Abstract
Propofol is widely applied in general anesthesia owing to its short effect and rapid recovery. Apart from its anesthetic advantages, propofol has also been observed to inhibit the growth of several types of cancer cells. Breast cancer is the most diagnosed cancer in females worldwide and triple negative breast cancer (TNBC) constitutes 15–20% of all breast cancer cases. TNBC is characterized by a high recurrence rate, which is associated with its high mortality rate. The present study aimed to evaluate apoptosis in MDA-MB-468 cells treated with propofol. The Cell Counting Kit-8 assay was used to assess proliferation in cells treated with different concentrations of propofol. In addition, Annexin V-FITC was used to detect apoptosis. Furthermore, the generation of reactive oxygen species (ROS) was examined. The relative expression of proteins in the intrinsic apoptosis pathway, such as Bak, Bax, Bcl-2, Cytochrome c, apoptotic peptidase-activating factor 1 (Apaf-1), Caspase 3 and Caspase 9, were calculated relative to GAPDH with western blot analysis. A wound healing assay was performed to examine the effect of propofol on MDA-MB-468 cell migration. The present study revealed that propofol inhibited the proliferation and increased the level of ROS in MDA-MB-468 cells. The expression levels of Cytochrome c, Apaf-1, Bax, Bak and cleaved Caspase 3/9 were upregulated compared with GAPDH. The level of Bcl-2 protein was upregulated by propofol at a concentration of 5 µM and downregulated at concentrations of 10 and 20 µM. In the wound-healing assay, propofol reduced the scratch distance and area. Taken together, the results of the present study suggested that propofol may induce ROS-mediated intrinsic apoptosis and promote migration in TNBC cells.
Keywords: propofol, intrinsic apoptosis, reactive oxygen species, migration, triple negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) has distinct pathological features and poor prognosis due to the negative expression of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2) (1). Due to its high aggression, the presently available therapies have limited efficacy (2). TNBC comprises 15–20% of all newly-diagnosed breast cancer cases; however, TNBC accounts for a disproportionate number of breast cancer-associated mortalities, and is responsible for up to 5% of all cancer mortalities annually (3). Notably, the majority of the available studies on TNBC have been conducted in a Western population (4–6); however, TNBC has been reported to occur more frequently in African women. Therefore, identifying effective therapeutics against TNBC remains an urgent challenge. Although genomic profiling of TNBC has shown promise in aiding clinicians to develop personalized targeted agents against TNBC, this research is still in its infancy, and requires more development, prior to its widespread application in a clinical setting (7). At present, surgery, radiotherapy and chemotherapy are the primary treatments for TNBC; however, due to the complexity of the disease, and the rate of recurrence, the curative effects are not satisfactory (8).
Propofol has been used in surgery since 1977, and was created by Kay and Rolly (9). Due to advantages such as less toxicity, rapid induction of anesthesia and no accumulation in vivo, propofol is widely applied in general anesthesia (10). Experimental evidence has revealed that, not only does propofol have multiple anesthetic advantages, it also exerts a number of non-anesthesia effects (11), and the anti-tumor effect of propofol has been confirmed by numerous researchers (12,13). A previous study demonstrated that propofol actively inhibits proliferation and induces apoptosis in chronic myeloid leukemia KBM-7, KU812 and K562 cells (13). In addition, a previous report has revealed that propofol can inhibit the invasion and growth of ovarian cancer ES-2 cells by downregulating the expression of matrix metalloproteinase (MMP)-9 (14). Yang et al (15) suggested that propofol exerts an inhibitory effect on the growth and survival of gastric cancer cells by interfering with the degradation of inhibitor of growth family member 3 (ING3). In pancreatic cancer cells, propofol attenuates cell growth and invasion by inhibiting miR-21 and Slug in a dose- and time-dependent manner (16). Furthermore, extensive evidence has indicated that, in chronic breast cancer, propofol may serve as a novel therapeutic, that functions by inhibiting NF-κB and reducing MMP-2 and MMP-9 (17). In brief, propofol effectively influences several biological processes in the development of cancer, by regulating microRNAs and long non-coding RNAs and modulating signaling pathways, such as hypoxia inducible factor 1 subunit α, MAPK, NF-κB and NF-E2-related factor 2 pathways, which are essential for cell proliferation, invasion and apoptosis (18–20).
It has previously been reported that treatment of MDA-MB-231 cells with propofol resulted in increased cell proliferation and migration in a dose- and time-dependent manner (21). However, other researchers have hypothesized that propofol-induced cell migration and the suppression of invasion are partially mediated by downregulating H19 imprinted maternally expressed transcript (H19) in MDA-MB-231 cells in vitro (22). It is surprising that it shows an opposite effect in the same cell line. Therefore, the present study detected the effect of propofol on TNBC cells. The choice of anesthesia protocol has a specific relationship with the recurrence and metastasis of postoperative tumors (23); however, this mechanism has yet to be completely elucidated. Consequently, the role of propofol in TNBC cells remains unclear, and the aim of the present study was to investigate and explain how propofol influences TNBC cells.
Materials and methods
Reagents
Propofol was purchased from MedChemExpress and was dissolved in dimethyl sulfoxide (DMSO). DMSO was diluted at 1:1,000 in culture medium. N-acetyl-L-cysteine (NAC), which was purchased from Beyotime Institute of Biotechnology, was dissolved in the culture medium at a working concentration of 5 mM.
Cell lines and culture
The human triple-negative breast cancer MDA-MB-468 cell line was purchased from the Shanghai Cell Collection. MDA-MB-468 cells were cultured in Ham's F-12K (Kaighn's) Medium (Gibco; Thermo Fisher Scientific, Inc.) with L-glutamine, supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U ml−1 penicillin at 37°C in a 5% CO2 humidified atmosphere. Trypsin (0.02%)/EDTA (0.02%) solution was used when the confluence reached >80%. Aseptic technique was applied in all experiments.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.) was utilized to assess the cell proliferation of MDA-MB-468 cells according to the manufacturer's protocol. The MDA-MB-468 cells were seeded in 96-well plates at a density of 2×104 cells/well. Cell confluency was permitted to reach ~80%, and the medium was replaced with fresh medium containing 0.1% DMSO or varying concentrations of propofol (0, 5, 10 and 20 µM) or 20 µM propofol with 5 mM NAC. The cells were then incubated for 24 h, and a total of 10 µl CCK-8 solution was added to the cultures for 2 h at 37°C. The absorbance was measured at wavelength of 450 nm with an automatic enzyme analyzer. All experiments were performed in triplicate. The results are presented as the percentages of live cells over control cells.
Apoptosis assay
The Annexin V-FITC apoptosis detection kit (BD Biosciences) was used to detect cell apoptosis according to the manufacturer's protocol. Briefly, cells were treated with different concentrations of propofol (0, 5, 10 and 20 µM) or 20 µM propofol with 5 mM NAC for 24 h and washed twice with PBS. Subsequently, cells were stained with 5 µl Annexin V and 5 µl propidium iodide (BD Biosciences) for 15 min at room temperature. The cells were analyzed using a flow cytometer (BD Biosciences) and the percentage of apoptotic cells was determined using CellQuest software version 7.5.3 (BD Biosciences).
Measurement of the generation of reactive oxygen species (ROS)
The ROS assay kit (Beyotime Institute of Biotechnology) was used to detect the intracellular ROS. MDA-MB-468 cells were plated in 6-well plates at a density of 2×104 cells/well and incubated for 24 h at 37°C Following treatment with various concentrations of propofol (0, 5, 10 and 20 µM) or 20 µM propofol with 5 mM NAC for 24 h at 37°C, cells were washed with PBS three times and incubated with 10 µM DCFH-DA in serum-free medium for 25 min at 37°C in the dark. The cells were then washed with PBS three times to remove excess DCFH-DA that was unable to fully penetrate the cells. The cells were subsequently fixed in 4% paraformaldehyde for 30 min at room temperature, and observed under an inverted fluorescence microscope (magnification, ×200).
Wound healing assay
A wound healing assay was performed to examine the effect of propofol on MDA-MB-468 cell migration. Briefly, MDA-MB-468 cells were seeded in 6-well plates containing medium supplemented with 10% FBS and allowed to form an 80% confluent cell monolayer. A scratch was made using a 200 µl pipette tip and the complete medium was replaced with serum-free medium. The cells were subsequently incubated with various concentrations of propofol (0 or 20 µM) or 20 µM propofol with 5 mM NAC for 24 and 48 h at 37°C. In order to evaluate wound healing, the scratch area was monitored under an inverted Leica phase-contrast microscope (Leica DFC 300 FX; Leica Microsystems, Inc.), calculated using ImageJ software version 1.52a (National Institutes of Health) and analysed using GraphPad Prism software (version 5; GraphPad Software, Inc.). The assay was performed in triplicate.
Western blot analysis
MDA-MB-468 cells were treated with different concentrations of propofol (0, 5, 10 and 20 µM) for 24 h at 37°C. Subsequently, total protein was extracted from cells using the RIPA kit and protein concentrations were quantified using the BCA protein quantification kit (both from Beyotime Institute of Biotechnology). A total of 30 µg protein/lane was separated via SDS-PAGE on a 10% gel and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk in TBST for 1 h at room temperature, prior to incubation with primary antibodies against: Caspase 3 (cat. no. sc-5273; 1:1,000; Santa Cruz Biotechnology, Inc.), Caspase 9 (cat. no. sc-56076; 1:1,000; Santa Cruz Biotechnology Inc.), Bax (cat. no. sc-7480; 1:1,000; Santa Cruz Biotechnology Inc.), Bak (cat. no. 12105; 1:1,000; Cell Signaling Technology, Inc.), Bcl-2 (cat. no. 15071; 1:1,000; Cell Signaling Technology, Inc.), cytochrome c (cat. no. 11940; 1:1,000; Cell Signaling Technology, Inc.) Apaf-1 (cat. no. 8969; 1:1,000; Cell Signaling Technology, Inc.) and GAPDH (cat. no. 5174; 1:5,000; Cell Signaling Technology, Inc.) overnight at 4°C. Membrane was washed three times with TBST and subsequently incubated with anti-rabbit (cat. no. sc-2030) or anti-mouse (cat. no. sc-2031) secondary antibodies (both 1:5,000 and from Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. Protein bands were visualized using the enhanced chemiluminescence reagent (BD Biosciences) and analyzed using Image Lab software v3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
The data are presented as mean ± standard deviation. Statistical analyses were performed using SPSS software (v20; IBM Corp.). The Student's t-test was used to detect the difference between two groups. The one-way ANOVA followed by Tukey's post hoc test was used to compare several groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of propofol on MDA-MB-468 cell proliferation and apoptosis
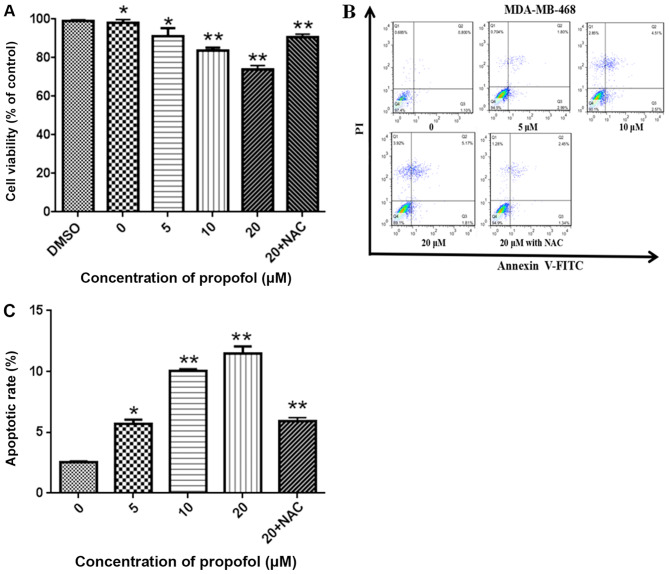
MDA-MB-468 cells were treated with 0.1% DMSO or propofol (5, 10 and 20 µM) or 20 µM propofol with 5 mM NAC for 24 h. As illustrated in Fig. 1A, propofol significantly decreased cell proliferation in a concentration-dependent manner, and this effect was reversed, following treatment with 5 mM NAC (P<0.05). Additionally, treatment with 0.1% DMSO did not affect cell viability (Fig. 1A). Apoptosis was detected MDA-MB-468 cells using an Annexin V-FITC apoptosis detection kit and flow cytometry. The rates of apoptosis following treatment with 5, 10 and 20 µM propofol for 24 h were 5.5±2.1, 9.9±3.4 and 10.9±2.9%, respectively, and were significantly increased compared with control cells (2.6±1.5%; P<0.05). Furthermore, treatment with 5 mM NAC was revealed to attenuate apoptosis induced by 20 µM propofol (Fig. 1B and C).
Figure 1.
Propofol inhibits cell proliferation and induces apoptosis in human triple negative breast cancer cells. (A) A Cell Counting Kit-8 assay was conducted to detect cell proliferation after exposure to propofol for 24 h. Propofol inhibited the proliferation of MDA-MB-468 cells in a dose-dependent manner. (B) Apoptosis in cells treated with propofol was tested using an Annexin V-FITC and PI staining assay and flow cytometry. The rate of apoptosis in cells treated with propofol (5, 10, 20 and 20 µM + NAC) for 24 h was 5.5, 9.5, 10.1 and 5.1%, respectively. (C) The percentage of apoptotic cells in each treatment group was quantified. Values are reported as the mean ± SD (n=3). *P<0.05 and **P<0.01 vs. the control group. PI, propidium iodide; NAC, N-acetyl-L-cysteine.
Effect of propofol on the level of cellular ROS
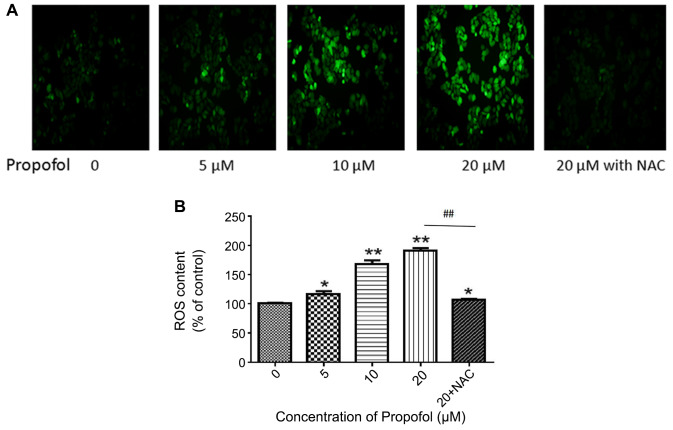
In order to examine the ROS accumulation induced by propofol, and fluorescence microscopy were used to measure the generation of intracellular ROS. The results demonstrated that propofol significantly induced ROS accumulation in MDA-MB-468 cells in a dose-dependent manner, when compared with the control group treated with PBS (P<0.05; Fig. 2). In addition, treatment with 5 mM NAC reduced the amount of ROS induced by 20 µM propofol (P<0.01). The results demonstrated that the brighter cells appeared under the inverted fluorescence microscope, the more ROS were produced, which was indicative of heavier damage to the cells.
Figure 2.
ROS generation in MDA-MB-468 cells is increased following propofol exposure. (A) Intracellular ROS production in MDA-MB-468 cells was measured using a DCF-DA assay. MDA-MB-468 cells were treated with PBS (control) or propofol (5, 10, 20 and 20 µM + NAC) for 24 h. Cellular ROS production was observed using an inverted fluorescence microscope. (B) Quantification of ROS production following propofol treatment. Data are presented as the mean ± SD (n=3). *P<0.05 and **P<0.01 vs. the control group. Compared with the 20 µM group, the 20 µM + NAC group showed a significant difference (##P<0.01). ROS, reactive oxygen species; NAC, N-acetyl-L-cysteine.
Effect of propofol on the endogenous apoptotic pathway
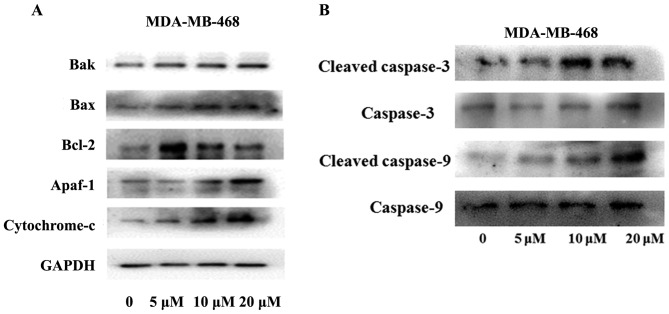
As shown in Fig. 3A the expression of Bax and Bak was increased while that of Bcl-2 was decreased in cells treated with propofol (P<0.05). Apaf-1 and Cytochrome c were increased in a concentration-dependent manner after propofol treatment (Fig. 3B; P<0.05). Fig. 3B revealed that the expression of cleaved caspase-3 and cleaved caspase-9 in MDA-MB-468 cells was increased in a concentration-dependent manner following treatment with propofol (P<0.05).
Figure 3.
MDA-MB-468 cells were treated with PBS (control) or propofol for 24 h. The expression levels of (A) Bak, Bax, Bcl-2, Cytochrome C, Apaf-1 and (B) Cleaved caspase- 3/9 and Caspase- 3/9 were measured by western blotting. In MDA-MB-468 cells treated with 5, 10 and 20 µM propofol for 24 h, the protein expression of the apoptotic proteins Bax, Bak, Apaf-1 and Cytochrome C was upregulated compared with the control group treated with PBS for the same time. Similarly, propofol increased the expression of cleaved caspase- 3/9 in MDA-MB-468 cells in a dose-dependent manner. Propofol-induced Bcl-2 upregulation; however, expression was decreased at high concentrations. The ratio of Bax to Bcl-2 suggested that propofol has exhibits a proapoptotic effect. Figures showed that the GAPDH was used as an internal control. Data are presented as the mean ± standard deviation (n=3). Apaf-1, apoptotic peptidase-activating factor 1.
Effect of propofol on MDA-MB-468 cell migration
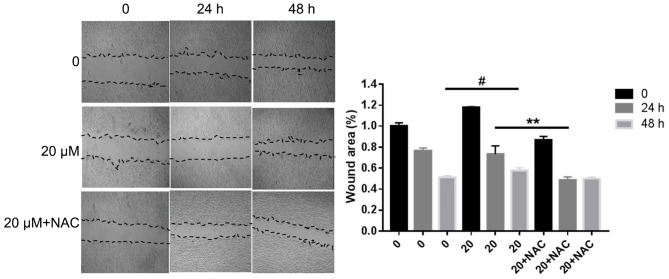
In order to examine the effect of propofol on MDA-MB-468 cell migration, a wound healing assay was performed. The experimental results revealed that the scratch distance became narrower and the scratch area became smaller in a dose and time-dependent manner (P<0.05). However, treatment with 5 mM NAC partially reversed the effect of 20 µM propofol. The analysis was performed relative to the starting wound width at 0 h. The results shown in Fig. 4 suggested that propofol promoted the migration of MDA-MB-468 cells.
Figure 4.
MDA-MB-468 cells were seeded in 6-well plates and allowed to reach a confluent monolayer. The cells were incubated with various concentrations of propofol for 0, 24 or 48 h. Cells were also incubated with 20 µM propofol + NAC for 48 h. (A) Wound healing assay and (B) analysis performed relative to the starting wound width at 0 h. Data are represented as the mean ± SD (n=3). #P<0.05 (0 vs. 20 µM propofol for 48 h) **P<0.01 (20 µM propofol vs. 20 µM propofol + NAC for 48 h). NAC, N-acetyl-L-cysteine.
Discussion
The treatment of TNBC is challenging due to its aggressiveness, cellular and histological heterogeneity and complexity of its molecular mechanisms (24). Recent studies have demonstrated that, not only do certain anesthetics function as sedatives and analgesics, but can also serve a pivotal role in antitumor and immunoregulation (25). Among these, propofol has been the focus of an increasing amount of interest, due to its correlative studies. The active component in propofol is 2, 6-diisopropylphenol (26). The present study investigated the effect of propofol on the proliferation and apoptosis of MDA-MB-468 cells, and attempted to elucidate the underlying molecular mechanism of action.
The results of the present study revealed that propofol inhibited proliferation and promoted the ROS-mediated endogenous apoptosis pathway in MDA-MB-468 cells. Initially, cell proliferation was inhibited following treatment with propofol; however, this effect was reversed by NAC. In addition, the rate of apoptosis was increased with increasing propofol concentration, and 5 mM NAC attenuated apoptosis induced by 20 µM propofol. The present study examined the expression of important proteins in the endogenous apoptotic pathway. It is well established that there are two general categories of apoptotic pathways, namely endogenous and exogenous. In the endogenous apoptotic pathway, Cytochrome c is released into the cytoplasm by enhanced mitochondrial transition pore opening under the effect of drugs. Cytochrome c in the cytoplasm combines with Apaf-1, contributing to the formation of a polymer (27). There is an upstream and downstream relationship between the initiators of apoptosis (Caspase 2, 8, 9 and 10) and the executors (Caspase 3, 6 and 7), and a previous study demonstrated that the initiators could activate the executors (28). Caspase 3 and Caspase 9 are closely associated with apoptosis, and once apoptosis is initiated, the caspase cascade is initiated by apoptotic proteases, which induces irreversible apoptosis. Apoptosis is strictly regulated, and the normal cell caspases are in the non-activated zymogen state (29). In the present study, following treatment with propofol, the expression of the pro-apoptotic protein Bax, was increased, and as the concentration of propofol was increased, the apoptotic effect was enhanced accordingly. Notably, the level of Bcl-2 was increased with 5 µM propofol; however, the expression of Bcl-2 was decreased when cells were treated with 10 and 20 µM propofol. It was hypothesized that propofol may partially result in the activation of antiapoptotic systems at a concentration of 5 µM.
ROS generated by cellular metabolism readily oxidize adjacent molecules and participate in various signal transduction pathways in cells (30). An excessive amount of ROS can cause oxidative damage to important molecules in the cell. When ROS are excessively produced, or ROS scavenging is reduced, the redox level is unbalanced, resulting in oxidative stress damage to the body (31). In the present study, the level of ROS increased with increasing concentrations of propofol, and NAC reduced the level of ROS. ROS may promote the formation of a monolayer on the mitochondrial surface of the antiapoptotic protein Bcl-2, and activates the proapoptotic protein Bax, which results in the formation of pores in the mitochondrial membranes, resulting in the leakage of Cytochrome c. Cytochrome c binds to Apaf-1 to form apoptotic bodies, which then bind to and activate Caspase-9, leading to cell death (32). Apoptosis and cell proliferation are essential cellular processes, and are also the basic measurements used to evaluate cell dynamics and cell numbers in vivo (33,34). The present study investigated the effect of propofol on the migration MDA-MB-468 and revealed that propofol increased migration. It has been confirmed that ROS from various sources serve an important role in the malignant proliferation and migration of tumor cells; however, its mechanism of action has not been completely elucidated (35,36). ROS may activate ataxia telangiectasia mutated directly or indirectly, thereby activating IL-8 and promoting tumor cell migration (37).
To the best of our knowledge, the present study was the first to report that propofol may promote the activation of the endogenous apoptotic pathway by inducing the production of ROS, leading to decreased MDA-MB-468 cell proliferation and increased migration. The results suggested that propofol may be a promising therapeutic choice for patients with TNBC following surgery. Future studies should explore whether there are other signaling pathways and molecules involved in the effects of propofol on apoptosis and migration in TNBC cells, and whether it has an antitumor effect in the body. Once this information is obtained and verified, propofol may be considered for use as an anticancer agent in the clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was funded by the project from Shanghai Municipal Health Commission and Shanghai Municipal Administrator of Traditional Chinese Medicine Planning (grant no. ZY(2018–2020) CCCX-1002), the Shanghai Foundation for Development of Science and Technology (grant no. 17401901200), the National Natural Science Foundation of China (grant no. 81570293) and the Scientific Research Foundation of Shanghai Songjiang District Committee of Science and Technology (grant no. 2019sjkjgg012).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
SW and JH designed the present study. HW, LZ and JW performed the experiments, while HW, LZ and JH analyzed the data. HW, LZ and SW drafted the initial manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ha GH, Kim DY, Breuer EK, Kim CK. Combination treatment of polo-like kinase 1 and tankyrase-1 inhibitors enhances anticancer effect in triple-negative breast cancer cells. Anticancer Res. 2018;38:1303–1310. doi: 10.21873/anticanres.12352. [DOI] [PubMed] [Google Scholar]
- 2.Wahdan-Alaswad R, Fan Z, Edgerton SM, Liu B, Deng XS, Arnadottir SS, Richer JK, Anderson SM, Thor AD. Glucose promotes breast cancer aggression and reduces metformin efficacy. Cell Cycle. 2013;12:3759–3769. doi: 10.4161/cc.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yardley DA, Brufsky A, Coleman RE, Conte PF, Cortes J, Gluck S, Nabholtz JM, O'Shaughnessy J, Beck RM, Ko A, et al. Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): Study protocol for a randomized controlled trial. Trials. 2015;16:575. doi: 10.1186/s13063-015-1101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukong KE, Ogunbolude Y, Kamdem JP. Breast cancer in Africa: Prevalence, treatment options, herbal medicines, and socioeconomic determinants. Breast Cancer Res Treat. 2017;166:351–365. doi: 10.1007/s10549-017-4408-0. [DOI] [PubMed] [Google Scholar]
- 5.Abdulrahman GJ, Jr, Rahman GA. Epidemiology of breast cancer in europe and Africa. J Cancer Epidemiol. 2012;2012:915610. doi: 10.1155/2012/915610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz H, Hussain F, Sohn C, Mediavillo R, Saitta A, Hussain A, Brandys M, Homel P, Rotman M. Early onset of breast carcinoma in African American women with poor prognostic factors. Am J Clin Oncol. 1999;22:436–440. doi: 10.1097/00000421-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Cao J, Chen Z, Chi J, Sun Y, Sun Y. Recent progress in synergistic chemotherapy and phototherapy by targeted drug delivery systems for cancer treatment. Artif Cells Nanomed Biotechnol. 2018;46:817–830. doi: 10.1080/21691401.2018.1436553. [DOI] [PubMed] [Google Scholar]
- 8.Wilson TR, Udyavar AR, Chang CW, Spoerke JM, Aimi J, Savage HM, Daemen A, O'Shaughnessy JA, Bourgon R, Lackner MR. Genomic Alterations Associated with recurrence and TNBC subtype in high-risk early breast cancers. Mol Cancer Res. 2019;17:97–108. doi: 10.1158/1541-7786.MCR-18-0619. [DOI] [PubMed] [Google Scholar]
- 9.Kay B, Rolly G. I.C.I. 35868, a new intravenous induction agent. Acta Anaesthesiol Belg. 1977;28:303–316. [PubMed] [Google Scholar]
- 10.Dietrich SK, Mixon MA, Rogoszewski RJ, Delgado SD, Knapp VE, Floren M, Dunn JA. Hemodynamic effects of propofol for induction of rapid sequence intubation in traumatically injured patients. Am Surg. 2018;84:1504–1508. [PubMed] [Google Scholar]
- 11.Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, Papadimitriou L. Propofol: A review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2017;124:1697–1708. doi: 10.1213/ANE.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 13.Tan Z, Peng A, Xu J, Ouyang M. Propofol enhances BCR-ABL TKIs' inhibitory effects in chronic myeloid leukemia through Akt/mTOR suppression. BMC Anesthesiol. 2017;17:132. doi: 10.1186/s12871-017-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Teng Y, Yang H, Ma J. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-κB signal. Braz J Med Biol Res. 2016;49:e5717. doi: 10.1590/1414-431x20165717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Gao J, Yan N, Wu B, Ren Y, Li H, Liang J. Propofol inhibits the growth and survival of gastric cancer cells in vitro through the upregulation of ING3. Oncol Rep. 2017;37:587–593. doi: 10.3892/or.2016.5218. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Zhang J, Hong G, Quan J, Zhang L, Yu M. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res. 2016;8:4120–4133. [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Zhang L, Han Y, Jiang Z, Wang Q. Propofol reduces MMPs expression by inhibiting NF-κB activity in human MDA-MB-231 cells. Biomed Pharmacother. 2012;66:52–56. doi: 10.1016/j.biopha.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Yang N, Liang Y, Yang P, Ji F. Propofol suppresses LPS-induced nuclear accumulation of HIF-1α and tumor aggressiveness in non-small cell lung cancer. Oncol Rep. 2017;37:2611–2619. doi: 10.3892/or.2017.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Wang Y, Zhu Z, Zheng Y, Song B. Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. Int J Biol Macromol. 2018;120:975–984. doi: 10.1016/j.ijbiomac.2018.08.173. [DOI] [PubMed] [Google Scholar]
- 20.Du QH, Xu YB, Zhang MY, Yun P, He CY. Propofol induces apoptosis and increases gemcitabine sensitivity in pancreatic cancer cells in vitro by inhibition of nuclear factor-κB activity. World J Gastroenterol. 2013;19:5485–5492. doi: 10.3748/wjg.v19.i33.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng C, Song L, Wang J, Li D, Liu Y, Cui X. Propofol induces proliferation partially via downregulation of p53 protein and promotes migration via activation of the Nrf2 pathway in human breast cancer cell line MDA-MB-231. Oncol Rep. 2017;37:841–848. doi: 10.3892/or.2016.5332. [DOI] [PubMed] [Google Scholar]
- 22.Bai JJ, Lin CS, Ye HJ, Guo PP, Wang W. Propofol suppresses migration and invasion of breast cancer MDA-MB-231 cells by down-regulating H19. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:1255–1259. (in Chinese) [PubMed] [Google Scholar]
- 23.Grandhi RK, Lee S, Abd-Elsayed A. The relationship between regional anesthesia and cancer: A metaanalysis. Ochsner J. 2017;17:345–361. [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez BR, Ortega GA, Hidalgo MA, Zentella DA, Villarreal-Garza C, Avila-Moreno F, Arrieta O. Long non-coding RNAs: Implications in targeted diagnoses, prognosis, and improved therapeutic strategies in human non- and triple-negative breast cancer. Clin Epigenetics. 2018;10:88. doi: 10.1186/s13148-018-0514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Liu H, Dilger JP, Lin J. Effect of Propofol on breast Cancer cell, the immune system, and patient outcome. Bmc Anesthesiol. 2018;18:77. doi: 10.1186/s12871-018-0543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker MT. The anticonvulsant effects of propofol and a propofol analog, 2,6-diisopropyl-4-(1-hydroxy-2,2,2-trifluoroethyl)phenol, in a 6 Hz partial seizure model. Anesth Analg. 2011;112:340–344. doi: 10.1213/ANE.0b013e3182025b30. [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim Biophys Acta. 1998;1366:139–149. doi: 10.1016/S0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 28.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2015;7(pii):a026716. doi: 10.1101/cshperspect.a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopeina GS, Prokhorova EA, Lavrik IN, Zhivotovsky B. Alterations in the nucleocytoplasmic transport in apoptosis: Caspases lead the way. Cell Prolif. 2018;51:e12467. doi: 10.1111/cpr.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23(pii):E965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Kim YJ, Seo YR. An overview of carcinogenic heavy metal: Molecular Toxicity mechanism and prevention. J Cancer Prev. 2015;20:232–240. doi: 10.15430/JCP.2015.20.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seervi M, Rani A, Sharma AK, Santhosh Kumae TR. ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine. Biomed Pharmacother. 2018;106:200–209. doi: 10.1016/j.biopha.2018.06.123. [DOI] [PubMed] [Google Scholar]
- 33.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Lin XL, Yang L, Fu SW, Lin WF, Gao YJ, Chen HY, Ge ZZ. Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget. 2017;8:33586–33600. doi: 10.18632/oncotarget.16829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park GB, Kim D. PI3K catalytic isoform alteration promotes the LIMK1-related metastasis through the PAK1 or ROCK1/2 activation in cigarette smoke-exposed ovarian cancer cells. Anticancer Res. 2017;37:1805–1818. doi: 10.21873/anticanres.11515. [DOI] [PubMed] [Google Scholar]
- 37.Chen WT, Ebelt ND, Stracker TH, Xhemalce B, Van Den Berg CL, Miller KM. ATM regulation of IL-8 links oxidative stress to cancer cell migration and invasion. Elife. 2015;4 doi: 10.7554/eLife.07270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.