Abstract
Previous studies using mouse liver tumor models have indicated that coexpression of CD38 and CD101 in programmed cell death-1 (PD-1)+CD8+ T cells may reflect fixed dysregulation of CD8+ T cells and thus indicate a poor response to anti-PD-1 immunotherapy. However, whether CD38 and CD101 expression in PD-1+CD8+ T cells can predict the clinical status and efficacy of chemotherapy for various cancer types, including ovarian cancer (OC), remains unclear. In the present study, peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Hypaque gradient centrifugation from 96 fresh samples from healthy adult volunteers and patients with epithelial OC, aged 55.21±9.91 years. Additionally, tumor-infiltrating lymphocytes (TILs) were separated using a combination of mechanical, chemical and enzymatic digestion from fresh surgically removed tumor tissues from 15 patients with epithelial OC. The expression of CD38 and CD101 in PD-1+CD8+ T cells or TILs was detected by flow cytometry or immunofluorescence (IF) staining, respectively. The association between the level of CD38/CD101 expression and clinicopathological parameters or postoperative chemotherapy in patients with OC was statistically analyzed. The levels of CD38/CD101-coexpressing PD-1+CD8+ T cells were significantly increased in PBMCs and TILs of patients with OC compared with those of healthy volunteers. The frequency of PD-1+CD38+CD101+CD8+ T cells among the total PD-1+CD8+ T cell subpopulation was negatively associated with clinical stage, lymph node metastasis and postoperative chemotherapy prognosis in patients with OC. Furthermore, IF staining confirmed colocalization of CD38 and CD101 on the majority of TILs in OC tissues. Thus, the present study suggests that coexpression of CD38 and CD101 in peripheral PD-1+CD8+ T cells and TILs may serve as a new indicator for diagnosis and treatment efficacy in patients with epithelial OC.
Keywords: ovarian cancer, programmed cell death-1, CD38, CD101, tumor-infiltrating lymphocytes, diagnosis
Introduction
The incidence of ovarian cancer (OC) is third highest among female genital malignancies, and has the highest fatality rate; for example, ~14,080 women died from OC in the United States in 2017 (1). Moreover, the mortality rate of OC ranks first among all types of gynecological tumors, and these malignant tumors seriously threaten the lives of women (2). In recent decades, there have been three major advances in the diagnosis and treatment of OC, namely, scientific surgical pathology staging, maximum tumor reduction surgery, and combination chemotherapy with platinum and paclitaxel, which have increased the 5-year survival rate of patients from ~35 to 50% (1). However, there are no effective biomarkers for the early diagnosis of OC; thus, 70–80% of patients are at the late stage of the disease upon hospital admission and miss the optimal time window for radical surgery, chemotherapy and radiotherapy (3). Therefore, it is crucial to identify suitable prognostic biomarkers of OC to improve outcomes in patients.
CD8+ cytotoxic T lymphocytes (CTLs) specifically kill tumor cells and play an important role in antitumor immunity. However, numerous factors in the tumor microenvironment (TME) can induce CD8+ T cells to upregulate the expression of the inhibitory receptor programmed cell death-1 (PD-1) (4). The binding of PD-1 to programmed cell death-ligand 1 (PD-L1) significantly decreases the ability of CD8+ CTLs to kill tumor cells (5). Although several basic studies and clinical trials have shown that anti-PD-1 or anti-PD-L1 antibodies can elicit significant antitumor immunity, ~80% of patients fail to respond to treatment with antibodies against PD-1 or PD-L1 (6). Based on the available clinical data, PD-1 blockade is effective in only a small number of patients with certain types of tumors. Furthermore, the molecular mechanisms that underlie this phenomenon remain to be clarified.
Recently, Schietinger et al (7) reported that tumor-infiltrating lymphocytes (TILs) in mice strongly express PD-1 and that their differentiation involved two separate chromatin states, i.e., a therapeutically reversible state (state 1) and a fixed state (state 2) (7). These authors further demonstrated that the CD8+ T cells in state 1 exhibit a low level of CD38 and CD101 coexpression, and can recover the ability to secrete interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) following in vitro stimulation, thus restoring the ability of the CD8+ T cells to kill tumor cells. By contrast, state 2 is an irreversible dysfunctional state in which the CD8+ T cells strongly coexpress CD38 and CD101, and cannot recover IFN-γ or TNF-α secretion despite several rounds of stimulation (8). Therefore, the coexpression of CD38 and CD101 on PD-1+CD8+ T cells can predict the therapeutic effect of anti-PD-1/PD-L1 blockade (8). Nonetheless, whether the coexpression of CD38 and CD101 in PD-1+CD8+ T cells can predict the clinical prognosis of OC remains unclear.
In the present study, the expression and distribution of CD38/CD101 in PD-1+CD8+ T cells was examined in peripheral blood and cancer tissues of patients with OC. Furthermore, the association of PD-1+CD8+ T cell subsets with patient clinical parameters, postoperative chemotherapy outcome indexes, serum cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) values was analyzed to explore the predictive value of CD38/CD101 coexpression in PD-1+CD8+ T cells on the prognosis of patients with OC.
Materials and methods
Patients
A total of 96 women with epithelial OC aged 37–68 years (mean age, 55.21±9.91 years) were enrolled in the present study, as well as 26 age-matched healthy volunteers. All patients were admitted to the Department of Obstetrics and Gynecology at Southwest Hospital of the Third Military Medical University (Chongqing, China) between March 2017 and February 2018. On the basis of histological stage, 45 cases had high and moderate differentiation, and 51 cases had low differentiation. Tumour staging was performed by the International Federation of Gynecology and Obstetrics classification (https://www.figo.org). Inclusive criteria were as follows: Ovarian cancer as the first tumor; no radiotherapy or chemotherapy treatment before admission; and all specimens histologically confirmed with complete clinical data. Exclusive criteria were as follows: Borderline OC or OC in combination with other malignant tumors or abnormal liver and kidney; severe blood coagulation disorders; and primary cardiovascular and cerebrovascular diseases. The detailed clinical characteristics of these patients and the healthy controls are shown in Table I. Written, informed consent was obtained from all subjects prior to participation, and the study was approved by the ethics committee of the Third Military Medical University in Chongqing, China.
Table I.
Patient clinicopathological parameters.
| Clinicopathological parameters | Peripheral blood samples, n=96 | Tissue samples, n=15 |
|---|---|---|
| Age (years) | 37-68 | 37-68 |
| Pathological grade, well + moderately differentiated/poorly differentiated | 45/51 | 4/11 |
| Tumor grade, T1-T2/T3-T4 | 25/71 | 3/12 |
| Lymph node metastasis, N0/N1 | 26/70 | 3/12 |
| Distant metastasis, M0/M1 | 93/3 | 15/0 |
| Serum CA125 (U/ml), ≤35/>35 | 25/71 | 4/11 |
| Serum HE4 (pmol/ml), ≤76.2/>76.2 | 31/65 | 4/11 |
CA125, cancer antigen 125; HE4, human epididymis protein 4.
Cell separation
Fresh peripheral blood mononuclear cells (PBMCs) were obtained from the peripheral blood of healthy adult volunteers and patients with OC by Ficoll-Hypaque gradient centrifugation (Axis-Shield), according to the manufacturer's instructions. TILs were separated from the fresh tumor tissue of 15 patients with epithelial OC by a combination of mechanical, chemical and enzymatic digestion. Briefly, tissues were cut into small pieces with a surgical scissor, and were digested with type IV collagenase and pancreatin (Gibco; Thermo Fisher Scientific, Inc.). The TILs were then obtained by Ficoll-Hypaque gradient centrifugation.
Flow cytometric analysis
The PBMCs or TILs were stained with the following fluorochrome-labeled monoclonal antibodies (mAbs) at 4°C for 30 min in the dark: Anti-CD3-PerCP (1:100; cat. no. 300428; BioLegend, Inc.), anti-CD8-FITC (1:100; cat. no. 11-0086.42; eBioscience; Thermo Fisher Scientific, Inc.), anti-PD-1-PE/Cy7 (1:100; cat. no. 329918; BioLegend, Inc.), anti-CD38-PE (1:100; cat. no. 4322550; Invitrogen; Thermo Fisher Scientific, Inc.) and anti-CD101-APC (1:100; cat. no. 331007; BioLegend, Inc.). Isotype control antibodies were used to ensure accurate compensation and to set gates. The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed with FlowJo 10 software (FlowJo LLC).
Immunofluorescence (IF) analysis
The colocalization of CD38 and CD101 was assessed by IF staining of fresh tumor tissues and adjacent non-tumor tissues from five patients with epithelial OC representative of the whole cohort. For double-staining with anti-CD38 (cat. no. ZM-0422; OriGene Technologies, Inc.) and anti-CD101 (cat. no. AG09158376; BIOSS) antibodies, anti-CD38 (50 µl) and anti-CD101 (1:100) were mixed and applied to slides, and subsequently incubated for 12 h at 4°C in a humidified chamber. Next, DyLight 488-labeled anti-rabbit (1:500; cat. no. A-11008; Invitrogen, Thermo Fisher Scientific, Inc.) and DyLight 555-labeled anti-mouse antibodies (1:500; cat. no. A-31570; Invitrogen; Thermo Fisher Scientific, Inc.) were applied for 60 min at 37°C. Nuclear counterstaining was performed by incubating the slides in DAPI (1:100 in PBS; Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min at room temperature. Normal mouse, goat or rabbit IgG (1:100; cat. no. bs-0296P; BIOSS) was used as an isotype control. Images were obtained using an upright fluorescence microscope at ×200 magnification (Olympus Corporation).
Statistical analysis
All data were analyzed using SPSS statistics software (v24.0; IBM Corp.) and GraphPad Prism software (v6.0; GraphPad Software, Inc.). All experiments were repeated three times and all data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference. The patients were divided into high- and low-expression groups according to the median value. Student's t-test was used to analyze comparisons between two groups, and the χ2 test was applied to analyze the associations between each cell subset and the clinicopathological parameters of patients. The associations between CD38/CD101 coexpression on PD-1+CD8+ T cells in PBMCs or TILs and serum CA125 or HE4 levels following surgery and first chemotherapy in OC patients were analyzed using nonparametric Kruskal-Wallis test.
Results
CD38/CD101 were markedly upregulated on peripheral PD-1+CD8+ T cells of patients with OC
PD-1 is a key checkpoint factor in immunosuppressive pathways and an acceptable marker of immune failure. The overexpression of PD-1 has been reported in patients with OC compared with healthy donors (9). Therefore, the expression of PD-1 on peripheral CD8+ T lymphocytes was investigated in patients with OC by flow cytometry, using the indicated gating strategies (Fig. 1A). Statistical analysis revealed that the frequency of PD-1+CD8+ T cells in the peripheral blood of patients with OC was significantly higher than that in the peripheral blood of healthy controls (Fig. 1B). Further analysis of CD38 and CD101 expression on PD-1+CD8+ T cells demonstrated that the frequencies of CD38−CD101+ and CD38+CD101+PD-1+ CD8+ T cells were higher in samples from patients with OC than in samples from healthy controls. In contrast, the frequency of CD38−CD101−PD-1+CD8+T and CD38+CD101−PD-1+CD8+T cells was dramatically decreased in samples from patients with OC compared with those from healthy controls (Fig. 1C).
Figure 1.
PD-1+CD8+ T cell subsets in the peripheral blood of patients with ovarian cancer. (A) CD38 and CD101 expression on PD-1+CD8+ T cells was detected by flow cytometry with the indicated gating strategies. (B) PD-1 expression on peripheral CD8+ T cells. (C) CD38 and CD101 expression on PD-1+CD8+ T cells from patients with OC (n=96) and HCs (n=26). PD-1, programmed death-1; OC, ovarian cancer; HCs, healthy controls.
Clinical significance of PD-1 and CD38/CD101 expression on peripheral CD8+ T cells in patients with OC
In order to explore the clinical significance of CD38 and CD101 expression on PD-1+CD8+ T cell subpopulations in OC patients, the associations between the expression frequency of total peripheral PD-1+CD8+ T or CD38/CD101-expressing peripheral PD-1+CD8+ T cells and clinical parameters or postoperative chemotherapy outcomes were analyzed. According to the results, total PD-1+CD8+ T cells and all PD-1+CD8+ T cell subsets showed no association with age or histopathological grade of patients (Table II and Table SI). Moreover, the frequency of total PD-1+CD8+ T cells and positive expression of CD38 or CD101 on PD-1+CD8+ T cell subsets were not associated with the clinicopathological parameters of patients (Table SI). However, the double-positive or double-negative expression of CD38/CD101 on PD-1+CD8+ T cell subsets was significantly associated with tumor grade and lymph node metastasis (Table II).
Table II.
Association between CD38/CD101 coexpression in PD-1+CD8+ T cells and clinical parameters of patients with ovarian cancer.
| PD-1+CD38+ CD101+ | PD-1+CD38− CD101− | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological parameters | n | Low | High | P-value | Low | High | P-value |
| Age (years) | 0.537 | 0.537 | |||||
| <55 | 54 | 29 | 25 | 25 | 29 | ||
| ≥55 | 42 | 19 | 23 | 23 | 19 | ||
| Pathological grade | 0.101 | 0.220 | |||||
| Well/moderately differentiated (G1-G2) | 45 | 27 | 18 | 19 | 26 | ||
| Poorly differentiated (G3) | 51 | 21 | 30 | 29 | 22 | ||
| Tumor grade | 0.019 | 0.019 | |||||
| T1-T2 | 25 | 18 | 7 | 7 | 18 | ||
| T3-T4 | 71 | 30 | 41 | 41 | 30 | ||
| Lymph node metastasis | 0.002 | 0.038 | |||||
| N0 | 26 | 20 | 6 | 8 | 18 | ||
| N1 | 70 | 28 | 42 | 40 | 30 | ||
P-values were all calculated by the χ2 test. PD-1, programmed cell death-1.
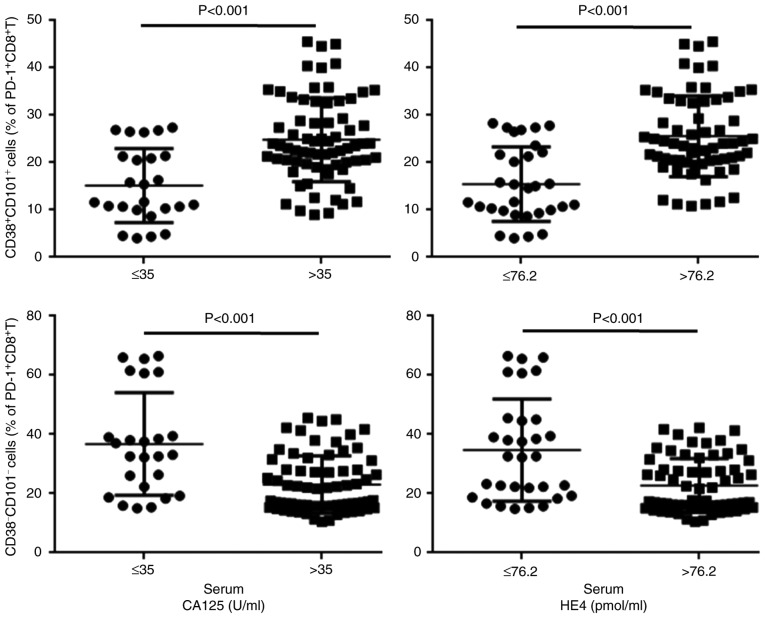
Similarly, the frequency of total PD-1+CD8+ T cells and positive expression of CD38 or CD101 on PD-1+CD8+ T cell subsets was not associated with the therapeutic effect of chemotherapy following surgical resection (Fig. S1), whereas double-positive or double-negative expression of CD38/CD101 on PD-1+CD8+ T cell subsets were significantly associated with the therapeutic effect (Fig. 2).
Figure 2.
Association between CD38/CD101 coexpression on PD-1+CD8+ T cells and serum CA125 and HE4 levels following surgery and first chemotherapy in patients with ovarian cancer. PD-1, programmed death-1; CA125, cancer antigen 125; HE4, human epididymis protein 4.
Expression of CD38/CD101 on CD8+ T cells among TILs from patients with OC and clinical significance
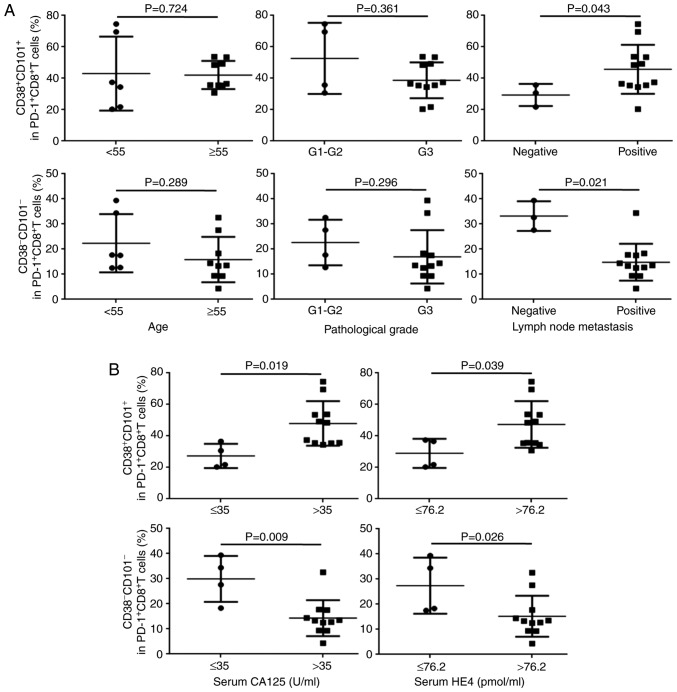
TILs were isolated from OC tissues, and expression frequencies of PD-1, CD38 and CD101 on CD8+ T cells from OC TILs were determined separately by flow cytometry. Similar to the observations in peripheral blood, the frequency of total PD-1+CD8+ T cells and positive expression of CD38 or CD101 on PD-1+CD8+ T cell subsets were not associated with the clinicopathological parameters of patients except that CD38 expression on PD-1+CD8+ T cells was associated with the pathological grade (P=0.037; Figs. 3A and S2A). In contrast, double positive or double negative expression of CD38/CD101 on PD-1+CD8+ T cell subsets was significantly associated with lymph node metastasis (Fig. 3A).
Figure 3.
Association between CD38/CD101 coexpression on PD-1+CD8+ T cell subsets among TILs. CD38/CD101 coexpression on PD-1+CD8+ T cell subsets among TILs based on (A) clinical parameters of patients with OC and (B) serum CA125 and HE4 levels following treatment. PD-1, programmed death-1; OC, ovarian cancer; CA125, cancer antigen 125; HE4, human epididymis protein 4; TILs, tumor-infiltrating lymphocytes.
Additionally, the frequency of total PD-1+CD8+ T cells and positive expression of CD38 or CD101 on PD-1+CD8+ T cell subsets was not associated with the therapeutic effect of chemotherapy following surgical resection (Fig. S2B). However, both double-positive and double-negative expression of CD38/CD101 on PD-1+CD8+ T cell subsets demonstrated significant association with the therapeutic effect (Fig. 3B).
Colocalization of CD38 and CD101 on TILs from OC tissues
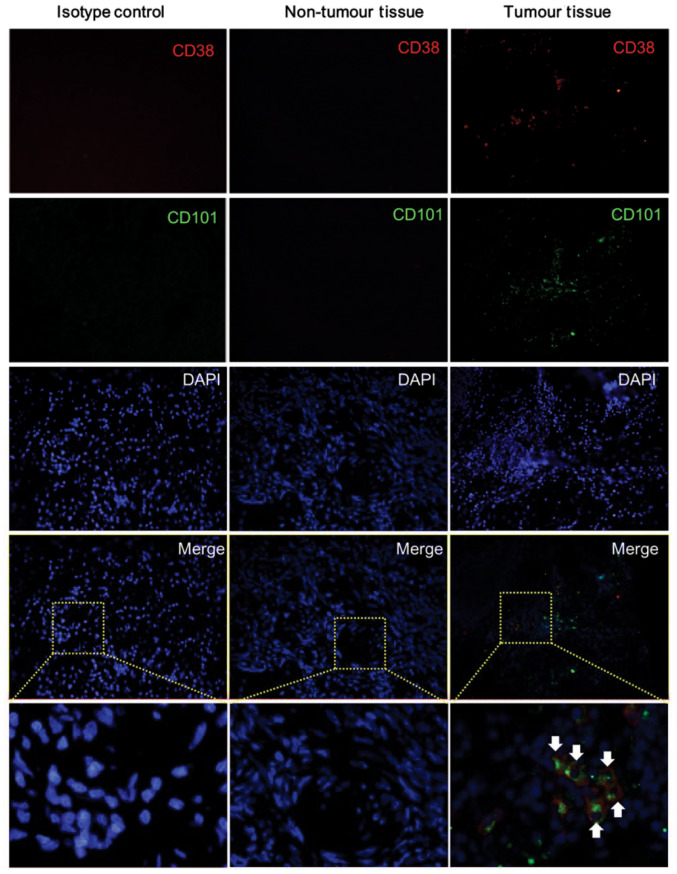
The flow cytometry results indicated that the levels of CD38+CD101+ T cells were significantly increased in OC tissues. Thus, double-IF staining was conducted in order to assess whether CD38 and CD101 are able to colocalize on TILs from OC tissues. The results showed that both CD38 and CD101 were highly expressed in OC tissues, and some staining revealed colocalization of the two markers on TILs. In contrast, almost no CD38 and CD101 expression was observed in adjacent tissues (Fig. 4).
Figure 4.
CD38/CD101 colocalization on TILs from ovarian cancer tissues. Five fresh samples of OC tissue and matched paracancerous tissue were analyzed by immunofluorescence staining with anti-human CD38 (red), anti-human CD101 (green) antibodies and counterstained with DAPI (blue). Representative results of the five IF samples are shown. Boxes indicate enlarged areas. The arrows indicate the CD38 and CD101 co-expressing cells. PD-1, programmed death-1; OC, ovarian cancer; TILs, tumor-infiltrating lymphocytes. Magnification, x200.
Discussion
The immune system plays a crucial role in the antitumor process. On one hand, the immune system can monitor and eliminate tumor cells and prevent the development of tumors. On the other hand, in a complex TME, tumor cells exert an inhibitory effect on the immune system, allowing tumor cells to evade immune surveillance, and rapidly multiply and metastasize in vivo (10). T cells differentiate from lymphatic stem cells in the thymus, and are the most complex type of lymphocytes (11). Among T cells, killer T cells, which are also called cytotoxic T cells, release perforin/granzyme, and cytokines such as IFN-γ and TNF-α to kill target cells, including cells infected by pathogens and cells expressing tumor-specific antigens (12). To ensure that T cells are not overstimulated, activated T cells also express coinhibitory molecules, mainly cytotoxic T-lymphocyte-associated protein 4-B7 and PD-1/PD-L1, which regulate their activity (13–15).
However, the TME usually induces CD8+ T cells to overexpress immunosuppressive molecules such as PD-1; binding of PD-1 with PD-L1, a ligand on the surface of tumor cells, significantly inhibits the ability of CD8+ CTLs to eliminate tumor cells (5). It is widely known that the binding of PD-1 with PD-L1 activates a critical immune checkpoint, leading to T cell dysfunction, exhaustion and tolerance (16–18). Moreover, high-affinity anti-PD-1 or anti-PD-L1 mAbs, which block the PD-1-PD-L1 interaction, can reverse the immune checkpoint, releasing the repression of T cell responses (19). However, previous studies have also shown that certain CD8+ T cells can enter an irreversible dysfunctional state that cannot be rescued by PD-1/PD-L1 blockade. In the majority of advanced tumors, with the exception of Hodgkin's lymphoma and melanoma, the objective response rate with anti-PD-1/PD-L1 monotherapy is only ~20% (20), which strongly suggests that PD-1+CD8+ T cells may comprise a highly heterogeneous population, of which a certain subpopulation may determine the effects of PD-1 blockade immunotherapy.
Recently, Schietinger et al (7) demonstrated that tumor-transformed tissues express tumor-specific antigens during tumorigenesis. In the noninflammatory TME, a small number of inhibitory receptors expressed in original tumor-specific CD8+ T cells disrupt CD8+ T cell function; this dysfunction is reversible during the early stages of malignancy (state 1). However, during persistent antigen stimulation, CD8+ T cells continuously upregulate the expression of inhibitory receptors such as PD-1, thus causing irreversible dysfunction of CD8+ T cells and loss of the killing effect of CD8+ T cells against tumors, which contributes to tumor immune escape and unregulated growth (state 2) (7,21). Notably, Schietinger et al (7) further reported that CD8+ T cells in state 1 exhibit low levels of CD38 and CD101 coexpression, and can recover the ability to secrete IFN-γ and TNF-α following in vitro stimulation to kill tumors, and that CD8+ T cells in state 2 strongly coexpress CD38 and CD101, and lose their ability to secrete IFN-γ and TNF-α despite several rounds of stimulation (8). These results indicate that PD-1+CD8+ T cells can be subgrouped into several subpopulations according to their CD38 and CD101 expression. This hypothesis may explain the observation that, although PD-1/PD-L1 blockade with neutralizing antibodies has achieved great clinical success in combating certain cancer types, and long-lasting responses can be achieved in patients (22,23), clinical trials have demonstrated that the majority of patients with PD-L1+/PD-1+ expression do not respond to PD-1/PD-L1 blockade (20).
The aforementioned studies also suggest that the frequency of PD-1+CD8+ T cell subsets with different CD38/CD101 expression patterns may be a key indicator of tumor prognosis. To verify this hypothesis, the association of CD38/CD101 expression in PD-1+CD8+ T cells with clinical parameters and postoperative chemotherapy outcomes in patients OC was analyzed in the present study. The results demonstrated that the proportion of CD38/CD101-coexpressing PD-1+CD8+ T cells significantly increased and the proportion of CD38/CD101 double-negative PD-1+CD8+ T cells was markedly decreased in peripheral blood and tumor tissues of patients with OC compared with the proportions in paracancerous tissues. The frequency of PD-1+CD38+CD101+CD8+ T cells was associated with clinical stage, lymph node metastasis and postoperative chemotherapy outcomes in patients with OC but not with age or histological grade. A higher level of CD38 and CD101 expression was associated with a higher degree of malignancy in OC and worse postoperative chemotherapy outcomes. Therefore, the coexpression of CD38 and CD101 in PD-1+CD8+ T cells may be used as an indicator of adjuvant diagnosis and prognosis in patients with OC, and may serve as an effective adjuvant biological index for determining the applicability of anti-PD-1 immunotherapy in patients with OC.
CD38 is a 45-kDa single-chain transmembrane glycoprotein that localizes to the cell membrane. The expression of CD38 in human peripheral blood lymphocytes varies with age (24). The first indicator that CD38 may be involved in signal transduction was due to the fact that the interaction between CD38 and ligands on PBMCs and T cell lines can induce activation and proliferation signals (24). Furthermore, studies on hepatitis B virus and human immunodeficiency virus have shown that, since CD38 induces T cell activation via external signals, CD38+ can be used as a T cell activation marker (25,26). CD101 is a 240-kDa disulfide-linked homodimeric type I glycoprotein (27) that was considered to play a costimulatory role in T cell receptor/CD3-mediated T cell activation. A mAb against CD101 inhibits allogeneic T cell responses (28). Therefore, CD38 and CD101 are viewed as markers of T cell activation (24,29); however, the overexpression of these factors may also be considered an exhaustion marker due to overactivation of T cells, particularly when these factors are highly expressed in PD-1+CD8+ T cells. The expression of PD-1 was significantly increased following T cell activation to limit T cell overactivation; thus, sustained high expression of PD-1 in T cells has been viewed as a marker of T cell exhaustion. It is reasonable to propose that high CD38, CD101 and PD-1 expression in CD8+ T cells represents the irreversible state 2 of CD8+ T cells. Hence, a significant increase in CD38+CD101+PD-1+CD8+T cells would predict poor clinical status and treatment outcomes for patients with OC, which is supported by the data in the present study, i.e., CD38/CD101 coexpression levels in PBMCs and TILs in patients with terminal-stage OC were much higher than those in patients with early-stage OC.
CA125 is currently the most commonly used biomarker for OC (30,31); it is employed as a diagnostic tool to assess the effect of OC treatment and to monitor recurrence (32,33). HE4 is considered a potential biomarker for OC because it is expressed in 32% of patients with OC without CA125 expression, and the Food and Drug Administration has approved HE4 for monitoring disease recurrence in patients with OC (34). Thus, the combination of serum CA125 and HE4 measurements may improve evaluations of malignancy prognosis in ovarian tumors (35,36). The present study demonstrated that patients with OC and a higher frequency of CD38+CD101+PD-1+CD8+ T cells exhibited higher CA125 and HE4 levels upon chemotherapy following surgical resection compared with those of patients with a lower frequency of CD38+CD101+PD-1+CD8+ T cells. Thus, the hypothesis that coexpression of CD38/CD101 on PD-1+CD8+ T cells is an indicator of worse prognosis in patients with OC is further supported.
In conclusion, the present study is the first to demonstrate an association between the coexpression of CD38/CD101 in PD-1+CD8+ T cells and the clinical stage, lymph node metastasis of patients with OC; by contrast, total PD-1+CD8+ T cells alone among TILs or PBMCs do not reflect disease status or prognosis. These observations are in line with a previous study, in which CD38/CD101 protein coexpression was suggested to represent a more useful predictor of diagnosis and prognosis for patients with pancreatic cancer compared with PD-1 alone (37). However, the present study further suggested that the coexpression of CD38/CD101 by peripheral PD-1+CD8+ T cells and TILs may serve as a new indicator for the treatment efficacy of patients with OC and as an indicator for the selection of patient populations for further immunotherapy by immune checkpoint blockade. However, the detection of CD38/CD101 coexpression in PD-1+CD8+T cells in the present study relied on flow cytometry analysis, and therefore this method would be more expensive than routine ELISA detection of levels of CA125, HE4 and other serum biomarkers. Accordingly, cost is one of the limitations of the strategy for OC diagnosis described in this study.
Although the coexpression of CD38/CD101 in PD-1+CD8+ T cells is a potential novel biomarker for the diagnosis and treatment of OC, which is strongly associated with serum levels of CA125 and HE4, other helpful biomarkers of OC diagnosis and treatment exist (38). For instance, osteopontin (OPN) has been used as a biomarker for the diagnosis and treatment of several cancer types, such as ovarian cancer, breast cancer, colorectal cancer and osteosarcoma (39–41). OPN is a secreted extracellular matrix glycoprotein that is involved in a number of cellular processes, including wound healing, inflammation, immune response and tumorigenesis (40). As OPN is usually overexpressed in several cancer types, including OC, an increase in serum OPN is often used to assess the diagnosis and prognosis of various human cancer types, including breast, lung, gastric and ovarian cancer, as well as melanoma (39–41). Therefore, in the future, the combined application of several biomarkers, such as CA125 and OPN, as well as the expression of CD38/CD101 in PD-1+CD8+ T cells, may provide more accurate and sensitive diagnoses and/or treatment indicators for patients with OC.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by the National Key Research and Development Project (grant no. 2016YFA0502203).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request
Authors' contributions
JZ and WW performed the main experiments in this study. WH and DW performed the clinical data collection and statistical analysis. ZL provided the clinical samples and interpreted the clinical data of patients. BN, WH and DW designed the study and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the ethics committee of the Third Military Medical University (Chongqing, China). Written, informed consent was obtained from all subjects prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Miller EM, Tymon-Rosario J, Strickler HD, Xie X, Xue X, Kuo DYS, Makhija SK, Nevadunsky NS. Racial differences in survival from epithelial ovarian cancer are associated with stage at diagnosis and use of neoadjuvant therapy: A 10-year single-institution experience with a racially diverse urban population. Int J Gynecol Cancer. 2018;28:749–756. doi: 10.1097/IGC.0000000000001238. [DOI] [PubMed] [Google Scholar]
- 3.Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51(Suppl 3):e72–e76. doi: 10.4103/0019-509X.154049. [DOI] [PubMed] [Google Scholar]
- 4.Santoiemma PP, Powell DJ., Jr Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16:807–820. doi: 10.1080/15384047.2015.1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol. 2012;51:263–272. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, Melero I, Ascierto PA. Cancer treatment with anti-PD-1/PD-L1 agents: Is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76:925–945. doi: 10.1007/s40265-016-0588-x. [DOI] [PubMed] [Google Scholar]
- 7.Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hämmerling GJ, et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rådestad E, Klynning C, Stikvoort A, Mogensen O, Nava S, Magalhaes I, Uhlin M. Immune profiling and identification of prognostic immune-related risk factors in human ovarian cancer. Oncoimmunology. 2018;8:e1535730. doi: 10.1080/2162402X.2018.1535730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ada G. The enunciation and impact of Macfarlane Burnet's clonal selection theory of acquired immunity. Immunol Cell Biol. 2008;86:116–118. doi: 10.1038/sj.icb.7100156. [DOI] [PubMed] [Google Scholar]
- 12.Reimann J, Böhm W, Schirmbeck R. Alternative processing pathways for MHC class I-restricted epitope presentation to CD8+ cytotoxic T lymphocytes. Biol Chem Hoppe Seyler. 1994;375:731–736. doi: 10.1515/bchm3.1994.375.11.731. [DOI] [PubMed] [Google Scholar]
- 13.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA. 2013;110:E2480–E2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arasanz H, Gato-Cañas M, Zuazo M, Ibañez-Vea M, Breckpot K, Kochan G, Escors D. PD1 signal transduction pathways in T cells. Oncotarget. 2017;8:51936–51945. doi: 10.18632/oncotarget.17232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan P, Wu X, Basu M, Rossi C, Sandler AD. PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease. PLoS Med. 2018;15:e1002497. doi: 10.1371/journal.pmed.1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 20.Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597. doi: 10.3389/fimmu.2017.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellström I, Hellström KE, Pierce GE, Yang JP. Cellular and humoral immunity to different types of human neoplasms. Nature. 1968;220:1352–1354. doi: 10.1038/2201352a0. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 24.Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F. CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom. 2013;84:207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- 25.d'Ettorre G, Ceccarelli G, Serafino S, Giustini N, Cavallari EN, Bianchi L, Pavone P, Bellelli V, Turriziani O, Antonelli G, et al. Dominant enrichment of phenotypically activated CD38(+) HLA-DR(+) CD8(+) T cells, rather than CD38(+) HLA-DR(+) CD4(+) T cells, in HIV/HCV coinfected patients on antiretroviral therapy. J Med Virol. 2016;88:1347–1356. doi: 10.1002/jmv.24475. [DOI] [PubMed] [Google Scholar]
- 26.Mao X, Peng L, Liu X, Yang Y, Wang Q, Wang D, Xiao J, Leng J. TLR9 expression is positively correlated with the levels of CD38, HLA-DR and CD95 on peripheral blood mononuclear cells in chronic HBV infected patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:660–665. (In Chinese) [PubMed] [Google Scholar]
- 27.Rivas A, Ruegg CL, Zeitung J, Laus R, Warnke R, Benike C, Engleman EG. V7, a novel leukocyte surface protein that participates in T cell activation. I. Tissue distribution and functional studies. J Immunol. 1995;154:4423–4433. [PubMed] [Google Scholar]
- 28.Soares LR, Tsavaler L, Rivas A, Engleman EG. V7 (CD101) ligation inhibits TCR/CD3-induced IL-2 production by blocking Ca2+ flux and nuclear factor of activated T cell nuclear translocation. J Immunol. 1998;161:209–217. [PubMed] [Google Scholar]
- 29.Gouttefangeas C, Jacquot S, Meffre E, Schmid M, Boumsell L, Bensussan A. Differential proliferative responses in subsets of human CD28+ cells delineated by BB27 mAb. Int Immunol. 1994;6:423–430. doi: 10.1093/intimm/6.3.423. [DOI] [PubMed] [Google Scholar]
- 30.Urban N, Mcintosh MW, Andersen M, Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. 2003;17(ix):989–1005. doi: 10.1016/S0889-8588(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 31.Urban N, Drescher C. Current and future developments in screening for ovarian cancer. Womens Health (Lond) 2006;2:733–742. doi: 10.2217/17455057.2.5.733. [DOI] [PubMed] [Google Scholar]
- 32.Kafali H, Artunc H, Erdem M. Evaluation of factors that may be responsible for cyclic change of CA125 levels during menstrual cycle. Arch Gynecol Obstet. 2007;275:175–177. doi: 10.1007/s00404-006-0242-z. [DOI] [PubMed] [Google Scholar]
- 33.Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):S274–S281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, Hellstrom I, Mok SC, Liu J, Bast RC., Jr Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–277. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Hu W. CA125 and HE4: Measurement tools for ovarian cancer. Gynecol Obstet Invest. 2016;81:430–435. doi: 10.1159/000442288. [DOI] [PubMed] [Google Scholar]
- 36.Xi QP, Pu DH, Lu WN. Research on application value of combined detection of serum CA125, HE4 and TK1 in the diagnosis of ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21:4536–4541. [PubMed] [Google Scholar]
- 37.Zhang M, Yang J, Zhou J, Gao W, Zhang Y, Lin Y, Wang H, Ruan Z, Ni B. Prognostic values of CD38+CD101+PD1+CD8+ T cells in pancreatic cancer. Immunol Invest. 2019;48:466–479. doi: 10.1080/08820139.2019.1566356. [DOI] [PubMed] [Google Scholar]
- 38.Kumari S. Serum biomarker based algorithms in diagnosis of ovarian cancer: A review. Indian J Clin Biochem. 2018;33:382–386. doi: 10.1007/s12291-018-0786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu ZD, Wei TT, Yang M, Ma N, Tang QQ, Qin BD, Fu HT, Zhong RQ. Diagnostic value of osteopontin in ovarian cancer: A meta-analysis and systematic review. PLoS One. 2015;10:e0126444. doi: 10.1371/journal.pone.0126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei R, Wong JPC, Kwok HF. Osteopontin-a promising biomarker for cancer therapy. J Cancer. 2017;8:2173–2183. doi: 10.7150/jca.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han X, Wang W, He J, Jiang L, Li X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol Lett. 2019;17:2592–2598. doi: 10.3892/ol.2019.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request