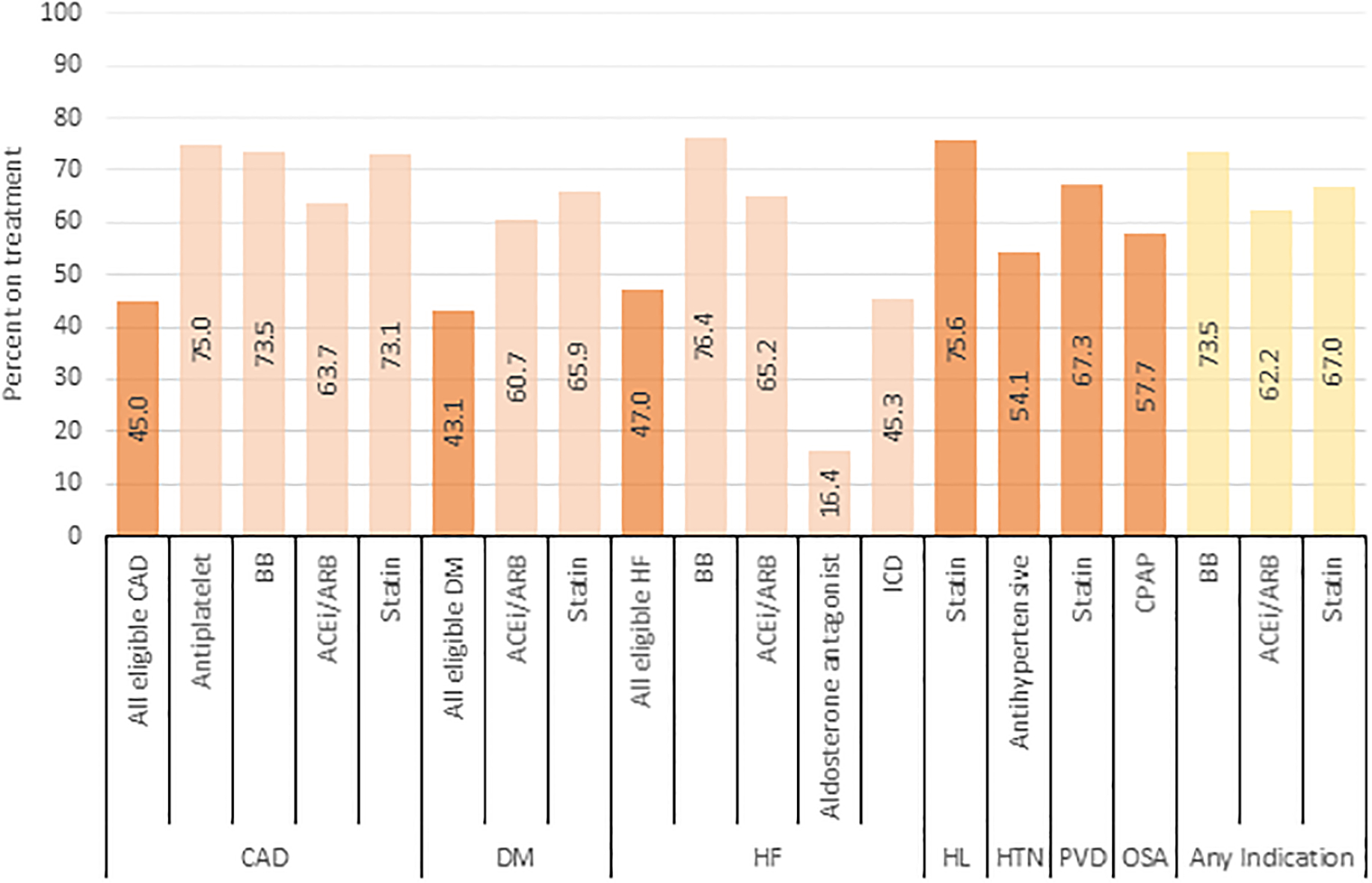
GDT use by comorbidity. Abbreviations: ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB=beta blocker; CAD = coronary artery disease; HF = congestive heart failure; CPAP = continuous positive airway pressure; DM = diabetes mellitus: HL = hyperlipidemia; HTN = hypertension; ICD = implantable cardioverter defibrillator; OSA= obstructive sleep apnea; PVD = peripheral vascular disease.
The association between the use of “all eligible” vs “not all eligible” comorbidity specific GDT and outcomes is shown in Figure 3. None of the comorbidity specific GDT were associated with the primary endpoint (MACNE). For the end point of all-cause mortality, use of all eligible HF related GDT was associated with a lower hazard of mortality (HR 0.77 [0.67–0.89], p<0.001). Use of all CAD related GDT was associated with a higher odds of AF progression (OR 1.17 [1.01–1.34]). Use of CPAP for OSA was associated with a lower odds of AF progression (OR 0.75 [0.62–0.90]). Use of all GDT for DM, HL HTN and PVD was not associated with any of the outcomes.
Figure 3:
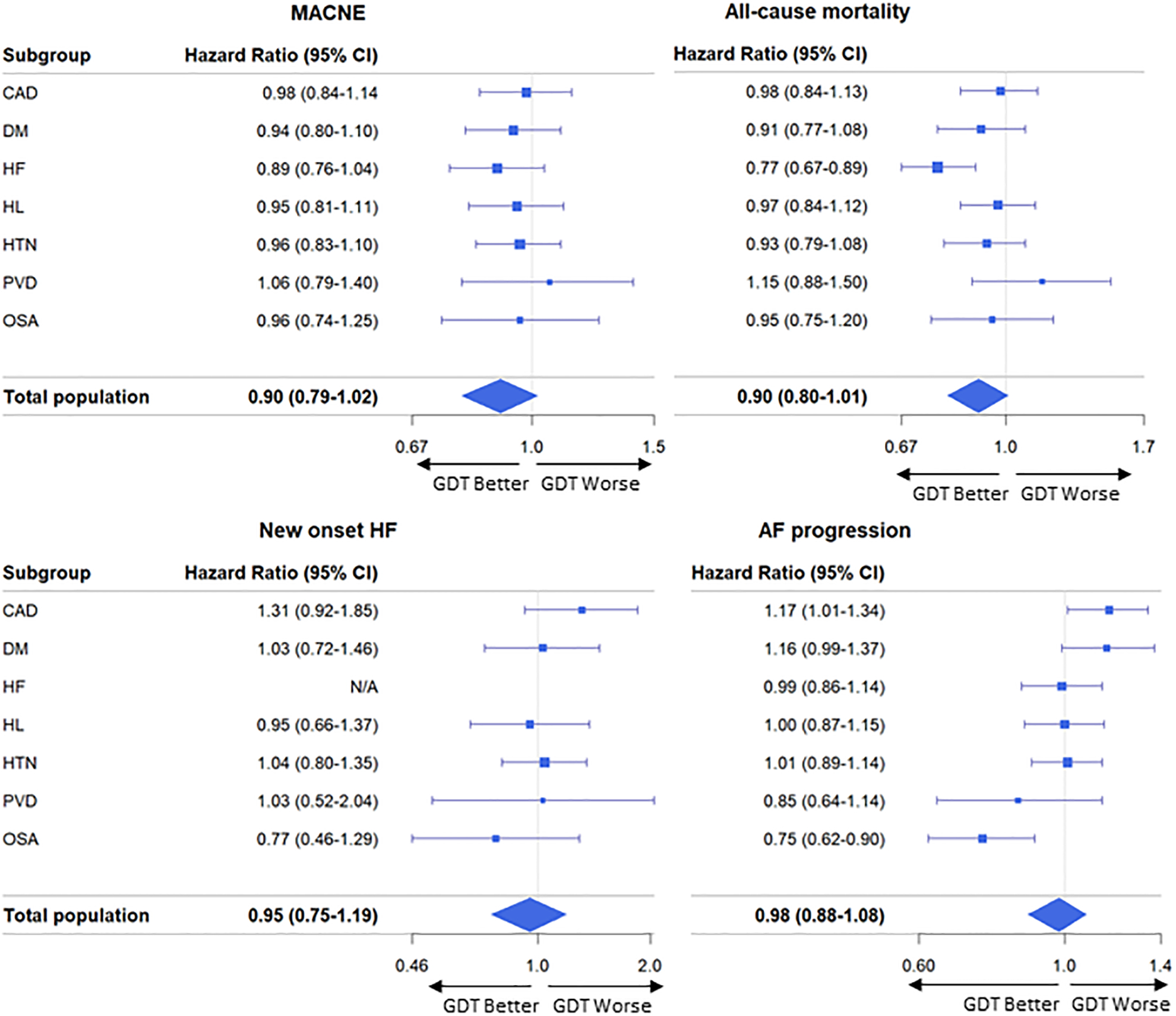
Comorbidity-specific GDT use and MACNE (upper left): all-cause mortality (upper right): new onset HF (lower left), and AF progression (lower right). Abbreviations: MACNE = Major adverse cariac/neurovascular events; CI= confidence interval; CAD = coronary artery disease; DM = diabetes mellitus; HF = heart failure; HL = hyperlipidemia; HTN = hypertension; PVD = peripheral vascular disease; OSA = obstructive sleep apnea; GDT = guideline-directed therapies.
Site level analyses
A total of 322 sites were included in this analysis including 199 cardiology practices, 62 internal medicine/primary care practices, 60 electrophysiology practices and 1 neurology practice. The mean %GDT at the site level was 59.9 ± 9.5% and was similar across site types (60.1% for cardiology, 56.7% for internal medicine/primary care, 62.2% for electrophysiology, and 67.0% for neurology). The association between site level %GDT use and outcomes is shown in the Supplemental Table. There was a non-linear relationship between site level %GDT use and outcomes so a linear spline was used and results were derived separately for sites with %GDT above and below 60% (the approximate level where risk changed, which also corresponds with the mean value). At low utilizing sites (sites with %GDT below the mean), increasing %GDT use was associated with a higher hazard of MACNE (adjusted HR 1.37, 95% confidence interval [CI] 1.15–1.64 per 10% increase), and new onset HF (adjusted HR 1.71 [1.14–2.58]). There were no statistically significant relationships between increasing %GDT and clinical outcomes at high utilizing sites (sites with %GDT above the mean); however, there was a trend towards lower hazards of all-cause mortality (HR 0.89 [0.77–1.03] per 10% increase) with higher %GDT use.
Discussion
In this analysis of a large nationwide registry of patients with atrial fibrillation, receiving all eligible GDT was infrequently achieved. Only 33% of patients received all the GDT for which they were eligible. Patients who received “all eligible” GDT were more often white and had fewer of the evaluated comorbidities. Comorbidity specific GDT use was highest for patients with HL (75.6% of patients appropriately treated with a statin) and similar to rates reported in other outpatient registries.21, 22 Comorbidity specific GDT use was lowest for patients with DM (43.1% of patients on all eligible diabetic therapies), but the rates of statin and ACEi/ARB use were similar to large registry studies.23, 24 When examining individual comorbidities, use of HF related GDT was associated with a 23% lower hazard of all-cause mortality and use of CPAP in OSA patients was associated with a 25% lower odds of AF progression. Use of CAD related GDT was associated with a higher hazard of AF progression.
Concurrent comorbidities and risk factors are common in AF and contribute to the risk of adverse outcomes associated with AF.25, 26 Prior work, such as the ARREST-AF Cohort Study,10 has demonstrated that aggressive management of risk factors such as blood pressure, weight, cholesterol, glycemic control, sleep apnea and use of alcohol and/or tobacco can result in improved AF control and less symptom burden than standard care. Yet, two thirds of the AF patients in this study were not receiving all the GDT for which they were eligible. We also found that a lower proportion of African American patients received “all eligible” GDT compared to whites. This may be due to an overall higher burden of disease (patients in the “all eligible” GDT group had fewer of the comorbidities evaluated) or a manifestation of racial disparities in cardiovascular care.27 This highlights the importance of quality improvement efforts like Get With The Guidelines- AFIB which aim to assist hospitals in providing up to date evidence based treatment for AF patients.28
The relationship between GDT use and clinical outcomes is influenced both by the type of patients who received GDT as well as the direct effect of GDT on outcomes. While patients receiving “all eligible” GDT had a trend towards lower hazards of MACNE or all-cause mortality, we also found that these patients had fewer of the evaluated comorbidities than those on “not all eligible” therapies. The association between GDT use and outcomes is likely influenced in part by confounding if the lower event rates among patients on “all eligible” GDT was reflective of their lower overall disease burden rather than an effect of the therapies themselves. To explore this further, we evaluated the use of GDT at the site level and found that at low utilizing sites (sites where GDT was used in ≤60% of situations in which it was indicated), GDT use was associated with higher hazard of MACNE, major bleeding, all-cause and CV hospitalization as well as new onset HF. It is possible that at these sites, the studied therapies were allocated only to patients with high disease severity and thus GDT use was associated with higher rates of adverse outcomes. In high utilizing sites, where GDT use was likely more uniformly used across patient risk groups, there was a trend towards lower all-cause mortality and bleeding hospitalizations. The consistency of the relationship across analyses suggests a more direct relationship between GDT use and all-cause mortality; however any causal relationship cannot be inferred from this observational analysis.
The comorbidity specific analyses provide a more detailed look into the drivers behind the relationships between GDT use and clinical outcomes. Use of HF related GDT was associated with 23% lower hazards of all-cause mortality. This is consistent with previously published estimates of the reduction in mortality risk attributable to heart failure therapies ranging from 17% for ACEi/ARB to 34% for beta blockers.29 Despite the aggregate evidence for the benefit of GDT in HF, it is notable that our data estimate that for patients with HF and AF, 24% of eligible patients are not on a BB, 35% of eligible patients are not on an ACEi or ARB, and 55% of eligible patients do not have an ICD. These rates are similar to the general HF population in whom underuse of GDT and device therapy among eligible patients has been well documented.30, 31 We also observed that CPAP use among OSA patients was associated with a lower odds of AF progression, which is consistent with prior analyses.32, 33
The association of CAD related GDT use with AF progression is less straightforward. CAD related GDT included beta blocker, statin and in some patients ACEi/ARB and an antiplatelet agent. Many of these therapies were components of GDT for other evaluated comorbidities (beta blockers and ACEi/ARB were components of HF related GDT and statin use was considered HL GDT) none of which were associated with AF progression. While this may be due to a unique relationship between these medications and AF progression in the setting of CAD, the association may also be due to chance.
The results of this study highlight a need for improved implementation of GDT for AF patients. An integrated management approach to atrial fibrillation that incorporates nurse-based, physician supervised clinic model has been shown to be cost-effective and may reduce cardiovascular morbidity and mortality.34, 35 This approach may provide the ideal platform for optimizing both direct AF care as well as improve the implementation of all eligible GDT for comorbidities in AF patients both of which may improve clinical outcomes.
Limitations:
This study included patients who were part of a voluntary registry and may not be fully representative of all AF patients or patterns of care in the US. Not all guideline recommended therapies were measured in this registry (including use of hydralazine/nitrates, angiotensin receptor-neprilysin inhibitors, or cardiac resynchronization therapy) nor were non-pharmacologic/device based interventions such as smoking cessation, diet and exercise evaluated. ACC/AHA Guidelines for the management of heart failure were updated in 2013 during the study period and expanded the class I recommendation for aldosterone antagonists in HF to include patients with NYHA class II symptoms.36 We chose to consider patients with HF and NYHA class II-IV symptoms as eligible for aldosterone antagonists as the pivotal trial prompting this expansion (EMPHASIS-HF)37 was presented in 2010 and published in 2011 near the beginning of the study period, which may have overestimated the number of patients eligible for aldosterone antagonists based on published guidelines. CPAP use for OSA was included as a component of GDT as part of the hypothesis that evidence-based therapies may reduce AF progression; however, there is no robust evidence that CPAP use for OSA improves cardiovascular outcomes and thus its inclusions as a component of GDT for the MACNE, mortality and new-onset heart failure endpoints may have biased those analyses towards the null.38 As mentioned above, patients “not all eligible” category were older with greater comorbidity. Our analyses were stratified on both the type and number of comorbid conditions to compare patients with similar risk profiles and attempt to isolate the effect of GDT use on outcomes. However, this statistical technique is unable to account for unmeasured confounding factors nor the severity of each of these conditions. Thus some component of the trend towards worse outcomes in the “undertreated” patients may reflect the greater overall illness burden in this group. While medication use was documented, adherence and dosing information was not available. Additionally, information about contraindications or intolerances to the therapies evaluated in this study was not available. Comorbidity presence and medication use was assessed at the time of registry enrollment; thus, the development of comorbid conditions or starting/stopping of GDT after registry enrollment are not accounted for in the presented models and would have biased the result towards the null. Registry patients may be more likely to receive GDT than the general population due to their more frequent contact with the health system so estimates of GDT use in the general population may be lower than reported here. Finally, the retrospective, observational nature of this study limits any causal inference and may be impacted by residual, unmeasured confounding.
Conclusions
GDT for treatment of comorbidities in AF patients in routine practice is under-utilized. The association between receiving all eligible GDT use and clinical outcomes was strongest in those with HF and OSA, where use of GDT was associated with lower all-cause mortality and less AF progression, respectively. Future efforts to improve the comprehensive care of AF patients including management of their comorbid conditions may improve clinical outcomes.
Supplementary Material
Supplemental Table: Association between %GDT use and clinical outcomes at the site-level. The adjusted and unadjusted hazard/odds ratios between %GDT use and clinical outcomes are shown. Low and high utilizing sites refer to sites that have a percent GDT use above or below the mean value for all sites (60%). HR and OR refer to risk per 10% increase in percent GDT use at the site level.
Sources of Funding:
Primary funding was provided by Ortho-McNeil Janssen Scientific Affairs, LLC.
Disclosures:
ZL is supported in part by an NIH T32 training grant (#5T32HL069749). PS has no relationships to disclose. LAA received consulting fees from Janssen, ACI Clinical, Amgen, and Boston Scientific and grant funding from AHA, PCORI, and NIH. RB has no relationships to disclose. PSC discloses consulting for Optum Rx. MDE disclosers consulting/advisory board for Boehringer Ingelheim, Diachi Sanko, Pfizer, Bristol Myers Squibb, and Janssen Scientific Affairs. GCF discloses consulting for Abbott, Amgen, Bayer, Janssen, Medtronic, and Novartis. JVF serves as a consultant on the advisory board for Janssen Scientific, Medtronic, Biosense Webster, Boston Scientific and receives salary support from the American College of Cardiology. BJG discloses membership of a data safety monitoring board for Mount Sinai St Lukes, Boston Scientific Corporation, Teva Pharmaceutical Industries, St. Jude Medical, Janssen Research & Development, Baxter Healthcare Corporation, and Cardiovascular Research Foundation as well as consulting/advisory board for Janssen Scientific Affairs, Cipla Limited, Armetheon Inc and Medtronic. KWF’s financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. GVN discloses research support from Janssen and consults for Janssen, Omeicos, Acesion, and Milestone. KP has no relationships to disclose. JAR has been an investigator and consultant for Medtronic, Janssen, Gilead, and Sanofi; a consultant for Portola, Acesion, InCardia Therapeutics; and a member of speaker’s bureaus for Janssen and Boehringer Ingelheim. DES discloses consulting/advisory board for Boehringer Ingelheim, Johnson and Johnson, Merck, Pfizer and Bristol-Myers Squibb and conducts contracted research with Bristol-Myers Squibb and Boehringer Ingelheim. BAS is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156; receives research support from Boston Scientific and Janssen; consulting to Janssen and Merit Medical; speaking for NACCME (funded by Sanofi). LET receives grants from Jansen and BMS. EDP receives grants from Janssen Pharmaceuticals and Eli Lilly, discloses consulting for Janssen Pharmaceuticals and Boehringer Ingelheim. JPP receives grants for clinical research from Abbott, American Heart Association, Boston Scientific, Gilead, Janssen Pharmaceuticals, and the NHLBI and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, Johnson & Johnson, LivaNova, Medtronic, Milestone, Oliver Wyman Health, Sanofi, Philips, and Up-to-Date.
References:
- 1.Alkhouli M, Alqahtani F, Aljohani S, Alvi M and Holmes DR. Burden of Atrial Fibrillation-Associated Ischemic Stroke in the United States. JACC Clinical electrophysiology. 2018;4:618–625. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P and McGavigan AD. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. European heart journal. 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 3.Bassand JP, Accetta G, Al Mahmeed W, Corbalan R, Eikelboom J, Fitzmaurice DA, Fox KAA, Gao H, Goldhaber SZ, Goto S, Haas S, Kayani G, Pieper K, Turpie AGG, van Eickels M, Verheugt FWA and Kakkar AK. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation. PloS one. 2018;13:e0191592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayor M, Enserro DM, Xanthakis V, Larson MG, Benjamin EJ, Aragam J, Mitchell GF and Vasan RS. Comorbidities and Cardiometabolic Disease: Relationship With Longitudinal Changes in Diastolic Function. JACC Heart failure. 2018;6:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmqvist F, Kim S, Steinberg BA, Reiffel JA, Mahaffey KW, Gersh BJ, Fonarow GC, Naccarelli GV, Chang P, Freeman JV, Kowey PR, Thomas L, Peterson ED and Piccini JP. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart (British Cardiac Society). 2015;101:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappone C, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Sacchi S, Mazzone P, Paglino G, Gulletta S, Sala S and Santinelli V. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart rhythm. 2008;5:1501–7. [DOI] [PubMed] [Google Scholar]
- 7.Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD and Filippatos GS. Physicians’ guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. European journal of heart failure. 2017;19:1414–1423. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen JN, Chong A and Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Jama. 2007;297:177–86. [DOI] [PubMed] [Google Scholar]
- 9.Gaede P, Lund-Andersen H, Parving HH and Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. The New England journal of medicine. 2008;358:580–91. [DOI] [PubMed] [Google Scholar]
- 10.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP and Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. Journal of the American College of Cardiology. 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 11.Hess PL, Kim S, Piccini JP, Allen LA, Ansell JE, Chang P, Freeman JV, Gersh BJ, Kowey PR, Mahaffey KW, Thomas L, Peterson ED and Fonarow GC. Use of evidence-based cardiac prevention therapy among outpatients with atrial fibrillation. The American journal of medicine. 2013;126:625–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM and Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. American heart journal. 2011;162:606–612.e1. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg BA, Blanco RG, Ollis D, Kim S, Holmes DN, Kowey PR, Fonarow GC, Ansell J, Gersh B, Go AS, Hylek E, Mahaffey KW, Thomas L, Chang P, Peterson ED and Piccini JP. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II: rationale and design of the ORBIT-AF II registry. American heart journal. 2014;168:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr., Smith SC Jr., Spertus JA and Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:2199–2269.29146533 [Google Scholar]
- 16.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr., Watson K and Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of the American College of Cardiology. 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]
- 18.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D and Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 19.Epstein LJ, Kristo D, Strollo PJ Jr., Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM and Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 20.Enders CK. Applied missing data analysis: Guilford press; 2010. [Google Scholar]
- 21.Pokharel Y, Tang F, Jones PG, Nambi V, Bittner VA, Hira RS, Nasir K, Chan PS, Maddox TM and Oetgen WJ. Adoption of the 2013 American College of Cardiology/American Heart Association cholesterol management guideline in cardiology practices nationwide. JAMA cardiology. 2017;2:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar AM, Wang TY, Li S, Robinson JG, Goldberg AC, Virani S, Roger VL, Wilson PW, Elassal J and Lee LV. Lipid management in contemporary community practice: results from the Provider Assessment of Lipid Management (PALM) Registry. American heart journal. 2017;193:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borden WB, Maddox TM, Tang F, Rumsfeld JS, Oetgen WJ, Mullen JB, Spinler SA, Peterson ED and Masoudi FA. Impact of the 2014 Expert Panel Recommendations for Management of High Blood Pressure on Contemporary Cardiovascular Practice: Insights From the NCDR PINNACLE Registry. Journal of the American College of Cardiology. 2014;64:2196–2203. [DOI] [PubMed] [Google Scholar]
- 24.Pokharel Y, Gosch K, Nambi V, Chan PS, Kosiborod M, Oetgen WJ, Spertus JA, Ballantyne CM, Petersen LA and Virani SS. Practice-Level Variation in Statin Use Among Patients With Diabetes: Insights From the PINNACLE Registry. Journal of the American College of Cardiology. 2016;68:1368–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lip GYH, Nieuwlaat R, Pisters R, Lane DA and Crijns HJGM. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach: The Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 26.Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE and Albert CM. Risk of Death and Cardiovascular Events in Initially Healthy Women With New-Onset Atrial Fibrillation. Jama. 2011;305:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewey J and Choudhry NK. The current state of ethnic and racial disparities in cardiovascular care: lessons from the past and opportunities for the future. Current cardiology reports. 2014;16:530. [DOI] [PubMed] [Google Scholar]
- 28.Lewis WR, Piccini JP, Turakhia MP, Curtis AB, Fang M, Suter RE, Page RL 2nd and Fonarow GC. Get With The Guidelines AFIB: novel quality improvement registry for hospitalized patients with atrial fibrillation. Circulation Cardiovascular quality and outcomes. 2014;7:770–7. [DOI] [PubMed] [Google Scholar]
- 29.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA and Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. American heart journal. 2011;161:1024–30.e3. [DOI] [PubMed] [Google Scholar]
- 30.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C and Peterson ED. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF and Fonarow GC. Medical Therapy for Heart Failure With Reduced Ejection Fraction. Journal of the American College of Cardiology. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 32.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, Holmes DN, Peterson ED, Piccini JP and Gersh BJ. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). American heart journal. 2015;169:647–654.e2. [DOI] [PubMed] [Google Scholar]
- 33.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS and Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. [DOI] [PubMed] [Google Scholar]
- 34.Hendriks J, Tomini F, van Asselt T, Crijns H and Vrijhoef H. Cost-effectiveness of a specialized atrial fibrillation clinic vs. usual care in patients with atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15:1128–35. [DOI] [PubMed] [Google Scholar]
- 35.Carter L, Gardner M, Magee K, Fearon A, Morgulis I, Doucette S, Sapp JL, Gray C, Abdelwahab A and Parkash R. An Integrated Management Approach to Atrial Fibrillation. Journal of the American Heart Association. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 37.Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ and Pitt B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. New England Journal of Medicine. 2010;364:11–21.21073363 [Google Scholar]
- 38.Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, Elgendy A, Mahmoud AN, Mentias A, Barakat A and Lal C. Meta-Analysis of Cardiovascular Outcomes With Continuous Positive Airway Pressure Therapy in Patients With Obstructive Sleep Apnea. The American journal of cardiology. 2017;120:693–699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table: Association between %GDT use and clinical outcomes at the site-level. The adjusted and unadjusted hazard/odds ratios between %GDT use and clinical outcomes are shown. Low and high utilizing sites refer to sites that have a percent GDT use above or below the mean value for all sites (60%). HR and OR refer to risk per 10% increase in percent GDT use at the site level.


