Abstract
Purpose
Women with Li-Fraumeni syndrome (LFS), a cancer predisposition syndrome caused by germline mutations in TP53, have an over 50% risk of developing breast cancer by age 70. Patients with LFS are at risk for radiation-induced malignancies; however, only small case series have prior investigated radiation risks in the treatment of breast cancer. We therefore aimed to investigate the risk of malignancy in breast cancer patients with LFS following adjuvant radiotherapy.
Methods
A single-institution retrospective chart review was conducted for female breast cancer patients with confirmed germline TP53 mutation. The frequency of radiation-induced malignancies in LFS patients were compared to non-LFS breast cancer cases reported in the Penn Medicine Cancer Registry via statistical analyses.
Results
We identified 51 female LFS breast cancer patients with 74 primary diagnoses. Fifty-seven% had a history of breast cancer only, and 25% had breast cancer as their presenting diagnosis of LFS. LFS-associated breast cancers were predominantly invasive ductal carcinoma (48%) and HER2+ (58%). Twenty patients underwent adjuvant radiotherapy with a median follow up of 12.5 (2–20) years. Of 18 patients who received radiation in a curative setting, one (6%) patient developed thyroid cancer, and one (6%) patient developed sarcoma in the radiation field. This risk for radiation-induced malignancy associated with LFS was higher for both sarcoma and thyroid cancer in comparison to our control cohort.
Conclusions
We found a lower risk of radiation-induced secondary malignancies in LFS breast cancer patients than previously reported in the literature (33% risk of radiation-induced sarcoma). These findings suggest that LFS may not be an absolute contraindication for radiotherapy in breast cancer. The potential risk for locoregional recurrence without radiotherapy must be weighed against the long-term risk for radiation-induced malignancies in consideration of adjuvant radiotherapy for LFS breast cancer patients.
Keywords: Li-Fraumeni syndrome, breast cancer, radiotherapy, radiation-induced malignancy
INTRODUCTION
Li-Fraumeni syndrome (LFS) is an autosomal dominant inherited cancer predisposition syndrome, often due to germline mutations in TP53. LFS patients have an 80–90% risk of acquiring cancer over their lifetimes often starting in childhood [1]. The aggressiveness of this syndrome is attributed to the disruption of normal functions of TP53, a pivotal tumor suppressor gene that encodes the p53 protein, a DNA binding transcription factor that regulates DNA repair, oxidant metabolism, cell cycle arrest, apoptosis, and senescence.
Classic presentation of LFS includes multiple primary LFS-associated carcinomas, i.e., sarcomas, rare childhood cancers, and early onset breast cancer, where 54% of women with LFS develop breast cancer before age 70, with a median age of onset of 33 years [2]. Chompret criteria for LFS were revised in 2015 to account for individuals with breast cancer before age 31 independent of family history [3]. Given this increased breast cancer risk, female LFS patients are recommended to undergo biannual breast examination and annual breast MRI starting at age 20, as well as consideration of risk-reducing bilateral mastectomy [4]. To reduce radiation exposure from mammography, breast MRI is used exclusively between the ages of 20–30, given the utility of breast MRI in identifying clinically significant lesions in younger women with denser breasts [5]. For management of LFS-associated breast cancer, mastectomy is often favored over lumpectomy to reduce the risks of a second primary breast cancer [6,7]. Providers often have concerns using adjuvant radiation for locoregional control, where it would be otherwise indicated, due to a perceived high risk of radiation-induced sarcoma.
Previous studies have described increased chromosomal aberrations in in vitro mutant TP53 fibroblasts [8], as well as genotoxicity in mouse models with deficient germline TP53 due to radiation exposure [9]. However, there is a paucity of corresponding clinical data on the incidence of radiation-induced malignancies after adjuvant radiotherapy for breast cancer in LFS patients. Here we evaluate the clinical and pathological characteristics of LFS-associated breast cancers and the frequency of second malignancies within and outside of the irradiated field in a single-institution retrospective cohort of LFS patients presenting with breast cancer.
METHODS
LFS patients who presented at Penn Medicine between 2007 and 2019 were ascertained under a University of Pennsylvania institutional review boards approved protocol [10]. Briefly, patients were identified via a query of the medical record for individuals with a germline mutation in TP53; and cases were cross-referenced with cancer predisposition registry records. Eligibility criteria for the current analysis included: (i) confirmed germline mutation in TP53 and (ii) diagnosis of female breast cancer. Medical records and pathology reports were reviewed for clinical history and treatment.
Data on non-LFS breast cancer patients from the Penn Medicine Cancer Registry (PMCR) includes all breast cancer patients who were either diagnosed and/or treated at Penn Medicine from 2009 to 2017 and their historical treatments. We queried the PMCR for all primary carcinomas of the breast with a ductal, lobular, medullary, or mixed histology and excluded any cases with a diagnosis of LFS. Only cases where radiotherapy was administered at least two years prior to the last contact date recorded in either the PMCR or the Penn Cancer Service line in the EHR were included the analysis of radiation-induced malignancies to ensure a minimum of two-year follow-up. Pathological data were downloaded from the Surveillance, Epidemiology, and End Results (SEER) database [11] which include breast cancer cases from 1975 to 2016 nation-wide. Data on the LFS cohort from the International Agency for Research on Cancer (IARC) database were downloaded for individuals with a pathogenic or likely pathogenic TP53 mutation [10].
Statistical comparisons of the frequency of radiation-induced malignancies and pathological characteristics of breast cancer were performed with two-tailed Fisher’s exact tests and Chi-Square tests. In addition, we estimated propensity scores using logistic regression on the following covariates and all pairwise interactions: age at diagnosis, stage, and type of surgery received. We used standardized mean differences to assess the covariate balance before and after propensity score weighting and overlap of propensity scores was evaluated. We then used the propensity score to calculate the inverse probability weights for the patients. These weights were one divided by the propensity score for the LFS patients and one divided by one minus the propensity score for the non-LFS patients. These weights were included in a Poisson regression model with robust standard errors in order to estimate relative risks and corresponding 95% confidence intervals and p-values.
RESULTS
We identified 94 patients with LFS presenting to Penn Medicine, including 74 (79%) female patients from 42 families (Online Resource, Supplementary Figure 1a). Fifty-one patients (69%) and 33 probands (79%) had at least one breast cancer with a total of 74 primary breast cancer diagnoses (Table 1). The rate of breast cancer among female probands was similar to IARC data (73% of n=183, p=0.56).
Table 1.
Clinical and pathological characteristics of the cohort
| Clinical characteristics of patients (n=51) | |
| Age of onset | 33.6 ± 8.6 |
| Self-reported race, n (%) | |
| Caucasian | 42 (82) |
| African American | 4 (8) |
| Asian | 1 (2) |
| Mixed Race | 2 (4) |
| Not Reported | 2 (4) |
| Self-reported religion, n (% of n=44) | |
| Ashkenazi Jewish | 7 (16) |
| LFS classification, n (%) | |
| Classical | 12 (24) |
| Chompret | 31 (61) |
| LFL (Birch or Eeles) | 31 (61) |
| De novo | 2 (4) |
| Vital status, n (%) | |
| Alive | 42 (84) |
| Personal cancer history, n (%) | |
| Multiple primaries, BC 1st Dgx | 14 (27) |
| Multiple primaries, BC Sub. dgx | 7 (14) |
| Multiple BC | 11(22) |
| Single BC | 19 (37) |
| Disease progression, n (%) | |
| Local recurrence | 5 (10) |
| Distant metastasis | 9 (18) |
| Pathological characteristics of Tumors (n=74) | |
| Histology, n (%) | |
| IDC | 37 (48) |
| DCIS | 22 (29) |
| MDLC | 3 (4) |
| NOS | 12 (16) |
| Grade, n (% of n=41) | |
| High | 35 (71) |
| Intermediate | 14 (29) |
| Low | 0 (0) |
| Hormone receptor status, n (% of n=38) | |
| ER+/HER2+ | 10 (26) |
| ER−/HER2+ | 12 (32) |
| ER+/HER2− | 13 (34) |
| ER−/HER2− | 3 (8) |
| Nodal status, n (% of n=44) | |
| pN0 | 25 (57) |
| pN1 | 10 (23) |
| pN2 | 6 (13) |
| pN3 | 3 (7) |
LFL, Li-Fraumeni-like criteria including Birch and Eeles criteria; BC, breast cancer; 1st Dgx, first diagnosis; Sub. Dgx, subsequent diagnosis; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; MDLC, mixed ductal and lobular carcinoma; NOS, not otherwise specified.
The average onset age of breast cancer was 33.6 (±8.6) years ranging from 17 to 59 in all female patients. Age of onset among breast cancer probands in our cohort was nearly identical to IARC breast cancer probands (32.1±7.8 vs 30.9±6.7, p=0.39, Online Resource, Supplementary Figure 1b). Twenty-nine patients (57%) had breast cancer only, 13 (25%) patients had breast cancer as their first diagnosis and developed a subsequent cancer, and nine patients (18%) developed breast cancer after a non-breast cancer diagnosis, with seven of nine having had a pediatric malignancy (Figure 1). The majority of breast cancers were ductal histology (either invasive or DCIS) and 43% had positive lymph node. As expected for an LFS breast cancer cohort [12], we observed an overrepresentation of HER2+ breast cancers (58%, n=38) as compared to breast cancers in SEER (16%, n=549268, X2(3, N=549306) = 78.6, p<0.001) and PMCR (15%, n=7748, X2(3, N=7748) = 65.3, p<0.001). In our LFS cohort, 25% of breast cancers presented at stage 0, I or II each, 16% presented at stage III, and 9% were metastatic at diagnosis (Table 2).
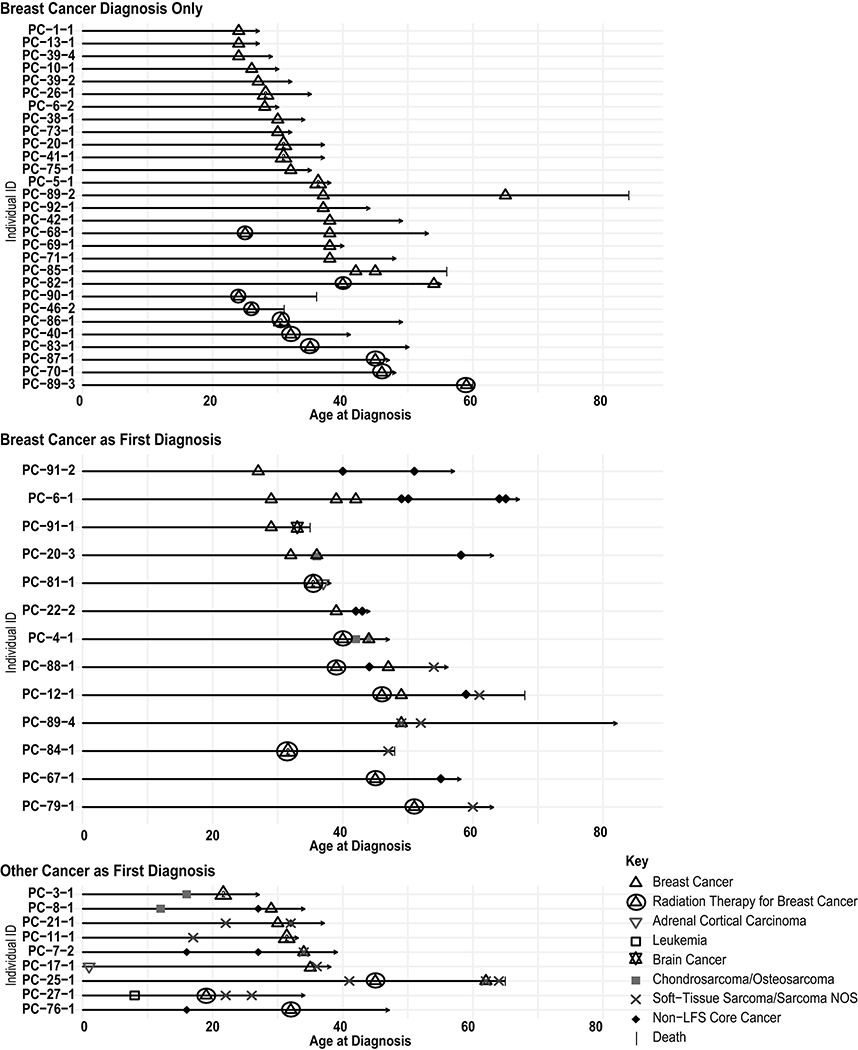
Fig. 1. Personal cancer trajectory of breast cancer patients with LFS.
Cancer diagnoses are depicted as a function of age for patients with one or more breast cancers only, multiple primary tumors with breast cancer as first diagnosis, and multiple primary tumors with another cancer as first diagnosis. PC-11–1 and PC-21–1 presented with a malignant phyllodes tumor in the breast as their first diagnosis, coded as a soft-tissue sarcoma. PC-27–1 developed a radiation-induced sarcoma 4 years after radiotherapy for breast cancer. PC-67–1 developed thyroid cancer 10 years following radiotherapy for breast cancer
Table 2.
Pathological characteristics of LFS-associated breast cancers in comparison to non-LFS breast cancers and breast cancers in the general population
| LFS |
General Population (SEER) |
Non-LFS (PMCR) |
|
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total | 38 (-) | 549268 (-) | 7710 (-) |
| ER+/HER2+ | 10 (26) | 58083 (11) | 782 (10) |
| ER−/HER2+ | 12 (32) | 24898 (5) | 412 (5) |
| ER+/HER2− | 13 (34) | 405475 (74) | 5462 (71) |
| ER−/HER2− | 3 (8) | 60812 (11) | 1054 (14) |
| Chi-Square test | N/A | X2 (3, N=549306) = 78.617, p<0.001 | X2 (3, N=7748) = 65.342, p<0.001 |
| Total | 44 (-) | 606467 (-) | 9818 (-) |
| Stage 0 | 11 (25) | 121527 (20) | 1849 (19) |
| Stage I | 11 (25) | 239562 (40) | 4048 (41) |
| Stage II | 11 (25) | 159265 (26) | 2504 (25) |
| Stage III | 7 (16) | 61693 (10) | 957 (10) |
| Stage IV | 8 (9) | 24420 (4) | 460 (5) |
| Average age onset | 34 | 61 | 57 |
| Median age onset | 31 | 61 | 57 |
| Positive Node | 19/44 (43) | 153301/597188 (26) | 2267/8260 (27) |
LFS, Li Fraumeni Syndrome; ER, estrogen receptor. Comparison analyses were performed for all non-LFS groups against LFS using Chi-Square tests. Significant results suggest that hormone receptor status differs upon diagnosis of LFS.
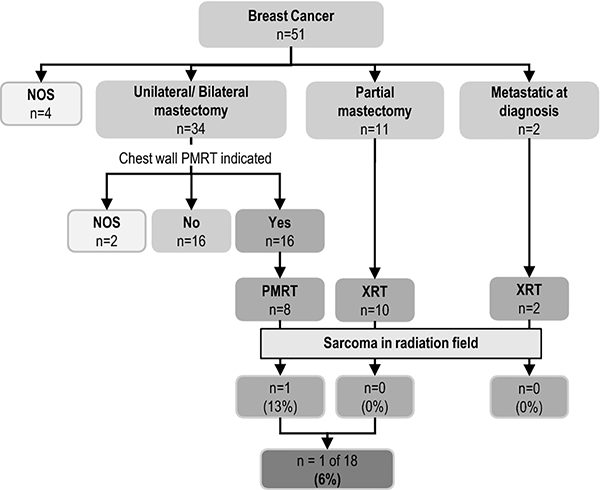
Treatment data for the first LFS-associated breast cancer were available for 47 patients (Figure 2). Thirty-four (72%) had unilateral or bilateral mastectomy, 11 (23%) had partial mastectomy, and two were metastatic at diagnosis. Twenty patients received radiotherapy for their breast cancer. Based on breast cancer stage (Online Resource, Supplementary Table 1), post-mastectomy radiotherapy (PMRT) in the chest wall was indicated in 16 of the 34 patients where 14 had positive lymph node invasion and/or large tumor size, and two had a positive surgical margin. Eight of 16 (50%) patients received radiation; one (13%) developed locoregional recurrence (LRR), specifically in the chest wall. T wo patients with metastatic disease both received palliative radiation treatment to the chest wall.
Fig. 2. Incidence of radiation-induced sarcoma and recurrence by radiation treatment regimen.
Flow sheet depicting the treatment history of 51 patients in the LFS breast cancer cohort. Data shows the number of patients that developed of sarcoma in the radiation field. BC, breast cancer; NOS, not specified otherwise; PMRT, post-mastectomy radiotherapy; XRT, radiotherapy
Of the combined 18 patients who received radiation for breast cancer treatment in the curative setting, one (6%) patient (PC-67–1) developed thyroid cancer 10 years post-radiotherapy, and one (6%) patient (PC-27–1) treated with PMRT developed sarcoma in the radiation field (Figure 1, Table 3). Follow-up ranged from 2 to 20 years with a median of 12.5 years. The radiation-induced sarcoma occurred with a latency of 4 years post-radiotherapy in a classic LFS patient who had also had childhood leukemia. The patient is alive with no evidence of recurrence at 12 years after surgical resection for the sarcoma.
Table 3.
Frequency of radiation-induced malignancies between LFS and non-LFS patients
| Non-LFSa |
LFS |
|||
|---|---|---|---|---|
| n / Median | % / range | n / Median | % / range | |
| BC tumors | 7050 | - | 86b | - |
| BC patients | 6607 | - | 51 | - |
| XRT recommended | 3898 | 59% | 29 | 57% |
| Definitive XRT for BC | 3863/3898 | 99%** | 20/29 | 69% |
| XRT in metastatic settings | 85/3816c | 3% | 2/20 | 10% |
| XRT in curative settings | 3731/3816c | 97% | 18/20 | 90% |
| XRT follow up years | 4 | 2–10 | 12.5 | 2–20 |
| Post-XRT thyroid cancer | 17/3731 | 0.5% | 1/18 | 6% |
| Post-XRT sarcoma in radiation field | 1/3731 | 0.03%* | 1/18 | 6% |
| Relative risk of thyroid cancer post-XRTd | N/A | 23.82 (95% CI=3.47–163.25, p=0.001) 5.93 | ||
| Relative risk of sarcoma post-XRTd | N/A | 5.93 (95% CI=0.34–103.04, p=0.22) | ||
LFS, Li Fraumeni Syndrome; BC, breast cancer; XRT, radiation therapy.
The PMCR cohort represents the non-LFS breast cancer patient population seen at Penn Medicine.
Includes primary and recurrent breast tumors
Patients with sufficient data on clinical stage
Relative risk associated with LFS in comparison to the non-LFS cohort in propensity weighted analyses.
p=0.01
p<0.001 by Fisher’s exact test
There were a total of 6607 non-LFS breast cancer patients in the PMCR. Radiotherapy was indicated in 3898 patients; 3863 (99%) received definitive radiotherapy. In comparison, 69% of patients in the LFS cohort received radiation when indicated (p<0.001). Of the 3731 patients whose treatment was in the curative setting, 17 (0.5%) and one (0.03%) patients developed thyroid cancer and radiation-induced sarcoma respectively. The frequencies of radiation-induced sarcoma and thyroid cancer post-radiotherapy in LFS breast cancer patients were 6% and 6% respectively. By a Fisher exact test, there was a significantly lower prevalence of radiation-induced sarcoma (0.03%, p<0.001) and a trend towards a lower prevalence for thyroid cancer (0.5%, p=0.08) in non-LFS breast cancer patients in the PMCR. In propensity score weighted analyses (Online Resource, Supplementary Table 2), diagnosis with LFS was associated with a non-significant increase in risk of sarcoma following radiation for primary breast cancer than no diagnosis with LFS (RR=5.93, 95% CI=0.34–103.04, p=0.22). Diagnosis with LFS was associated with a significant increase in risk of thyroid cancer following radiation for primary breast cancer than no diagnosis with LFS (RR=23.82, 95% CI=3.47–163.25, p=0.001) (Table 3).
DISCUSSION
To our knowledge, we studied the largest cohort of LFS related breast cancer patients with regards to radiation outcomes. There have been few reports on radiation-induced malignancies in breast cancer patients with LFS, mainly restricted to case studies [13,14] and one retrospective study with a small sample size [15]. A wide range of cancer types have been postulated to associate with radiotherapy in LFS breast cancer patients, including small cell lung cancer, colon cancer [13], thyroid cancer [14], and especially sarcoma within the radiation field [14,15]. One study reported nine radiation-induced cancers in 28 LFS families [16,17]; only one case was post-radiotherapy for breast cancer specifically. Another study found a 30% risk of developing a second malignancy after radiation exposure [3], although the number of breast cancer cases was not specified. Heyman et al. [15] reported that two of six (33%) breast cancer patients with LFS developed radiation-induced sarcomas after treatment. In our cohort with similar characteristics to LFS breast cancer patients reported in IARC, we studied 18 LFS breast cancer patients post-radiotherapy and found that one patient (6%) developed radiation-induced sarcoma and one patient (6%) developed radiation-induced thyroid cancer. In comparison to a non-LFS control cohort, there was a consistent upward trend in the rates of radiation-induced sarcoma and thyroid cancer in LFS patients, although the statistical significance was inconsistent. Larger cohorts are therefore needed to confirm our results.
It is important to note that LFS is a rare condition, which constitutes difficulties in assembling a large enough sample size. In addition, the PMCR only includes historic cases of patients who were being actively treated and followed at Penn Medicine. This introduced a potential selection bias due to the lack of exact time lapse between the LFS and control cohorts, leading to a longer follow-up time in the LFS cohort which consisted of all identified historic cases of LFS-associated breast cancers. To increase the data integrity of the non-LFS control cohort, we minimized the number of control cases that were lost to follow up in the PMCR by cross-checking the last contact date within the PMCR with that of the Penn Cancer Service line and eliminated cases with a discrepancy of over five years. However, it is theoretically possible that the non-LFS cohort includes TP53 mutation carriers that do not have a diagnosis of LFS documented in the electronic health record.
Our data have significant management implications, suggesting that radiotherapy should not be ruled out when indicated for breast cancer treatment in LFS. PMRT in the chest wall is normally indicated in high risk cases for LRR including four or more nodes positive, large tumor size of over 5 cm, or positive margins [6]. PMRT is also strongly considered for patients with 1–3 positive nodes and other risk factors such as medial/ central location, extensive lymphovascular invasion, or receptor negative tumors. In these cases, there is consensus that radiotherapy reduces LRR and breast cancer mortality [18]. The consensus also leaves open the possibility that the decision for radiation requires clinical judgement when the risks may outweigh the benefits. Per our data, adherence to standard of care in treatment of breast cancer was significantly lower in the LFS cohort (69% vs 99%, p<0.001). It is important to note, however, that one patient (13%) who received radiation where clinically indicated developed LRR afterwards.
CONCLUSIONS
Although the risks of radiation-induced sarcoma and thyroid cancer are magnified by LFS as compared to the general population, the frequency of subsequent radiation-induced sarcoma in LFS (6%) is lower than previously believed (33%). We recommend that radiation be considered in LFS breast cancer patients when the potential risk for LRR, or the mortality benefit to radiation, is greater than the risk for serious adverse events.
Supplementary Material
Supplementary Figure 1 Overall LFS Adult cohort. A) LFS cohort stratification by vital status and personal cancer history. LFS patients were ascertained from the Cancer Risk Evaluation Program at Penn Medicine and by a query of the Penn Medicine electronic health records between 2007 and 2019 under a University of Pennsylvania institutional review boards approved protocol. Clinical data abstraction was conducted for 51 patients with a personal history of breast cancer. B) Kaplan Meier estimates of breast cancer development in LFS patients at Penn Medicine and in the IARC database. Data were downloaded from the IARC database for 1990 individuals (727 families) with confirmed pathogenic/likely pathogenic germline TP53 mutation
Supplementary Tables 1–2 (.doc). Table S1, Detailed clinical data for LFS-associated breast cancer cohort. Table S2, Covariates of matched-control analysis.
ACKNOWLEDGMENTS AND FUNDING INFORMATION
Funding. This work is supported by the Institute for Translational Medicine and Therapeutics at the University of Pennsylvania (ANL, SPM, KNM), the National Cancer Institute (K08CA21531, KNM), and the Burroughs Wellcome Foundation (KNM).
ABBREVIATIONS
- LFS
Li-Fraumeni syndrome
- LFL
Li-Fraumeni-like
- BC
breast cancer
- LRR
locoregional recurrence
- IDC
invasive ductal carcinoma
- DCIS
ductal carcinoma in situ
- MDLC
mixed ductal and lobular carcinoma
- NOS
not otherwise specified
- HR
hormone receptor
- ER
estrogen receptor
- XRT
radiotherapy
- n/a
not applicable
- BLM
bilateral mastectomy
- ULM
unilateral mastectomy
- PM
partial mastectomy
- CW
chest wall
- NED
no evidence of disease
- Decd
deceased
- Met
metastatic
- OC
other cause
- Sp
splicing
- trunc
truncating
- DBD
DNA binding domain
- TD
tetramerization domain
- LGR
large genomic rearrangement
- PMRT
post-mastectomy radiotherapy
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Disclosure of potential conflicts of interest. Author SMD has received honoraria from Clovis, Bristol-Myers Squibb, and AstraZeneca. Author AB has an advisory role at AstraZeneca and Merck and has received research fund not related to this manuscript from AstraZeneca and Merck. All other authors declare that they have no potential conflict of interest.
Ethical approval. All studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. This single-institution human subjects retrospective chart review is qualified for exempt status and does not require informed consent or waiver of consent.
Availability of data and material. The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, Bremer RC, Rosenberg PS, Savage SA (2016) Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 122 (23):3673–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadou A, Waddington Achatz MI, Hainaut P (2018) Revisiting tumor patterns and penetrance in germline TP53 mutation carriers: temporal phases of Li-Fraumeni syndrome. Curr Opin Oncol 30 (1):23–29. doi: 10.1097/CCO.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 3.Bougeard G, Renaux-Petel M, Flaman J-M, Charbonnier C, Fermey P, Belotti M, Gauthier-Villars M, Stoppa-Lyonnet D, Consolino E, Brugieres L, Caron O, Benusiglio PR, Bressac-de Paillerets B, Bonadona V, Bonaiti-Pellie C, Tinat J, Baert-Desurmont S, Frebourg T (2015) Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33 (21):2345–2352 [DOI] [PubMed] [Google Scholar]
- 4.Kratz CP, Achatz MI, Brugieres L, Frebourg T, Garber JE, Greer MC, Hansford JR, Janeway KA, Kohlmann WK, McGee R, Mulli ghan CG, Onel K, Pajtler KW, Pfister SM, Savage SA, Schiffman JD, Schneider KA, Strong LC, Evans DGR, Wasserman JD, Villani A, Malkin D (2017) Cancer Screening Recommendations for Individuals with Li-Fraumeni Syndrome. Clin Cancer Res 23 (11):e38–e45. doi: 10.1158/1078-0432.CCR-17-0408 [DOI] [PubMed] [Google Scholar]
- 5.Abdel Razek AAK, Gaballa G, Denewer A, Tawakol I (2010) Diffusion weighted MR imaging of the breast. Acad Radiol 17 (3):382–386 [DOI] [PubMed] [Google Scholar]
- 6.Network NCC (2019) Breast Cancer (Version 2.2019). https://www.nccn.org/professionals/physiciangls/pdf/breast.pdf. Accessed July 18, 2019
- 7.Schon K, Tischkowitz M (2018) Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Res Treat 167 (2):417–423. doi: 10.1007/s10549-017-4531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Moura J, Kavalec FL, Doghman M, Rosati R, Custodio G, Lalli E, Cavallari GMD, Santa Maria J, Figueiredo BC (2010) Heterozygous TP53stop146/R72P fibroblasts from a Li-Fraumeni syndrome patient with impaired response to DNA damage. Int J Oncol 36 (4):983–990 [DOI] [PubMed] [Google Scholar]
- 9.Kasper E, Angot E, Colasse E, Nicol L, Sabourin J-C, Adriouch S, Lacoume Y, Charbonnier C, Raad S, Frebourg T, Flaman J-M, Bougeard G (2018) Contribution of genotoxic anticancer treatments to the development of multiple primary tumours in the context of germline TP53 mutations. Eur J Cancer 101:254–262 [DOI] [PubMed] [Google Scholar]
- 10.MacFarland SP, Zelley K, Long JM, McKenna D, Mamula P, Domchek SM, Nathanson KL, Brodeur GM, Rustgi AK, Katona BW, Maxwell KN (2019) Earlier Colorectal Cancer Screening May Be Necessary In Patients With Li-Fraumeni Syndrome. Gastroenterology 156 (1):273–274. doi: 10.1053/j.gastro.2018.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program released April 2019, based on the November 2018 submission. edn.,
- 12.Melhem-Bertrandt A, Bojadzieva J, Ready KJ, Obeid E, Liu DD, Gutierrez-Barrera AM, Litton JK, Olopade OI, Hortobagyi GN, Strong LC, Arun BK (2012) Early onset HER2-positive breast cancer is associated with germline TP53 mutations. Cancer 118 (4):908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limacher JM, Frebourg T, Natarajan-Ame S, Bergerat JP (2001) Two metachronous tumors in the radiotherapy fields of a patient with Li-Fraumeni syndrome. International journal of cancer 96 (4):238–242 [DOI] [PubMed] [Google Scholar]
- 14.Salmon A, Amikam D, Sodha N, Davidson S, Basel-Vanagaite L, Eeles RA, Abeliovich D, Peretz T (2007) Rapid development of post-radiotherapy sarcoma and breast cancer in a patient with a novel germline ‘de-novo’ TP53 mutation. Clin Oncol (R Coll Radiol) 19 (7):490–493 [DOI] [PubMed] [Google Scholar]
- 15.Heymann S, Delaloge S, Rahal A, Caron O, Frebourg T, Barreau L, Pachet C, Mathieu M-C, Marsiglia H, Bourgier C (2010) Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol 5:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans DGR, Birch JM, Ramsden RT, Sharif S, Baser ME (2006) Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. Journal of medical genetics 43 (4):289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birch JM, Alston RD, McNally RJ, Evans DG, Kelsey AM, Harris M, Eden OB, Varley JM (2001) Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene 20 (34):4621–4628 [DOI] [PubMed] [Google Scholar]
- 18.Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, Hudis CA, Hwang ES, Kirshner JJ, Morrow M, Salerno KE, Sledge GW Jr., Solin LJ, Spears PA, Whelan TJ, Somerfield MR, Edge SB (2017) Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Annals of surgical oncology 24 (1):38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Overall LFS Adult cohort. A) LFS cohort stratification by vital status and personal cancer history. LFS patients were ascertained from the Cancer Risk Evaluation Program at Penn Medicine and by a query of the Penn Medicine electronic health records between 2007 and 2019 under a University of Pennsylvania institutional review boards approved protocol. Clinical data abstraction was conducted for 51 patients with a personal history of breast cancer. B) Kaplan Meier estimates of breast cancer development in LFS patients at Penn Medicine and in the IARC database. Data were downloaded from the IARC database for 1990 individuals (727 families) with confirmed pathogenic/likely pathogenic germline TP53 mutation
Supplementary Tables 1–2 (.doc). Table S1, Detailed clinical data for LFS-associated breast cancer cohort. Table S2, Covariates of matched-control analysis.