Abstract
Objective:
To determine whether visual aids help people recall quantitative efficacy information in direct-to-consumer (DTC) prescription drug advertisements, and if so, which types of visual aids are most helpful.
Methods:
Individuals diagnosed with high cholesterol (n = 2504) were randomized to view a fictional DTC print or television advertisement with no visual aid or one of four visual aids (pie chart, bar chart, table, or pictograph) depicting drug efficacy. We measured drug efficacy and risk recall, drug perceptions and attitudes, and behavioral intentions.
Results:
For print advertisements, a bar chart or table, compared with no visual aid, elicited more accurate drug efficacy recall. The bar chart was better at this than the pictograph and the table was better than the pie chart.
For television advertisements, any visual aid, compared with no visual aid, elicited more accurate drug efficacy recall. The bar chart was better at this than the pictograph or the table.
Conclusion:
Visual aids depicting quantitative efficacy information in DTC print and television advertisements increased drug efficacy recall, which may help people make informed decisions about prescription drugs.
Practice implications:
Adding visual aids to DTC advertising may increase the public’s knowledge of how well prescription drugs work.
Keywords: quantitative information, direct-to-consumer advertising, communication
Introduction
Efficacy claims in direct-to consumer (DTC) prescription drug advertisements (ads) are often simply variants of the drug’s indication that do not inform patients of the likelihood that they will benefit from taking the drug [1, 2]. Previous research has demonstrated that people who receive quantitative drug efficacy information (for instance, how many clinical trial participants experienced the drug’s intended outcome compared with placebo) can use it to recall the drug’s effectiveness and make better treatment decisions [3-7]. Thus, including visual aids in DTC ads might increase patients’ knowledge of the advertised drug’s efficacy, as visual aids have been shown to help in other contexts [8, 9]. Patients, in consultation with a healthcare professional, must make an assessment of the benefits and the risks of taking the drug, by weighing the likelihood that the drug will be effective versus the likelihood of adverse events or side effects caused by the drug. Knowledge of drug efficacy is crucial to this benefit-risk assessment.
Numerous studies in the risk communication field have examined visual aids. This literature suggests that the addition of visual aids, such as pictographs (icon arrays showing the number of people at risk in a population) [10], often increases individuals’ understanding of quantitative risk information [8, 9]. It also suggests that not all visual aids are equally helpful; for instance, pie charts may only help when people are comparing proportions [11]. Nevertheless, many studies have focused on risk avoidance rather than on weighing risks versus benefits [12-14]. Because prescription drugs have both risks (e.g., side effects such as joint pain) and benefits (e.g., lowering bad cholesterol levels), the goal in this context is not risk avoidance but rather an accurate understanding of the product’s benefits and risks that can lead to an informed decision. Moreover, the literature focuses almost exclusively on communicating risk information, and it is important to determine whether these risk findings translate to the communication of efficacy.
Additionally, to our knowledge, this previous research was conducted exclusively with static modalities (e.g., print materials). Given the frequency of DTC advertising on television and in online videos, it is important to determine if visual aids work across static and dynamic modalities. The mental processing required may differ between print and television ads because television ads include both audio and visuals and display at a pre-determined, rather than self-paced, speed [15].
Finally, the literature suggests a complicated relationship between visual aids and numeracy. Although some studies have shown visual aids to be particularly helpful to individuals with low numeracy skills [16], others have shown that visual aids were not helpful to these individuals [17]. However, past research [18] has generally shown that simplifying visual displays makes logical sense when presenting it to individuals with different levels of numeracy.
The purpose of this study was to expand this evidence base by evaluating visual aids that present quantitative efficacy information within DTC drug advertisements. The study was guided by the following research questions:
Do visual aids help people recall quantitative efficacy information in DTC print and television ads?
If so, which types of visual aids are most helpful?
We hypothesized that bar charts, tables, and pictographs would improve knowledge of efficacy information, whereas pie charts would not [11]. We also hypothesized that numeracy would moderate the effects of visual aids.
2. Methods
2.1. Design overview
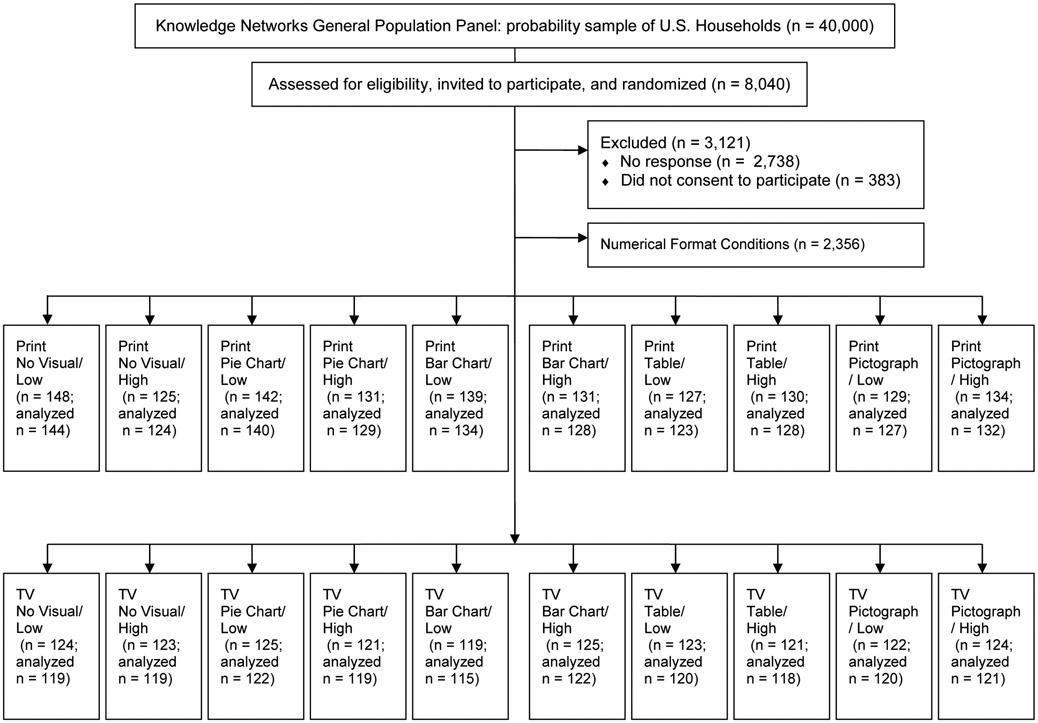
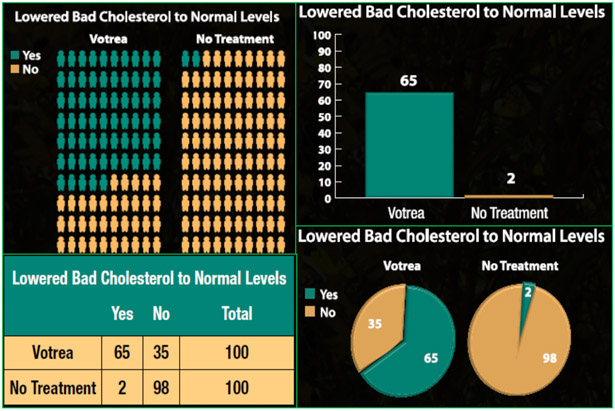
We implemented a randomized, controlled study (Fig. 1) exposing participants to 1 of 20 experimental conditions, each containing a DTC ad for a fictitious prescription cholesterol drug (Votrea). The ads contained quantitative information on the drug’s efficacy (for examples see Figs. 2 and 3). The independent variables in this study were ad type (print, television), efficacy level (high, low), and visual aid (pie chart, bar chart, table, pictograph, no visual aid; Fig. 4). We selected these visual aids based on previous research examining the effect of various visual aids on risk perceptions and comprehension [16, 17, 19].
Figure 1.
Study Flow Diagram
Note. All differences between number allocated and number analyzed were due to participants who were unable to view the ad. Low = low efficacy. High = High efficacy. Numerical format conditions were part of a separate study not reported here.
Figure 2.
Sample Fictitious Drug Print Advertisement
Figure 3.
Sample Screenshot from Fictitious Drug Television Advertisement
Figure 4.
Sample Visual Aids. Clockwise from top left: pictograph, bar chart, pie chart, table.
2.2. Setting and participants
The study’s sampling frame was Knowledge Networks’ (now GfK) nationally representative online consumer panel of U.S. adults [20, 21]. We simultaneously invited a total of 8,040 panelists who had previously reported a diagnosis of high cholesterol to participate in this study or another study reported elsewhere [7]. These panelists were randomly assigned to an experimental condition prior to response or consent. Of those panelists, 4,919 provided informed consent for one of the two separate studies, including 2,563 panelists who were randomly assigned to the conditions in this study. For this study, we analyzed data for 2,504 participants who were able to view the ads (Fig 1). The sample size was based on a priori power analyses. The study was conducted in November and December 2010.
2.3. Procedure
Participants with broadband Internet connections were eligible for random assignment to either the print or television conditions. Because of bandwidth and speed restrictions, participants without confirmed broadband Internet (n = 349) were assigned to the print conditions only. Within ad type, participants were randomly assigned to one of the visual aid and efficacy conditions.
Participants viewed either a print ad or a 90-second television ad for Votrea. The two-page print ad contained a display page with a photo and the study manipulations and a page listing important information about the drug (the brief summary). The actors and visual setting were identical in the print and television ads.
All ads contained quantitative efficacy information presented in an absolute frequency format. Because the drug’s efficacy level could influence how people process and use the information, we varied whether the ad presented a drug with high efficacy or low efficacy: “65 out of 100 people [10 out of 100 people] who used Votrea lowered their bad cholesterol to normal levels (compared with 2 out of 100 who received no treatment).” We varied whether the ad contained no visual aid, a pie chart, a bar chart, a table, or a pictograph. The drug risk information, a section listing the risks, was the same across conditions and contained no quantitative information (see the “Important risk information” section of Fig. 2).
In the print ad conditions, participants could navigate back and forth between the pages without time restrictions. In the television conditions, participants viewed the ad twice in succession. After viewing the ad, participants completed a cognitively tested and pretested questionnaire. Participants were not allowed to proceed to the questionnaire without viewing the ad, and they were not permitted to view the ad again after beginning the questionnaire. Finally, we debriefed participants about the experiment by explaining that Votrea is fictitious.
2.4. Outcomes
2.4.1. Efficacy accuracy
We measured whether participants could accurately report (1) the number of people who received the drug who benefited (“absolute frequency”), (2) the percentage of people who received the drug who benefited (“percent”), (3) the number of people in the control group who benefited (“placebo”), and (4) the ratio of the number of people who received the drug who benefited to the number of people in the control group who benefited (“relative frequency.”) Note that absolute frequency and placebo questions corresponded to the information participants were given; thus, these were recall questions. Percent and relative frequency questions required participants to transform information they were given to determine the correct answer. All questions were open-ended, and we coded responses to these efficacy accuracy measures as correct or incorrect (see Table 2 for question wording and correct responses).
Table 2.
Drug efficacy accuracy by visual aids.a
| Correct Response | Percent Who Gave the Correct Response b | |||||||
|---|---|---|---|---|---|---|---|---|
| Question | Low Efficacy |
High Efficacy |
Pie Chart |
Bar Chart |
Table | Pictograph | No Visual |
|
| Print Ad | ||||||||
| N | 269 | 262 | 251 | 259 | 268 | |||
| Absolute frequency | If 100 people take Votrea, how many will lower their bad cholesterol to normal levels? | 10 | 65 | 41.5 | 52.8*♦ | 51.9 | 36.5 | 37.7 |
| Percent | What percentage (%) of people who take Votrea will lower their bad cholesterol to normal levels? | 10 | 65 | 38.5 | 51.0*♦ | 48.1* | 35.1 | 34.1 |
| Relative frequency | How many times more effective is Votrea than no treatment in lowering bad cholesterol? | 5 | 32-33 | 17.4 | 21.0* | 22.0* | 15.8 | 11.1 |
| Placebo | If 100 people receive no treatment, how many will lower their bad cholesterol to normal levels? | 2 | 2 | 38.5^ | 49.2* | 58.0* | 46.8 | 33.0 |
| Television Ad | ||||||||
| N | 241 | 237 | 238 | 241 | 238 | |||
| Absolute frequency | If 100 people take Votrea, how many will lower their bad cholesterol to normal levels? | 10 | 65 | 56.2* | 68.6*♦^ | 52.2* | 47.9* | 27.8 |
| Percent | What percentage (%) of people who take Votrea will lower their bad cholesterol to normal levels? | 10 | 65 | 52.4* | 65.6*♦ | 51.0* | 44.9* | 26.6 |
| Relative frequency | How many times more effective is Votrea than no treatment in lowering bad cholesterol? | 5 | 32-33 | 28.2 | 31.9*♦ | 19.8 | 19.2 | 19.8 |
| Placebo | If 100 people receive no treatment, how many will lower their bad cholesterol to normal levels? | 2 | 2 | 61.7* | 72.0* | 66.7* | 61.9* | 31.4 |
Accuracy was measured as the percentage who reported the correct response.
Percentages are based on weighted data.
Significant difference compared with the no visual (control) condition (P < 0.005).
Significant difference compared with the pictograph condition (P < 0.005).
Significant difference compared with the table condition (P < 0.005).
2.4.2. Perceived efficacy and perceived risk
We asked two questions about perceived drug efficacy (“Based on the information in the ad, how effective would Votrea be for you?” and “Based on the information in the ad, how well would Votrea work for you?”) and perceived drug risk (“Based on the information in the ad, how safe would Votrea be for you?” and “Based on the information in the ad, how risky would Votrea be for you?”) on 7-point Likert scales (1 = not at all, 7 = very). Cronbach’s alphas were high for perceived efficacy (0.96, print; 0.97, television) and perceived risk (0.82, print; 0.79, television) measures; therefore, we created composite measures by averaging the two items for perceived efficacy and the two items for perceived risk.
2.4.3. Benefit-risk assessment and attitude toward the drug
We measured benefit-risk assessment and attitude toward the drug, respectively, by asking participants to respond to the statements “Thinking overall about the risk and benefits, would you say Votrea has…” (1 = more risks than benefits, 7 = more benefits than risks) and “How good or bad do you feel about this product?” (1 = very bad, 5 = very good).
2.4.4. Behavioral intentions
Behavioral intentions were measured by asking participants how likely they were to do three behaviors (1 = not at all likely, 4 = extremely likely): “Talk to your doctor about Votrea,” “Look for more information about Votrea,” and “Take Votrea if prescribed.”
2.4.5. Unaided and aided risk recall
We measured participants’ recall of drug risks (see the “Important risk information” section of Fig. 2). As a measure of unaided risk recall, participants listed the risks of Votrea in an open-ended text box. Independent raters coded responses into three categories: no risks recalled, incorrect risks recalled only, or one or more correct risks recalled (inter-rater reliability: 0.96). As a measure of aided risk recall, participants saw eight statements about Votrea, such as “People with liver problems should not take Votrea,” and reported whether each statement was mentioned in the ad as a risk of taking Votrea (yes/no). We summed the number of correct responses.
2.4.6. Numeracy
We measured numeracy by averaging the number of correct responses to three fill-in-the-blank math questions, which have been used to measure numeracy in previous research [22]. Participants also reported their demographic characteristics.
2.5. Analysis
All analyses were conducted with weighted data using SAS 9.2 and SUDAAN 10.0. Weighting was used to ensure that the study data more accurately represented the target population (U.S. adults with high cholesterol) and helped account for nonresponse, noncoverage, underrepresentation of minority groups, and other types of sampling and survey error. Because the samples were not comparable (e.g., participants without confirmed broadband Internet were assigned to the print conditions only), we analyzed the data separately for print and television ads.
We used chi-square tests and logistic regression models to assess whether visual aids and drug efficacy level affected efficacy accuracy and unaided risk recall, and we examined t-test pairwise comparisons to identify differences between visual aid conditions. We used ANOVAs to test whether perceived efficacy, perceived risk, attitude toward the drug, benefit-risk assessment, behavioral intentions, and aided risk recall differed by visual aid type and drug efficacy level. The logistic regressions and ANOVAs were fitted with both main effects and their interaction. Because efficacy level did not change the effect of visual aids, we present only a summary of the efficacy level results. Significance was defined as P < 0.05. For contrasts comparing among visual aid conditions, significance was defined with a Bonferroni adjusted P < 0.005 (0.05/10 comparisons).
To test whether numeracy moderated the effect of visual aids, we repeated the relevant analyses with numeracy included as a covariate and examined an interaction term between numeracy and visual aids.
3. Results
The following section summarizes the study’s results, including participant characteristics and the effects of visual aids, drug efficacy level, and numeracy.
3.1. Participants
All participants were U.S. adults who self-reported that they had been medically diagnosed with high cholesterol. Most participants were 45 years of age or older, White and Non-Hispanic, and had some college education or more. Approximately half were female (Table 1).
Table 1.
Weighted and non-weighted participant characteristics.
| 2,504 (100%) | ||
|---|---|---|
| Print Ad Conditions n (%) |
Television Ad Conditions n (%) |
|
| High Cholesterol Diagnosis | 1,309 (100%) | 1,195 (100%) |
| Currently Taking Prescription Drug for High Cholesterol | 964 (73.8%) 989 (75.7%) |
837 (70.2%) 864 (72.4%) |
| Sex | ||
| Male | 650 (49.7%) 647 (49.4%) |
583 (48.8%) 555 (46.4%) |
| Female | 659 (50.3%) 662 (50.6%) |
612 (51.2%) 640 (53.6%) |
| Age | ||
| 18–24 | 1 (<1%) 1 (<1%) |
9 (<1%) 5 (<1%) |
| 25–34 | 31 (2.4%) 16 (1.2%) |
37 (3.1%) 29 (2.4%) |
| 35–44 | 122 (9.3%) 82 (6.3%) |
112 (9.4%) 89 (7.4%) |
| 45–54 | 308 (23.5%) 194 (14.8%) |
273 (22.8%) 245 (20.5%) |
| 55–64 | 365 (27.9%) 491 (37.5%) |
330 (27.6%) 420 (35.2%) |
| 65–74 | 339 (25.9%) 375 (28.6%) |
336 (28.1%) 316 (26.4%) |
| 75+ | 143 (10.9%) 150 (11.5%) |
97 (8.1%) 91 (7.6%) |
| Race/Ethnicity | ||
| White, Non-Hispanic | 988 (75.5%) 1,125 (85.9%) |
897 (75.1%) 997 (83.4%) |
| Black, Non-Hispanic | 163 (12.5%) 86 (6.6%) |
106 (8.9%) 73 (6.1%) |
| Other, Non-Hispanic | 46 (3.5%) 29 (2.2%) |
64 (5.4%) 41 (3.4%) |
| Hispanic | 68 (5.2%) 44 (3.4%) |
85 (7.1%) 55 (4.6%) |
| Multiracial, Non-Hispanic | 44 (3.4%) 25 (1.9%) |
43 (3.6%) 29 (2.4%) |
| Education* | ||
| Less than High School | 76 (5.8%) 45 (3.4%) |
25 (2.1%) 23 (1.9%) |
| High School | 266 (20.3%) 223 (17%) |
200 (16.7%) 206 (17.2%) |
| Some College | 460 (35.1%) 475 (36.3%) |
428 (35.8%) 408 (34.1%) |
| Bachelor’s Degree or Higher | 507 (38.7%) 566 (43.2%) |
543 (45.4%) 558 (46.7%) |
Note: Non-weighted data are italicized.
Education level is significantly different between the print and television ad conditions (P < 0.001).
3.2. Did visual aids help people recall quantitative efficacy information in DTC print and television ads?
In the print ad conditions, participants who viewed an ad with a bar chart or table were more likely to report the efficacy information accurately in response to the absolute frequency (bar chart only), percent, relative frequency, and placebo questions than participants who did not view a visual aid (Tables 2 and 3). The pie chart and pictograph conditions did not significantly differ from the no visual conditions.
Table 3.
Test statistics from analyses comparing each visual aid with the no visual condition on accuracy measures.
| Accuracy Measure | Pie chart vs. No visual |
Bar chart vs. No visual |
Table vs. No visual |
Pictograph vs. No visual |
|---|---|---|---|---|
| Print Ad | ||||
| Absolute frequency | 0.80, P = 0.42 | 2.95, P = 0.003 | 2.74, P = 0.006 | −0.01, P = 0.92 |
| Percent | 0.96, P = 0.34 | 3.40, P = 0.001 | 2.81, P = 0.005 | 0.36, P = 0.72 |
| Relative frequency | 2.07, P = 0.04 | 2.93, P = 0.003 | 3.11, P = 0.002 | 1.70, P = 0.09 |
| Placebo | 0.91, P = 0.36 | 2.85, P = 0.004 | 4.23, P < 0.001 | 2.38, P = 0.02 |
| Television Ad | ||||
| Absolute frequency | 5.45, P < 0.001 | 7.57, P < 0.001 | 4.68, P < 0.001 | 3.99, P < 0.001 |
| Percent | 5.01, P < 0.001 | 7.26, P < 0.001 | 4.70, P < 0.001 | 3.69, P < 0.001 |
| Relative frequency | 1.91, P = 0.06 | 2.81, P = 0.005 | −0.01, P = 0.99 | −0.13, P = 0.90 |
| Placebo | 5.66, P < 0.001 | 7.26, P < 0.001 | 6.57, P < 0.001 | 5.73, P < 0.001 |
Note: Accuracy (absolute frequency, percentage, relative frequency, and placebo) was measured as the percentage who reported the correct quantitative efficacy information. Bonferroni-adjusted significance was defined as P < 0.005. Print condition t-tests had 1,308 degrees of freedom. Television condition t-tests had 1,194 degrees of freedom.
In the television ad conditions, participants who viewed an ad with any visual aid were more likely to report the efficacy information accurately in response to the absolute frequency, percent, and placebo questions than participants who did not view a visual aid (Tables 2 and 3). Participants who viewed an ad with a bar chart were also more likely to report the efficacy information accurately in response to the relative frequency question than participants who did not view a visual aid.
3.3. Which visual aids were most helpful?
In the print conditions, the overall Pearson chi-square test was significant for the absolute frequency (χ2(4) = 3.83, P = 0.004), percent (χ2(4) = 3.88, P = 0.004), and placebo questions (χ2(4) = 5.58, P < 0.001); therefore, we examined pairwise comparisons among visual aids for each of these questions. Participants who viewed an ad with a bar chart, compared with participants who viewed an ad with a pictograph, were more likely to report the efficacy information accurately in response to the absolute frequency and percent questions (Tables 2 and 4). Participants who viewed an ad with a table, compared with participants who viewed an ad with a pie chart, were more likely to report the efficacy information accurately in response to the placebo question.
Table 4.
Test statistics from analyses comparing among visual aids on accuracy measures.
| Pie chart vs. | Bar chart vs. | Table vs. | ||||
|---|---|---|---|---|---|---|
| Accuracy Measures | Bar chart | Table | Pictograph | Table | Pictograph | Pictograph |
| Print Ad | ||||||
| Absolute frequency | −2.10, P = 0.04 | −1.84, P = 0.07 | 0.94, P = 0.35 | 0.15, P = 0.88 | 2.99, P = .003 | 2.69, P = 0.007 |
| Percent | −2.36, P = 0.02 | −1.73, P = 0.08 | 0.67, P = 0.50 | 0.50, P = 0.62 | 2.95, P = 0.003 | 2.30, P = 0.02 |
| Relative frequency | −0.91, P = 0.36 | −1.04, P = 0.30 | 0.44, P = 0.66 | −0.22, P = 0.83 | 1.31, P = 0.19 | 1.40, P = 0.16 |
| Placebo | −2.03, P = 0.04 | −3.55, P < 0.001 | −1.54, P = 0.12 | −1.54, P = 0.12 | 0.41, P = 0.68 | 1.91, P = 0.06 |
| Television Ad | ||||||
| Absolute frequency | −2.39, P = 0.02 | 0.76, P = 0.44 | 1.58, P = 0.12 | 3.15, P = 0.002 | 3.99, P < 0.001 | 0.80, P = 0.42 |
| Percent | −2.50, P = 0.01 | 0.26, P = 0.79 | 1.41, P = 0.16 | 2.75, P = 0.006 | 3.92, P < 0.001 | 1.14, P = 0.26 |
| Relative frequency | −0.75, P = 0.46 | 1.86, P = 0.06 | 2.08, P = 0.04 | 2.56, P = 0.01 | 2.79, P = 0.005 | 0.15, P = 0.88 |
| Placebo | −2.00, P = 0.04 | −0.97, P = 0.33 | −0.03, P = 0.98 | 1.06, P = 0.29 | 1.99, P = 0.05 | 0.95, P = 0.34 |
Note. Accuracy (absolute frequency, percentage, relative frequency, and placebo) was measured as the percentage who reported the correct quantitative benefit information. Bonferroni-adjusted significance was defined as P < 0.005. Print ad condition t-tests had 1,304 degrees of freedom. Television ad condition t-tests had 1,190 degrees of freedom.
In the television conditions, the overall Pearson chi-square test was significant for absolute frequency (χ2(4) = 158.12, P < 0.001), percent (χ2(4) = 14.01, P < 0.001), relative frequency (χ2(4) = 3.09, P = 0.01), and placebo questions (χ2(4) = 15.68, P < 0.001); therefore, we examined pairwise comparisons among visual aids for each of these questions. Participants who viewed an ad with a bar chart, compared with participants who viewed an ad with a pictograph, were more likely to report the efficacy information accurately in response to the absolute frequency, percent, and the relative frequency questions (Tables 2 and 4). Participants who viewed an ad with a bar chart, compared with participants who viewed an ad with a table, were more likely to report the efficacy information accurately in response to the absolute frequency question.
Visual aids did not affect perceived risk, perceived efficacy, benefit-risk assessment, attitude toward the drug, or risk recall in the print or television conditions (P > 0.05; Table 3).
3.4. Did numeracy moderate the effect of visual aids?
The median number of correct responses on the numeracy measure was two out of three in both the print and television ad conditions. Participants’ numeracy skills did not moderate the relationship between visual aids and efficacy information accuracy in the print or television ad conditions (P > 0.05). However, numeracy was directly associated with all accuracy measures in both the print and television ad conditions, such that accuracy increased as numeracy increased (P < 0.001).
3.5. Did participants distinguish between low and high efficacy drugs?
In the print and television conditions, participants who viewed an ad for a high efficacy drug, compared with participants who viewed an ad for a low efficacy drug, had higher perceived efficacy, lower perceived risk, a more positive perception of the benefit-risk assessment, and a more positive attitude toward the drug (Table 3). In the television conditions only, participants who viewed an ad for a high efficacy drug, compared with participants who viewed an ad for a low efficacy drug were more likely to intend to ask their doctor about the drug and take the drug if prescribed. Accurate recall of efficacy information did not vary by drug efficacy level.
4. Discussion and conclusion
This section summarizes the study’s results, limitations, future directions, and practice implications.
4.1. Discussion
Presenting a visual aid appears to help participants accurately recall how well a drug works, although it does not necessarily change their attitude toward the drug, their perceptions of how well the drug works, how risky the drug is, or their intentions to research or take the drug. These results suggest that visual aids that truthfully convey quantitative efficacy information in DTC print and television ads may aid patients.
4.1.1. Visual aids
Comparing among visual aids, the bar chart and (for some questions) the table led to more accurate responses. Importantly, we found that only participants in the bar chart and table print ad conditions and the bar chart television ad conditions more accurately reported relative frequency compared with the no visual aid condition. Accurately reporting the relative frequency went beyond simple recall; participants had to perform a calculation (e.g., 10/2 = 5 times more likely) to report the relative frequency accurately. These findings suggest that the bar chart and table may help people process numerical information more fully.
The finding that the bar chart and table were more helpful in this study than the pie chart is not surprising given previous research showing that pie charts are more useful when communicating proportions [11]. The finding that the bar chart and table were in some cases more helpful than the pictograph is surprising given that previous research suggests that pictographs help individuals understand numerical or medical information [10, 12-14, 23-25]. However, bar charts have outperformed pictographs when communicating effects greater than 10 percentage points, as was the case in this study [26]. The format and design of the visual aids also may play a role in individuals’ comprehension [27]. For instance, the pictograph in this study was the only format that did not include actual numbers within the visual aid, although all participants encountered the absolute frequency and placebo rate in the text (print ad) or voiceover (television ad). In light of this, it is important to note that even without numbers in the visual, the pictograph still significantly increased participants’ recall of the absolute frequency and placebo rate in the television conditions.
Our data suggest that visual aids depicting quantitative efficacy information do not influence perceptions, attitudes, or intentions, contrary to previous research that found visual aids do have such effects [16, 19]. There may be several explanations for this finding. First, unlike previous research, our measures addressed visual aids within DTC advertisements. It is important to note that having quantitative information about a drug’s efficacy is only one element of drug decision making, and individuals must consider additional sources of information, such as their healthcare provider’s recommendation, before significantly changing their intentions. Second, it is possible that with repeated exposure to a novel product, as in traditional advertising campaigns, people may develop different perceptions, attitudes, and intentions. Finally, these outcomes might have been affected had we changed the presentation of the risk information. Because participants’ perceptions, attitudes, and intentions are likely influenced by the presentation of both the benefits and the risks, keeping the risks constant may have limited our ability to detect these effects.
4.1.2. Numeracy
Previous research was unclear about whether visual aids help individuals with low numeracy differently than individuals with high numeracy [16, 17]. Our study supports the findings of Keller and Siegrist who found no particular advantage of pictographs for those with low numeracy; in our case, none of the visual aids provided this particular advantage. This contrasts with more recent research demonstrating advantages for those with low numeracy [28]. The visuals we tested did not differentially aid accurate recall among low numeracy populations, but may assist individuals in other related areas we did not measure, such as perceived understanding or time necessary to process the information.
4.1.3. Print and television modalities
A major contribution of this research is that it is one of the first studies to systematically examine graphics depicting quantitative information in DTC television ads. To our knowledge, the risk communication literature has focused only on static modalities. It is important to study quantitative information in multiple modalities before applying the risk communication literature to DTC advertising more generally.
Because the sampling frame differed in television and print conditions, we did not directly compare these two modalities. However, in general, we found that our results were similar but stronger for participants who viewed the television ad. This could have resulted from the modalities themselves: the television ad may have drawn participants’ attention to the visual aids more than the print ad did because of peripheral factors such as music, dialog, and movement. Likewise, the presence of two kinds of communication, audio and visual, may have created additional memory traces that allowed for better recall and processing of visual aids in the television ad. Interestingly, when no visual aid was presented, overall accuracy was somewhat higher in the print conditions than in the television conditions. Future research should examine directly whether visual aids may mitigate the potentially distracting effects of dynamic features in television ads.
Alternatively, these differences may have been due to sample selection. Only individuals with broadband Internet access could be randomly assigned to the television ad conditions because dial-up connections could not reliably download the television ad. This difference in administration mode accounts for the fact that participants in the television ad conditions had more years of education than those in the print ad conditions. Thus, the difference in education levels may account for the higher accuracy in the television conditions. Future research designed to compare print and television ads directly could shed light on these issues.
4.1.4. Limitations and future directions
As with any research study, this study had limitations. The study was conducted via the Internet and is subject to many of the limitations inherent in this mode of administration, such as the lack of control over testing conditions [29, 30]. However, the Internet panel from which we drew our participants was not a typical opt-in, or self-selected, panel, but rather a panel specifically designed to be nationally representative. Therefore, the sample suffers from fewer biases than a typical opt-in panel [31, 32]. As noted above, the television conditions were limited to participants with broadband access. Despite this limitation, conducting the study via the Internet enabled us to increase the power needed to detect differences and allowed for greater geographical distribution.
To limit the scope of the study, only quantitative efficacy information was depicted in visual aids. Because both efficacy and risk information are important for decision-making, future research should test the simultaneous presentation of quantitative efficacy and risk information in DTC ads. Furthermore, this study focused on one medical condition (high cholesterol) and one print and television ad for a fictitious prescription drug. Different effects may be found with different medical conditions or with different ads. Studies that examine many visuals with different quantitative information are needed to ensure that results are not specific to any particular layout or choice of numerical information.
4.2. Conclusion
Our work demonstrates that including quantitative information with visual aids increases accurate recall of efficacy information in DTC prescription drug ads. Almost all visual aids had some benefit over no visual information, and bar charts and tables seemed most successful at increasing accuracy. Although we saw no differences in perceptions or attitudes toward the drug, repeated exposure to an advertising campaign may evoke such changes. Given our findings, providing quantitative information visually in DTC ads, if implemented appropriately, may help individuals have better conversations with their healthcare providers.
4.3. Practice implications
Providing quantitative prescription drug efficacy information in the form of visual aids improved the recall of efficacy information but did not detract from the understanding of risk information. This study provides evidence that adding certain types of visual aids to DTC advertising may increase the public’s understanding of how well prescription drugs work. Consequently, these visual aids may better inform consumers about the benefit and risk tradeoffs inherent in these treatments, which may lead to more informed treatment decisions and discussions. Healthcare providers and industry may wish to use visual aids to convey quantitative efficacy information when communicating prescription drug information to patients and consumers.
We confirm that all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Table 5.
Weighted means (95% confidence intervals) and percentages of dependent variables by visual aids.
| Visual Aid |
Efficacy Level |
||||||
|---|---|---|---|---|---|---|---|
| Dependent Variables | Pie Chart | Bar Chart | Table | Pictograph | No Visual | Low | High |
| Print Ad | |||||||
| N | 269 | 262 | 251 | 259 | 268 | 668 | 641 |
| Intention: Talk to doctor | 2.1 (1.9-2.2) | 1.8 (1.7-2.0) | 2.1 (1.9-2.2) | 1.9 (1.8-2.1) | 2.1 (1.9-2.2) | 2.0 (1.9-2.1) | 2.0 (1.9-2.2) |
| Intention: Search for information | 2.0 (1.9-2.2) | 1.91 (1.8-2.0) | 2.0 (1.8-2.2) | 2.1 (1.9-2.2) | 2.1 (2.0-2.3) | 2.0 (1.9-2.1) | 2.1 (2.0-2.2) |
| Intention: Take drug | 2.5 (2.3-2.6) | 2.3 (2.2-2.5) | 2.3 (2.1-2.5) | 2.4 (2.2-2.5) | 2.4 (2.3-2.6) | 2.4 (2.3-2.5) | 2.4 (2.3-2.5) |
| Attitude toward the drug | 3.2 (3.0-3.3) | 2.9 (2.8-3.1) | 3.0 (2.9-3.2) | 3.1 (3.0-3.2) | 3.0 (2.9-3.2) | 2.9 (2.8-3.0) | 3.2* (3.1-3.3) |
| Benefit-risk assessment | 4.0 (3.8-4.3) | 3.8 (3.5-4.0) | 3.8 (3.5-4.0) | 4.0 (3.8-4.3) | 3.9 (3.7-4.2) | 3.7 (3.6-3.8) | 4.1* (4.0-4.3) |
| Perceived efficacy | 4.3 (4.0-4.6) | 4.3 (4.0-4.5) | 4.3 (4.0-4.6) | 4.4 (4.1-4.7) | 4.3 (4.0-4.5) | 4.0 (3.9-4.2) | 4. 6* (4.4-4.7) |
| Perceived risk | 4.1 (3.9-4.3) | 4.2 (4.0-4.5) | 4.2 (4.0-4.5) | 4.2 (4.0-4.5) | 4.3 (4.0-4.5) | 4.4 (4.2-4.5) | 4.1* (3.9-4.2) |
| Aided risk recall | 5.9 (5.7-6.1) | 5.8 (5.6-6.0) | 6.1 (5.8-6..4) | 5.9 (5.7-6.1) | 5.8 (5.6-6.0) | 5.9 (5.7-6.0) | 6.0 (5.8-6.1) |
| Unaided risk recall (%) | 80.8 | 84.5 | 87.6 | 83.6 | 83.4 | 82.5 | 85.4 |
| Television Ad | |||||||
| N | 241 | 237 | 238 | 241 | 238 | 596 | 599 |
| Intention: talk to doctor | 1.9 (1.8-2.0) | 1.9 (1.7-2.0) | 2.0 (1.8-2.1) | 1.9 (1.8-2.1) | 1.9 (1.7-2.0) | 1.8 (1.7-1.9) | 2.0* (1.9-2.1) |
| Intention: search for information | 1.9 (1.8-2.0) | 2.0 (1.9-2.2) | 2.0 (1.8-2.1) | 2.1 (1.9-2.2) | 2.0 (1.8-2.1) | 1.9 (1.8-2.0) | 2.0 (1.9-2.1) |
| Intention: take drug | 2.4 (2.2-2.5) | 2.4 (2.3-2.6) | 2.3 (2.2-2.5) | 2.4 (2.2-2.5) | 2.4 (2.2-2.5) | 2.2 (2.1-2.3) | 2.5* (2.4-2.6) |
| Attitude toward the drug | 3.0 (2.9-3.1) | 2.9 (2.8-3.1) | 3.0 (2.9-3.1) | 3.1 (2.9-3.2) | 3.1 (2.9-3.2) | 2.9 (2.8-3.0) | 3.2* (3.1-3.2) |
| Benefit-risk assessment | 3.8 (3.6-4.0) | 3.9 (3.6-4.1) | 3.9 (3.7-4.1) | 4.0 (3.7-4.2) | 3.8 (3.6-4.0) | 3.5 (3.4-3.6) | 4.2*(4.1-4.4) |
| Perceived efficacy | 4.3 (4.0-4.5) | 4.4 (4.1-4.7) | 4.4 (4.2-4.6) | 4.5 (4.3-4.8) | 4.3 (4.1-4.6) | 4.0 (3.9-4.2) | 4.8* (4.6-4.9) |
| Perceived risk | 4.1 (3.9-4.3) | 4.0 (3.8-4.3) | 4.2 (4.0-4.4) | 4.0 (3.8-4.2) | 4.2 (4.0-4.4) | 4.3 (4.2-4.4) | 3.9* (3.8-4.0) |
| Aided risk recall | 6.6 (6.3-6.8) | 6.7 (6.5-6.8) | 6.4 (6.2-6.7) | 6.5 (6.3-6.7) | 6.4 (6.2-6.6) | 6.5 (6.4-6.6) | 6.5 (6.4-6.6) |
| Unaided risk recall (%) | 85.6 | 87.4 | 85.3 | 87.4 | 85.8 | 88.3 | 84.2 |
Note:
Denotes significant difference compared with the low efficacy condition (P < 0.05).
Intention measures were assessed on a scale from 1 = not at all likely to 4 = extremely likely. Attitude toward the drug was assessed on a scale from 1 = very bad to 5 = very good. Benefit-risk assessment was assessed on a scale from 1= more risks than benefits to 7 = more benefits than risks. Perceived efficacy was assessed on a scale from 1 = not at all effective/well to 7 = very effective/well. Perceived risk was assessed on a scale from 1 = very safe/not at all risky to 7 = not at all safe/very risky. Aided risk recall is the sum of correct responses (0 to 8). Unaided risk recall is the percent who recalled one or more correct risks in an open-ended format. There were no significant differences among visual aid conditions on these measures.
Acknowledgements
Financial support for this study was provided entirely by a contract with the U.S. Food and Drug Administration. We thank John Swasy, American University Kogod School of Business, for his assistance with the development of the design. We also thank Karol Krotki, Harley Rohloff, and Jennifer Gard Read of RTI International and Rick Li of GfK for their help collecting and preparing the study data. Finally, we thank Kayla Gray, Maria Ashbaugh, Cliff Haac, and John Bollenbacher of RTI International for creating the fictitious drug advertisements.
References
- 1.Frosch DL, Krueger PM, Hornik RC, Cronholm PF, Barg FK. Creating demand for prescription drugs: A content analysis of television direct-to-consumer advertising. Ann Fam Med., 2007;5:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woloshin S, Schwartz L, Tremmel J, Welch HG. Direct to consumer advertisements for prescription drugs: what are Americans being told. Lancet. 2001;358:1141–46. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz LM, Woloshin S, Welch HG. The drug facts box: Providing consumers with simple tabular data on drug benefit and harm. Med Decis Making. 2007;27:655–92. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz LM, Woloshin S, Welch HG. Communicating drug benefits and harms with a drug facts box: Two randomized trials. Ann of Intern Med. 2009;150:516–27. [DOI] [PubMed] [Google Scholar]
- 5.Woloshin S, Schwartz LM. Communicating data about the benefits and harms of treatment: A randomized trial. Ann Intern Med. 2011;155:87–96. [DOI] [PubMed] [Google Scholar]
- 6.Woloshin S, Schwartz LM, Welch HG. The value of benefit data in direct-to-consumer dug ads. Health Aff. 2004; W4 (Suppl):234–45. [DOI] [PubMed] [Google Scholar]
- 7.O’Donoghue AC, Sullivan HW, Aikin KJ, Chowdhury D, Moultrie RR, Rupert DJ. Presenting efficacy information in direct-to-consumer prescription drug advertisements. Patient Educ Couns. 2014;95:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancker JS, Senathirajah Y, Kukafka R, Starren JB. Design features of graphs in health risk communication: A systematic review. J Am Med Inform Assoc. 2006;13:608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipkus I, Hollands JG. The visual communication of risk. J Natl Cancer Inst Monogr. 1999;25:149–63. [DOI] [PubMed] [Google Scholar]
- 10.Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: Is a picture worth a thousand statistics? Med Decis Making. 2005;25:398–405. [DOI] [PubMed] [Google Scholar]
- 11.Lipkus I. Numeric, verbal, and visual formats of conveying health risks: Suggested best practices and future recommendations. Med Decis Making. 2007;27:697–713. [DOI] [PubMed] [Google Scholar]
- 12.Stone ER, Yates JF, Parker AM. Effects of numerical and graphical displays on professed risk-taking behavior. J Exp Psychol-Appl. 1997;3:243–56. [Google Scholar]
- 13.Schirillo JA, Stone ER. The greater ability of graphical versus numerical displays to increase risk avoidance involves a common mechanism. Risk Anal. 2005;25:555–66. [DOI] [PubMed] [Google Scholar]
- 14.Chua HF, Yates JF, Shah P. Risk avoidance: Graphs versus numbers. 2006;34:399–410. [DOI] [PubMed] [Google Scholar]
- 15.Dijkstra M, Buijtels HEJJM, van Raaij WF. Separate and joint effects of medium type on consumer responses: A comparison of television, print, and the internet. J Bus Res. 2006;58:377–86. [Google Scholar]
- 16.Galesic M, Garcia-Retamero R, Gigerenzer G. Using icon arrays to communicate medical risks: Overcoming low numeracy. Health Psychol. 2009;28:210–16. [DOI] [PubMed] [Google Scholar]
- 17.Keller C, Siegrist M. Effect of risk communication formats on risk perception depending on numeracy. Med Decis Making. 2009;29:483–90. [DOI] [PubMed] [Google Scholar]
- 18.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Improving understanding of adjuvant therapy options by using simpler risk graphics. Cancer. 2008;113:3382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman-Stewart D, Brundage MD, Zotov V. Further insight into the perception of quantitative information: Judgments of gist in treatment decisions. Med Decis Making. 2007;27:34–43. [DOI] [PubMed] [Google Scholar]
- 20.DiSogra CA, Cobb C, Chan E, Dennis JM. Calibrating non-probability Internet samples with probability samples using early adopter characteristics JSM Proceedings, Survey Methods Section. 2011. Alexandria, VA: American Statistical Association, pp. 4501–15. [Google Scholar]
- 21.GfK Custom Research. KnowledgePanel Design Summary Report. Available from: http://www.knowledgenetworks.com/knpanel/docs/knowledgePanel%28R%29-design-summary-description.pdf. Accessed May 7, 2014.
- 22.Schwartz L, Woloshin S, Black W, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127:966–72. [DOI] [PubMed] [Google Scholar]
- 23.Hess R, Visschers CHM, Siegrist M. Risk communication with pictographs: The role of numeracy and graph processing. Judgm Decis Mak. 2001;6:263–74. [Google Scholar]
- 24.Houts PS, Witmer JT, Egeth HE, Loscalzo MJ, Zabora JR. Using pictographs to enhance recall of spoken medical instructions. Patient Educ Couns. 2001;43:231–42. [DOI] [PubMed] [Google Scholar]
- 25.Zikmund-Fisher BJ, Ubel PA, Smith DM, Derry HA, McClure JB, Stark A, et al. Communicating side effect risks in a tamoxifen prophylaxis decision aid: The debiasing influence of pictographs. Patient Educ Couns. 2008;73:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCaffery KJ, Dixon A, Hayen A, Jansen J, Smith S, Simpson JM. The influence of graphic display format on the interpretations of quantitative risk information among adults with lower education and literacy: A randomized experimental study. Med Decis Making. 2012;32:532:44. [DOI] [PubMed] [Google Scholar]
- 27.Price M, Cameron R, Butow P. Communicating risk information: The influence of graphical display format on quantitative information perceptions—accuracy, comprehension, and preferences. Patient Educ Couns. 2007;69:121–8. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Retamero R, Cokely ET. Communicating health risks with visual aids. Curr Dir Psychol Sci, 2013;22:392–9. [Google Scholar]
- 29.Heerwegh D, Loosveldt G. Face-to-face versus web surveying in a high-Internet-coverage population: Differences in response quality. Public Opin Q. 2008;72:836–46. [Google Scholar]
- 30.Klovning A, Sandvik H, Hunskaar S. Web-based survey attracted age-biased sample with more severe illness than paper-based survey. J Clin Epidemiol. 2009;62:1068–74. [DOI] [PubMed] [Google Scholar]
- 31.Chang L, Krosnick JA. National surveys via RDD telephone interviews versus the internet: Comparing sample representativeness and response quality. Public Opin Q. 2009;73:641–78. [Google Scholar]
- 32.Dennis M KnowledgePanel: Processes and procedures contributing to sample representativeness and tests for self-selection bias. 2010. Accessed at http://www.knowledgenetworks.com/ganp/docs/KnowledgePanelR-Statistical-Methods-Note.pdf on May 7, 2014.