Abstract
The present study aimed to evaluate the total progression-free survival (PFS) time of the 1st-line chemotherapy (CHT)/2nd-line tyrosine kinase inhibitor (TKI) and 1st-line TKI/2nd-line CHT therapeutic regimens. Data from patients with non-small-cell lung cancer (NSCLC) harboring sensitizing epidermal growth factor receptor (EGFR) mutations, who had received both TKI and platinum CHT were retrieved from the Shandong Cancer Hospital (Jinan, China) database. A total of 89 patients were included, 50 of whom were treated with the 1st-line CHT/2nd-line TKI regimen and the remaining 39 patients underwent a 1st-line TKI/2nd-line CHT regimen. The differences in total PFS time between the two regimens were analyzed. The median total PFS time was 14.28 months with the 1st-line CHT/2nd-line TKI regimen and 17.77 months with the 1st-line TKI/2nd-line CHT regimen (adjusted hazard ratio, 0.96; 95% confidence interval (CI), 0.56–1.66; P=0.886). A significant difference in PFS time was revealed between the two strategies when comparing only the 1st-line or 2nd-line treatments (all P<0.001). The objective response rate (RR) was 52.0% for those treated with 1st-line CHT/2nd-line TKI and 38.5% for the reverse regimen. After adjusting for associated factors, the odds ratio for the RR was 2.77 (95% CI: 0.77–9.90; P=0.117). The current results revealed that there was no significant difference between the total PFS time of patients with NSCLC undergoing the 1st-line CHT/2nd-line TKI regimen compared with patients with NSCLC undergoing the 1st-line TKI/2nd-line CHT regimen.
Keywords: EGFR mutation, TKI, chemotherapy, NSCLC, PFS
Introduction
Mutations in the epidermal growth factor receptor (EGFR) gene are present in ~17 (Caucasians) and 40% (East Asians) of lung adenocarcinoma in 2015 (1). Deletions in exon 19 and a point mutation in exon 21 (L858R) are common and account for 90% of all EGFR mutations in Asians with non-small-cell lung cancer (NSCLC) (2). Patients with advanced EGFR-mutant NSCLC may experience notable tumor reduction and continued responses following treatment with EGFR tyrosine kinase inhibitors (TKIs) (3,4). EGFR and its cognate ligands regulate tumor proliferation and growth. EGFR TKIs are successful in the treatment of in EGFR-mutant NSCLC patients because of the oncogene-addicted biology of this disease (4,5). Nevertheless, 10–14 months after peroral administration of TKIs, specific drug resistance occurs and results in progressive disease (3–6). A previous study investigated the best treatment method following EGFR TKI-acquired resistance (AR); EGFR T790M is the most common mutation associated with AR to EGFR TKI therapy, and AZD9291 is an oral third-generation TKI that inhibits T790M (7,8).
The clinical benefits of TKI treatment of NSCLC are more likely to be observed in patients with an EGFR mutation, non-smokers, women, patients with adenocarcinoma and patients of Asian ethnicity (9,10). Patients with advanced NSCLC may receive 1st-line TKIs or chemotherapy (CHT) followed by 2nd-line CHT or TKIs as an alternative treatment (3–6). The choice between administration of CHT or TKIs as the 1st-line therapy is controversial. Despite the improved progression-free survival (PFS) time in 1st-line TKI therapy, the overall survival (OS) was revealed to be similar in several prospective trials (4,6). To date, there is little available data on the most effective sequence of TKI and CHT treatment in NSCLC. The order delivery of these systemic therapies may result in different PFS times.
The present study investigated the total PFS time (1st-line PFS plus 2nd-line PFS) of the 1st-line CHT/2nd-line TKI regimen. The 1st-line CHT/2nd-line TKI regimen is sometimes utilized in clinical practice, but the associated total PFS time has not yet been evaluated prospectively. Although a TKI is recommended as 1st-line treatment in patients with EGFR-mutant NSCLC by clinical guidelines (11), the benefits of TKIs have only been observed with 1st-line treatment and have not affected total PFS time (1st-line PFS plus 2nd-line PFS) and OS time. A number of randomized controlled trials, including EURTAC, WJTOG3405 and IPASS, have also reported that OS data of patients receiving different therapeutic strategies (1st-line CHT/2nd-line TKI and 1st-line TKI/2nd-line CHT) is similar (3–6,12). Notably, in clinical practice, certain patients cannot tolerate chemotherapy after the development of TKI resistance due to low performance status and tumor progression. Therefore, the present retrospective study of patients with NSCLC harboring sensitizing EGFR mutations aimed to elucidated the total PFS time of a 1st-line CHT/2nd-line TKI regimen compared with the 1st-line TKI/2nd line CHT regimen.
Patients and methods
Patient selection
The authors reviewed internal databases from Shandong Cancer Hospital (Jinan, China) between January 2012 (when 1st-line treatment was initiated) and April 2016 with a hospital review board approved protocol. The medical histories of patients with Stage IV NSCLC harboring EGFR mutations were reviewed to confirm which patients had received 1st-line CHT/2nd-line TKI or 1st-line TKI/2nd-line CHT treatment regimens (13). The lung tumor staging in the present study was conducted according to the 8th edition of the TNM stage classification (13). The 1st-line treatment was terminated after progressive disease occurred and patients then received 2nd-line treatment. Disease progression was defined according to radiographic identification of significant tumor growth, and resulted in a change in therapy. Included patients received either the 1st-line CHT/2nd-line TKI or the 1st-line TKI/2nd-line CHT treatment regimen. In the present study, only patients harboring confirmed EGFR mutations were included. EGFR mutation testing was performed by the molecular diagnostic core laboratory of the Department of Pathology in Shandong Cancer Hospital. Patients were treated with erlotinib or gefitinib, because both TKIs are commercially available in China (14). Moreover, no patients who had participated in other clinical trials associated with the 1st-line CHT/2nd-line TKI regimen, or the reverse regimen, were included in the present study. The exclusion criteria for patients were: i) Incomplete patient records, ii) TKIs and CHT treatment given simultaneously, iii) a treatment interval more than one month between two regimens, iv) if TKI therapy or CHT was terminated due to toxicity rather than tumor progression, v) if they had another active malignancy and vi) if they exhibited an NSCLC histology at diagnosis (15,16). In total, the present study included 89 patients according to the inclusion criteria. Of the total patients, 50 received the 1st-line CHT/2nd-line TKI regimen and the remaining 39 were treated with the 1st-line TKI/2nd-line CHT regimen. In the 1st-line CHT/2nd-line TKI group, 26 men (52.0%) and 24 women (48.0%) were included with a median age of 54 years (age range, 37–81). The 1st-line TKI/2nd-line CHT group included 18 men (46.2%) and 21 women (53.8%) with a median age of 54 years (age range, 41–71). A total of 84.0% from the 1st-line CHT/2nd-line TKI group and 92.3% of the 1st-line TKI/2nd-line CHT group were administered pemetrexed as chemotherapy (Table I). The present study was approved by The Institutional Review Board of Shandong Cancer Hospital (Jinan, China; IRB number: SDCH20170136).
Table I.
Demographics and treatment regime of patients with non-small cell lung cancer with EGFR mutations.
| Treatment regime | |||
|---|---|---|---|
| Patient characteristics | 1st-line CHT + 2nd-line TKI (n=50) | 1st-line TKI + 2nd-line CHT (n=39) | P-value |
| Sex, n (%) | 0.584 | ||
| Male | 26 (52.0) | 18 (46.2) | |
| Female | 24 (48.0) | 21 (53.8) | |
| Median age, years (range) | 54 (37–81) | 54 (41–71) | 0.180 |
| Smoking status, n (%) | 0.919 | ||
| Never-smoker | 38 (76.0) | 30 (76.9) | |
| Former/current smoker | 12 (24.0) | 9 (23.1) | |
| Mean body mass index, kg/m2 (SD) | 23.69 (2.57) | 25.06 (2.27) | 0.329 |
| Mean prealbumin level, g/l (SD) | 0.24 (0.06) | 0.26 (0.06) | 0.880 |
| Performance status 0–1, n (%) | 24 (48.0) | 12 (30.8) | 0.100 |
| Pathological subtype, n (%) | 0.206 | ||
| Adenocarcinoma | 48 (96.0) | 39 (100.00) | |
| Other | 2 (4.0) | 0 (0.0) | |
| Tumor status ≥3, n (%) | 24 (48.0) | 15 (38.5) | 0.368 |
| Lymph node status=3, n (%) | 24 (48.0) | 17 (43.6) | 0.679 |
| Metastasis sites ≥3, n (%) | 12 (24.0) | 12 (30.8) | 0.475 |
| Brain metastasis, n (%) | 15 (30.0) | 8 (20.5) | 0.310 |
| Lung metastasis, n (%) | 16 (32.0) | 18 (46.2) | 0.173 |
| EGFR mutation, n (%) | 0.173 | ||
| Exon 19 deletion | 16 (32.0) | 18 (46.2) | |
| L858R | 34 (68.0) | 21 (53.8) | |
| Chemotherapeutic regimen, n (%) | 0.237 | ||
| Pemetrexed-containing regimen | 42 (84.0) | 36 (92.3) | |
| Other | 8 (16.0) | 3 (7.7) | |
| Palliative radiotherapy, n (%) | 18 (36.0) | 12 (30.8) | 0.604 |
| Initial TKI type, n (%) | 0.463 | ||
| Erlotinib | 27 (54.0) | 18 (46.2) | |
| Gefitinib | 23 (46.0) | 21 (53.8) | |
The χ2 test was used to compare patient characteristics between the 1st-line CHT/2nd-line TKI and 1st-line TKI/2nd-line CHT strategies. SD, standard deviation; EGFR, epidermal growth factor receptor; CHT, chemotherapy; TKI, tyrosine kinase inhibitor.
Data collection
Medical data of patients were obtained from medical records, including age, sex, body mass index, smoking status, prealbumin levels, performance status, tumor status, lymph node status, metastasis sites, brain and lung metastasis status, Tumor-Node-Metastasis staging (13), EGFR mutation status, chemotherapeutic regimen and history of palliative radiotherapy. The authors reviewed and abstracted treatment courses, including treatment of EGFR TKIs, time of receiving 1st- and 2nd-line treatment (defined as the period from the initiation of 1st- or 2nd-line treatment until tumor progression). The Eastern Cooperative Oncology Group (ECOG) performance status (PS) and basal information were recorded at the beginning of treatment (17). Official EGFR mutation reports of all cases were reviewed from the clinical records, according to a PCR-based allele-specific assay or direct sequencing (18,19).
Response and survival evaluation
The objective response rate (RR) to 1st-line or 2nd-line treatment was assessed in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) by two radiologists who were blinded to the therapeutic regimen (20). A partial response to RECIST was defined as a ≥30% decrease in the sum of the longest diameter for all target lesions. Additionally, disease progression was defined as a ≥20% increase in the sum of the longest diameter for all target lesions or newly found lesions. RR was determined by the percentage of partial response to RECIST when analyzing the patients' best response during treatment. Total RR was defined as the percentage of RECIST's partial response during the whole regimen including 1st- and 2nd-line treatment. The baseline scan was obtained prior to treatment initiation. The 2nd-line treatment was detected after the identification of clinical disease progression during 1st-line treatment. The authors estimated PFS time in both groups with different therapeutic strategies. PFS time was defined as the period from treatment initiation to the date of disease progression or death. Both 1st- and 2nd-line progress-free survival (PFS) time were recorded. Total PFS time was calculated by the addition of 1st- and 2nd-line PFS time. High resolution CT was performed every 2 months to assess the patient's response to the treatment regimes. The longest follow-up of patients in the present study was 48 months. Additionally, the treatment response and PFS time of all patients with sufficient clinical data were evaluated.
Statistical analyses
The χ2 test was performed to analyze baseline data and characteristics from the 1st-line CHT/2nd-line TKI and the 1st-line TKI/2nd-line CHT groups. Continuous numeric variables expressed as the mean ± standard deviation were analyzed using the unpaired Student's t-test. A model was also constructed including potential confounding variables, such as age, sex, body mass index prior to treatment, prealbumin level prior to treatment, PS before treatment, smoking status, primary tumor status, lymph node status, metastasis sites, brain metastasis, lung metastasis, EGFR mutations, chemotherapeutic regimen and palliative radiotherapy. Survival probabilities between treatments were calculated using the Kaplan-Meier method and the Log-rank test. Multivariable Cox analysis was performed to adjust for the same covariates as in the logistic regression analysis of RR. In the present study, univariate and multivariate logistic regression analysis were used to calculate OR. SPSS version 17.0 (SPSS, Inc.) was used to conduct all analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
Overall, there were 1,028 patients with EGFR-mutant NSCLC who received CHT and EGFR TKI treatment. Moreover, 927 patients were excluded for the following reasons: A total of 348 had insufficient clinical records, 235 changed therapy once stable disease or remission was reached, 173 received TKIs and CHT simultaneously, 93 had a treatment interval of more than one month between the two regimens, 57 discontinued therapy because of toxicity or reasons other than disease progression or acquired resistance (AR), 12 had another concurrent active malignancy, 9 developed small-cell lung cancer at progression or the time of AR and 12 continued treatment with AZD9291 after the T790M mutation was identified at the time of AR to TKIs. Ultimately, analysis included 89 patients who met all the inclusion criteria. At initial diagnosis of patients with NSCLC harboring EGFR mutations, 50 (56.2%) were treated with 1st-line CHT/2nd-line TKI and 39 (43.8%) received 1st-line TKI/2nd-line CHT (Table I). The median age of all patients was 54 and the majority of patients had never been smokers (Table I). EGFR mutations, including the L585R point mutation and exon 19 deletion were observed in all patients (Table I). More patients with poor tumor and lymph node status received 1st-line CHT/2nd-line TKI compared with 1st-line TKI/2nd-line CHT (Table I). Overall, 18 (36.0%) patients in the 1st-line CHT/2nd-line TKI group and 12 (30.8%) patients in the 1st-line TKI/2nd line CHT group received palliative radiotherapy (Table I).
Associations of different therapeutic strategies with response
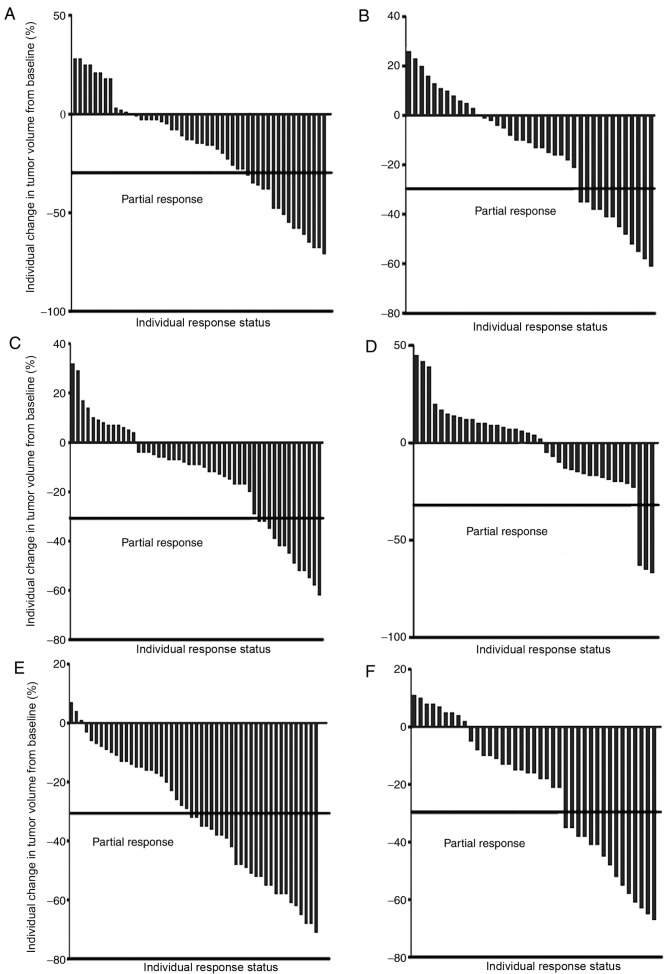
Overall, 89 patients were evaluated for their treatment response. Partial responses were observed in 26/50 (52.0%) patients in the 1st-line CHT/2nd-line TKI group and in 15/39 (38.5%) patients in the 1st-line TKI/2nd-line CHT group (Table II; Fig. 1), with an odds ratio (OR) of 1.73 (95% confidence interval [CI], 0.74–4.06; P=0.204; data not shown). Upon adjusting for clinical confounders, the adjusted OR was 2.77 (95% CI, 0.77–9.90; P=0.117; data not shown). The partial RRs in 1st-line treatment were 32.0% in the 1st-line CHT/2nd-line TKI group and in 30.8% patients in the 1st-line TKI/2nd-line CHT group (adjusted OR, 1.06; 95% CI, 0.43–2.61; P=0.901; data not shown). A significant difference was identified in the partial response in 2nd-line treatment between the two groups (adjusted OR, 8.16; 95% CI, 1.82–36.67; P=0.012; data not shown).
Table II.
Response to different orders of treatment regime in patients with non-small cell lung cancer with epithelial growth factor receptor mutations.
| 1st-line CHT + 2nd-line TKI, n=50 | 1st-line TKI + 2nd-line CHT, n=39 | |||||
|---|---|---|---|---|---|---|
| Patient outcome | 1st-line CHT | 2nd-line TKI | Total efficacy | 1st-line TKI | 2nd-line CHT | Total efficacy |
| Partial response, n (%) | 16 (32.0) | 13 (26.0) | 26 (52.0) | 12 (30.8) | 3 (7.7) | 15 (38.5) |
| Stable disease, n (%) | 28 (56.0) | 35 (70.0) | 24 (48.0) | 24 (61.5) | 32 (82.0) | 24 (61.5) |
| Progressive disease, n (%) | 6 (12.0) | 2 (4.0) | 0 (0.0) | 3 (7.7) | 4 (10.3) | 0 (0.0) |
CHT, chemotherapy; TKI, tyrosine kinase inhibitor.
Figure 1.
Partial responses to different therapeutic strategies. Response to (A) 1st-line CHT, (B) 1st-line TKI, (C) 2nd-line TKI, (D) 2nd-line CHT, (E) 1st-line CHT/2nd-line TKI and (F) 1st-line TKI/2nd-line CHT. The partial response to RECIST was defined as a ≥30% decrease in the sum of the longest diameter for all target lesions. TKI, tyrosine kinase inhibitor; CHT, chemotherapy.
Association of different therapeutic strategies with PFS time
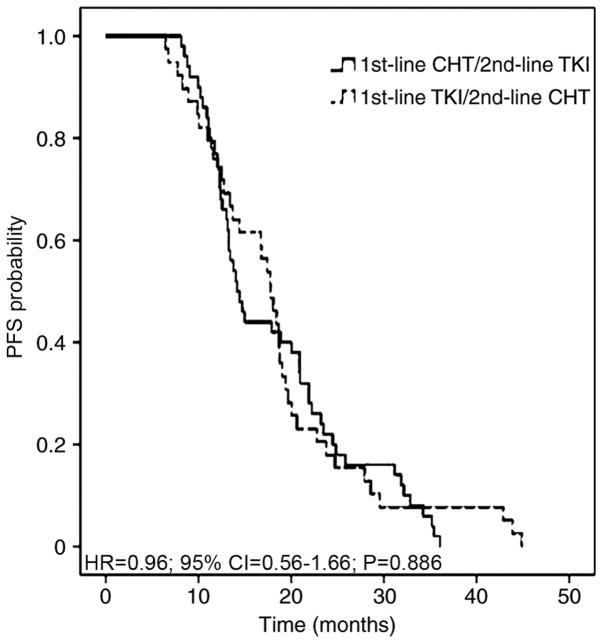
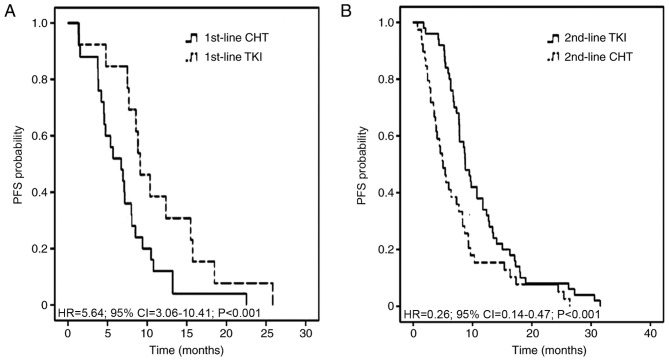
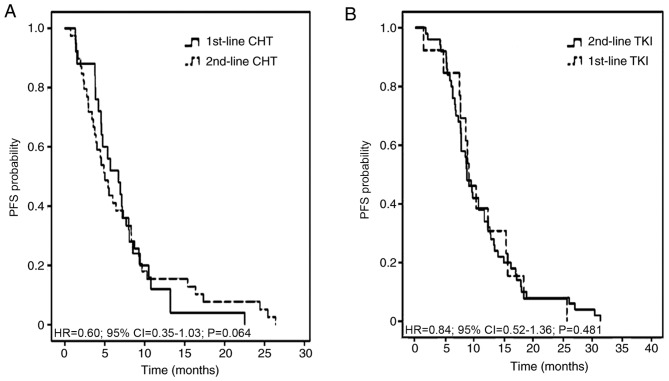
Chemotherapeutic treatment, including pemetrexed and a platinum agent, resulted in the most favorable partial responses in patients. The 1st-line, 2nd-line and total PFS time were assessed in all 89 patients, according to 1st- and 2nd-line therapy. The median total PFS time was not significantly different between the two therapeutic strategies, at 14.28 months in 1st-line CHT/2nd-line TKI regimen and 17.77 months in 1st-line TKI/2nd-line CHT regimen with an adjusted HR of 0.96 (95% CI, 0.56–1.66; P=0.886; Fig. 2). The median 1st-line PFS time was 6.72 months in the 1st-line CHT/2nd-line TKI group and 9.11 months in the 1st-line TKI/2nd-line CHT group (adjusted HR, 5.64; 95% CI, 3.06–10.41; P<0.001). The median 2nd-line PFS time was 8.74 and 5.02 months, respectively (adjusted HR, 0.26; 95% CI, 0.14–0.47; P<0.001; Fig. 3). The adjusted HR of PFS time from individuals receiving chemotherapy was 0.60 (95% CI, 0.35–1.03; P=0.064) between the 1st-line CHT/2nd-line TKI and the other groups. The adjusted HR of PFS time from patients receiving TKIs was 0.84 (95% CI, 0.52–1.36; P=0.481) between the two groups (Fig. 4).
Figure 2.
PFS time in patients with non-small cell lung cancer with EGFR mutations who received 1st-line CHT/2nd-line TKI or 1st-line TKI/2nd-line CHT strategies. PFS, progression-free survival; TKI, tyrosine kinase inhibitor; CHT, chemotherapy; HR, hazard ratio; CI, confidence interval.
Figure 3.
Kaplan-Meier plot exhibiting 1st-line and 2nd-line PFS curves in patients with non-small cell lung cancer with epithelial growth factor receptor mutations who received either 1st-line CHT/2nd-line TKI or 1st-line TKI/2nd-line CHT strategies. (A) 1st-line CHT and 1st-line TKI. (B) 2nd-line TKI and 2nd-line CHT. TKI, tyrosine kinase inhibitor; CHT, chemotherapy; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival.
Figure 4.
Kaplan-Meier curves exhibiting 1st- and 2nd-line PFS time in patients who received either 1st-line CHT/2nd-line TKI or 1st-line TKI/2nd-line CHT strategies. (A) 1st-line CHT and 2nd-line CHT. (B) 1st-line TKI and 2nd-line TKI. TKI, tyrosine kinase inhibitor; CHT, chemotherapy; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival.
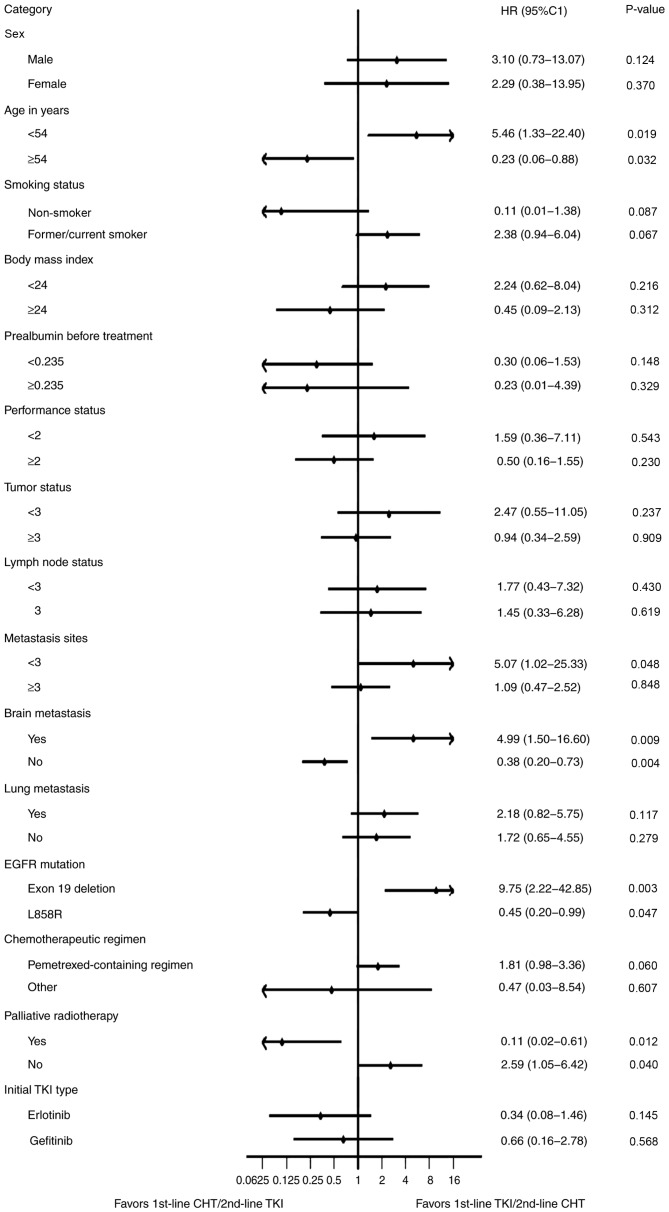
Subgroup analyses
After adjustment for confounding variables, a significant benefit was identified for 1st-line CHT/2nd-line TKI compared with 1st-line TKI/2nd-line CHT in patients who were >54 years old, had no brain metastasis, harbored L858R or other mutations and received palliative radiotherapy. Subgroup analyses of other clinical factors revealed no improved outcome in total PFS time with 1st-line CHT/2nd-line TKI regimen (Fig. 5).
Figure 5.
Forest plot of subgroup multivariate analyses on all patients with non-small cell lung cancer harboring EGFR mutations. The plot exhibited progression-free survival after adjustment for confounding variables. The tumor staging was conducted according to the 8th edition of the TNM stage classification. P<0.05 was considered to indicate a statistically significant difference. TKI, tyrosine kinase inhibitor; CHT, chemotherapy; HR, hazard ratio; CI, confidence interval.
Discussion
To the best of our knowledge, the present study is the first retrospective study describing whether the total PFS time (1st- plus 2nd-line PFS) of patients with EGFR-mutant NSCLC treated by both CHT and TKI is influenced by the order of the regimen administration. It was identified that the 1st-line CHT/2nd-line TKI regimen had a significantly higher partial RR to the 2nd-line treatment compared with the 1st-line TKI/2nd-line CHT regimen. Nevertheless, this significant improvement did not translate into a difference in the total RR or PFS among this cohort.
Previously, Iressa Pan-Asia Study (IPASS) data revealed that patients with NSCLC harboring mutant EGFRs had a high RR in the 1st-line TKI treatment group (12). However, 30% of patients with lung adenocarcinoma and EGFR mutation exhibited no response to 1st-line EGFR TKI treatment (12,21). Despite several prospective trials reporting that PFS is more favorable in 1st-line TKI compared with CHT, the OS was not significantly different (3–6,12). This may be a result of additional systemic and combined treatments, which can influence OS outcomes (6,21). The Tarceva or Chemotherapy (TORCH) study (22) reported that 1st-line erlotinib/2nd-line cisplatin-gemcitabine was significantly associated with a less favorable OS compared with the standard strategy of 1st-line CHT/2nd-line erlotinib in unselected patients (with either sensitive or non-sensitive EGFR mutation) with advanced NSCLC. Further subgroup analysis of EGFR-mutant patients was conducted in the TORCH study and the results revealed that the 1st-line CHT/2nd-line TKI regimen conferred a longer total PFS time, without statistical significance. Furthermore, the combination of TKI and platinum doublet CHT may not improve PFS time in patients with advanced NSCLC, compared with CHT monotherapy (23–26). Therefore, establishing the most effective 1st-line treatment in patients with advanced NSCLC harboring EGFR mutations remains a pertinent clinical challenge. Recently, a meta-analysis of randomized controlled trials revealed that the OS of patients receiving 1st-line TKI/2nd-line platinum-based CHT is not different from the reverse sequence of the same regimen in patients with NSCLC and an EGFR-mutation (27). Notably, chemotherapy was still necessary in the treatment of NSCLC (27).
The present study demonstrated that delivering CHT prior to TKI therapy in patients with EGFR-mutant advanced NSCLC did not result in a poorer total PFS time compared with the 1st-line TKI/2nd-line CHT regimen (P=0.886). Establishing the total PFS time of 1st- and 2nd-line treatment is crucial for advanced NSCLC, as it influences patient survival and life quality. Patients with EGFR-mutant NSCLC may receive the 1st-line CHT/2nd-line TKI regimen for more effective CHT or to avoid incomplete treatment. PFS analysis of the two strategies revealed that EGFR TKIs exhibited significantly better efficiency compared with CHT (both P<0.001) in only 1st- or 2nd-line treatment. The current data indicate that EGFR TKI is a crucial treatment for patients with advanced NSCLC harboring EGFR mutations. To explore the influence of treatment sequence on the effect of CHT and TKI treatment, the PFS time of CHT or TKI therapy from one group was compared with the same treatment from the other in the present study. The results demonstrated that the effect of a particular treatment (CHT or TKI) was not influenced by the delivery sequence.
Although the percentage of gefitinib administration was similar to that of erlotinib in the present study, the efficacy may be slightly different compared with previously reported values (28,29). According to previous studies, erlotinib has benefits over gefitinib in patients with EGFR-mutated patients with leptomeningeal NSCLC metastases that progressed during gefitinib treatment but responded to erlotinib (28,29). Additionally, recent studies demonstrated that erlotinib-treated patients have better PFS and OS time compared with the gefitinib-treated group (29,30). At present, this is no definite conclusion of TKI type choice in 1st-line treatment of EGFR-mutant NSCLC patients with brain metastases (31).
EGFR T790M accounts for >50% of all instances of resistance to gefitinib and erlotinib and the recently developed, covalently binding, irreversible inhibitor AZD9291 can effectively target T790M (32,33). As T790M detection started later in China compared with the USA and EU (15,33), only 23 patients in the present study exhibited T790M and 12 patients were mutation-positive. The PFS time was prolonged by administration of AZD9291 before disease progression with 1st-line TKI, and was not included in the total PFS time in the present study. In China, the National Health Insurance provides TKIs for patients with EGFR-mutant NSCLC; however, the duration of the detection of EGFR mutations and the application of TKIs usually requires nearly two weeks, often resulting in a delay to the initial therapy. 1st-line CHT can decrease the timing of initial treatment. The further decision regarding TKI use can be made after planned four- to six-cycles of CHT according to the American Society of Clinical Oncology Clinical Practice Guidelines and the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (11,34).
CHT as a follow-up to TKI treatment may not be as potent as 1st-line CHT (3–6,12); however, a significant difference in PFS time between the two therapeutic regimens was not observed in the present study. A number of patients did not complete the standard course of 2nd-line CHT after 1st-line TKI treatment due to the natural course of disease progression and tumor flare. Incomplete treatment may result in performance deterioration, decondition, malnutrition, tumor enlargement and metastasis. All of the above reasons will affect the treatment interval, dose, regimen and tolerance to chemotherapy's toxicity, which are associated with the response to chemotherapy (12). In the present study, after adjusting for clinical confounding variables, Patients with the following characteristics exhibited a significant benefit in PFS time from 1st-line CHT/2nd-line TKI treatment: Age >54 years old, L858R mutation, no brain metastasis and palliative radiotherapy. Although 1st-line CHT/2nd-line TKI revealed beneficial effects, subgroup analyses of other clinical factors did not exhibit a more favorable outcome in total PFS time between 1st-line CHT/2nd-line TKI and 1st-line TKI/2nd-line CHT.
The present retrospective study must be interpreted carefully due to inherent bias of the research method. For example, the decision on whether a patient should receive the 1st-line CHT/2nd-line TKI or the reverse regimen was made by the attending physician instead of randomization. The effect of confounding variables was minimized by controlling for several clinical factors which may influence PFS: Performance status, smoking, tumor status, lymph node status, metastasis status, EGFR mutation types, chemotherapeutic regimen and palliative radiotherapy. After controlling for these factors, no significant difference was observed between the two treatment strategies. Although, the present study was retrospective, this analysis was balanced by demographic and clinical features. However, the present study is not without limitations. The improved performance status observed in patients in the 1st-line TKI/2nd-line CHT group compared with the 1st-line CHT/2nd-line TKI group may have been due to the fact that patients in the former group were able to tolerate CHT following disease progression, or AR of TKIs. Furthermore, the present study excluded patients who were not able to tolerate 2nd-line CHT due to low performance status and tumor progression and this may influence the accuracy of the results. Time to failure of strategy (TFS) may represent a better surrogate endpoint for OS. TFS was used in the research of NSCLC patients with EGFR mutations by Shinno et al (35). Additionally, the small population size in the present study is another limitation. Median cutoff values were used in the present study due to the relatively small number size used. Cut-off points of age, pre-albumin and BMI would cause quite imbalance of two group numbers with bias (36).
The standard 1st-line therapy for patients with EGFR-mutant advanced NSCLC is an EGFR-directed oral TKI. The findings of the present study were consistent with previous clinical trials (EURTAC, WJTOG3405 and IPASS), EGFR TKIs exert an unmatched advantage in terms of the 1st-line PFS time of EGFR-mutant NSCLC patients (3–6,18). When 1st-line PFS time is analyzed, EGFR TKIs plus CHT in EGFR-mutant lung cancer should be considered. A phase III randomized trial in India revealed that adding pemetrexed and carboplatin chemotherapy to gefitinib significantly prolonged 1st-line PFS and OS time but increased toxicity in patients with NSCLC (37). However, in this clinical trial, fewer subsequent therapeutic methods and increased toxicity may restrict this strategy (37).
The authors do not recommend the 1st-line CHT/2nd-line TKI regimen in patients with wild-type EGFR, as that disease subtype lacks oncogene addiction (22). Further validation in clinical trials is needed. A phase II randomized, double-blind trial is being designed by Shandong Cancer Hospital (Jinan, China). This prospective trial will address the efficacy in terms of total PFS time of NSCLC patients harboring EGFR mutants treated with CHT followed by TKI, compared with that of the reverse regimen.
In conclusion, in the present study, no significant difference was observed between 1st-line CHT/2nd-line TKI and the reverse strategy in terms of the total PFS time in patients with NSCLC harboring an EGFR-mutation. Therefore, 1st-line CHT/2nd-line TKI may represent an alternative therapeutic regimen in specific patients undergoing precision treatment.
Acknowledgements
The authors of the present study would like to sincerely thank Dr Bairu Le (Department of Radiation Oncology, Shandong Cancer Hospital and Institute Affiliated to Shandong University) and Dr Jialiang Huang (Department of Radiation Oncology, Shandong Cancer Hospital and Institute Affiliated to Shandong University) for the collection of data and Dr Joan Chen (Department of Radiation Oncology, Shandong Cancer Hospital and Institute Affiliated to Shandong University) for the data analysis.
Glossary
Abbreviations
- PFS
progression-free survival
- CHT
chemotherapy
- TKI
tyrosine kinase inhibitor
- EGFR
epidermal growth factor receptor
- NSCLC
non-small-cell lung cancer
- OS
overall survival
- AR
acquired resistance
- RR
response rate
- HR
hazard ratio
Funding
The present study was supported by the National Health and Family Planning Commission of China (grant no. 201402011) and Innovation Project of Shandong Academy of Medical Science (grant no. SD20150023).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JH, YX and YZ performed the statistical analysis, interpreted the data, drafted and revised the article. HL, JH and JY participated in the study design, the revision of the manuscript and led the operation of the study. JC, AY, PC, HZ, XZ and CS participated in the study design and assisted in the collection of data. All the authors were involved in the conception of the study, read the manuscript and ensure the integrity of this work.
Ethics approval and consent to participate
The present study was approved by The Institutional Review Board of Shandong Cancer Hospital and Institute (Jinan, China; IRB no.: SDCH20170136).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016;107:1179–1186. doi: 10.1111/cas.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura S, Tanaka K, Harada T, Liu R, Shibahara D, Kawano Y, Nakanishi Y, Okamoto I. Sensitivity of epidermal growth factor receptor with single or double uncommon mutations to afatinib confirmed by a visual assay. Cancer Sci. 2018;109:3657–3661. doi: 10.1111/cas.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa C, Molina MA, Drozdowskyj A, Giménez-Capitán A, Bertran-Alamillo J, Karachaliou N, Gervais R, Massuti B, Wei J, Moran T, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 2014;20:2001–2010. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Zhao X, He D, Wang J, Li W, Liu Y, Ma L, Jiang M, Teng Y, Wang Z, et al. Loss of EGFR confers acquired resistance to AZD9291 in an EGFR-mutant non-small cell lung cancer cell line with an epithelial–mesenchymal transition phenotype. J Cancer Res Clin Oncol. 2018;144:1413–1422. doi: 10.1007/s00432-018-2668-7. [DOI] [PubMed] [Google Scholar]
- 8.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17:5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Kang S, Fang W, Hong S, Liang W, Yan Y, Qin T, Tang Y, Sheng J, Zhang L. Impact of Smoking Status on EGFR-TKI Efficacy for Advanced Non-Small-Cell Lung Cancer in EGFR Mutants: A Meta-analysis. Clin Lung Cancer. 2015;16:144–151.e1. doi: 10.1016/j.cllc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Wu YL, Chan V, Kurnianda J, Nakagawa K, Saijo N, Fukuoka M, McWalter G, McCormack R, Mok TS. Epidermal growth factor receptor mutation analysis in previously unanalyzed histology samples and cytology samples from the phase III Iressa Pan-ASia Study (IPASS) Lung Cancer. 2014;83:174–181. doi: 10.1016/j.lungcan.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) Edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Jin B, Chu T, Dong X, Yang H, Zhang Y, Wu D, Lou Y, Zhang X, Wang H, Han B. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer. 2016;96:87–92. doi: 10.1016/j.lungcan.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida T, Kuroda H, Oya Y, Shimizu J, Horio Y, Sakao Y, Hida T, Yatabe Y. Clinical outcomes of platinum-based chemotherapy according to T790M mutation status in EGFR-positive non-small cell lung cancer patients after initial EGFR-TKI failure. Lung Cancer. 2017;109:89–91. doi: 10.1016/j.lungcan.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA, Ladanyi M. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neeman E, Gresham G, Ovasapians N, Hendifar A, Tuli R, Figlin R, Shinde A. Comparing physician and nurse eastern cooperative oncology group performance status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncologist. 2019;24:e1460–e1466. doi: 10.1634/theoncologist.2018-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen C, Kehl KL, Zhao B, Simon GR, Zhou S, Giordano SH. Utilization patterns and trends in epidermal growth factor receptor (EGFR) mutation testing among patients with newly diagnosed metastatic lung cancer. Clin Lung Cancer. 2017;18:e233–e241. doi: 10.1016/j.cllc.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias-Santagata D, Akhavanfard S, David SS, Vernovsky K, Kuhlmann G, Boisvert SL, Stubbs H, McDermott U, Settleman J, Kwak EL, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanoue LT. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. Yearbook Pulmon Dis. 2010;2010:149–151. doi: 10.1016/S8756-3452(09)79377-6. [DOI] [Google Scholar]
- 22.Cesare G, Fortunato C, Ciro G, Feld R, Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: The TORCH randomized trial. J Clin Oncol. 2012;30:3002–3011. doi: 10.1200/JCO.2011.41.2056. [DOI] [PubMed] [Google Scholar]
- 23.Jänne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, Capelletti M, Edelman MJ, Villalona-Calero MA, Kratzke R, et al. Randomized Phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 Trial. J Clin Oncol. 2012;30:2063–2069. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Giuseppe G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial--INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/jco.2004.22.90140.7011. [DOI] [PubMed] [Google Scholar]
- 26.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, et al. TRIBUTE: A Phase III Trial of Erlotinib Hydrochloride (OSI-774) Combined With Carboplatin and Paclitaxel Chemotherapy in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 27.Qiao L, Jin W, Long G, Jiang Y. Sequential treatment of tyrosine kinase inhibitor and platinum-based doublet chemotherapy on EGFR mutant non-small cell lung cancer: A meta-analysis of randomized controlled clinical trials. Onco Targets Ther. 2017;10:1279–1284. doi: 10.2147/OTT.S128187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardona AF, Arrieta O, Zapata MI, Rojas L, Wills B, Reguart N, Karachaliou N, Carranza H, Vargas C, Otero J, et al. Acquired resistance to erlotinib in EGFR mutation-positive lung adenocarcinoma among hispanics (CLICaP) Target Oncol. 2017;12:513–523. doi: 10.1007/s11523-017-0497-2. [DOI] [PubMed] [Google Scholar]
- 29.Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, Wang Z, Xu CR, Su J, Wang BC, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116:568–574. doi: 10.1038/bjc.2016.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen YC, Tseng GC, Tu CY, Chen WC, Liao WC, Chen WC, Li CH, Chen HJ, Hsia TC. Comparing the effects of afatinib with gefitinib or Erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer. 2017;110:56–62. doi: 10.1016/j.lungcan.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Chang CH, Lee CH, Wang JY. Gefitinib or Erlotinib for Previously Treated Lung Adenocarcinoma: Which Is Superior? J Clin Oncol. 2017;35:1374–1375. doi: 10.1200/JCO.2016.68.5842. [DOI] [PubMed] [Google Scholar]
- 32.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, Papadimitrakopoulou V, Solomon BJ, Oxnard GR, Dziadziuszko R, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 33.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 34.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S, Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, Smith TJ, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Oncol Pract. 2015;33:2488–3515. [Google Scholar]
- 35.Shinno Y, Goto Y, Watanabe S, Sato J, Morita R, Matsumoto Y, Murakami S, Kanda S, Horinouchi H, Fujiwara Y, et al. Evaluation of time to failure of strategy as an alternative surrogate endpoint in patients with lung cancer with EGFR mutations. ESMO Open. 2018;3:e000399. doi: 10.1136/esmoopen-2018-000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Jeon H, Shim B. Prognostic value of ferritin-to-hemoglobin ratio in patients with advanced non-small-cell lung cancer. J Cancer. 2019;10:1717–1725. doi: 10.7150/jca.26853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in -mutated lung cancer. J Clin Oncol. 2020;18:124–136. doi: 10.1200/JCO.19.01154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JH, YX and YZ performed the statistical analysis, interpreted the data, drafted and revised the article. HL, JH and JY participated in the study design, the revision of the manuscript and led the operation of the study. JC, AY, PC, HZ, XZ and CS participated in the study design and assisted in the collection of data. All the authors were involved in the conception of the study, read the manuscript and ensure the integrity of this work.