Abstract
Background
Autoimmune hypophysitis is a rare disease characterized by the infiltration of lymphocytic cells into the pituitary gland. 18F-fluorodeoxyglucose (FDG) and 18F-2-fluorodeoxy sorbitol (FDS) positron emission tomography (PET) are well-established and emerging techniques, respectively, which may aid in the diagnosis and classification of autoimmune hypophysitis.
Case presentation
Here, we report a 40-year-old female diagnosed with central diabetes insipidus and multiple pituitary hormone deficiencies, and MRI revealed homogeneous signals in the pituitary gland as well as thickened in the pituitary stalk. FDG PET localized the pituitary and pituitary stalk lesions and displayed an SUVmax of 5.5. FDS, a sensitive radiotracer for bacterial infections but remains unproven under aseptic inflammation, also demonstrated elevated radioactivity, with an SUVmax of 1.1 at 30 min and 0.73 at 120 min. Transnasal biopsy suggested a diagnosis of autoimmune hypophysitis, and the patient displayed radiological and clinical improvement after treatment with glucocorticoids and hormone replacement.
Conclusions
Autoimmune hypophysitis can display elevated FDG uptake, which aids in the localization of the lesions. In addition to revealing bacterial infection specifically, FDS can also accumulate under autoimmune conditions, suggesting that it could serve as a potential radiotracer for both bacterial and aseptic inflammation.
Trial registration
The patient was enrolled in study NCT02450942 (clinicaltrials.gov, Registered May 21, 2015).
Keywords: Autoimmune hypophysitis, PET, FDG, FDS, Case report
Background
Autoimmune hypophysitis, also known as lymphocytic hypophysitis, is characterized by the infiltration of lymphocytic cells into the pituitary gland due to an autoimmune etiology that leads to pituitary dysfunction and occurs in pituitary patients with an incidence ranging from 0.24 to 0.87% [1–4]. Positron emission tomography (PET) is a well-established molecular imaging technique that assesses cellular metabolism using radiolabeled substrates [5]. 18F-fluorodeoxyglucose (FDG) is the most widely used radiotracer to aid in the localization and diagnosis of diseases. However, altered glucose metabolism can occur in multiple circumstances [6–8], and FDG alone may be insufficient to differentiate neoplastic, infectious, or autoimmune diseases. 18F-2-fluorodeoxy sorbitol (FDS) is an analog of sorbitol that can be taken up by Enterobacteriaceae [9, 10], leading to its use as a sensitive radiotracer for infections. However, the utility of FDS under aseptic inflammation faces controversies [10, 11]. This article reports a case of pathologically diagnosed autoimmune hypophysitis that displayed FDG and FDS activity, which highlights the significance of PET in disease localization and the potentialities of FDS in sterile inflammation.
Case presentation
A 40-year-old female with a 4-month history of polydipsia, polyuria, headache, menstrual disorder and fatigue was admitted to the hospital. Examinations revealed a urine osmolality of 63 mOsm/kg when plasma osmolality reached 307 mOsm/kg and serum sodium was 148 mmol/L, and deficiencies of adrenocorticotropic hormone, thyroid-stimulating hormone and gonadotropins. Magnetic resonance imaging (MRI) revealed homogeneous signals in the pituitary gland as well as thickening of the pituitary stalk (Fig. 1), which supported a diagnosis of central diabetes insipidus. FDG PET was performed with a dose of 5.55 MBq (0.15 mCi) per kilogram of body weight to localize the pituitary and/or the pituitary stalk lesions and revealed radioactivity at both sites (Fig. 2). However, the elevated FDG uptake was inadequate for an etiological diagnosis, and FDS (5.55 MBq/kg) was utilized to further explore the nature of the disease. The lesions also showed increased FDS uptake at 30 min and 120 min after FDS injection (Fig. 2), both of which were significantly higher than normal brain uptake. The patient was suspected to have autoimmune or infectious pituitary inflammation based on FDG and FDS activity, and both tracers also excluded possible extracranial involvement.
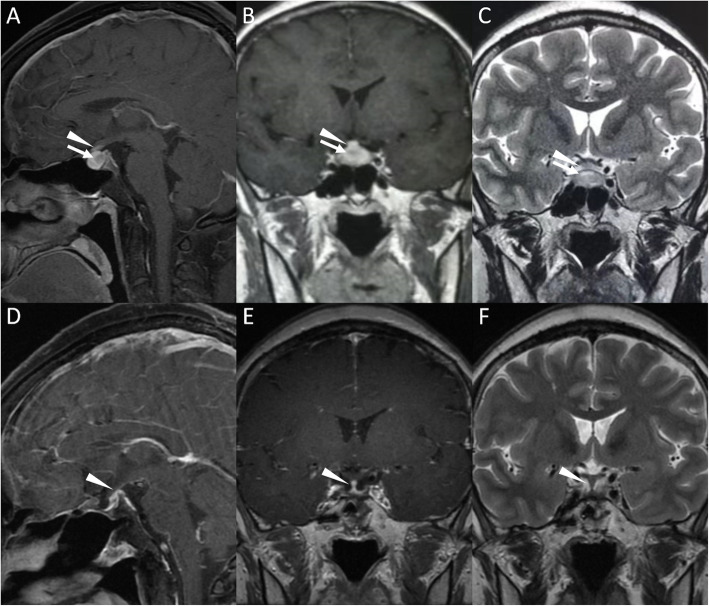
Fig. 1.
Contrast-enhanced T1-weighted and T2-weighted MR images of the lesions. a-b. Pituitary with a size of 18.6 mm × 8.2 mm × 9.9 mm displayed a contrast-enhanced signal, and a lesion with a size of 6.5 mm × 5.2 mm × 4.6 mm and a relative hypointense contrast-enhanced signal was located (arrow noted). The pituitary stalk with a size of 5.1 mm × 1.7 mm showed an isointense contrast-enhanced signal (arrowhead noted) compared with the normal pituitary stalk. c. The pituitary lesion presented a hyperintense T2-weighted signal (arrow noted), and the thickened pituitary stalk exhibited an isointense T2-weighted signal in comparison with the normal pituitary. d-f. Contrast-enhanced T1-weighted and T2-weighted MR images of the lesions 3 years after surgery. The pituitary displayed postsurgical changes with no significant hypointense contrast-enhanced signal or hyperintense T2-weighted signal, and the pituitary stalk thickness was reduced (3.0 mm × 2.0 mm, arrowhead noted) compared with pretreatment MRI
Fig. 2.
FDG and FDS activity of the lesions. a-d. Both the pituitary and pituitary stalk lesions displayed FDG activity with an SUVmax of 5.5 after 40 min of radiotracer injection. e-f. Both lesions displayed FDS activity with a SUVmax of 1.1 at 30 min after FDS injection; in comparison, the SUVmax of the normal brain tissue was 0.15, and the T/N ratio was 7.3. g-h. The lesions remained FDS active after 120 min of FDS administration with a SUVmax of 0.73; the SUVmax of the normal brain and the T/N ratio were 0.08 and 9.1, respectively
Transnasal biopsy was performed for a definitive diagnosis. A lesion was found locating at posterior pituitary, while the anterior pituitary remained normal. Histopathology of the posterior pituitary lesion revealed lymphocyte infiltration and fibrosis of the pituitary gland, while no evidence of malignant elements or infection was seen, which indicated a diagnosis of autoimmune hypophysitis. The patient was then treated with oral prednisone (starting dosage of 40 mg/day, gradually decreased every 3 weeks to a maintenance dosage of 10 mg/day), desmopressin acetate (0.2 mg/8 h) and levothyroxine (50 μg/day) and showed an improvement in clinical symptoms and on radiological examination (Fig. 1). However, hypopituitarism remained stable and hormonal replacement was consistently needed.
Discussion and conclusions
This case reports the FDG and FDS activity in a patient with autoimmune hypophysitis, with the results suggesting the role of FDG PET in differential diagnosis and disease classification as well as the potential application of FDS PET. Autoimmune hypophysitis, according to its infiltrating range, can be classified as lymphocytic adenohypophysitis (involving the anterior pituitary but not the infundibulum or posterior pituitary), lymphocytic infundibuloneurophypophysitis (infiltrating the infundibulum or posterior pituitary or pituitary stalk without anterior pituitary involvement) or lymphocytic panhypophysitis (infiltrating both the anterior pituitary and infundibulum or posterior pituitary or pituitary stalk). In addition to obtaining tissue for definitive diagnosis and classification, the clinical and neuroradiological characteristics of autoimmune hypophysitis together with their improvement during treatment can establish a clinical diagnosis of autoimmune hypophysitis if alternative pituitary diseases and other causes can be excluded [4]. In our case, the MRI results showed abnormal signals of a pituitary lesion and a thickened pituitary stalk, but the signals from the pituitary lesion and thickened pituitary stalk were inconsistent, which caused difficulties in narrowing down the clinical characteristics into a diagnosis of autoimmune hypophysitis. Moreover, alternative disease possibilities such as pituitary abscess, central nervous system germ cell tumor or Langerhans cell histiocytosis needed to be excluded. Thus, metabolic imaging was applied to further investigate the nature and relationship of the lesions.
Although the role of FDG PET is somewhat limited by the high background activity when evaluating central nervous system diseases, it provides considerable information for the evaluation of pituitary diseases since the normal pituitary gland does not typically take up FDG (Fig. 3) [12]. The usefulness of FDG PET has been demonstrated in neoplastic diseases such as pituitary adenoma, Langerhans cell histiocytosis and nonneoplastic disorders such as primary hypothyroidism [13–15]. However, only one previous case report described that primary hypophysitis had moderate uptake of FDG and displayed an SUVmax of 4.7 [16]. Recently, owing to the development of immunotherapy, immunotherapy-induced hypophysitis has also been reported [17]. Both anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) and anti-programmed cell death protein 1 (PD1) treatments can result in secondary hypophysitis [18–20] and display elevated FDG radioactivity. In addition, a normalization of FDG uptake can also be observed after hypophysitis treatment [18]. In the present case, mild FDG activity was located in both the pituitary and pituitary stalk lesions, providing substantial evidence for disease classification.
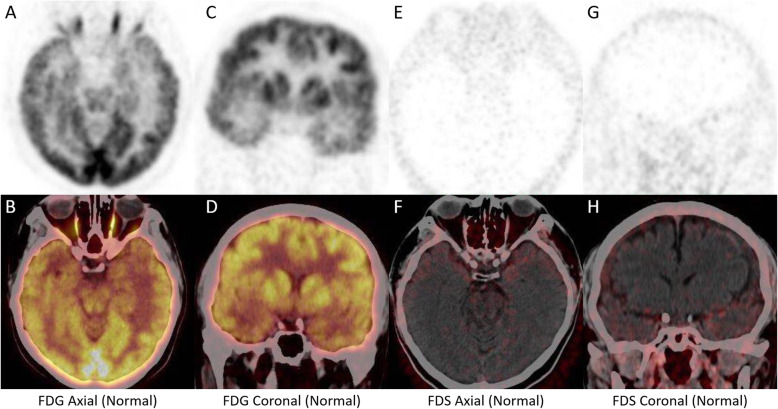
Fig. 3.
FDG and FDS activity in a patient with a normal pituitary. a-d. Both the pituitary and pituitary stalks did not display FDG activity. e-h. FDS was also not absorbed in normal pituitary and pituitary stalks
FDS can be synthesized through a three-step automated reaction from 18F ions or reduced from FDG with NaBH4 [9, 11] and shares a similar structure with FDG. FDS can be rapidly eliminated from organs and blood through the renal-urinary system [9], and due to its favorable renal kinetics, it is also considered a potential tracer for renal functional imaging [21]. Previously, FDS was reported to be absorbed by Enterobacteriaceae, leading to its use as a promising tracer for bacterial infection [9–11]. In our patient, FDS was initially given to exclude pituitary abscesses that can occur without a clear sign of infection [22], and negative results would further eliminate the possibility of infectious diseases. However, the uptake of FDS under aseptic inflammation faces controversies. Li et al. induced sterile inflammation by 12-O-tetradecanoyl-phorbol-13-acetate (TPA) and displayed increased FDS uptake in animal models [11], while Weinstein et al. inoculated mice with heat-killed E. coli which did not demonstrate FDS activity [10]. Thus, the increased radioactivity in our case may be the result of either autoimmune hypophysitis or abscess, and biopsy was performed for a definitive diagnosis. Our results demonstrated that aseptic inflammation can also display elevated FDS uptake, and radioactivity can be seen on both the 30 min and 120 min scans, which excluded blood pool effects. To the best of our knowledge, this is the first report of FDS radioactivity in autoimmune conditions in humans, suggesting FDS as a potential radiotracer for both bacterial and aseptic inflammation. Nevertheless, the diagnostic role and underlying mechanism of FDS in inflammatory circumstances need to be further investigated, and a comparison of diagnostic performances with widely applied radiotracers (e.g., FDG) is required.
In conclusion, autoimmune hypophysitis is a rare disease whose diagnosis and classification remain difficult. FDG and FDS PET may provide additional radiological information to aid in the localization and diagnosis of disease. In addition to specifically revealing bacterial infection, FDS can also accumulate under autoimmune inflammation, indicating another potential application.
Acknowledgements
The authors thank American Journal Experts for providing language help.
Abbreviations
- CTLA4
Cytotoxic T-lymphocyte antigen 4
- FDG
18F-fluorodeoxyglucose
- FDS
18F-2-fluorodeoxy sorbitol
- MRI
Magnetic resonance imaging
- PD1
Programmed cell death protein 1
- PET
Positron emission tomography
- TPA
12-O-tetradecanoyl-phorbol-13-acetate
Authors’ contributions
ZK and XC acquired and interpreted the imaging data. YW and WM performed the surgery, made the clinical diagnosis and clinical treatment of the patient. ZK and YW drafted the manuscript. WM and XC critically revised the manuscript. All authors read and approved the final manuscript as well as submission for publication.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81201121), the Beijing Municipal Natural Science Foundation (Grant No. 7202150), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant No. 2016-I2M-2-001 and No. 2018-I2M-3-001). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used during the current study are available on request from the corresponding author.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (committee name: Institute Review Board of Peking Union Medical College Hospital, approval number: S-766) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The manuscript was prepared in accordance with CARE guidelines.
Consent for publication
Written consent is provided from the participant for the publication of this case study and accompanying data.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ziren Kong, Email: kongziren@pumc.edu.cn.
Yu Wang, Email: ywang@pumch.cn.
Wenbin Ma, Email: mawb2001@hotmail.com.
Xin Cheng, Email: pumch_chengxin@126.com.
References
- 1.Sautner D, Saeger W, Ludecke DK, Jansen V, Puchner MJ. Hypophysitis in surgical and autoptical specimens. Acta Neuropathol. 1995;90(6):637–644. doi: 10.1007/BF00318578. [DOI] [PubMed] [Google Scholar]
- 2.Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26(5):599–614. doi: 10.1210/er.2004-0011. [DOI] [PubMed] [Google Scholar]
- 3.Johnston PC, Chew LS, Hamrahian AH, Kennedy L. Lymphocytic infundibulo-neurohypophysitis: a clinical overview. Endocrine. 2015;50(3):531–536. doi: 10.1007/s12020-015-0707-6. [DOI] [PubMed] [Google Scholar]
- 4.Chiloiro S, Tartaglione T, Angelini F, et al. An overview of diagnosis of primary autoimmune Hypophysitis in a prospective single-center experience. Neuroendocrinology. 2017;104(3):280–290. doi: 10.1159/000446544. [DOI] [PubMed] [Google Scholar]
- 5.Basu S. Personalized versus evidence-based medicine with PET-based imaging. Nat Rev Clin Oncol. 2010;7(11):665–668. doi: 10.1038/nrclinonc.2010.121. [DOI] [PubMed] [Google Scholar]
- 6.Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18) F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013;2013:623036. doi: 10.1155/2013/623036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balink H, Bennink RJ, Veeger NJ, van Eck-Smit BL, Verberne HJ. Diagnostic utility of (18) F-FDG PET/CT in inflammation of unknown origin. Clin Nucl Med. 2014;39(5):419–425. doi: 10.1097/RLU.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 8.Kong Z, Jiang C, Zhu R, et al. (18) F-FDG-PET-based radiomics features to distinguish primary central nervous system lymphoma from glioblastoma. NeuroImage. Clinical. 2019;23:101912. doi: 10.1016/j.nicl.2019.101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao S, Xing H, Zhu W, et al. Infection imaging with (18) F-FDS and first-in-human evaluation. Nucl Med Biol. 2016;43(3):206–214. doi: 10.1016/j.nucmedbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein EA, Ordonez AA, DeMarco VP, et al. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med. 2014;6(259):259ra146. doi: 10.1126/scitranslmed.3009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZB, Wu Z, Cao Q, et al. The synthesis of 18F-FDS and its potential application in molecular imaging. Mol Imaging Biol. 2008;10(2):92–98. doi: 10.1007/s11307-007-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesias P, Cardona J, Diez JJ. The pituitary in nuclear medicine imaging. Eur J Intern Med. 2019;68:6–12. doi: 10.1016/j.ejim.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Hou B, Lu L, et al. PET/MRI in the diagnosis of hormone-producing pituitary microadenoma: a prospective pilot study. J Nucl Med. 2018;59(3):523–528. doi: 10.2967/jnumed.117.191916. [DOI] [PubMed] [Google Scholar]
- 14.Obert J, Vercellino L, Van Der Gucht A, et al. (18) F-fluorodeoxyglucose positron emission tomography-computed tomography in the management of adult multisystem Langerhans cell histiocytosis. Eur J Nucl Med Mol Imaging. 2017;44(4):598–610. doi: 10.1007/s00259-016-3521-3. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Wu S, Xu J, Wang H, Ma C. Pituitary 18F-FDG uptake correlates with serum TSH levels in thyroid cancer patients on 18F-FDG PET/CT. Nucl Med Commun. 2019;40(1):57–62. doi: 10.1097/MNM.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju H, Zhou J, Pan Y, Lv J, Zhang Y. Evaluation of pituitary uptake incidentally identified on (18) F-FDG PET/CT scan. Oncotarget. 2017;8(33):55544–55549. doi: 10.18632/oncotarget.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncology. 2018;4(2):173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Hiel B, Blank CU, Haanen JB, Stokkel MP. Detection of early onset of hypophysitis by (18) F-FDG PET-CT in a patient with advanced stage melanoma treated with ipilimumab. Clin Nucl Med. 2013;38(4):e182–e184. doi: 10.1097/RLU.0b013e3182639765. [DOI] [PubMed] [Google Scholar]
- 19.Wachsmann JW, Ganti R, Peng F. Immune-mediated disease in Ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol. 2017;24(1):111–115. doi: 10.1016/j.acra.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Mekki A, Dercle L, Lichtenstein P, et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer (Oxford, England : 1990) 2018;96:91–104. doi: 10.1016/j.ejca.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Werner RA, Wakabayashi H, Chen X, et al. Functional renal imaging with 2-Deoxy-2-(18) F-Fluorosorbitol PET in rat models of renal disorders. J Nucl Med. 2018;59(5):828–832. doi: 10.2967/jnumed.117.203828. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Guo X, Tian R, et al. Pituitary abscess: clinical manifestations, diagnosis and treatment of 66 cases from a large pituitary center over 23 years. Pituitary. 2017;20(2):189–194. doi: 10.1007/s11102-016-0757-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available on request from the corresponding author.