Abstract
Some respiratory viruses have long been known to cause neurological involvement. A novel coronavirus, leading to severe acute respiratory syndrome, also called coronavirus disease 19 (COVID-19), seems to be a new member of neuroinvasive viruses. While severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) keeps on spreading around the world rapidly, reports about the neurological manifestations associated with SARS-CoV-2, increases day by day. It is reported that a variety of symptoms and syndromes such as headache, dizziness, confusion, ataxia, epilepsy, ischemic stroke, neuropathic pain and myopathy are common especially in more severe COVID-19 patients. It is also suggested that the development of neurological complications is strongly associated with a poor outcome. On the other hand, hyposmia can be the unique symptom in COVID-19 carriers and this can serve as a marker for identifying the otherwise asymptomatically infected patients. It is thought that SARS-CoV-2 may cause neurological symptoms through direct or indirect mechanisms. Nevertheless, neuroinvasion capability of SARS-CoV2 is confirmed by the presence of the virus, in the cerebrospinal fluid of a COVID-19 patient with encephalitis, and this is proven by gene sequencing. In conclusion, during the COVID-19 pandemic, it is crucial to be aware of the possible neurological complications of the disease. Therefore, in this review, we aimed to report neurological manifestations associated with SARS-CoV-2 and possible underlying pathophysiological mechanisms. Due to the high homology of SARS-CoV-2 with other human coronaviruses such as SARS-CoV or Middle East Respiratory Syndrome (MERS)-CoV, reviewing the neurological involvement also associated with these coronaviruses will provide an idea about the long-term complications of COVID-19.
Keywords: Coronavirus disease 19, COVID-19, acute respiratory distress syndrome, SARS-CoV-2, neurological symptom
INTRODUCTION
The outbreak of acute severe respiratory failure syndrome (SARS) coronavirus 2 (SARS-CoV-2), or more commonly known as coronavirus disease (COVID-19), is rapidly spreading worldwide since December 2019. More than 1.5 million people in a total of 184 countries have been confirmed to be infected. The infection is shown to affect not only the respiratory or cardiovascular system but also many systems and organs, including the central nervous system (CNS) and peripheral nervous system (PNS). Nowadays, while all clinical symptoms and signs of the COVID-19 are being determined, the diversity of neurological involvement draws the attention of neurologists. A potential of neurovirulence of the virus seems to exist. The fact that the single symptom of decreased sense of smell in many COVID-19 carriers even suggests that this symptom can be considered as a disease marker. It is recommended by some authors to investigate the patient at this stage for the presence of CNS involvement (1).
The epidemic is increasing in our country. The reports from long-term strugglers, such as China and Italy, are guiding us in understanding the disease and recognizing the possible neurological signs and symptoms that can be related to COVID-19. Also, the data from other highly homologous human coronaviruses such as SARS-CoV or Middle East Respiratory Syndrome (MERS)-CoV may allow us to have an idea about the long-term complications of the pandemic.
In this review, we aim to present the definite and likely neurological symptoms and neuropathogenesis of COVID-19 with the guidance of recently published data.
SARS-CoV-2 and Possible Neuro-invasion Mechanisms
SARS-CoV-2 is a novel positive-sense, single-stranded RNA virus and is located in the genus Betacoronavirus, known for its neuro-invasion potential (2). The virus can enter the cell by binding to angiotensin-converting enzyme 2 (ACE2), which acts as a receptor (3). ACE-2 is present in many tissues in the human body, including the nervous system and skeletal muscle (4–6). The connection of this receptor with some drugs used in the treatment of hypertension also raises question marks. However, given the potential benefits, it is not currently recommended by cardiologists to discontinue this group of drugs in patients with controlled hypertension (7).
It is thought that SARS-CoV-2 can cause neurological symptoms through direct and indirect mechanisms (8). The first of the possible pathological mechanisms is the direct effect of the virus to the CNS as shown in SARS-CoV and MERS-CoV (9–13). Invasion of respiratory viruses into the CNS can occur in two ways: through bloodstream or via retrograde neuronal route. The virus in the bloodstream first infects the endothelial cells in the blood-brain barrier (BBB) or the epithelial cells in the blood-cerebrospinal fluid (CSF) barrier in the choroid plexus or reaches the CNS through leukocytes. Relatively slow blood flow in the brain microcirculation probably increases the interaction of the virus with the ACE2 in the capillary endothelium, and thus facilitates the virus to attach to ACE2 and enter the CNS after capillary endothelial damage (14). As a second route, it is assumed that the virus can reach the CNS via retrograde axonal transport through several cranial (such as olfactory, trigeminal, glossopharyngeal and vagus) or the peripheral nerves (15, 16).
Myositis which is supposed to be another form of neurological involvement of the coronavirus, might be due to the direct invasion of the virus into the muscle tissue through ACE2. Although the virus binds to the same receptor, in post-mortem muscle tissue studies conducted in patients with SARS-CoV, the virus has not been isolated by in-situ hybridization or viral culture. Myopathy findings might be related to immune damage mechanism due to cytokine release (17).
Neurological symptoms may also occur with indirect mechanisms as a result of systemic adverse outcomes (multiple organ failure and/or disseminated intravascular coagulation and sepsis) due to increased immune response to the coronavirus infection or as a result of respiratory failure due to pathological changes in the lungs (8).
SARS-CoV-2 Related Neurological Involvements Reported up to Date
There is only one study in the literature regarding this subject, in addition to reviews, letters to the editor, and case reports. Neurological symptoms reported to date in COVID-19 patients and their distribution are shown in Figure 1.
Figure 1.
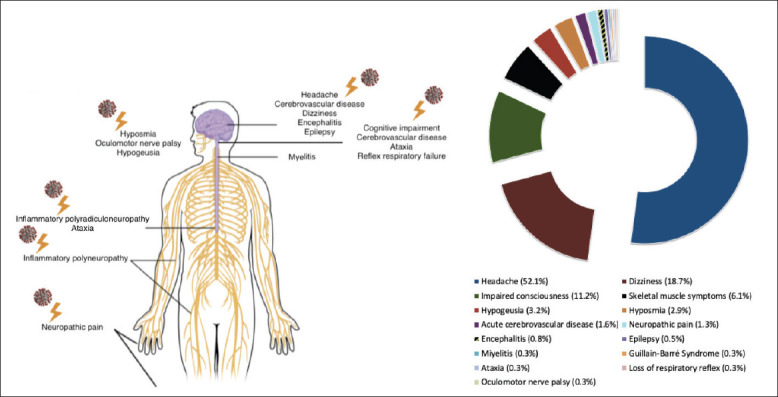
The neurological symptoms reported to date in COVID-19 patients and their distribution (%).
In order to investigate the neurological findings of the virus, the medical data of 214 patients who were hospitalized with the diagnosis of SARS-CoV-2 related SARS between January 16, 2020, and February 19, 2020, at the Union Hospital of Huazhong University of Science and Technology in Wuhan, China, where the outbreak first appeared, were analyzed retrospectively. Neurological findings were detected in 78 (36.4%) patients. In this study, neurological symptoms were classified in three categories:
CNS symptoms or signs (headache, dizziness, impaired consciousness, ataxia, acute cerebrovascular disorder or epilepsy) were detected in 53 (24.8%) patients,
Neuropathy symptoms (hypogeusia, hyposmia or neuropathic pain) were detected in 19 (8.9%) patients,
Skeletal muscle symptoms were detected in 23 (10.7%) patients.
Dizziness (36; 16.8%) and headache (28; 13.1%) were the most frequent symptoms in patients with CNS involvement. On the other hand, hypogeusia (12; 5,6%) and hyposmia (11; 5.1%) were the most common symptoms related to PNS involvement. It was noted that severe patients with comorbid diseases like hypertension were more likely to have nervous system involvement, without typical symptoms, such as fever and coughing. In these patients, prominent inflammatory response with lower lymphocyte counts and increased C-reaction protein levels were found. Moreover, D-dimer levels were higher in this group and were thought to be the reason for the cerebrovascular diseases frequency (8). It was also observed that muscle involvement could be very severe and may result in rhabdomyolysis (CK levels: 525-12216 U/L). These patients frequently had accompanying organ dysfunction, such as liver or kidney failure. Since the hospitalization of the patients continued during this study, the effects of neurological findings on disease outcomes could not be evaluated (8).
In a retrospective study, examining the clinical features of 799 cases with moderate-severe COVID-19 at the Tongji Hospital in Wuhan Province, China, impaired consciousness during hospital admission was 22% in the deceased cases, and only, 1% in the recovered cases. It was observed that the frequency of headache (12% vs. 10%) and dizziness (10% vs. 7%) was similar between the deceased and the recovered cases on admission. Although the neurological complications were not included, the development of secondary neurological symptoms was reported to be associated with poor outcome in this report. It was also noted that the levels of D-dimer and pro-inflammatory cytokines such as interleukin (IL) -2, IL-6, IL-8, IL-10, and tumor necrosis factor-α were significantly higher in deceased cases (18). IL-6 probably causes the initiation and the development of inflammatory damage in the brain and microvascular endothelial cells in ischemic strokes, and the release of this cytokine also disrupts cerebrovascular autoregulation (19). It is well known that the immune system activation and related to inflammatory processes that develop at the post-infectious stage, play an important role in the development of both atherothrombotic and cardioembolic ischemic strokes. Vascular damage can occur directly due to endothelial dysfunction during the inflammation process, and autoimmune processes may cause cardiac dysfunction. It was reported that coronary artery disease, myocardial infarction, dilated cardiomyopathy, and atrial fibrillation may develop due to immune mechanisms, and this entire pathological process which ends up with a cardioembolic stroke is defined as “cardiopathy” (20). COVID-19 infection was also observed to cause severe cardiovascular system involvement due to acute myocardial damage and endothelial dysfunction (21). Along with an increase in cytokine levels, widespread microvascular thrombosis with prothrombotic activation was also reported, and consequently these patients had increased D-dimer levels. In the new guideline of AHA/ASA, it was emphasized that 5.9% of the patients with COVID-19 had a stroke on average ten days after the onset of symptoms (22). Depending on first observations, cerebrovascular diseases were frequently seen in the group with poor prognosis (23).
In another report from China, reflex respiratory function in a 24-year-old medical school student from Wuhan University was lost, and she constantly needed to be kept awake at the intensive care unit to survive. Based on this observation and previous studies with experimental animal models, it was emphasized that COVID-19 can also cause severe respiratory symptoms by affecting the CNS, especially the cardiorespiratory center of the brainstem. Nevertheless, this should be assessed cautiously since the obsevation is based on a single case and controversial data. It was also stated that the virus could protect itself from the immune response inside the neurons and may not be completely cleared from the body even after the signs of acute infection have ended, similar to Herpesviruses that cause latent infection (24). However, the data is still insufficient to conclude that SARS-CoV-2 has a neurotropic feature like Herpesviruses.
The gene sequencing of the virus from the CSF of a 56-year-old patient in Beijing hospital, suggested that the virus infects the CNS. Along with respiratory symptoms, this patient also developed altered consciousness while CSF examination confirmed the diagnosis of viral encephalitis. After the treatment of encephalitis, the patient improved and finally was discharged from the hospital (25). The increasing number of case reports and systematic clinical studies will of course improve our understanding of CNS involvement of COVID-19.
Thirty-year-old Iranian female patient without any history of previous seizures or family history presented with new-onset seizures. COVID-19 was accused to be the etiology of her seizures although brain magnetic resonance imaging (MRI) and CSF examination including microscopy, protein, glucose, and SARS-CoV-2 testing by polymerase chain reaction (PCR) turned out to be normal. Authors claimed that direct invasion of brain tissue and production of toxins by the virus or production of inflammatory mediators by the brain might be the possible mechanisms for the etiology of seizures (26). Future studies are needed to clarify the relation between COVID-19 and epilepsy. As a reference center in neurology, according to our experience, an increased tendency of status epilepticus exists, even in patients with epilepsy associated with different etiologies such as high-grade glial tumors during the pandemic period. Clinical research is now planned to disclose this relationship.
The first case with acute hemorrhagic necrotizing encephalopathy associated with COVID-19 is published on 31st of March, 2020. A 50-year-old female airline worker presented with confusion and headache (27). Her MRI demonstrated widespread lesions affecting bilateral mesial temporal lobes, subinsular region and thalamus. The clinical prognosis is unknown in this patient. Previous studies identify acute necrotizing encephalopathy as a rare complication associated with other respiratory viruses, probably caused by loss of blood-brain barrier integrity due to intracranial cytokine storm. Additionally, the isolation of the virus from the neuronal tissue was absent in these cases (28). Following the previous one, a case with meningoencephalitis is published on 4th of April, 2020. In this patient, the nasopharyngeal swab was negative for SARS-CoV2 while PCR analysis of the CSF confirmed the diagnosis. The patient, published by Moriguchi et al, is a 24-year-old male without a history of traveling abroad, developed headache and sore throat on the fifth day of his fatigue and fever. Later, on day 9, he was found unconscious by his relatives and had a generalised tonic-clonic seizure on his way to the hospital. His examination on admission, revealed neck stiffness and 6/15 points according to the Glasgow Coma Scale. Ground-glass opacities were present at his chest computerized tomography (CT). Cranial MRI showed hyperintensities in the right mesial temporal lobe and hippocampus in fluid-attenuated inversion recovery (FLAIR) images with slight hippocampal atrophy and no definite dural enhancement existed during contrast-enhanced imaging. The patient was then intubated and the outcome was unknown when the article was published. Interestingly, nasopharyngeal swabs were negative for the virus in this patient (29).
Zhao et al have published the first case with post-infectious myelitis associated with COVID-19. This patient was a 66-year-old male presented with fever and fatigue. After his chest CT revealed patchy changes in both lungs and he was nasopharyngeal-swab-positive, and he was admitted to the hospital with a diagnosis of COVID-19. During follow-up, after an attack of high fever (40°C), he developed flask tetraparesis with incontinence. His neurological examination showed bilateral weakness in the upper (MRC 3/5), and lower limbs (MRC 0/5) and a global sensory loss at T10 level with decreased deep tendon reflexes, with no pathological reflexes. Cranial CT revealed multiple bilateral basal ganglia and paraventricular lacunar infarcts with brain atrophy. Acute myelitis was diagnosed. MRI of the spine and CSF analysis could not be performed. After the treatment with antiviral agents and intravenous immunoglobulin (15 gr/day for 7 days), his neurological symptoms improved parallel with his infectious state and then he was transferred to a rehabilitation clinic (30).
Wei et al, recently published a case report in which they present a 62-year-old patient with diplopia and unilateral ptosis. Neurological examination of the patient revealed unilateral oculomotor nerve palsy and laboratory tests showed elevated CRP levels and sedimentation rates. MRI and MR angiogram were normal without any lesions associated with the clinical picture. Although the patient was diabetic, the authors reported that the diabetic peripheral neuropathy was excluded due to the well controlled blood glucose levels of the patient. The viral respiratory panel was negative. However, the chest CT showed bilateral ground-glass opacities, in accordance with COVID-19. There were no symptoms associated with COVID-19 initially. On day 2, the patient developed respiratory distress with high fever and SARS-CoV-2 was detected in the throat swab sample. The patient died because of respiratory failure at day 12 after admission. Although lumbar puncture was not performed and the virus could not be isolated from CSF, authors claimed that oculomotor nerve palsy was associated with COVID-19 because all possible but etiologies causing this neurological presentation were discarded. The mechanism underlying the involvement of the third cranial nerve was thought to be due to the invasion of the virus, and inflammatory mediators or it could be related to the disruption of the myelin and the axon of the cranial nerve caused by the virus (31). These mechanisms need to be clarified by future research.
While autopsy studies of COVID-19 patients were reported to have severe hypoxia and viremia (32), another study reported edema in the brain tissue (33). These findings suggest that toxic encephalopathy may also play a role in the etiology of confusion, especially in severe cases (34).
As we know, Italy is one the countries having the highest mortality rates due to COVID-19. In Brescia University of Italy, an 18-bed neuro-COVID-19 unit was established due to the high percentage of patients with neurological symptoms and signs, such as stroke, delirium, epileptic seizure, and encephalitis (35). The frequency of ischemic stroke and thrombosis increased dramatically in COVID-19 patients, and in some cases, the virus is thought to affect the coagulation mechanisms because no risk factors existed previously for these patients. They performed tissue plasminogen activator administration and/or mechanical thrombectomy in acute stroke patients positive for COVID-19 if they did not have respiratory involvement. Authors stated that the majority of the doctors dealing with COVID-19 have observed neurological symptoms, and the most common were headache, confusion, taste and smell impairment, myalgia, and prominent weakness.
Zhao et al. reported a 61-year-old woman with a complaint of bilateral lower extremity weakness. She had returned from Wuhan four days ago. Her initial blood tests revealed lymphopenia and thrombocytopenia without any signs or symptoms of an infection. Seven days later, she was diagnosed as Guillain-Barre syndrome (GBS) and started to receive IVIG. One day later, fever and cough developed, while chest imaging and nasopharyngeal swab PCR sampling showed COVID-19 infection (36). Authors stated that, because of a recent Wuhan journey and abnormal lymphocyte and thrombocyte counts on admission, the patient should be assumed to be infected before admission. In this case, GBS is a para-infectious phenomenon secondary to COVID-19 infection. The authors refer to a report of a para-infectious GBS during Zikavirus infection, regarding this case. They also pointed out that another possibility could be the presence of an unknown co-incidental infection leading to GBS, and COVID-19 infection was nosocomial. However, COVID-19 was not detected in the hospital staff who were in charge of this patient. This case report is worth mentioning, as this is the first case to show GBS and COVID-19 association, but more reports are needed to see whether the association is causal or random. Currently, screening the patients with peripheral nervous system involvement symptoms and abnormal blood tests might be useful, even when they do not have a classical COVID-19 presentation.
Another study suggests that, COVID-19 patients with neurological symptoms may not have fever and cough at the beginning (37). Some may develop respiratory failure while some may not, later in the course of the disease. Insidious and mild neurological symptoms can be overlooked, especially in patients presenting with severe systemic findings and respiratory failure who first receive medical intervention for vital functions. The frequency of mild neurological symptoms such as hyposmia or hypogeusia in the home quarantine cases is currently unknown.
Additionally, unpublished reports indicate that the frequency of admission of peripheral facial palsy and even trigeminal neuralgia cases increased relatively during the pandemic period.
Possible Neurological Involvements in COVID-19 Regarding the Other Coronavirus Infections
The first data showing that coronavirus could infect the CNS goes back to 1980 when the virus was detected in the brain tissue at the autopsy of a multiple sclerosis (MS) patient (38). In a study conducted in 2000, the presence of coronavirus in brain autopsies of various neurological diseases was investigated, and the frequency of coronavirus in MS was significantly higher than other diseases (39). The murine coronavirus (M-CoV) that causes epidemics with high mortality, especially in laboratory mice, is genetically similar to the human coronavirus OC43 (HCoV-OC43). CNS involvement findings of both viruses that lead to demyelination are similar (16). Experimentally microglia cells are important in M-CoV infection and the virus replicates faster by decreasing their number. Besides, when inoculated intracranially in mice, the virus can cause meningitis, focal acute encephalitis, and optic neuritis in a short period of time, especially in 3 days (40). The COVID-19 infection data shows us that the virus may cause chronic inflammation and brain damage by activating immune cells in CNS, so an increase in the prevalence of demyelinating diseases can be expected.
Studies and case reports conclude that coronavirus can cause encephalitis, especially in immunosuppressed individuals. Two different publications showed that two immunosuppressed children have developed HCOV-OC43 related fatal encephalitis after upper respiratory complaints, but the presence of the virus could only be shown in the brain tissue not in the CSF (41, 42). In another study published in 2017, 22 (12%) of 183 children were positive for the presence of anti-CoV immunoglobulin M (IgM), who were hospitalized with acute encephalitis findings and respiratory tract infections in China. Inflammatory cytokines and chemokine levels such as IL-6, IL-8, and monocyte chemotactic protein 1 (MCP-1) were found to be significantly higher in the CSF of anti-CoVIgM positive patients, and it was stated that these cytokines might be the cause of brain damage in these patients (43).
Neurological syndromes such as encephalitis, polyneuropathy, and ischemic stroke were reported during the SARS-CoV epidemic during the 2002-2003 period, in which a total of 8098 cases were infected, and 774 of them died. Cases with myopathy and rhabdomyolysis developed either due to the direct damage to the nerve or muscle tissue by the virus or due to critical illness (44). Epileptic seizures and encephalopathy were also reported, genetic material of the virus was detected in CSF samples of these patients (16). Similar to the recent reports in the current COVID-19 pandemic, it was also pointed out that during the SARS epidemic, coronavirus could cause hypercoagulation leading to ischemic stroke, especially in patients with critical illness (45).
In a retrospective study conducted during the MERS-CoV epidemic in Saudi Arabia in 2012, 25.7% of patients developed confusion, and 8.6% had epileptic seizures (46). Additionally, 4 patients had CNS involvement (acute disseminated encephalomyelitis, stroke and encephalitis), and also critical illness polyneuropathy (47). In 2015, during the MERS-CoV pandemic in South Korea, neurological symptoms were reported to be present in 20% of the infected patients. Four patients were published with overlapping neurological manifestations like Bickerstaff encephalitis, GBS and critical illness polyneuropathy after 2-3 weeks of MERS-CoV pneumonia (48).
In a latest report by Dr. Padovani, (46) from Italy, published at the website of “European Academy of Neurology” on 28th of March, 2020, a patient with Bickerstaff cerebellitis was already admitted to the neuro-COVID-19 inpatient clinic of the Brescia University (49). Immune-mediated complications like acute disseminated encephalomyelitis and GBS associated with COVID-19 should be kept in mind.
CONCLUSION
SARS-CoV-2 may have neuro-invasion and neurotrophic ability like other coronaviruses and the presence of neurological symptoms and signs should be thoroughly looked for when evaluating the COVID-19 cases. Isolation of the virus from the cerebral microcirculation, endothelial cells, CSF, nerve and muscle tissue in autopsy examinations is also crucial. Thus, neurological spectrum of the virus and the underlying pathophysiological mechanisms can further be clarified.
Consequently, it should be kept in mind that the patients may have COVID-19 regardless of the presence of respiratory infection symptoms, especially those presenting to the emergency departments or neurology outpatient clinics during the pandemic with the above-mentioned neurological complaints and symptoms. This approach is of great importance in terms of protecting healthcare workers from infection as well as preventing late or misdiagnosis. Investigations of accompanying neurological involvement in patients admitted with severe respiratory tract infection and impaired consciousness are mandatory for correct management.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - BB; Design - ANÖA; Supervision - ANÖA, BS, EE, AÇ, NGŞ, TG, YP, BB; Resource - ANÖA, BS, TG, EE; Materials - ANÖA, BS; Data Collection and/ or Processing - ANÖA, BS, EE, AÇ; Analysis and/or Interpretation - ANÖA, BS, YP, BB; Literature Search - ANÖA, BS, BB; Writing - ANÖA, BM, EE, AÇ, NGŞ, TG, YP, BB; Critical Reviews -ANÖA, BS, EE, AÇ, NGŞ, TG, YP, BB.
Conflicts of interest: There were no conflicts of interest in this study.
Financial Disclosure: No financial support was received for this study.
REFERENCES
- 1.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 Virus Targeting the CNS. Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, Epidemiology, Pathogenesis, and Control of Covid-19. Viruses. 2020;12:E372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA Expression Profiling of ACE 2, a Novel Homologue of Angiotensin Converting Enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 6.Xia H, Lazartigues E. Angiotensin-Converting Enzyme 2 in the Brain:Properties and Future Directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. France: European Society of Cardiology; 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang . [Google Scholar]
- 8.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;2020:1127. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, Lu Y, Wu D, He L, Yao K. The Clinical Pathology of Severe Acute Respiratory Syndrome (SARS):A Report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y. Detection of Severe Acute Respiratory Syndrome Coronavirus in the Brain:Potential Role of the Chemokine Mig in Pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desforges M, Favreau DJ, Brison É, Desjardins J, Meessen-Pinard M, Jacomy H, Talbot PJ. Human Coronaviruses:Respiratory Pathogens Revisited as Infectious Neuroinvasive, Neurotropic, and Neurovirulent Agents. In: Singh SK, Ruzek D, editors. Neuroviral Infections:RNA Viruses and Retroviruses. 1st ed. Boca Raton, FL: CRC Press; 2013. pp. 93–122. [Google Scholar]
- 13.Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, Al-Omari A, Hajeer AH, Senga M, Denison MR, Nguyen-Van-Tam JS, Shindo N, Bermingham A, Chappell JD, Van Kerkhove MD, Fowler RA. Middle East Respiratory Syndrome. N Engl J Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath A. Neurologic Complications of Coronavirus Infections. Neurology. 2020 doi: 10.1212/WNL.0000000000009455. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human Coronaviruses and Other Respiratory Viruses:Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses. 2020;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohmwald K, Galvez NMS, Ríos M, Kalergis AM. Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To KF, Tong JH, Chan PK, Au FW, Chim SS, Allen Chan KC, Cheung JL, Liu EY, Tse GM, Lo AW, Lo YM, Ng HK. Tissue and Cellular Tropism of the Coronavirus Associated with Severe Acute Respiratory Syndrome:an in-situ Hybridization Study of Fatal Cases. J Pathol. 2004;202:157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical Characteristics of 113 Deceased Patients with Coronavirus Disease 2019:Retrospective Study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasiorek P, Banach M, Sakowicz A, Glabinski A, Sosnowska B, Maciejewski M, Bielecka-Dabrowa A. The Potential Role of Inflammation in Cryptogenic Stroke. Adv Med Sci. 2019;64:381–387. doi: 10.1016/j.advms.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Satta N, Vuilleumier N. Auto-antibodies as Possible Markers and Mediators of Ischemic, Dilated, and Rhythmic Cardiopathies. Curr Drug Targets. 2015;16:342–360. doi: 10.2174/1389450115666141125122416. [DOI] [PubMed] [Google Scholar]
- 21.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the Cardiovascular System. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AHA/ASA Stroke Council Leadership. Temporary Emergency Guidance to US Stroke Centers During the COVID-19 Pandemic. Stroke. 2020 [Google Scholar]
- 23.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YC, Bai WZ, Hashikawa T. The Neuroinvasive Potential of SARS-CoV2 May Play a Role in the Respiratory Failure of COVID-19 Patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beijing Hospital Confirms Nervous System Infections by Novel Coronavirus. China.org.cn. 2020. http://www.china.org.cn/china/2020-03/05/content_75777888.htm .
- 26.Karimi N, Sharifi Razavi A, Rouhani N. Frequent Convulsive Seizures in an Adult Patient with COVID-19:A Case Report. Iran Red Crescent Med J. 2020;22:e102828. [Google Scholar]
- 27.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy:CT and MRI Features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi A. Imaging of Acute Disseminated Encephalomyelitis. Neuroimaging Clin N Am. 2008;18:149–161. doi: 10.1016/j.nic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A First Case of Meningitis/Encephalitis Associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute Myelitis After SARS-CoV-2 Infection:A Case Report. medRxiv. 2020;2020:20035105. [Google Scholar]
- 31.Wei H, Yin H, Huang M, Guo Z. The 2019 Novel Cornoavirus Pneumonia with Onset of Oculomotor Nerve Palsy:A Case study. J Neurol. 2020;267:1550–1553. doi: 10.1007/s00415-020-09773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The Origin, Transmission and Clinical Therapies on Coronavirus Disease 2019 (COVID-19) Outbreak-An Update on the Status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous System Involvement After Infection with COVID-19 and Other Coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talan J. COVID-19:Neurologists in Italy to Colleagues in US:Look for Poorly-Defined Neurologic Conditions in Patients with the Coronavirus. Neurology Today 2020 merican Academy of Neurology. https://journals.lww.com/neurotodayonline/blog/breakingnews/pages/post.aspx?PostID=920 .
- 36.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-BarréSyndrome Associated with SARS-CoV-2 Infection:Causality or Coincidence?Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HY, Li XL, Yan ZR, Sun XP, Han J, Zhang BW. Potential Neurological Symptoms of COVID-19. Ther Adv Neurol Disord. 2020;13:1–2. doi: 10.1177/1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burks JS, DeVald BL, Jankovsky LD, Gerdes JC. Two Coronaviruses Isolated from Central Nervous System Tissue of Two Multiple Sclerosis Patients. Science. 1980;209:933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- 39.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by Human Respiratory Coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes?A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul Immunol Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morfopoulou S, Brown JR, Davies EG, Anderson G, Virasami A, Qasim W, Chong WK, Hubank M, Plagnol V, Desforges M, Jacques TS, Talbot PJ, Breuer J. Human Coronavirus OC43 Associated with Fatal Encephalitis. N Engl J Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson A, Edner N, Albert J, Ternhag A. Fatal Encephalitis Associated with Coronavirus OC43 in an Immunocompromised Child. Infect Dis (Lond) 2020;52:419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, Wang C, Song Z, Li S, Li X, Lv X, Qu X, Huang R, Liu W. Coronavirus Infections in the Central Nervous System and Respiratory Tract Show Distinct Features in Hospitalized Children. Intervirology. 2016;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai LK, Hsieh ST, Chang YC. Neurological Manifestations in Severe Acute Respiratory Syndrome. Acta Neurol Taiwan. 2005;14:113–119. [PubMed] [Google Scholar]
- 45.Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, Lee CC, Lim PL, Ponnudurai K, Chuah KL, Tan PH, Tai DY, Ang SP. Large Artery Ischaemic Stroke in Severe Acute Respiratory Syndrome (SARS) J Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MAA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM. Clinical Aspects and Outcomes of 70 patients with Middle East Respiratory Syndrome Coronavirus Infection:A Single-Center Experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Algahtani H, Subahi A, Shirah B. Neurological Complications of Middle East Respiratory Syndrome Coronavirus:A Report of Two Cases and Review of the Literature. Case Rep Neurol Med. 2016;2016:3502683. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, Ahn JY, Kim MK, Choi JP. Neurological complications during treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padovani A. Special Report COVID-19:Neurologists adapt in Northern Italy. EANpages. 2020. https://www.eanpages.org/2020/04/01/special-report-covid-19-neurologists-adapt-in-northern-italy/


