Abstract
Objective
Although the molecular components of circadian rhythms oscillate in discrete cellular components of the vasculature and many aspects of vascular function display diurnal variation, the cellular connections between the molecular clock and inflammatory cardiovascular diseases remain to be elucidated. Previously we have shown that pre- vs post-natal deletion of Bmal1 (brain and muscle aryl hydrocarbon receptor nuclear-like 1), the non-redundant core clock gene has contrasting effects on atherogenesis. Here we investigated the effect of myeloid cell Bmal1 deletion on atherogenesis and abdominal aortic aneurysm formation in mice.
Approach and Results
Mice lacking Bmal1 in myeloid cells were generated by crossing Bmal1 flox/flox mice with lysozyme 2 promoter driven Cre recombinase mice on a hyperlipidemic low-density lipoprotein receptor deficient background and were fed on a high fat diet to induce atherosclerosis. Atherogenesis was restrained, concomitant with a reduction of aortic pro-inflammatory gene expression in myeloid cell Bmal1 knockout mice. Body weight, blood pressure, blood glucose, triglycerides, and cholesterol were unaltered. Similarly, myeloid cell depletion of Bmal1 also restrained angiotensin II induced formation of abdominal aortic aneurysm in hyperlipidemic mice. In vitro, RNA-Seq analysis demonstrated a pro-inflammatory response in cultured macrophages in which there was overexpression of Bmal1.
Conclusions
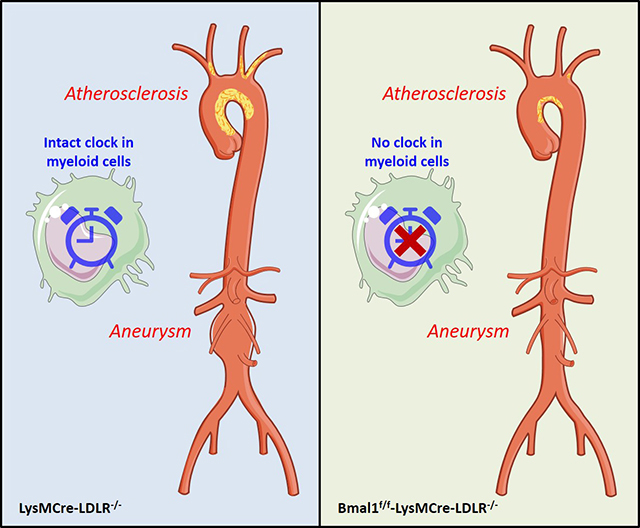
Myeloid cell Bmal1 deletion retards atherogenesis and restrains the formation of abdominal aortic aneurysm and may represent a potential therapeutic target for inflammatory cardiovascular diseases.
Keywords: Bmal1, atherosclerosis, abdominal aortic aneurysm, myeloid cells
Graphical Abstract
Introduction
Coronary artery disease and stroke arising from atherosclerosis are leading causes of death and morbidity worldwide. Circadian disruption by exposure to chronic jet lag or shift work is associated with increased cardiovascular diseases including atherosclerosis1 and rupture of abdominal aortic aneurysm (AAA)2. It has been demonstrated that hyperlipidemia altered both the rhythmicity and expression of circadian genes3, and the rhythmic expression of core clock genes, including Bmal1, Clock, Period 1–3, and Cryptochrome 1 and 2, is inhibited in human atherosclerotic plaque-derived cells4.
Growing evidence from animal studies indicates that the disruption of circadian machinery is closely associated with the progression of atherosclerosis. Overexpression of Cryptochrome 1 protected against the development of atherosclerosis via decreasing pro-inflammatory gene expression and inhibiting monocyte and macrophage adhesion5. ClockΔ19/Δ19 mutant mice had accelerated atherosclerosis with reducing cholesterol efflux from macrophages and increasing hepatic lipoprotein production6. Global or liver-specific Bmal1 deletion in mice increased hyperlipidemia and atherosclerosis7.
However, some contrasting observations have been reported8, 9. Thus, the transcription factor Clock might serve as a positive regulator of ICAM-1 (Intercellular Adhesion Molecule 1) to promote the adhesion of mononuclear cells to endothelial cells and contribute to the progression of atherosclerosis8. Furthermore, we found that, unlike conventional Bmal1 knockout (KO), adult-life deletion of Bmal1 (tamoxifen-induced deletion) restrains lesion development in hyperlipidemic mice9. This observation raises the possibility that in the conventional KO, the phenotype derives from loss of “off-target” effects of Bmal1 during development. Pertinent to this possibility, the Clock gene does not become available to partner with Bmal1 to drive oscillatory gene expression until late in development10. Finally, although aortic dissection and rupture exhibit circadian variability11, 12 and deletion of Bmal1 in vascular smooth muscle cells was protective against AAA13, limited information connects the circadian clock and formation of AAA.
Rhythmic oscillations of inflammatory monocytes and macrophages contribute to the pathogenesis of many inflammatory diseases14. Here we utilized lysozyme 2 (LysM) promoter driven Cre recombinase mice to interrogate the impact of myeloid cell depletion of the non-redundant core clock gene, Bmal1, on atherogenesis and AAA formation in mice.
Material and methods
The data that support the findings of this study are available from the corresponding authors on reasonable request.
Animals
Bmal1flox/flox mice were crossed with LysMCre mice to yield mice with Bmal1 selectively deleted in myeloid cells throughout development and postnatal life as previously reported15, 16. These mice were further crossed with low-density lipoprotein receptor KO mice (LDLR−/−) to generate Bmal1flox/flox-LysMCre-LDLR−/− mice, named KO. LysMCre-LDLR−/− mice were used as controls (Ctrl). Mice were kept under 12 h light/12 h dark conditions with free access to food and water. All animal studies were performed in accordance with the guidelines approved by the Institute for Animal Care and Use Committee at the University of Pennsylvania and Dalian Medical University.
Model and quantification of atherosclerosis
The atherosclerosis study adhered to the guidelines for experimental atherosclerosis studies described in the AHA Statement17. Both male and female eight- to ten-week-old mice were fed on a high fat diet (HFD, Harlan-Tekland TD 88137) for 3 months (3m) or 6 months (6m). Samples were collected at ZT4–5 (4–5 hours after lights on). Mouse aortic trees were prepared and stained, and atherosclerotic lesions were quantified as described18. Briefly, the entire aorta from the root to the iliac bifurcation was collected and fixed in formalin-free fixative Prefer (Anatech Ltd.). After removal of the adventitial fat, the aorta was opened longitudinally, stained with Sudan IV (Sigma), and pinned down on black wax to expose the intima. The extent of atherosclerosis was determined by the en face lesion area percentage to the entire intimal area.
Model and characterization of AAA
Eight- to ten-week-old male mice were fed on an HFD for 8 weeks, followed by angiotensin II (AngII, 1μg/min/kg body weight) infusion using a subcutaneously imbedded Alzet Minipump (Model 2004) for 4 weeks to induce AAA formation. Female mice were not used because they have a low incidence of AngII–induced AAA, as described in the ATVB Council statement19. After euthanization, the abdominal aorta was cleaned with removal of the adventitial fat. An increase of more than 50% in external diameter of the abdominal aorta was used to define the occurrence of an AAA. The severity of AAA formation was evaluated by the external diameter of the abdominal aorta and were classified based on the gross appearance of the aorta as described previously20 with modification In brief, the AAAs were scored into 4 categories: category I, no obvious dilation or slight dilated lumen in the supra-renal region of the aorta. II, clear dilated lumen with remodeled tissue in the supra-renal region. III, a form of type II that is pronounced bulbous or usually contains thrombus. IV, a form that shows multiple aneurysms containing thrombus, some overlapping, in the suprarenal area of the aorta. The classification was adjudged by two independent blinded observers. Notably, all the aortas are collected at ZT4–5 and the methods were adhered to the guidelines as described in the ATVB Council Statement19.
Morphologic analysis of aortic root lesions
Immunohistochemistry analysis of atherosclerotic lesion morphology from male mice was performed as previously described21. In brief, mouse hearts containing aortic roots were embedded in optimal cutting temperature compound (Tissue-Tek), and 20μm serial cryosections were cut and mounted on Superfrost Plus slides (Fisher Scientific). After fixation, sections were incubated with the desired primary antibody or IgG overnight at 4°C, followed by incubation with a secondary antibody for 1 hour at room temperature. Then the sections were incubated with Streptavidin-Horseradish Peroxidase for 30 minutes and developed using the DAB substrate kit as per the manufacturer’s protocol. Samples were counterstained with hematoxylin, dehydrated and mounted.
Primary culture of peritoneal macrophages
Thioglycollate-elicited peritoneal macrophages were harvested and cultured as previously described18. The migration assays were performed in duplicate by use of 24-well Transwell (Corning Incorporated Costar, REF3422). Cells were added to the upper chambers, and media with or without 10ng/ml MCP-1 (monocyte chemoattractant protein 1, Sigma) was placed in the lower chambers. Cells on the upper surface were removed 12 hours later, and the migrated cells were washed twice in PBS and fixed with methanol for 10min. Then, the cells were stained with 0.5% crystal violet solution for 30 min and counted.
For the DiI-Ac-LDL uptake assay, cells were incubated with 10μg/ml DiI-Ac-LDL for 4 hours at 37ºC, and then washed with PBS for twice and visualized under a fluorescence microscopy21. DAPI was used to stain the nuclei.
Blood pressure measurement
Systolic blood pressure was measured in conscious mice using a computerized noninvasive tail cuff system as we previously described22. After a three-day adaptation period, blood pressure was recorded as the mean value of 20 measurements at ZT4–5 each day for 5 consecutive days.
Plasma lipid analysis
Plasma total triglyceride and cholesterol levels were measured using commercial kits from Wako Chemicals (Richmond, VA) following manufacturer’s instruction.
Quantitative RT-PCR
Total RNA from aortae and macrophages was isolated using the Qiagen RNeasy Kit. Reverse transcription was performed using an RNA-cDNA kit (Applied Biosystems). Real-time PCR was performed using ABI TaqMan probes and reagents on an ABI Prism 7500 thermocycler. All mRNA measurements were normalized to 18s rRNA levels.
RNA-Seq
Primary cultured peritoneal macrophages with green fluorescent protein or Bmal1 adeno-associated virus infection (25MOI, 36 hours) were used for RNA-Seq experiments. Total RNA was isolated with TRIzol reagent (Invitrogen, CA) and cDNA was synthesized using SuperScript™ Double-Stranded cDNA Synthesis kit (Invitrogen, CA) following manufactural protocol. The resulting cDNA was fragmented by nebulization, and sequenced following the standard Illumina protocol (Novogene, Beijing, CN). For the data analysis, basecalls were performed using CASAVA. Reads are aligned to the genome using the split read aligner TopHat (v2.0.7) and Bowtie2. HTSeq is used for calculating their abundances. The resulting differentially expressed genes was used for enrichment analysis using Enrichr (http://amp.pharm.mssm.edu/Enrichr/).
Statistical Analysis
Statistical differences were determined by 2-way ANOVA, χ2 test, or Student’s t-test as indicated. All data for 2-way ANOVA and Student’s t-test were expressed as means ± SEM without prior analysis of normality and variance. A P value less than 0.05 was considered as significant difference.
Results
Effects of myeloid cell Bmal1 deletion on atherosclerotic lesion formation in mice
Mice of both genders were fed a HFD for 3m or 6m. Atherosclerotic lesion burden was markedly reduced in myeloid cell Bmal1 KOs in both male (Fig. 1A) and female mice (Fig. 1B) after 6m HFD feeding compared to controls. A small but significant decrease of lesion burden was observed in the male KO group at 3 months, while the trend towards a reduction in females failed to attain significance (Fig. 1). Plasma glucose and lipid levels (Fig.I), body weight, systemic blood pressure, and heart rate (Fig.II) did not differ by genotype.
Figure 1. Effects of myeloid cell Bmal1 deletion on atherosclerotic lesion formation in male (A) and female (B) hyperlipidemic mice.
The extent of aortic atherosclerotic lesion formation, represented by the ratio of lesion area to total aortic area, was quantified by en face analysis of aortae from mice after 3m or 6m on a HFD (Upper). Representative en face graphs are shown (lower) with each vertically matched to the corresponding data set in the upper panel (Student’s t-test, KO vs. Ctrl, * P<0.05, *** p<0.001, n=11–17).
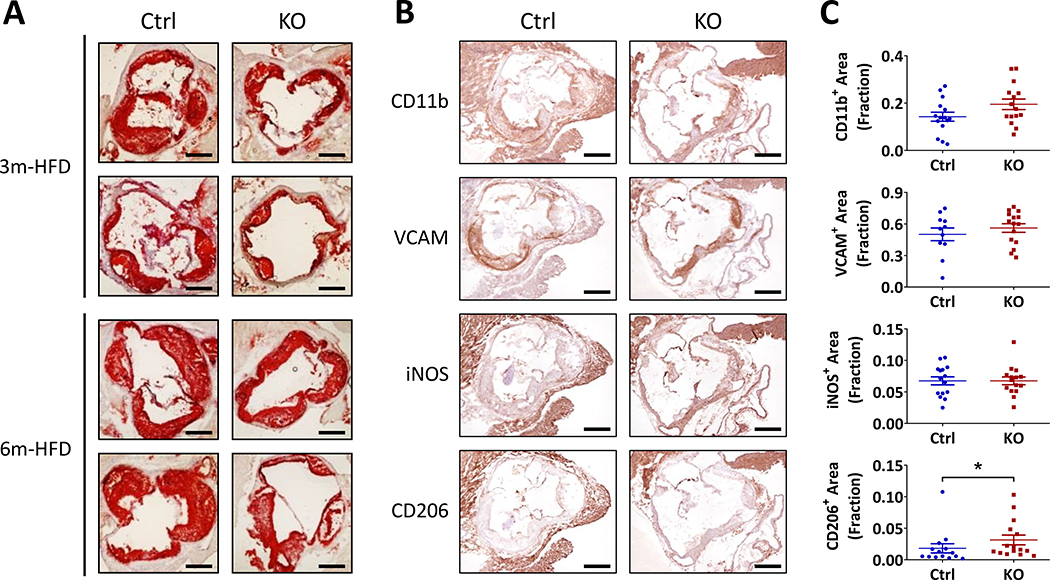
Morphometric consequences of myeloid cell Bmal1 deletion on lesion development
Lesion coverage was measured by en face Oil Red O staining of the aortic roots, and both lesion size and lipid content were reduced in the KOs at 3 and 6 months of age (Fig. 2A). Lesional morphology did not differ by genotype at 3 months (Fig.III). However, by 6 months, Ctrl lesions are complex with a mixture of smooth muscle cells, macrophages and necrotic layers (Fig.2B) while KO lesions are highly enriched for smooth muscle cells (VCAM staining) with macrophage rich (CD11b) regions generally on the surface. Detection of iNOS (inducible nitric oxide synthase) corresponds to VCAM rich areas and is observed in proximity to necrotic areas. No obvious difference was observed between KOs and Ctrls in lesional macrophage abundance, as stained by a pan macrophage marker CD11b. Notably, although CD11b is also expressed on monocytes, it is widely accepted that the majority, if not all, of the monocytes in atherosclerotic lesions are activated as they transition through the endothelial layer and become macrophages23, 24. Thus, total macrophage infiltration was not reduced despite depletion of Bmal1 in such myeloid cells restraining atherogenesis. However, CD206, a marker of anti-inflammatory type 2 macrophages, was significantly increased in the KO lesions. Fibronectin abundance and collagen-rich areas were also comparable (Fig.IV).
Figure 2. Morphometric consequences of myeloid cell Bmal1 deletion on lesion development.
(A) Representative Oil Red O staining of cross-sections of aortic roots from mice after 3m or 6m on a HFD (Scale bar, 500μm). (B) Representative images of immunohistochemistry analysis for CD11b, VCAM, iNOS, and CD206 in aortic roots from mice on 6m-HFD (Scale bar, 500μm). (C) Quantification of positive area of staining signals in immunohistochemistry (Student’s t-test, * p<0.05).
Myeloid Bmal1 deletion coincides with reduced aortic inflammatory responses during atherogenesis
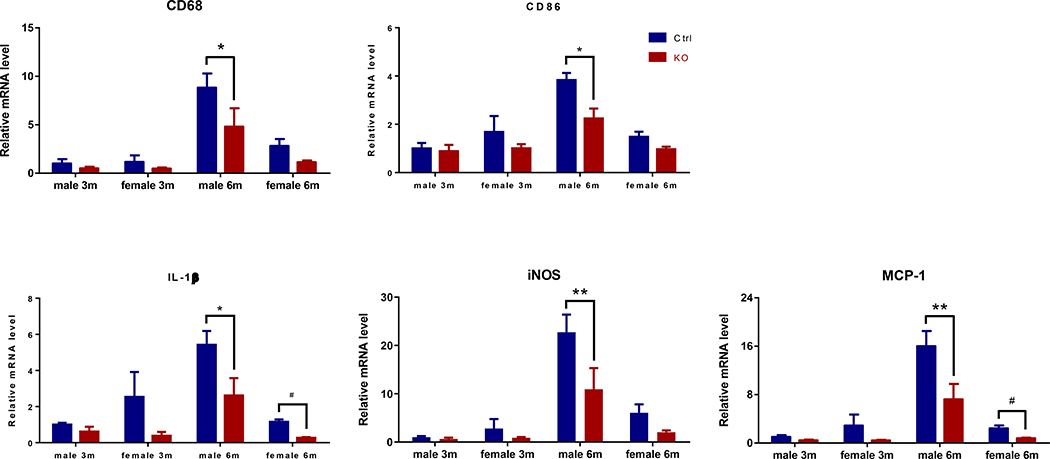
Despite these findings in the aortic roots, a reduced expression of the pan-macrophage marker CD68 and type 1 pro-inflammatory macrophage marker CD86 was observed in the freshly isolated thoracic aortas of the KOs. Expression of IL-1β, iNOS, and MCP-1 was also significantly suppressed (Fig.3). Thus, myeloid Bmal1 deletion results in a reduction in the aortic inflammatory signature during atherogenesis.
Figure 3. Myeloid cell Bmal1 deletion coincides with a reduced pro-inflammatory response in aortas during atherogenesis.
Real-time RT-PCR analysis of CD68, CD86, IL-1β, iNOS, and MCP-1 expression in thoracic aortas from mice treated with HFD for 3m or 6m (* p<0.05, ** p<0.01, 2-way ANOVA with Sidak’s multiple comparisons test; #p<0.05, student’s t-test; n=3).
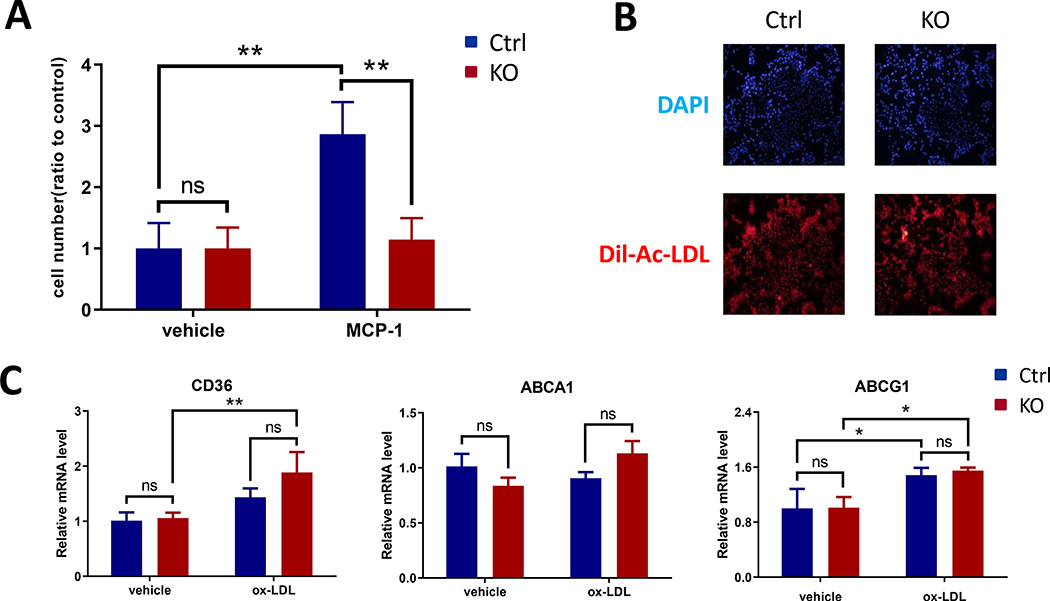
Bmal1 deficient macrophages showed reduced potential for migration without alteration of lipid handling
To study if the impaired inflammatory responses in the KO mice was due to reduced macrophage migration, peritoneal macrophages were harvested for a Transwell chamber assay. MCP-1-directed macrophage migration was significantly inhibited in Bmal1 deficient cells (Fig 4A). The KO cells showed undistinguishable ability to uptake DiI-Ac-LDL as Ctrl cells did (Fig. 4B), consistent with identical ox-LDL induced expression of lipid controlling genes CD36, ABCA1 and ABCG1 amongst the genotypes (Fig. 4C).
Figure 4. Bmal1 deficient macrophages showed reduced migration potential and unaltered lipid handling in vitro.
(A) Cells were placed in Transwell upper chambers and chemotaxis was assessed with or without exposure to 10ng/ml MCP-1, the KO cells showed significantly reduced migration to MCP-1. (B) Cells were treated with 10μg/ml DiI-Ac-LDL for 4h and uptake was assessed. Representative fluorescence images showed no difference between Ctrl and KO cells. DAPI staining was used as a control. (C) Cells were treated with or without 50μg/ml ox-LDL for 24h, real-time RT-PCR showed no difference between Ctrl and KO cells for CD36, ABCA1 and ABCG1 expression. (2-way ANOVA with Tukey’s multiple comparisons test, n=3, * p<0.05, ** p<0.01, ns, no statistical difference)
Effects of myeloid cell Bmal1 deletion on AAA formation in mice
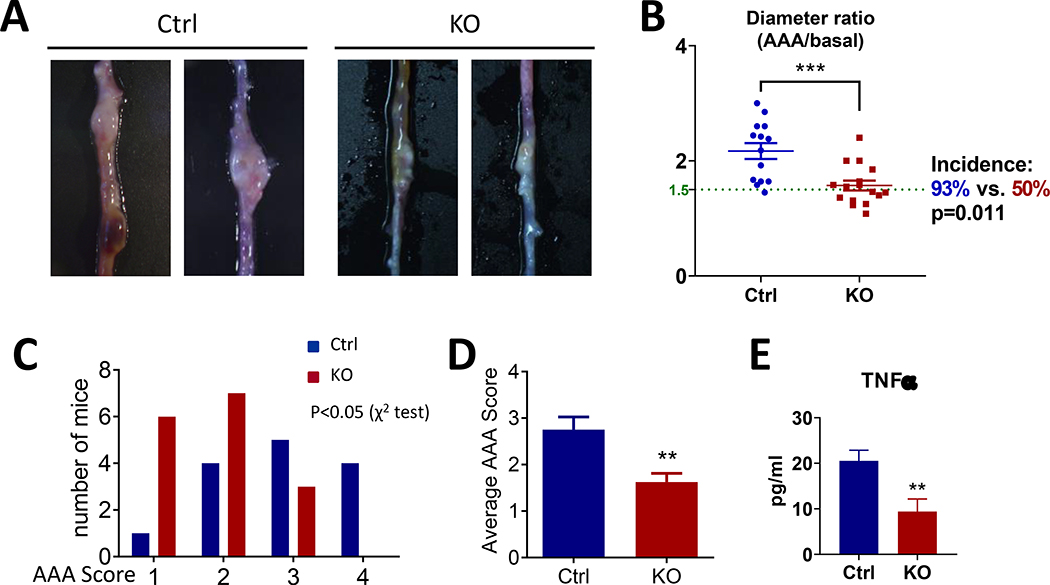
The molecular clock has been implicated in AAA. Deletion of Bmal1 in smooth muscle cells was reported to provide robust protection from aortic aneurysm formation13. To test the role of myeloid Bmal1 in AAA formation, we subjected the mice to HFD feeding and AngII infusion to induce formation of aneurysms predominantly in the abdominal aortae. Consistent with the protective effect on atherogenesis, myeloid Bmal1 deletion significantly decreased the incidence (93% in Ctrl versus 50% in KO) of AAA formation (Figure 5A&B), the ratio of maximal diameter of the abdominal aorta to baseline (Figure 5B), and the AAA score (Figure 5C&D). Sudden death due to rupture of the aneurysm was observed only in the Ctrl mice (2 of 16) but not in the KO mice. No obvious aneurysm was observed in the thoracic aorta of either Ctrl or KO mice.
Figure 5. Effects of myeloid cell Bmal1 deletion on AngII-induced abdominal aortic aneurysm formation.
(A) Representative images of AAAs in Ctrl and KO group. (B) The ratio of maximal diameter of the abdominal aorta to basal diameter (Student’s t-test, *** p<0.001) and the incidence of AAA (above the green line, ratio>1.5, χ2 test). (C) Comparison of mice number of the specific classification of aneurysms. (D) The average AAA score (Student’s t-test, ** p<0.01, n=14–16). (E) Plasma level of TNFα (Student’s t-test, **p<0.01, n=12–15).
Overexpression of Bmal1 elicits pro-inflammatory responses in cultured macrophages
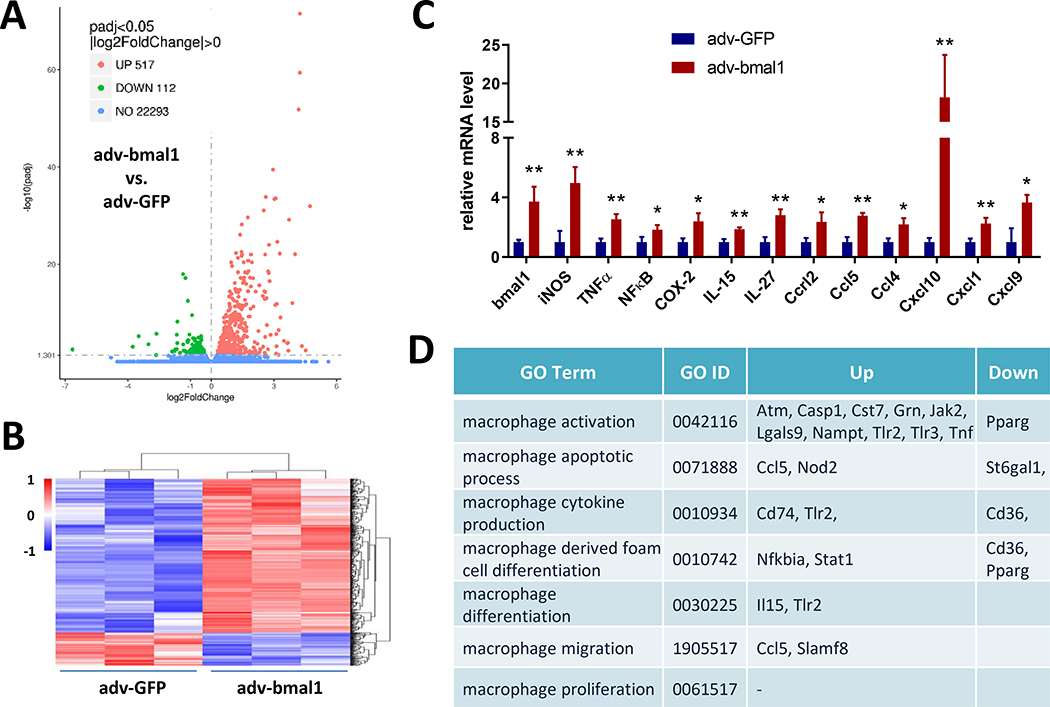
We performed RNA-Seq using Bmal1-overexpression in peritoneal macrophages to interrogate further the role of this transcription factor in driving inflammatory vascular diseases. In total, the expression of 22922 mRNAs were detected, of which 629 genes had an adjusted P-value less than 0.05 in Bmal1 overexpressed cells as compared with controls. Amongst them, 517 genes were markedly upregulated, and 112 genes were downregulated (Fig. 6A&B). Based on the gene ontology (GO) analysis of the 517 upregulated genes, we found that no category in GO molecular function or cell component was significantly enriched. GO biological process analysis showed that the most abundant functional pathways were associated with immune and inflammatory responses (Table 1). For example, iNOS, COX-2, NFκB, CCL5, all showed significantly induction with Bmal1 overexpression (Fig. 6C), consistent with reduced inflammatory responses in Bmal1 deficient macrophages and the consequent impact on atherogenesis and AAA formation. Besides, by comparing all 629 differentially expressed genes with the lists of macrophage function related GO terms, we found 20 genes fell into these categories, including activation, cytokine production, differentiation, migration, and proliferation (Fig. 6D).
Figure 6. Overexpression of Bmal1 elicits pro-inflammatory responses in macrophages.
(A) Volcano plot of the differentially expressed genes. Green indicates downregulation, red indicates upregulation, and blue means no significant difference. (B) Hierarchical clustering heatmap illustrates the all 629 differentially expressed mRNAs between Bmal1 adeno-associated virus infected cells and the GFP virus control. The map is color coded with red corresponding to up-regulation and blue to down-regulation. (C) The relative mRNA expression levels of bmal1 and classic inflammatory factors and chemokines (Student’s t-test, * p<0.05, ** p<0.01, n=3). (D) Differentially expressed genes related to macrophage function was classified based on gene ontology (GO) term.
Table I.
Gene ontology (GO) biological process classifications of the 517 upregulated genes. The top 20 pathways are given. Overlap, the number of appearing gene/number of background genes.
| GO Term | GO ID | Overlap (list/background) | Adjusted P-value |
|---|---|---|---|
| cellular response to type I interferon | 0071357 | 28/65 | 7.07E-24 |
| cytokine-mediated signaling pathway | 0019221 | 71/633 | 1.15E-22 |
| response to cytokine | 0034097 | 28/138 | 2.24E-14 |
| cellular response to interferon-gamma | 0071346 | 25/116 | 2.15E-13 |
| regulation of type I interferon production | 0032479 | 21/85 | 2.45E-12 |
| negative regulation of viral life cycle | 1903901 | 17/61 | 9.48E-11 |
| interferon-gamma-mediated signaling pathway | 0060333 | 18/70 | 9.66E-11 |
| negative regulation of type I interferon production | 0032480 | 14/44 | 1.60E-09 |
| regulation of viral genome replication | 0045069 | 16/63 | 1.93E-09 |
| negative regulation of viral genome replication | 0045071 | 14/50 | 9.26E-09 |
| response to interferon-gamma | 0034341 | 15/68 | 6.73E-08 |
| positive regulation of I-kappaB kinase/NF-kappaB signaling | 0043123 | 21/163 | 5.84E-07 |
| interleukin-1-mediated signaling pathway | 0070498 | 16/95 | 9.68E-07 |
| positive regulation of cytokine production | 0001819 | 24/220 | 1.05E-06 |
| regulation of I-kappaB kinase/NF-kappaB signaling | 0043122 | 23/204 | 1.11E-06 |
| positive regulation of NF-kappaB transcription factor activity | 0051092 | 18/128 | 1.54E-06 |
| positive regulation of type I interferon production | 0032481 | 13/62 | 1.55E-06 |
| regulation of defense response | 0031347 | 14/75 | 1.77E-06 |
| inflammatory response | 0006954 | 25/252 | 2.47E-06 |
| regulation of cytokine-mediated signaling pathway | 0001959 | 15/90 | 2.47E-06 |
Discussion
Accumulating evidence suggests that Bmal1, the only nonredundant gene in the core molecular clock, is involved in many vascular phenotypes, including arterial stiffness, vascular remodeling, inflammatory responses, and endothelial dysfunction25. Although we and others reported that conventional Bmal1 KO mice and hepatocyte-specific Bmal1 KO mice were prone to develop hyperlipidemia and atherosclerosis7. We recently found that global deletion of murine Bmal1 in adulthood protected mice from atherogenesis9. Thus, potentially “off target” effects during embryonic development of Bmal1 depletion, independent of its role in the molecular clock9, indicate that the impact of its role in the clock on cardiovascular disease phenotypes requires further investigation.
Indeed, the absence of a functional clock is beneficial for cardiovascular health in mice under certain conditions. For example, deletion of Bmal1 in vascular smooth muscle cells was protective against experimental aortic aneurysm induced by both DOCA- or Aldo-salt plus AngII infusion in hypercholesterolemic mice13. Per1 inhibition is associated with protection from high salt plus desoxycorticosterone pivalate induced hypertension26. Recently, we showed that global deletion of Bmal1 in adult mice facilitates adaptation to various light/dark schedules and protects mice from the insulin resistance induced by circadian disruption27. Thus, we speculated that lacking the intrinsic clock may be protective by rendering the mice impervious to environmental disturbances or high-fat diet induced circadian disruption28.
In the current experimental setting, we show that deletion of Bmal1 in myeloid cells in mice is protective from both atherogenesis and AAA induced by HFD feeding and/or AngII infusion in hypercholesterolemia condition. Mechanistically, the protection might be attributable to inhibition of pro-inflammatory responses in mouse aortas and macrophages. Our data contrasts with another recent report29. Generating Bmal1-LysMCre mice on an apolipoprotein E depleted background, Huo et al. showed that Bmal1 deletion in myeloid cells increased the size of atherosclerotic lesions with an increased total number of onsite monocytes and macrophages, the latter skewed from M2 to the pro-inflammatory M1 phenotype. Here, we failed to detect an alteration of lesional macrophage abundance, as stained by CD11b. By contrast, reduced expression of M1 macrophage marker CD86 and increased M2 marker CD206 was observed.
One obvious difference between the two studies is the hyperlipidemic models deployed - (apolipoprotein E KO versus LDLR KO). These models differ in their patterns of hyperlipidemia and the sexual dimorphism of disease progression. They were studied only at an early stage of disease progression (3 months) in the study of Huo et al. However, it is unclear how depletion of Bmal1 during and after development in myeloid cells should have such contrasting effects on atherogenesis in the two backgrounds. Moreover, the observed athero-protective effect of prenatal myeloid Bmal1 KO mice in the current study was consistent with the adult global Bmal1 KO mice, but not with conventional KO mice, as we reported before9. Considering that overall development was not affected in the lysMcre-bmal1f/f mice, this may indicate that the overall premature aging phenotypes in conventional KO mice mask the athero-protective role of bmal1 deficiency in individual cell types, such as myeloid cells. However, the possible effect of embryonic Bmal1 deletion on cytokine production in myeloid cells and subsequently on atherosclerosis and aneurysm formation cannot be excluded. Further studies, including deletion of Bmal1 and other core clock genes in myeloid cells in adulthood are required to clarify the role of the molecular clock in this lineage as a modulator of inflammatory vascular diseases.
Another distinct, more technical explanation may be the controls used in the two sets of experiments. Here, we used the LysMCre mice without flox as controls, while Huo et al. used the Bmal1flox/flox mice that do not express Cre as controls. The commonly used LysMCre (https://www.jax.org/strain/004781) mouse was constructed downstream of the endogenous LysM promoter, replacing the coding sequence of LysM, which compromises macrophage function30. Although they used heterozygous LysMCre mice, the effect of partial depletion of this myeloid cell marker gene on an inflammatory phenotype cannot be excluded.
Finally, Bmal1 is the only clock gene whose sole deletion leads to complete loss of circadian rhythmicity, however, direct evidence to link its role in circadian rhythms to atherogenesis and AAA development is still lacking. Further studies using animal models under disrupted light/dark conditions and cell specific postnatal gene deletion are required to clarify fully the role of circadian rhythm in these vascular diseases.
Taken together, our findings are consistent with the impact of adult global deletion of Bmal1 – where its role in the molecular clock is established - restraining atherogenesis. They are also consistent with our previous studies establishing the importance of myeloid cell Bmal1 in regulating the inflammatory response more broadly15, consistent with the anticipatory role of the molecular clock31. They raise the possibility that age dependent deterioration in myeloid cell clock function may contribute to cardiovascular disease and represent a novel approach to disease mitigation.
Supplementary Material
Highlights.
Myeloid cell Bmal1 deletion retards atherogenesis and restrains the formation of abdominal aortic aneurysm.
Myeloid Bmal1 deletion coincides with reduced aortic inflammatory responses without alteration of body weight, blood pressure, blood glucose, triglycerides, and cholesterol.
Overexpression of Bmal1 elicits pro-inflammatory responses in cultured peritoneal macrophages.
Acknowledgments
We thank C. A. Bradfield (University of Wisconsin, Madison, WI) for generously providing us Bmal1flox/flox mice.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (81670242 & 81570643), and the National Institutes of Health (U54TR001623). Dr. FitzGerald is the McNeil Professor in Translational Medicine and Therapeutics.
Nonstandard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- AngII
angiotensin II
- Bmal1
brain and muscle aryl hydrocarbon receptor nuclear-like 1
- Ctrl
control
- HFD
high fat diet
- iNOS
inducible nitric oxide synthase
- KO
knockout
- LDLR
low-density lipoprotein receptor
- LysM
lysozyme 2
- MCP-1
monocyte chemoattractant protein 1
Footnotes
Disclosures
None.
References
- 1.Haupt CM, Alte D, Dorr M, Robinson DM, Felix SB, John U, Volzke H. The relation of exposure to shift work with atherosclerosis and myocardial infarction in a general population. Atherosclerosis. 2008;201:205–211. [DOI] [PubMed] [Google Scholar]
- 2.Davis FM, Daugherty A, Lu HS. Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol. 2019;39:e83–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou L, Lu C, Huang Y, Chen S, Hua L, Qian R. Effect of hyperlipidemia on the expression of circadian genes in apolipoprotein e knock-out atherosclerotic mice. Lipids Health Dis. 2009;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C, Tang X, Zhu Z, Liao X, Zhao R, Fu W, Chen B, Jiang J, Qian R, Guo D. The rhythmic expression of clock genes attenuated in human plaque-derived vascular smooth muscle cells. Lipids Health Dis. 2014;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Chu Y, Wang L, Wang Y, Zhao X, He W, Zhang P, Yang X, Liu X, Tian L, Li B, Dong S, Gao C. Overexpression of cry1 protects against the development of atherosclerosis via the tlr/nf-kappab pathway. Int Immunopharmacol. 2015;28:525–530. [DOI] [PubMed] [Google Scholar]
- 6.Pan X, Jiang XC, Hussain MM. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation. 2013;128:1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan X, Bradfield CA, Hussain MM. Global and hepatocyte-specific ablation of bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat Commun. 2016;7:13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Meng D, Sun N, Zhu Z, Zhao R, Lu C, Chen S, Hua L, Qian R. Clock upregulates intercellular adhesion molecule-1 expression and promotes mononuclear cells adhesion to endothelial cells. Biochem Biophys Res Commun. 2014;443:586–591. [DOI] [PubMed] [Google Scholar]
- 9.Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G, FitzGerald GA. Timing of expression of the core clock gene bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umemura Y, Koike N, Ohashi M, Tsuchiya Y, Meng QJ, Minami Y, Hara M, Hisatomi M, Yagita K. Involvement of posttranscriptional regulation of clock in the emergence of circadian clock oscillation during mouse development. Proc Natl Acad Sci U S A. 2017;114:E7479–E7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale J, Manfredini R, Gallerani M, Mumoli N, Eagle KA, Ageno W, Dentali F. Chronobiology of acute aortic rupture or dissection: A systematic review and a meta-analysis of the literature. Chronobiol Int. 2015;32:385–394. [DOI] [PubMed] [Google Scholar]
- 12.Manfredini R, Fabbian F, Manfredini F, Salmi R, Gallerani M, Bossone E. Chronobiology in aortic diseases - “is this really a random phenomenon?”. Prog Cardiovasc Dis. 2013;56:116–124. [DOI] [PubMed] [Google Scholar]
- 13.Lutshumba J, Liu S, Zhong Y, Hou T, Daugherty A, Lu H, Guo Z, Gong MC. Deletion of bmal1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38:1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene bmal1 regulates diurnal oscillations of ly6c(hi) inflammatory monocytes. Science. 2013;341:1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis AM, Fagundes CT, Yang G, et al. Circadian control of innate immunity in macrophages by mir-155 targeting bmal1. Proc Natl Acad Sci U S A. 2015;112:7231–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha P, Chi HH, Kim HR, Clausen BE, Pederson B, Sercarz EE, Forster I, Moudgil KD. Mouse lysozyme-m knockout mice reveal how the self-determinant hierarchy shapes the t cell repertoire against this circulating self antigen in wild-type mice. J Immunol. 2004;173:1763–1771. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R, American Heart Association Council on Arteriosclerosis T, Vascular B, Council on Basic Cardiovascular S. Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the american heart association. Arterioscler Thromb Vasc Biol. 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 18.Yu Z, Crichton I, Tang SY, Hui Y, Ricciotti E, Levin MD, Lawson JA, Pure E, Fitzgerald GA. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to cox-2 deletion in mice. Proc Natl Acad Sci U S A. 2012;109:6727–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from atvb council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty A, Manning MW, Cassis LA. Antagonism of at2 receptors augments angiotensin ii-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Yang G, Monslow J, Todd L, Cormode DP, Tang J, Grant GR, Delong JH, Tang SY, Lawson JA, Pure E, Fitzgerald GA. Myeloid cell microsomal prostaglandin e synthase-1 fosters atherogenesis in mice. Proc Natl Acad Sci U S A. 2014;111:6828–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin e synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle JJ. Macrophage activation in atherosclerosis: Pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–68. [DOI] [PubMed] [Google Scholar]
- 24.Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. Macrophages and their role in atherosclerosis: Pathophysiology and transcriptome analysis. Biomed Res Int. 2016;2016:9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Pi W, Rudic RD. Old and new roles and evolving complexities of cardiovascular clocks. Yale J Biol Med. 2019;92:283–290. [PMC free article] [PubMed] [Google Scholar]
- 26.Alli A, Yu L, Holzworth M, Richards J, Cheng KY, Lynch IJ, Wingo CS, Gumz ML. Direct and indirect inhibition of the circadian clock protein per1: Effects on enac and blood pressure. Am J Physiol Renal Physiol. 2019;316:F807–F813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, Chen L, Zhang J, Ren B, FitzGerald GA. Bmal1 deletion in mice facilitates adaptation to disrupted light/dark conditions. JCI Insight. 2019;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. [DOI] [PubMed] [Google Scholar]
- 29.Huo M, Huang Y, Qu D, Zhang H, Wong WT, Chawla A, Huang Y, Tian XY. Myeloid bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017;31:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganz T, Gabayan V, Liao HI, Liu L, Oren A, Graf T, Cole AM. Increased inflammation in lysozyme m-deficient mice in response to micrococcus luteus and its peptidoglycan. Blood. 2003;101:2388–2392. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald GA, Yang G, Paschos GK, Liang X, Skarke C. Molecular clocks and the human condition: Approaching their characterization in human physiology and disease. Diabetes Obes Metab. 2015;17 Suppl 1:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.