Abstract
Purpose
Acute respiratory distress syndrome (ARDS) is characterized by its acute onset of symptoms such as bilateral pulmonary infiltrates, severe hypoxemia, and pulmonary edema. Many patients with ARDS survive in the acute phase, but then die from significant lung fibrosis.
Methods
The effect of combination therapy with polydeoxyribonucleotide (PDRN) and pirfenidone on ARDS was investigated using human lung epithelial A549 cells. ARDS environment was induced by treatment with lipopolysaccharide and transforming growth factor (TGF)-β. Enzyme-linked immunoassay for connective tissue growth factor (CTGF) and hydroxyproline were conducted. Western blot for collagen type I, fibroblast growth factor (FGF), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 was performed.
Results
In this study, 8-μg/mL PDRN enhanced cell viability. Combination therapy with PDRN and pirfenidone and pirfenidone monotherapy suppressed expressions of CTGF and hydroxyproline and inhibited expressions of collagen type I and FGF. Combination therapy with PDRN and pirfenidone and PDRN monotherapy suppressed expression of TNF-α and IL-1β.
Conclusions
The combination therapy with PDRN and pirfenidone exerted stronger therapeutic effect against lipopolysaccharide and TGF-β-induced ARDS environment compared to the PDRN monotherapy or pirfenidone monotherapy. The excellent therapeutic effect of combination therapy with PDRN and pirfenidone on ARDS was shown by promoting the rapid anti-inflammatory effect and inhibiting the fibrotic processes.
Keywords: Acute respiratory distress syndrome; Polydeoxyribonucleotide; Pirfenidone, Combination therapy
• HIGHLIGHTS
- The effect of combination therapy with PDRN and pirfenidone on ARDS environment was investigated.
- Combination therapy inhibited expressions of CTGF, hydroxyproline, collagen type I, FGF, TNF-α, and IL-1β.
- The therapeutic effect of combination therapy appeared by promoting anti-inflammatory effect as well as inhibiting fibrotic processes.
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is characterized by its acute onset of symptoms such as bilateral pulmonary infiltrates, severe hypoxemia, and pulmonary edema [1]. Although mortality from ARDS has some decreased due to the extracorporeal membrane oxygenation, ventilator bundles, and pharmacological agents, such as heparin and steroid, ARDS-related lethality remains high [2]. Even in patients who survive ARDS, there is evidence that their long-term quality of life is adversely affected [3].
The pro-inflammatory and pro-fibrotic response may become persistent or uncontrolled during mechanical ventilation, resulting in pulmonary fibrosis and deterioration of lung function [4]. Because of this process, many patients with ARDS survive in the acute phase, but then die from significant lung fibrosis [5,6].
Polydeoxyribonucleotide (PDRN), which is extracted from the sperm of salmon, stimulates tissue repair in chronic wound and burn [7]. PDRN stimulates expression of vascular endothelial growth factor by activating adenosine A2A receptor [8]. PDRN shows an anti-inflammatory effect by inhibiting the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, and by increasing the production of anti-inflammatory cytokines, such as IL-10 [9,10]. In addition, PDRN inhibits apoptosis by suppressing production of cytokines in lipopolysaccharide (LPS)-induced acute lung injury [11]. Although PDRN suppresses inflammatory lung symptoms, it does not inhibit the process of pulmonary fibrosis.
Pirfenidone is a drug used in the treatment of idiopathic pulmonary fibrosis. It works by reducing lung fibrosis through downregulation of growth factors and production of pro-collagen types I and II [12]. Pirfenidone attenuates fibrosis in numerous animal models, including fibrosis in the lungs, liver, heart, and kidneys [13,14].
In the present study, the effect of combination therapy with PDRN and pirfenidone on ARDS was investigated using human lung epithelial A549 cells. ARDS environment was induced by treatment with LPS and transforming growth factor (TGF)-β in human lung epithelial A549 cells. MTT assay and WST-8 assay were performed to determine the effective concentration of each drug. After determination of effective dosage of PDRN and pirfenidone, connective tissue growth factor (CTGF) and hydroxyproline were determined using an enzyme-linked immunoassay (ELISA). Western blot for collagen type I, fibroblast growth factor (FGF), TNF-α, and IL-6 was performed.
MATERIALS AND METHODS
Cell Culture
Human lung epithelial cells, A549 cells, were purchased from Korean Cell Line Bank (KCLB, Seoul, Korea). The cells were maintained in RPMI1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% inactivated fetal bovine serum, 100-μg/mL streptomycin, and 100-IU/mL penicillin. The cells were cultured under the condition of 37°C and 5% CO2, as the previously described method [15,16]. This experimental procedure was approved by the Institutional Care and Use Committee of Kyung Hee University (KHUASP[SE]-18-036).
MTT Assay
To determine the viability, A549 cells were plated at a density of 1×104 cells/well in a 96-well plate, as the previously described method [15,17]. For the induction of ARDS environment, 1-μg/mL LPS (Escherichia coli serotype 026:B6; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and 5-ng/mL TGF-β (Kolon Pharm., Seoul, Korea) were treated. Immediately, the drugs in each group, including PDRN (Kyongbo Pharm., Seoul, Korea) and pirfenidone (Kolon Pharma.) were treated as Table 1. After 48 hours the drug treatment, MTT solution (Sigma-Aldrich Chemical Co.) was added to a well plate treated with the drug at a final concentration of 0.05 mg/mL and incubated at 37°C for 1 hour. After media was removed, 100 μL of dimethylsulfoxide (Sigma-Aldrich Chemical Co.) was added and shaken for 15 minutes to dissolve the MTT formazan crystals formed. Each well was placed in an ELISA microplate reader (Thermo Fisher Scientific Inc., Waltham, MA, USA) and the optical density was measured at a wavelength of 570 nm.
Table 1.
The concentration of monotherapy in each drug
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| LPS | - | 1 μg/mL | 1 μg/mL | 1 μg/mL | 1 μg/mL | 1 μg/mL |
| TGF-β | - | 5 ng/mL | 5 ng/mL | 5 ng/mL | 5 ng/mL | 5 ng/mL |
| PDRN | - | - | 2 μg/mL | 4 μg/mL | 8 μg/mL | 16 μg/mL |
| Pirfenidone | - | - | 100 μg/mL | 200 μg/mL | 500 μg/mL | 1,000 μg/mL |
A, control group; B, ARDS-induced group; C, ARDS-induced and 2-μg/mL PDRN with 100-μg/mL pirfenidone teated group; D, ARDS-induced and 4-μg/mL PDRN with 200-μg/mL pirfenidone teated group; E, ARDS-induced and 8-μg/mL PDRN with 500-μg/mL pirfenidone teated group; F, ARDS-induced and 16-μg/mL PDRN with 1,000-μg/mL pirfenidone teated group.
LPS, lipopolysaccharide; TGF, tumor growth factor-β; PDRN, polydeoxyribonucleotide.
WST-8 Assay
To determine the viability, A549 cells were plated at a density of 1×104 cells/well in a 96-well plate, as the previously described method [15]. To induce the ARDS environment, 1-μg/mL LPS and 5-ng/mL TGF-β were treated. Immediately, the drugs in each group were treated as shown in Table 1. After 48 hours drug treatment, 10 μL of the WST-8 kit solution (Biomax, Seoul, Korea) was added to each well, and incubated for 1 hour. The WST-8 well was placed in an ELISA microplate reader (Thermo Fisher Scientific Inc.) and the optical density was measured at the 450 nm wavelength.
ELISA
The supernatants were collected to measure CTGF (Wuhan Fine Biotech Co. Ltd., Wuhan, China) and hydroxyproline (Wuhan Fine Biotech Co. Ltd.) concentration by ELISA, as the previously described method [15,18]. To induce the ARDS environment, 1-μg/mL LPS and 5-ng/mL TGF-β were treated. Immediately, the drugs in each group were treated as shown in Table 2. Standard, control, and sample were treated to each well and incubated at 37°C for 90 minutes, and washed plate. Biotin-labeled antibody working solution was treated to each well and incubated at 37°C for 60 minutes. Streptavidin conjugate working solution was incubated to each well for 30 minutes at 37°C. Tetramethylbenzidine substrate was added and incubated at 37°C for 20 minutes. Stop solution was added and calculated at 450 nm wavelength in microplate reader (Thermo Fisher Scientific Inc.).
Table 2.
The concentration of monotherapy and combination therapy in each drug
| A | B | C | D | E | |
|---|---|---|---|---|---|
| LPS | - | 1 μg/mL | 1 μg/mL | 1 μg/mL | 1 μg/mL |
| TGF-β | - | 5 ng/mL | 5 ng/mL | 5 ng/mL | 5 ng/mL |
| PDRN | - | - | 8 μg/mL | - | 8 μg/mL |
| Pirfenidone | - | - | - | 500 μg/mL | 500 μg/mL |
A, control group; B, ARDS-induced group; C, ARDS-induced and PDRN monotherapy group; D, ARDS-induced and pirfenidone monotherapy group; E, ARDS-induced and combination therapy with PDRN and pirfenidone group.
LPS, lipopolysaccharide; TGF, tumor growth factor-β; PDRN, polydeoxyribonucleotide.
Western Blot
A549 cells were plated at a density of 1×106 cells/dish in 100-mm dish, as the previously described method [15,19]. To induce the ARDS environment, 1-μg/mL LPS and 5-ng/mL TGF-β were added. Immediately, the drugs in each group were treated as shown in Table 2. A549 cells was washed in phosphate buffered saline to removed media. RIPA buffer (Cell Signaling Technology, Beverly, MA, USA) with 1mM PMSF (Sigma-Aldrich Chemical Co.) was added to 100 mm dish and incubated on ice for 5 minutes. The treated cells were collected in 1.5-mL tube and briefly sonicated. Lysate cells were centrifuged at 14,000 g for 20 minutes at 4°C and supernatants were collected. The sample was measured using a microdrop reader. Protein 20 μg was loaded on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, and transferred on nitrocellulose membrane (Amersham Pharmacia Biotech GmbH, Freiburg, Germany). The nitrocellulose membrane was blocked in 5% nonfat milk buffer for 1 hour. Primary antibodies were reacted overnight at 4°C as flows: mouse anti-TNF-α, mouse anti-IL-6, mouse collagen type I, mouse anti-β-actin, rabbit antiIL-6, and rabbit anti-bFGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Nitrocellulose membrane was developed using enhanced chemiluminescence detection kit (Bio-Rad, Hercules, CA, USA). Detected band was quantified by image pro plus version 6.0 (Media Cybernetics Inc., Silver Spring, MD, USA).
Statistical Analysis
Statistical analysis was conducted using 1-way analysis of variance followed by Duncan post hoc test using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). The results are expressed as the means ±standard errors of the mean. Significance was set as P<0.05.
RESULTS
Cell Viability
The percentage of cell viability in the control group of the MTT assay and WST-8 assay was set at 1.00. The results are presented in Fig. 1. Current results showed that cell viability was significantly reduced by the induction of ARDS in MTT assay and WST-8 assay (P<0.05). However, treatment with 8-μg/mL PDRN showed an enhanced effect on cell viability in ARDS-induced human lung epithelial A549 cells (P<0.05).
Fig. 1.
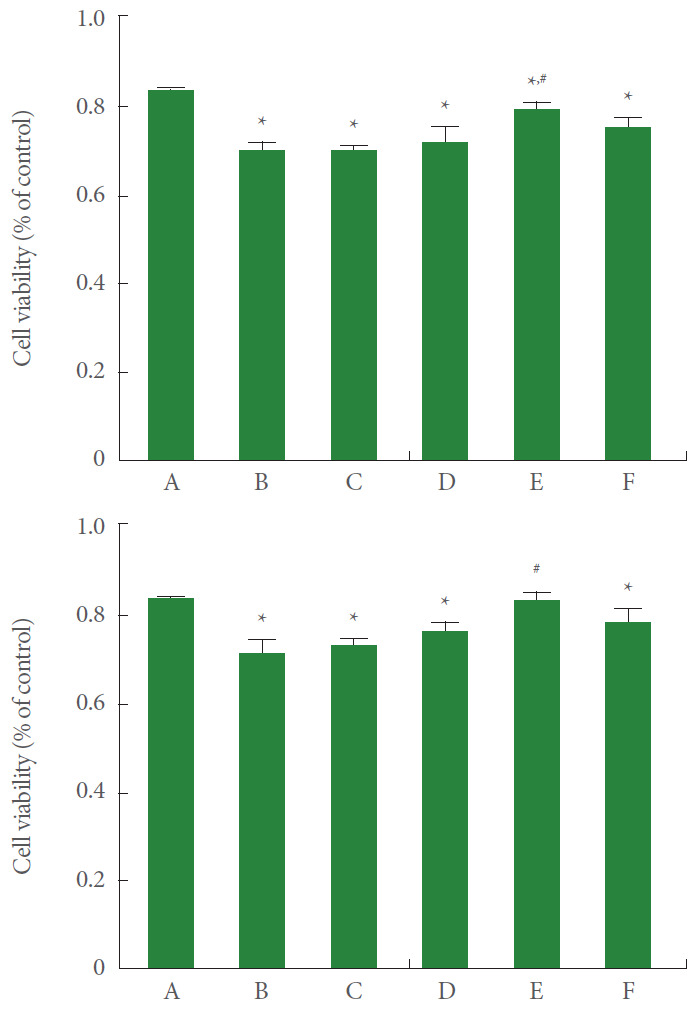
Effect of polydeoxyribonucleotide (PDRN) treatment on cell viability following acute respiratory distress syndrome (ARDS) environment induction in human lung epithelial A549 cells. Upper panel: The cells were stained using the MTT methods. Lower panel: The cells were stained using the WST-8 method. A, control group; B, ARDS-induced group; C, ARDS-induced and 2-μg/mL PDRN with 100-μg/mL pirfenidone teated group; D, ARDS-induced and 4-μg/mL PDRN with 200 μg/mL pirfenidone teated group; E, ARDS-induced and 8 μg/mL PDRN with 500-μg/mL pirfenidone teated group; F, ARDS-induced and 16-μg/mL PDRN with 1,000-μg/mL pirfenidone teated group. *P<0.05 compared to the control group. #P<0.05 compared to the ARDS-induced group.
CTGF and Hydroxyproline Expression
CTGF and hydroxyproline expression in A549 cells was measured using the ELISA kit. The results are presented in Fig. 2. Current results showed that the expression of CTGF and hydroxyproline was significantly increased by the induction of ARDS (P<0.05). However, pirfenidone monotherapy and combination therapy of PDRN with pirfenidone showed suppressing effect on CTGF and hydroxyproline expression in ARDS-induced human lung epithelial A549 cells (P<0.05).
Fig. 2.
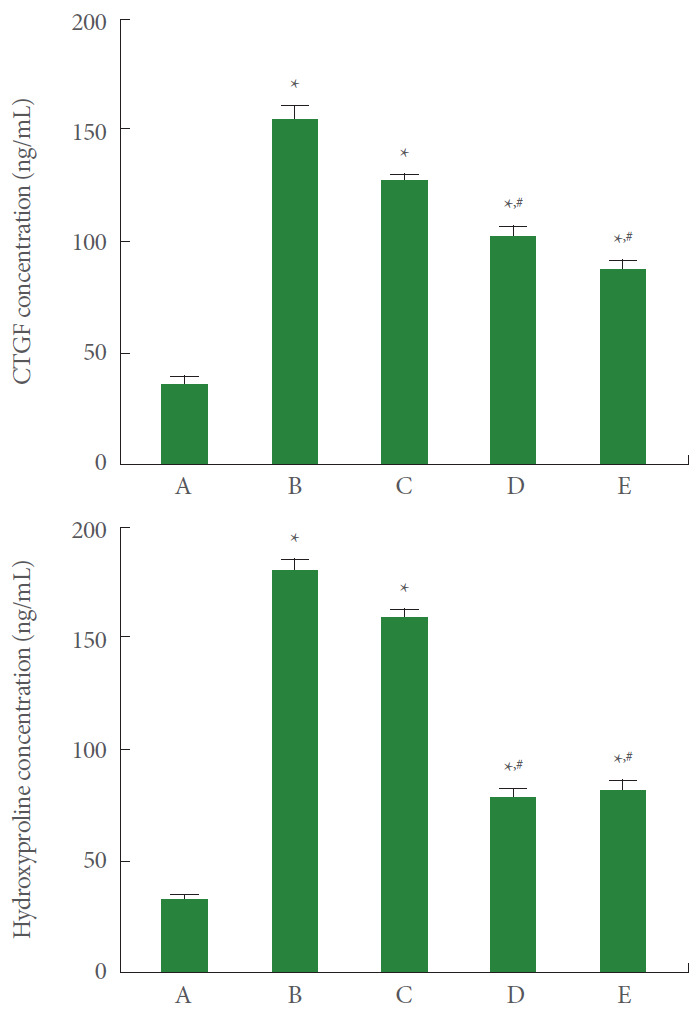
Effect of combination therapy with polydeoxyribonucleotide (PDRN) and pirfenidone on connective tissue growth factor (CTGF) and hydroxyproline expression following acute respiratory distress syndrome (ARDS) environment induction in human lung epithelial A549 cells. Upper panel: CTGF expression in enzyme assay using enzyme-linked immunoassay (ELISA) kit. Lower panel: Hydroxyproline expression in enzyme assay using ELISA kit. A, control group; B, ARDS-induced group; C, ARDS-induced and PDRN monotherapy group; D, ARDS-induced and pirfenidone monotherapy group; E, ARDS-induced and combination therapy with PDRN and pirfenidone group. *P<0.05 compared to the control group. #P<0.05 compared to the ARDS-induced group.
Collagen Type I Expression
Collagen type I expression in A549 cells was measured using the western blot. The results are presented in Fig. 3. Current results showed that collagen type I expression was significantly increased by the induction of ARDS (P<0.05). However, PDRN monotherapy, pirfenidone monotherapy, and combination therapy of PDRN with pirfenidone showed a suppressing effect on collagen type I expression in ARDS-induced human lung epithelial A549 cells (P<0.05). Pirfenidone monotherapy and combination therapy of PDRN with pirfenidone more potently suppressed collagen type I expression than PDRN monotherapy (P<0.05).
Fig. 3.
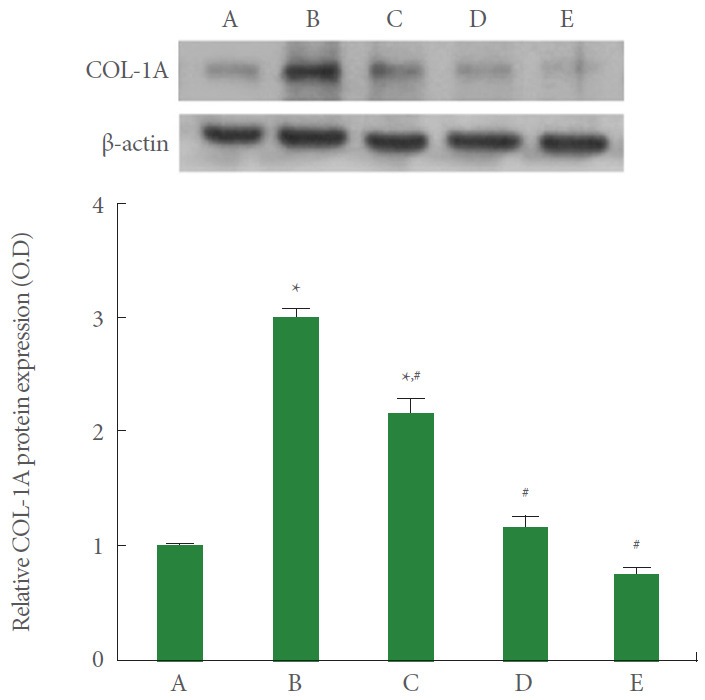
Effect of combination therapy of polydeoxyribonucleotide (PDRN) with pirfenidone on collagen type I expression following acute respiratory distress syndrome (ARDS) environment induction in human lung epithelial A549 cells. Upper panel: The results of band detection using the enhanced chemiluminescence (ECL) detection kit. Lower panel: The relative expression of collagen type I. A, control group; B, ARDS-induced group; C, ARDS-induced and PDRN monotherapy group; D, ARDS-induced and pirfenidone monotherapy group; E, ARDS-induced and combination therapy with PDRN and pirfenidone group. *P<0.05 compared to the control group. #P<0.05 compared to the ARDS-induced group.
FGF Expression
FGF expression in A549 cells was measured using the western blot. The results are presented in Fig. 4. Current results showed that FGF expression was significantly increased by the induction of ARDS (P<0.05). However, pirfenidone monotherapy and combination therapy of PDRN with pirfenidone showed suppressing effect on FGF expression in ARDS-induced human lung epithelial A549 cells (P<0.05).
Fig. 4.
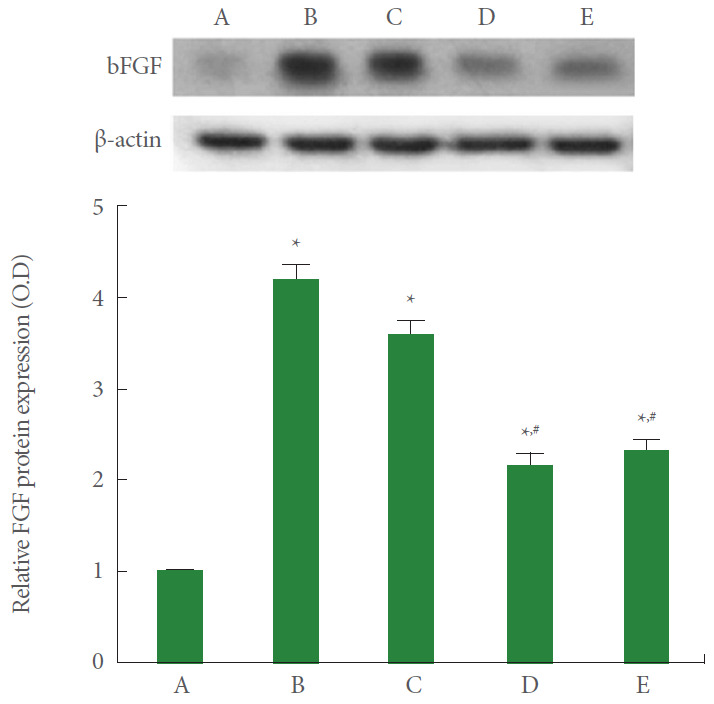
Effect of combination therapy with polydeoxyribonucleotide (PDRN) and pirfenidone on fibroblast growth factor (FGF) expression following acute respiratory distress syndrome (ARDS) environment induction in human lung epithelial A549 cells. Upper panel: The results of band detection using the enhanced chemiluminescence (ECL) detection kit. Lower panel: The relative expression of FGF. A, control group; B, ARDS-induced group; C, ARDS-induced and PDRN monotherapy group; D, ARDS-induced and pirfenidone monotherapy group; E, ARDS-induced and combination therapy with PDRN and pirfenidone group. *P<0.05 compared to the control group. #P<0.05 compared to the ARDS-induced group.
TNF-α Expression
TNF-α expression in A549 cells was measured using the western blot. The results are presented in Fig. 5. Current results showed that TNF-α expression was significantly increased by the induction of ARDS (P<0.05). However, PDRN monotherapy and combination therapy of PDRN with pirfenidone showed suppressing effect on TNF-α expression in ARDS-induced human lung epithelial A549 cells (P<0.05). Combination therapy of PDRN with pirfenidone more potently suppressed TNF-α expression compared to PDRN monotherapy (P<0.05).
Fig. 5.
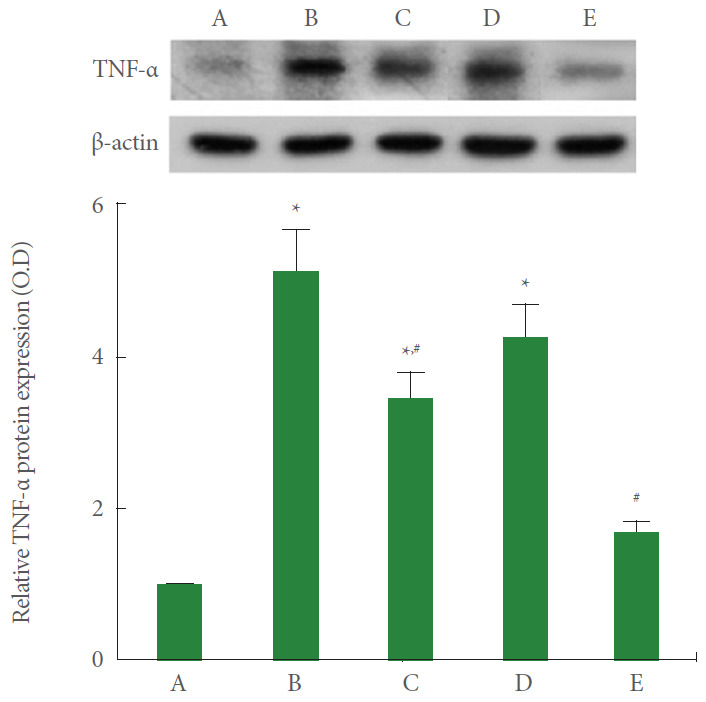
Effect of combination therapy with polydeoxyribonucleotide (PDRN) and pirfenidone on tumor necrosis factor (TNF)-α expression following acute respiratory distress syndrome (ARDS) environment induction in human lung epithelial A549 cells. Upper panel: The results of band detection using the enhanced chemiluminescence (ECL) detection kit. Lower panel: The relative expression of TNF-α. A, control group; B, ARDS-induced group; C, ARDS-induced and PDRN monotherapy group; D, ARDS-induced and pirfenidone monotherapy group; E, ARDS-induced and combination therapy with PDRN and pirfenidone group. *P<0.05 compared to the control group. #P<0.05 compared to the ARDS-induced group.
IL-6 Expression
IL-6 expression of A549 cells was measured using the western blot. The results are presented in Fig. 6. Current results showed that IL-6 expression was significantly increased by the induction of ARDS (P<0.05). However, PDRN monotherapy, pirfenidone monotherapy, and combination therapy of PDRN with pirfenidone showed suppressing effect on IL-6 expression in ARDS-induced human lung epithelial A549 cells (P<0.05). Combination therapy of PDRN with pirfenidone more potently suppressed IL-6 compared to PDRN monotherapy or pirfenidone monotherapy (P<0.05).
Fig. 6.
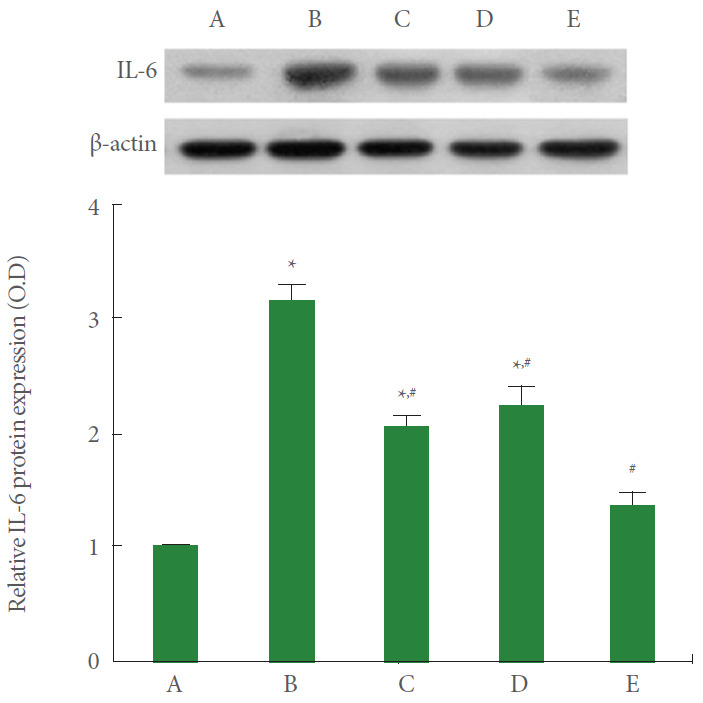
Effect of combination therapy with polydeoxyribonucleotide (PDRN) and pirfenidone on interleukin (IL)-6 expression following acute respiratory distress syndrome (ARDS) environment induction in human lung epithelial A549 cells. Upper panel: The results of band detection using the enhanced chemiluminescence (ECL) detection kit. Lower panel: The relative expression of IL-6. A, control group; B, ARDS-induced group; C, ARDS-induced and PDRN monotherapy group; D, ARDS-induced and pirfenidone monotherapy group; E, ARDS-induced and combination therapy with PDRN and pirfenidone group. *P<0.05 compared to the control group. #P<0.05 compared to the ARDS-induced group.
DISCUSSION
ARDS is a life-threatening condition associated with the migration of lethal inflammatory cells to the lungs, which can lead to the release of inflammatory mediators that obstruct alveolar capillary epithelial and endothelial barriers [20]. Corticosteroids are the preferred therapeutic agents to treat ARDS patients, but these agents are limited in use because they cause numerous side effects. To overcome this today, researchers are looking for new and safer drugs to treat ARDS symptoms. In the present study, 8-μg/mL PDRN enhanced cell viability in the MTT assay and WST-8 assay. However, pirfenidone treatment increased cytotoxicity as the high dose dependently, thus pirfenidone of high dose reduced cell viability [21].
The effect of adenosine is mediated by the four G proteincoupled receptors (A1, A2A, A2B, and A3) expressed in immune cells. Among them, adenosine A2A receptor agonists have strong anti-inflammatory property [22]. PDRN, adenosine A2A receptor agonist, suppressed the production of pro-inflammatory cytokines through promoting phosphorylation of cyclic adenosine-3,5′-monophosphate (cAMP) response element-binding protein through the cAMP-protein kinase A pathway [10,23]. Therefore, adenosine A2A receptor agonists are known to be effective in the treatment of inflammatory diseases [24]. Pulmonary fibrosis develops late in ARDS, and the morbidity and mortality rates of ARDS are particularly high when it causes persistent alveolar and interstitial fibrosis [4]. Pulmonary fibrosis results in diffuse interstitial inflammation and exaggerated collagen accumulation, which in turn leads to destruction and remodeling of the alveolar structure [25].
The present study showed that the expression of CTGF and hydroxyproline was significantly increased after LPS and TGF-β treatment for ARDS environment induction. CTGF and hydroxyproline are early markers of collagen synthesis during fibrosis [26,27]. Thus, an increase in CTGF and hydroxyproline means increased collagen synthesis for fibrosis. In the present study, combination therapy with PDRN and pirfenidone and pirfenidone monotherapy suppressed expressions of CTGF and hydroxyproline. PDRN monotherapy showed no significant effect on CTGF and hydroxyproline expression. In the present study, combination therapy with PDRN and pirfenidone and pirfenidone monotherapy inhibited fibrosis-related factors including collagen type I and FGF. PDRN also showed mild inhibitory effect on collagen type I but not FGF. Pirfenidone has a strong anti-fibrotic effect.
Enhanced pro-inflammatory cytokine levels activate multiple inflammatory signaling pathways that contribute to lung inflammation, such as ARDS. Among them, TNF-α has been implicated as the main inflammatory cytokine of ARDS [28,29]. The generation of reactive oxygen species with increasing intracellular Ca2+ concentration is associated with elevated level of TNF-α [30]. The present study showed that the expression of TNF-α and IL-6 was significantly increased after LPS and TGF-β treatment for ARDS environment induction. Pirfenidone reduces some inflammatory cytokines in addition to its anti-fibrotic action [31]. In the human peripheral blood lymphocytes, pirfenidone exerts a similar anti-inflammatory effect inhibiting staphylococcal enterotoxin B-induced proliferation and synthesis of TNF-α, IFN-γ, IL-1β, and IL-6 [12,32]. In the present study, combination therapy with PDRN and pirfenidone and PDRN monotherapy suppressed expression of TNF-α and IL-1β. Pirfenidone monotherapy decreased IL-6 expression, but not TNF-α. Combination therapy with PDRN and pirfenidone showed more potent suppressing effect on expression of TNF-α and IL-1β. PDRN showed a strong anti-inflammatory effect in this experiment.
In conclusion, the combination therapy with PDRN and pirfenidone exerted stronger therapeutic effect on LPS and TGF-β-induced ARDS environment compared to PDRN monotherapy or pirfenidone monotherapy. PDRN and pirfenidone combination therapy may offer the potential to provide new therapeutic techniques for refractory urogenital ulcers. The excellent therapeutic effect of combination therapy with PDRN and pirfenidone on ARDS was shown by promoting rapid anti-inflammatory effect and inhibiting the fibrotic processes. In conclusion, the combination therapy with PDRN and pirfenidone may provide new guideline for the treatment of ARDS.
Footnotes
Fund/Grant Support
This study was supported by a grant from the National Research Foundation of Korea (NRF-2018R1A2B2008421).
Research Ethics
The study was approved by the Institutional Care and Use Committee of Kyung Hee University (KHUASP[SE]-18-036).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: JJH, JWJ
· Formal Analysis: JJH, BSC, CWC
· Investigation: JJJ, LH, SHK. SSP
· Methodology: JJJ, LH, SHK. SSP
· Project Administration: CWC
· Writing – Original Draft: IGK
· Writing – Review & Editing: IGK
REFERENCES
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Gonzales JN, Lucas R, Verin AD. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2:1009. [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–52. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43:276–85. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabrera-Benitez NE, Laffey JG, Parotto M, Spieth PM, Villar J, Zhang H, et al. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121:189–98. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchioni A, Tonelli R, Ball L, Fantini R, Castaniere I, Cerri S, et al. Acute exacerbation of idiopathic pulmonary fibrosis: lessons learned from acute respiratory distress syndrome? Crit Care. 2018;22:80. doi: 10.1186/s13054-018-2002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, et al. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem. 2009;7:313–21. doi: 10.2174/187152509789541909. [DOI] [PubMed] [Google Scholar]
- 8.Jeon JW, Lee JI, Shin HP, Cha JM, Joo KR, Kim SH, et al. Adenosine A2A-receptor agonist polydeoxyribonucleotide promotes gastric ulcer healing in Mongolian gerbils. Animal Cells Syst. 2014;18:399–406. [Google Scholar]
- 9.Bitto A, Polito F, Irrera N, D’Ascola A, Avenoso A, Nastasi G, et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011;63:3364–71. doi: 10.1002/art.30538. [DOI] [PubMed] [Google Scholar]
- 10.Ko IG, Kim SE, Jin JJ, Hwang L, Ji ES, Kim CJ, et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018;203:12–9. doi: 10.1016/j.lfs.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 11.An J, Park SH, Ko IG, Jin JJ, Hwang L, Ji ES, et al. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced lung injury by inhibiting apoptotic cell death in rats. Int J Mol Sci. 2017;18:E1847. doi: 10.3390/ijms18091847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margaritopoulos GA, Vasarmidi E, Antoniou KM. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 2016;11:11–22. doi: 10.2147/CE.S76549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakugawa T, Mukae H, Hayashi T, Ishii H, Abe K, Fujii T, et al. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J. 2004;24:57–65. doi: 10.1183/09031936.04.00120803. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong H, Chung JY, Ko IG, Kim SH, Jin JJ, Hwang L, et al. Effect of polydeoxyribonucleotide on lipopolysaccharide and sevoflurane-induced postoperative cognitive dysfunction in human neuronal SH-SY5Y cells. Int Neurourol J. 2019;23(Suppl 2):S93–101. doi: 10.5213/inj.1938218.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EH, Choi YS, Kim YM. Antioxidative and anti-inflammatory effect of Phellinus igniarius on RAW 264.7 macrophage cells. J Exerc Rehabil. 2019;15:2–7. doi: 10.12965/jer.1938010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EH, Kim YM, Suh JH. Effect of type II collagen extract on immunosuppression induced by methotrexate in rats. J Exerc Rehabil. 2018;14:731–8. doi: 10.12965/jer.1836480.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin CH, Rhyu HS, Kim JY. The effects of combined aerobic and resistance training on inflammatory markers in obese men. J Exerc Rehabil. 2018;14:660–5. doi: 10.12965/jer.1836294.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ. Effects of preconditioning exercise on nitric oxide and antioxidants in hippocampus of epileptic seizure. J Exerc Rehabil. 2019;15:757–62. doi: 10.12965/jer.1938698.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson KC, Saukkonen JJ. Acute respiratory failure from abused substances. J Intensive Care Med. 2004;19:183–93. doi: 10.1177/0885066604263918. [DOI] [PubMed] [Google Scholar]
- 21.Molina-Molina M, Machahua-Huamani C, Vicens-Zygmunt V, Llatjós R, Escobar I, Sala-Llinas E, et al. Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm Med. 2018;18:63. doi: 10.1186/s12890-018-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Horikawa Y, et al. Attenuation of gastric mucosal inflammation induced by aspirin through activation of A2A adenosine receptor in rats. World J Gastroenterol. 2006;12:568–73. doi: 10.3748/wjg.v12.i4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milne GR, Palmer TM. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal. 2011;11:320–39. doi: 10.1100/tsw.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou WQ, Wang P, Shao QP, Wang J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem. 2016;419:19–28. doi: 10.1007/s11010-016-2745-7. [DOI] [PubMed] [Google Scholar]
- 26.Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 27.Chambers RC. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008;153:S367–78. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, et al. Tumor necrosis factor-α overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171:1363–70. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pooladanda V, Thatikonda S, Bale S, Pattnaik B, Sigalapalli DK, Bathini NB, et al. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation. Cell Death Dis. 2019;10:81. doi: 10.1038/s41419-018-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SS, Park HS, Jeong H, Kwak HB, No MH, Heo JW, et al. Treadmill exercise ameliorates chemotherapy-induced muscle weakness and central fatigue by enhancing mitochondrial function and inhibiting apoptosis. Int Neurourol J. 2019;23(Suppl 1):S32–9. doi: 10.5213/inj.1938046.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazato H, Oku H, Yamane S, Tsuruta Y, Suzuki R. A novel antifibrotic agent pirfenidone suppresses tumor necrosis factor-α at the translational level. Eur J Pharmacol. 2002;446:177–85. doi: 10.1016/s0014-2999(02)01758-2. [DOI] [PubMed] [Google Scholar]
- 32.Hale ML, Margolin SB, Krakauer T, Roy CJ, Stiles BG. Pirfenidone blocks the in vitro and in vivo effects of staphylococcal enterotoxin B. Infect Immun. 2002;70:2989–94. doi: 10.1128/IAI.70.6.2989-2994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


