Abstract
Ovarian cancer has a high incidence rate and mortality in gynaecologic malignancies. Epithelial ovarian cancer (EOC) accounts for >95% of ovarian cancer cases. Most of the patients with EOC are difficult to diagnose in early stage. The aim of the present study was to compare the long non-coding (lnc)RNA expression profiles of five ovarian cancer cell lines (IGROV1, A2780, SKOV3, ES2, and Hey) and an ovarian epithelial cell line (IOSE80) in order to identify differentially expressed lncRNAs and their associated microRNAs (miRNAs). The expression profiles of lncRNAs and mRNAs in these cell lines were determined by microarray gene analysis and reverse transcription-quantitative PCR. lncRNA neuropeptides B and W receptor 1–2 (NPBWR1-2) overexpression was induced in the SKOV3 cell line. Cell viability, proliferation, migration, invasion and apoptosis were evaluated using MTT, colony-formation, Transwell and flow cytometry assays, respectively. The microarray results indicated that several lncRNAs were differentially expressed in the five ovarian cancer cell lines compared with the normal ovarian epithelial cell line. Compared with IOSE80, lncRNA NPBWR1-2 was downregulated by more than two-fold in all five ovarian cancer cell lines. Moreover, NPBWR1-2 overexpression in the SKOV3 cell line decreased cell viability, inhibited proliferation, migration and invasion, and promoted apoptosis compared with the control cells. A total of 20 miRNAs, which are involved in tumorigenesis and development, were predicted to be associated with NPBWR1-2 by bioinformatics analysis. The results of the present study suggest that lncRNA NPBWR1-2 affects the occurrence and development of ovarian cancer via multiple miRNAs, providing a theoretical basis for the development of novel clinical treatments.
Keywords: lncRNA, miRNA, microarray, ovarian cancer, neuropeptides B and W receptor 1–2
Introduction
At present, ovarian cancer has the third highest incidence rate (3%) and the highest mortality rate (5%) worldwide among gynaecological malignancies (1). Epithelial ovarian cancer (EOC) accounts for >95% of ovarian malignancies and >50% of the patients with ovarian cancer are not diagnosed at an early stage. Furthermore, the recurrence rate of EOC is high (>80%) (2), which provides an explanation for the high mortality rate associated with the disease (3). Moreover, the mechanisms underlying the origin, pathogenesis and metastasis of EOC are not completely understood, which makes early diagnosis difficult.
Long non-coding (lnc)RNAs are non-coding RNAs with transcripts >200 nucleotides in length. lncRNAs are widely distributed in a number of tissues, for example brain, lung, heart and ovaries, and their expression is often tissue- and time-specific (4,5). Although the functions of a number of lncRNAs are unclear, it has been reported that they are involved either directly or in the regulation of development, cell differentiation, metabolism and other biological processes (6). lncRNAs can regulate gene expression at the epigenetic, transcriptional and post-transcriptional levels, which is closely associated with the occurrence, development and prevention of human diseases (6–8). Recently, an increasing number of studies have detected significant differences in the expression levels of lncRNAs between normal and tumour tissues (9–11). For example, brain cytoplasmic RNA1 is highly expressed during breast, lung, tongue and ovarian cancer, and HOX transcript antisense RNA expression is significantly higher in a number of tumour tissues compared with normal tissues (12).
MicroRNAs (miRNAs) are small non-coding RNA molecules, 18–22 nucleotides in length, that are present in eukaryotic cells. miRNAs bind to the 3′ or 5′ untranslated region (UTR) of specific target genes, by complete or incomplete complementary base pairing, in order to inhibit the translation of, or directly degrade the target mRNA, which ultimately affects downstream signalling. miRNA expression is important during cell proliferation, differentiation, apoptosis and autophagy (13). Several studies have demonstrated that lncRNAs can act as competitive endogenous (ce)RNAs to regulate the aggregation and biological function of miRNAs (14), thereby affecting mRNA expression (15,16). Bioinformatics analysis can clarify the regulatory mechanisms underlying lncRNAs by predicting the miRNAs that they compete for. Therefore, bioinformatics analysis can provide a theoretical basis for identifying novel markers for the early detection, timely diagnosis and clinical intervention of ovarian cancer (17).
A previous study reported that inorganic pyrophosphate (PPA1) is closely associated with the occurrence and development of ovarian cancer (18). Luo et al (19) demonstrated that PPA1 is associated with several genes [heat shock protein family B (small) member 1 (HSPB1), tumour protein p53 (TP53), unc-119 lipid binding chaperone (UNC119), small ubiquitin-like modifier 4 (SUMO4) and SET domain bifurcated histone lysine methyltransferase 1 (SETDB1)] in ovarian cancer cells. HSPB1 (20), TP53 (21) and UNC119 (22) are closely related to cell proliferation and apoptosis, whereas SUMO4 (23) and SETDB1 (24) are associated with regulating transcriptional activity. Therefore, the aim of the present study was to compare the lncRNA expression profiles of five ovarian cancer cell lines and an ovarian epithelial cell line, in order to identify differentially expressed lncRNAs and their associated miRNAs.
Materials and methods
Cell culture
The human EOC IGROV1, A2780, SKOV3, ES2 and Hey cell lines were purchased from the American Type Culture Collection. The human ovarian epithelial IOSE80 cell line was obtained from Wuxi Innovate Biomedical Technology Company (www.innovatbio.com/). All cell lines were cultured in RPMI-1640 medium (Corning, Inc.) supplemented with 10% foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at 37°C with 5% CO2.
RNA extraction and lncRNA microarray assay
Total RNA was extracted from EOC and IOSE80 cells using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and quantified using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Inc.). RNA integrity was assessed using the Agilent Bioanalyzer 2100 (Agilent Technologies, Inc.). Total RNA was reverse transcribed into double-stranded cDNAs (ds-cDNAs) using a Quick Amp Labeling kit (p/n 5190-0442; Agilent Technologies, Inc.) according to the manufacturer's protocol and oligo dT primers at 40°C for 2 h and 65°C for 15 min. The ds-cDNAs were then labelled with Cy3 and hybridized onto the Human lncRNA Microarray (version 4.0; Shanghai Kangcheng Biological Engineering Co., Ltd.), which contains probes for 40,173 lncRNAs, as previously described (25). Following hybridization, the microarray slides were washed using Gene Expression Wash Buffer 1 (p/n 5188–5325; Agilent Technologies, Inc.) and Gene Expression Wash Buffer 2 (p/n 5188–5326; Agilent Technologies, Inc.), scanned using the Agilent Microarray Scanner (p/n G2565BA; Agilent Technologies, Inc.), and analysed using Agilent Feature Extraction software (version 11.0.1.1; Agilent Technologies, Inc.). Quantile normalization and subsequent data processing were performed using GeneSpring GX software (version 12.2; Agilent Technologies, Inc). Subsequently, lncRNAs and mRNAs were selected for further data analysis. Differentially expressed lncRNAs and mRNAs with statistical significance between the EOC and IOSE90 cell lines were identified by P<0.05 and |fold change| >2.
Reverse transcription-quantitative PCR (RT-qPCR)
qPCR was performed using the SYBR Green RT-PCR Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used for reverse transcription: 5 min at 37°C, 60 min at 42°C, and 10 min at 70°C. Samples were stored at −20°C until further analysis. The following thermocycling conditions were used for qPCR: Initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 10 sec and a final extension at 60°C for 60 sec. The following primer pairs (Takara Bio, Inc.) were used for qPCR: Neuropeptides B and W receptor 1–2 (NPBWR1-2) forward, 5′-TTTTCATTTTTATGTATGGGCA-3′ and NPBWR1-2 reverse, 5′-ACAACAGAACTCGTTTTAAGTTAC-3′; and β-actin forward, 5′-GTGGCCGAGGACTTTGATTG3′ and β-actin reverse, 5′-CCTGTAACAACGCATCTCATATT-3′. lncRNA expression levels were quantified using the 2−ΔΔCq method (26) and normalized to the internal reference gene β-actin. qPCR was performed in triplicate.
Cell transfection
To induce NPBWR1-2 overexpression, five ovarian cancer cell lines were transfected with an NPBWR1-2 expression vector (lnc-NPBWR1-2; Genewiz, Inc.) or with a pcDNA3.1 negative control (NC) empty vector (Tianjin Saierbio, Inc.). Cells were plated in 6-well plates (3×105 cells/well) and incubated overnight at 37°C. Cells (~1.75×105 cells)with the plasmids (4 µg) were transfected using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Following transfection for 48 h, cells were collected and prepared for further experiments.
Cell viability assay
To determine cell viability, cells were examined using an MTT assay. Cells were seeded (1×103 cells/well; IGROV1, A2780, SKOV3, ES2, Hey cells and IGROV1, A2780, SKOV3, ES2, Hey/lnc-NPBWR1-2 cells) into 96-well plates. SKOV3 cell viability was assessed at different time points (24, 48 and 72 h) and others was assessed at 48 h using the MTT assay. A total of 100 µl DMSO was used to dissolve the purple formazan in each well. The optical density of each well, which represented cell proliferation, was measured daily for four consecutive days at a wavelength of 570 nm to estimate the number of viable cells at each time point. The cell viability rate (%)=experimental group A570 mean value/control group A570 mean value ×100%.
Cell proliferation assay
Cells were trypsinised using 0.25% trypsin and resuspended in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS, seeded 200 cells into 12-well plates and cultured in a humidified atmosphere containing 5% CO2 at 37°C for 7–14 days. Following incubation, cell colonies were washed with PBS, fixed with 4% methanol at 4°C for 30 min, and stained with 0.1% crystal violet (1 mg/ml) for 20 min at room temperature. Colonies containing >50 cells were counted, and the mean colony number was calculated.
Cell migration and invasion assays
Cell migration and invasion were assessed using Transwell assays. To assess cell invasion, matrigel was dissolved and plated into each well prior to seeding cells and medium into the Transwell plates for 1 h at room temperature. Cells were trypsinised using 0.25% trypsin and subsequently plated (1×105) in 100 µl serum-free RPMI-1640 medium into the upper chambers of the Transwell plates. RPMI-1640 medium supplemented with 20% FBS (500 µl) was plated into the lower chambers of the Transwell plates. Following incubation for 48 (migration assay) or 72 h (invasion assay), cells on the lower surface of the Transwell membrane were fixed with 75% methanol [a mixture of methanol and glacial acetic acid (3:1)] for 30 min at room temperature, and stained with 0.1% crystal violet for 15 min at room temperature. Stained cells were counted in three randomly selected fields of view using an fluorescence inverted microscope at ×200 magnification.
Cell apoptosis assays
The relative number of apoptotic cells was measured using an Annexin V-FITC/Propidium Iodide (PI) Apoptosis Detection kit according to the manufacturer's protocol (Shanghai Kaifeng Biotechnology). Briefly, cells were seeded (1×105 cells/well) into 6-well plates, 200 µl Annexin V followed by 10 µl PI was added to each well and cells were incubated for 10–20 min at room temperature in the dark. Subsequently, cells were washed twice with cold PBS. Early apoptotic cells were detected and analysed using a BD LSRFortessa™ flow cytometer (Becton, Dickinson and Company). Data were analysed using BD FACSDiva software (version 6.0; Becton, Dickinson and Company).
Western blotting
Cells were washed with PBS. Total protein was extracted using ProteoJET Mammalian Cell Lysis Reagent (Fermentas; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Protein levels were determined using a BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, proteins (20 µg) were separated via 10% gel by SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 0.05% Starting Block Blocking buffers (Thermo Fisher Scientific, Inc.) for 10 min at room temperature. Subsequently, the membranes were incubated at 4°C overnight with primary antibodies targeted against: IGFBP-7 (cat. no. GTX31152) and GAPDH (cat. no. GTX100118) (both 1:1,000; both from GeneTex, Inc.). Following primary incubation, the membranes were washed four times using TBS with 0.1% Tween-20. Membranes were then incubated with goat anti-rabbit IgG (1:10,000; cat. no. ab205718; Abcam) secondary antibodies for 1.5 h at room temperature. Protein bands were visualized using Western Lightning™ Chemiluminesence Reagent (PerkinElmer, Inc.) and photographed using a LabWorks™ gel imaging and analysis system (Analytik Jena AG). GAPDH was used as the loading control.
Bioinformatics analysis
The microRNA Target Prediction Database (miRDB) website (mirdb.org/index.html; human) was used to integrate the lncRNA/miRNA and miRNA-target networks, and predict miRNA/lncRNA relationships.
Statistical analysis
Data are expressed as the mean ± standard deviation (unless otherwise shown). Differences in the expression levels of NPBWR1-2 among the different experimental groups were analysed using an unpaired Student's t-test. In cell proliferation, migration, invasion and apoptosis assays, the SKOV3/NC group was set at 1. Statistical analyses were performed using SPSS software (version 17.0; SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
lncRNA microarray analysis
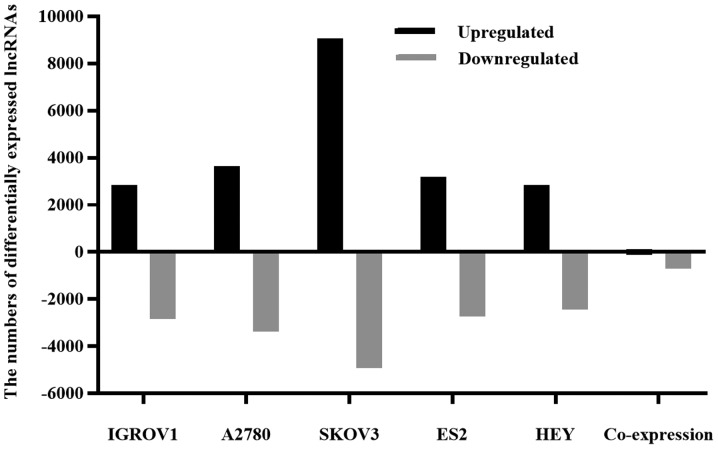
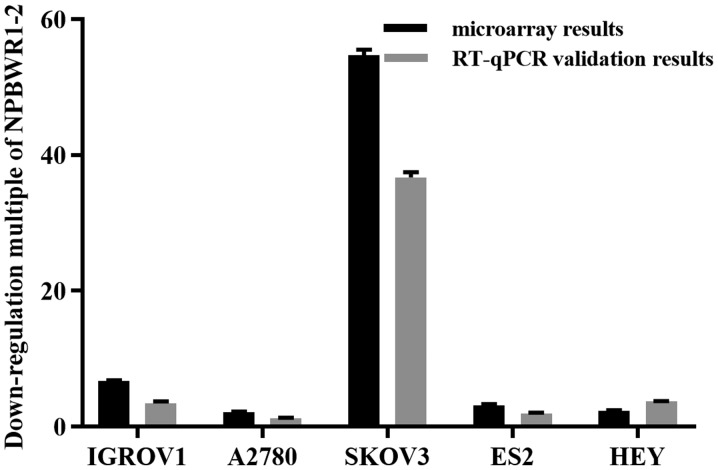
In order to identify differentially expressed lncRNAs between EOC and IOSE80 cells, the lncRNA expression profiles of the two cell types were compared (Fig. 1). Collectively, the five EOC cell lines displayed 110 upregulated and 699 downregulated lncRNAs, suggesting that these lncRNAs were associated with EOC formation and development. The expression profile of lncRNA NPBWR1-2 was assessed using RT-qPCR and microarray assays. Compared with IOSE80 cells, NPBWR1-2 was downregulated by more than two-fold in all five ovarian cancer cell lines, according to the microarray results. The RT-qPCR results were consistent with the microarray results (Fig. 2).
Figure 1.
Differentially expressed lncRNAs in EOC cells. Total RNA was isolated from five EOC cell lines (IGROV1, A2780, SKOV3, ES2 and Hey) and the IOSE80 cell line, reverse transcribed into double-stranded cDNAs and hybridized with a human lncRNA microarray. The microarray results were analysed using Agilent Feature Extraction software. P<0.05 and |fold change| >2 were considered to indicate differential expression. Black and gray bars represent the number of upregulated and downregulated lncRNAs, respectively, in the EOC cell line compared with the IOSE80 cell line. lncRNA, long non-coding RNA; EOC, epithelial ovarian cancer.
Figure 2.
Validation of RT-qPCR and microarray results. NPBWR1-2 was downregulated in the five ovarian cancer cell lines compared with IOSE80. RT-qPCR, reverse transcription-quantitative PCR; NPBWR1-2, neuropeptides B and W receptor 1–2.
NPBWR1-2 overexpression decreases cell viability, inhibits proliferation, migration and invasion, and promotes apoptosis
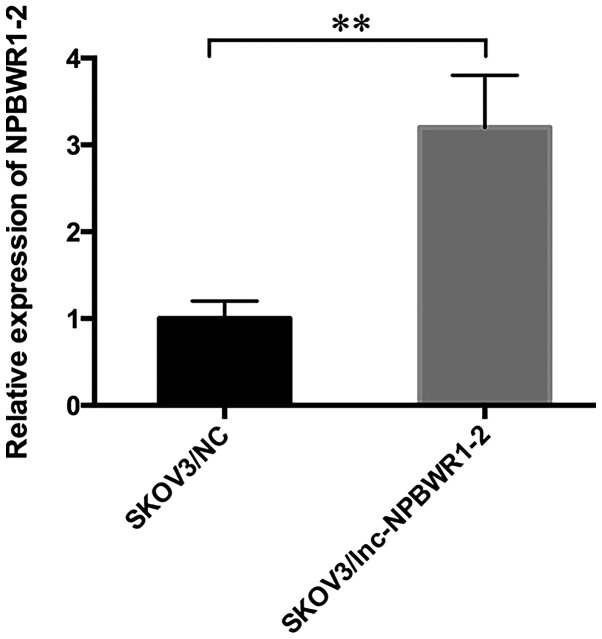
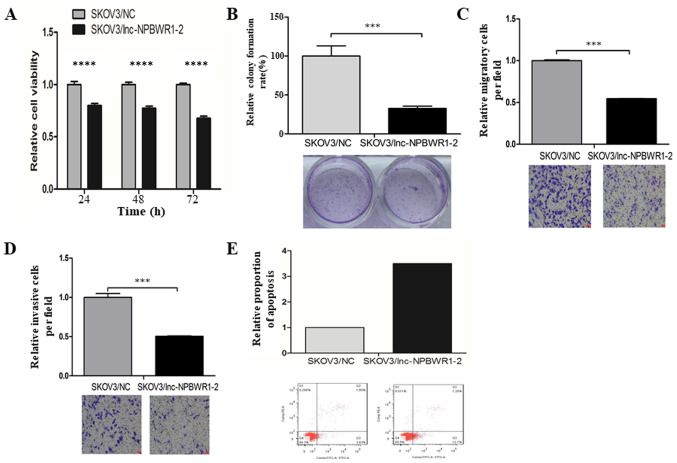
SKOV3 is a serous adenocarcinoma cell line, which displays one of the highest incidence rates of ovarian cancer (27). Among the five ovarian cancer cell lines, the expression levels of NPBWR1-2 were highest in the SKOV3 cell line; therefore, SKOV3 cells were chosen for subsequent experiments (Fig. 2). The expression level of NPBWR1-2 was significantly upregulated in the SKOV3/lnc-NPBWR1 group compared with the SKOV3/NC group, as determined by RT-qPCR (Fig. 3). Similar results were obtained for NPBWR1-2 expression in the other four ovarian cancer cell lines (Fig. S1). Cell viability was significantly decreased in the lnc-NPBWR1-2 group compared with the NC group for each of the five cell lines (IGROV1, P<0.0001; A2780, P<0.001; SKOV3, P<0.0001; ES2, P<0.0001; and Hey, P<0.0001, Fig. S2). The results of the cell viability assay in SKOV3 cells are displayed in Fig. 4A. The mean OD570 values of the SKOV3/NC and SKOV3/lnc-NPBWR1-2 groups were 0.328±0.018 and 0.41±0.030, 0.431±0.019 and 0.557±0.021, and 0.590±0.019 and 0.87±0.011 at 24, 48 and 72 h, respectively (P<0.0001; Fig. 4A). Cell proliferation was significantly decreased in the SKOV3/lnc-NPBWR1-2 group compared with the SKOV3/NC group (P<0.001; Fig. 4B), indicating that NPBWR1-2 overexpression inhibited SKOV3 cell proliferation in vitro. The mean number of migratory cells in the SKOV3/lnc-NPBWR1-2 and SKOV3/NC groups was 249.667±25.541 and 457.333±4.933, respectively (P<0.001; Fig. 4C). The number of invading cells was significantly lower in the SKOV3/lnc-NPBWR1-2 group compared with the SKOV3/NC group (P<0.001; Fig. 4D). Furthermore, the relative number of apoptotic cells was increased in the SKOV3/lnc-NPBWR1-2 group compared with the SKOV3/NC group (Fig. 4E).
Figure 3.
Expression levels of NPBWR1-2 in SKOV3/NC and SKOV3/lnc-NPBWR1-2 cells. Cells were transfected with an NPBWR1-2 overexpression vector or a nonsense pcDNA3.1 control vector. **P<0.01. NPBWR1-2, neuropeptides B and W receptor 1–2; NC, negative control; lncRNA, long non-coding RNA.
Figure 4.
Effects of NPBWR1-2 on SKOV3 cell viability, proliferation, migration, invasion and apoptosis. Cells were transfected with an NPBWR1-2 overexpression or a nonsense pcDNA3.1 control vector. (A) Cell viability was measured using the MTT assay. (B) Cell proliferation was assessed using a colony-formation assay. Cell (C) migration and (D) invasion were assessed using Transwell assays. (E) Apoptotic cells were detected by flow cytometry (the SKOV3/NC group was set at 1 in B, C, D and E). ***P<0.001 and ****P<0.0001. NPBWR1-2, neuropeptides B and W receptor 1–2; NC, negative control; lncRNA, long non-coding RNA.
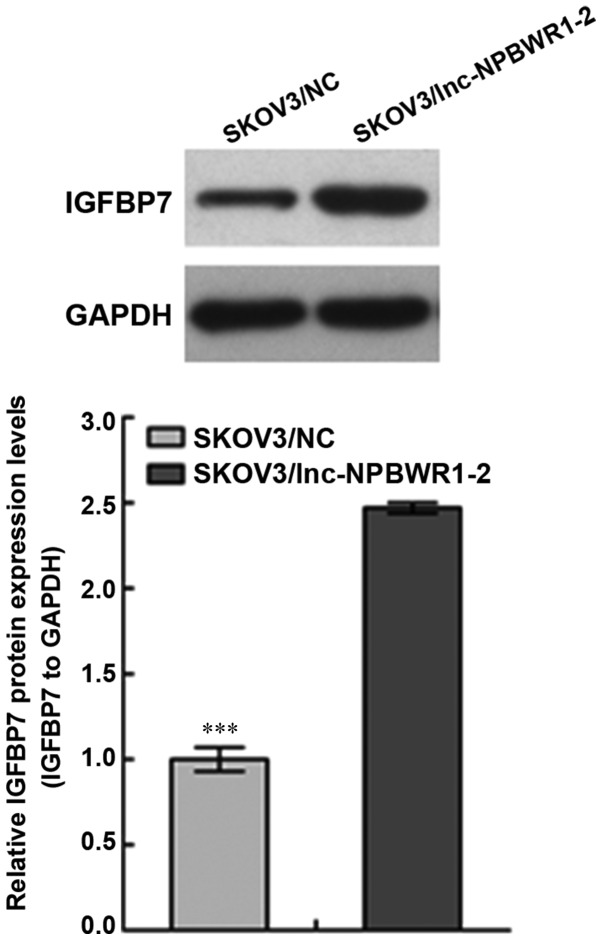
NPBWR1-2 overexpression affects IGFBP7 expression
The expression level of IGFBP7 in the SKOV3/NC group was significantly lower compared with the SKOV3/lnc-NPBWR1-2 group (P<0.001; Fig. 5).
Figure 5.
Effects of NPBWR1-2 on IGFBP7 expression in SKOV3 cells. The expression of IGFBP7 was analysed by western blotting in SKOV3/lnc-NPBWR1-2 and SKOV3/NC cells. ***P<0.001. NPBWR1-2, neuropeptides B and W receptor 1–2; IGFBP7, insulin-like growth factor binding protein 7; lncRNA, long non-coding RNA; NC, negative control.
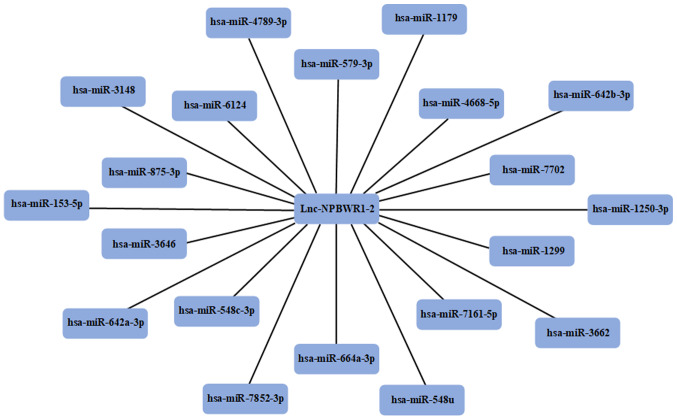
miRNA prediction
Using the miRDB, the miRNAs that competed with lncRNA NPBWR1-2 were predicted. A total of 20 miRNAs were identified as potential targets for NPBWR1-2 (Fig. 6).
Figure 6.
miRNAs predicted to be closely associated with lncRNA NPBWR1-2. Using the miRNA Target Prediction Database, 20 miRNAs that were predicted to target NPBWR1-2 were identified. miRNA/miR, microRNA; lncRNA, long non-coding RNA; NPBWR1-2, neuropeptides B and W receptor 1–2.
Discussion
Recently, an increasing number of studies investigating the roles of lncRNAs associated with cancer, immune signalling and the maintenance of stem cell biological characteristics have been conducted (12,28,29). lncRNAs display ‘one-to-many’ and ‘many-to-one’ regulatory functions and can regulate gene expression at multiple levels, including epigenetic, transcriptional and post-transcriptional levels (30). Previous studies have indicated that abnormal lncRNA expression is associated with tumour development, recurrence and metastasis (31–33). In clinical practice, patients administered with the same treatment often display different clinical responses, which may be explained in part by the differential expression of lncRNAs among patients (34). lncRNAs also function as competitive endogenous RNAs to regulate miRNA expression; however, the association between lncRNAs and miRNAs in ovarian cancer is not completely understood (35).
In patients with ovarian cancer, it is rarely possible to identify the histological type before surgery, and the lack of effective available biomarkers is a challenge for the detection and diagnosis of early-stage EOC without obvious symptoms (3,36). By identifying a marker that is sensitive to all types of EOC, ovarian cancer could be detected and diagnosed at an earlier stage, allowing patients to receive early treatment to maximize survival time. In the present study, five different ovarian cancer cell lines, which represent the most common ovarian cancer tissue types, were selected. Combined with the results of the present study, NPBWR1-2 was identified as a candidate lncRNA for ovarian cancer. The gene encoding NPBWR1-2 is located on chromosome 8, and to the best of our knowledge, the expression profile of NPBWR1-2 in ovarian cancer has not been previously reported (37). By performing a series of in vitro experiments, NPBWR1-2 expression levels in SKOV3 cells, following transfection with an NPBWR1-2 overexpression vector, were detected. The increased expression of NPBWR1-2 reduced the proliferation, invasion and migration of SKOV3 cells, suggesting that NPBWR1-2 overexpression inhibited the proliferation, invasion and migration of ovarian cancer cells. The results of the lncRNA microarray and in vitro experiments indicated that NPBWR1-2 was associated with ovarian cancer.
In various types of cancer, IGFBP7 is involved in a number of processes, including cell differentiation, cell adhesion, angiogenesis, cell proliferation and survival, aging and apoptosis (38). Moreover, it has been reported that IGFBP7 acts as a tumour suppressor gene (39). The western blotting results indicated that NPBWR1-2 overexpression significantly increased the expression of IGFBP7, which further suggested that NPBWR1-2 was associated with the occurrence and development of ovarian cancer.
Using bioinformatics analysis, 20 miRNAs that were predicted to bind to lncRNA NPBWR1-2 were identified, most of which are associated with the occurrence and development of tumours, including miR-153-5p, miR-548c-3p, miR-664a, miR-1299 and miR-1179. Recently, miRNA-153-5p was identified as an anticancer factor, which regulates tumour suppressor genes and participates in the growth, metastasis and infiltration of tumours (40). Some studies have reported that miRNA-153-5p is negatively regulated in hepatocellular carcinoma cell lines and tissues, and inhibits cell migration and invasion by binding to the 3′ UTR of Snail (41–43). Niu et al (44) demonstrated that miRNA-153 overexpression decreases the proliferation and invasion of osteosarcoma cells by inhibiting the transforming growth factor-β signalling pathway. Zhou et al (45) reported that miR-153 inhibits cell proliferation, suppresses epithelial-mesenchymal transition and reduces cell invasion in ovarian cancer cells by downregulating SET domain containing 7, histone lysine methyltransferase and zinc finger E-box binding homeobox 2, suggesting that miR-153 may serve as a therapeutic target for ovarian cancer.
The role of miR-548c-3p has been investigated in various types of cancer, including breast, prostate and Helicobacter pylori-negative gastric cancer (46–48). It has been hypothesized that miR-548c-3p mutations may drive tumorigenesis (49). In breast cancer tissues, miR-548c-3p expression is low; however, miR-548c-3p overexpression can induce apoptosis, which ultimately inhibits breast cancer cell proliferation (50). Furthermore, miR-548c-3p inhibits glioma tumorigenesis via MYB proto-oncogene, transcription factor (51). The results of the aforementioned studies indicated that miR-548-3p may serve as a therapeutic target in various types of cancer.
A number of studies have reported that miR-664a participates in the regulation of cancer cell proliferation and migration, primarily via increasing miR-664a expression (52–54). Sahin et al (55) demonstrated that miR-664a binds to and alters the expression of lncRNA maternally expressed 3, which alters the migration of osteosarcoma cells.
Previous studies have suggested that miRNA-1299 is associated with the occurrence and development of a number of different tumours; however, its precise role has not been previously reported. During prostate cancer, miR-1299 regulates the Pim-1 proto-oncogene, serine/threonine kinase-STAT3 signalling pathway (56). miR-1299 is also downregulated in retinoblastoma, alcoholic hepatitis and hepatocellular carcinoma tissues (57–59).
miRNA-1179 is located on chromosome 15q26.1, which has been identified as a cancer susceptibility locus (60). Several studies have reported the aberrant expression of miR-1179 in various types of human cancer, such as colorectal, familial breast, pancreatic and thyroid cancer, as well as glioma (61–65); however, the role of miR-1179 during cancer progression is not completely understood.
Although the results of the present study further indicated that numerous potential molecular markers were closely associated with the development of ovarian cancer, further investigation is required. To provide a theoretical basis for the development of novel clinical treatments, future studies should verify the molecular markers identified in the present study, perform functional studies on lncRNA NPBWR1-2 and explore the molecular mechanism underlying the interaction between lncRNA NPBWR1-2 and its associated miRNAs.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- lncRNA
long non-coding RNA
- EOC
epithelial ovarian cancer
- miRNAs
microRNAs
- ds-cDNAs
double-stranded cDNAs
- RT-qPCR
reverse transcription-quantitative PCR
Funding
The present study was supported by the State Key Laboratory of Medicinal Chemical Biology (grant. no. 2018003).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SL, CL and PQ made substantial contributions to the conception and design of the work. QD performed the experiments. YR collected, analysed and interpreted the data. SL and CL drafted the work and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Korkmaz T, Seber S, Basaran G. Review of the current role of targeted therapies as maintenance therapies in first and second line treatment of epithelial ovarian cancer; In the light of completed trials. Crit Rev Oncol Hematol. 2016;98:180–188. doi: 10.1016/j.critrevonc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Xiao N, Li Z, Wang Q. Expression of inorganic pyrophosphatase (PPA1) correlates with poor prognosis of epithelial ovarian cancer. Tohoku J Exp Med. 2017;241:165–173. doi: 10.1620/tjem.241.165. [DOI] [PubMed] [Google Scholar]
- 4.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci USA. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinger ME, Pang KC, Mercer TR, Mattick JS. Differentiating protein-coding and noncoding RNA: Challenges and ambiguities. PLOS Comput Biol. 2008;4:e1000176. doi: 10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonasio R, Shiekhattar R. Regulation of transcription by long noncoding RNAs. Annu Rev Genet. 2014;48:433–455. doi: 10.1146/annurev-genet-120213-092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 8.Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avgeris M, Tsilimantou A, Levis PK, Rampias T, Papadimitriou MA, Panoutsopoulou K, Stravodimos K, Scorilas A. Unraveling UCA1 lncRNA prognostic utility in urothelial bladder cancer. Carcinogenesis. 2019;40:965–974. doi: 10.1093/carcin/bgz045. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Liu YT, Tang H, Xie WQ, Yao H, Gu WT, Zheng YZ, Shang HB, Wang Y, Wei YX, et al. Exosome-transmitted lncRNA H19 inhibits the growth of pituitary adenoma. J Clin Endocrinol Metab. 2019;104:126345–6356. doi: 10.1210/jc.2019-00536. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Zhu G, Ma Y, Qu H. lncRNA CCAT1 contributes to the growth and invasion of gastric cancer via targeting miR-219-1. (Epub ahead of print) J Cell Biochem. 2017 Dec 12; doi: 10.1002/jcb.26560. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Zhang M, Qu P. Expression level and clinical significance of HOX transcript antisense intergenic RNA in cervical cancer: A meta-analysis. Sci Rep. 2016;6:38047. doi: 10.1038/srep38047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun GJ, Zhong GG, Ming ZS. miR-218 inhibits the proliferation of glioma U87 cells through the inactivation of the CDK6/cyclin D1/p21Cip1/Waf1 pathway. Oncol Lett. 2015;9:2743–2749. doi: 10.3892/ol.2015.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou RS, Zhang EX, Sun QF, Ye ZJ, Liu JW, Zhou DH, Tang Y. Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in squamous cell carcinoma of tongue. BMC Cancer. 2019;19:779. doi: 10.1186/s12885-019-5983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan CN, Ma L, Liu N. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J Transl Med. 2018;16:264. doi: 10.1186/s12967-018-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C, Yuan G, Hu Z, Zeng Y, Qiu X, Yu H, He S. Bioinformatics analysis of the interactions among lncRNA, miRNA and mRNA expression, genetic mutations and epigenetic modifications in hepatocellular carcinoma. Mol Med Rep. 2019;19:1356–1364. doi: 10.3892/mmr.2018.9728. [DOI] [PubMed] [Google Scholar]
- 18.Niu H, Zhou W, Xu Y, Yin Z, Shen W, Ye Z, Liu Y, Chen Y, Yang S, Xiang R, et al. Silencing PPA1 inhibits human epithelial ovarian cancer metastasis by suppressing the Wnt/β-catenin signaling pathway. Oncotarget. 2017;8:76266–76278. doi: 10.18632/oncotarget.19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo D, Wang G, Shen W, Zhao S, Zhou W, Wan L, Yuan L, Yang S, Xiang R. Clinical significance and functional validation of PPA1 in various tumors. Cancer Med. 2016;5:2800–2812. doi: 10.1002/cam4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JA, Lee S, Kim DE, Kim M, Kwon BM, Han DC. Fisetin, a dietary flavonoid, induces apoptosis of cancer cells by inhibiting HSF1 activity through blocking its binding to the hsp70 promoter. Carcinogenesis. 2015;36:696–706. doi: 10.1093/carcin/bgv045. [DOI] [PubMed] [Google Scholar]
- 21.Bodnar M, Luczak M, Bednarek K, Szylberg L, Marszalek A, Grenman R, Szyfter K, Jarmuz-Szymczak M, Giefing M. Proteomic profiling identifies the inorganic pyrophosphatase (PPA1) protein as a potential biomarker of metastasis in laryngeal squamous cell carcinoma. Amino Acids. 2016;48:1469–1476. doi: 10.1007/s00726-016-2201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei B, Chai W, Wang Z, Liu R. Highly expressed UNC119 promotes hepatocellular carcinoma cell proliferation through Wnt/β-catenin signaling and predicts a poor prognosis. Am J Cancer Res. 2015;5:3123–3134. [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang KW, Won TJ, Kim H, Chun HJ, Chun T, Park Y. Erratum to ‘Characterization of the regulatory roles of the SUMO’. Diabetes Metab Res Rev. 2012;28:196–202. doi: 10.1002/dmrr.2273. [DOI] [PubMed] [Google Scholar]
- 24.Rivière L, Gerossier L, Ducroux A, Dion S, Deng Q, Michel ML, Buendia MA, Hantz O, Neuveut C. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J Hepatol. 2015;63:1093–1102. doi: 10.1016/j.jhep.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Ye Z, Gu Y, Tian B, Wu B, Li J. Genomic analysis of drug resistant pancreatic cancer cell line by combining long non-coding RNA and mRNA expression profiling. Int J Clin Exp Pathol. 2015;8:38–52. [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Tudrej P, Olbryt M, Zembala-Nożyńska E, Kujawa KA, Cortez AJ, Fiszer-Kierzkowska A, Pigłowski W, Nikiel B, Głowala-Kosińska M, Bartkowska-Chrobok A, et al. Establishment and characterization of the novel High-grade serous ovarian cancer cell line OVPA8. Int J Mol Sci. 2018;19(pii):E2080. doi: 10.3390/ijms19072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadjicharalambous MR, Lindsay MA. Long Non-coding RNAs and the innate immune response. Noncoding RNA. 2019;5(pii):E43. doi: 10.3390/ncrna5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Wang Y, Wang C, Hu JF, Li W. LncRNA Functions as a new emerging epigenetic factor in determining the fate of stem cells. Front Genet. 2020;11:277. doi: 10.3389/fgene.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zampetaki A, Albrecht A, Steinhofel K. Long Non-coding RNA structure and function: Is There a Link? Front Physiol. 2018;9:1201. doi: 10.3389/fphys.2018.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and cancer: A New paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Yuan Y, Ma R, Xu B, Zhang R. lncRNA SNHG7 affects malignant tumor behaviors through downregulation of EZH2 in uveal melanoma cell lines. Oncol Lett. 2020;19:1505–1515. doi: 10.3892/ol.2019.11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: An emerging paradigm of cancer research. Tumour Biol. 2013;34:613–620. doi: 10.1007/s13277-013-0658-6. [DOI] [PubMed] [Google Scholar]
- 34.Malek E, Jagannathan S, Driscoll JJ. Correlation of long non-coding RNA expression with metastasis, drug resistance and clinical outcome in cancer. Oncotarget. 2014;5:8027–8038. doi: 10.18632/oncotarget.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Ding W, Xu Y, Tao E, Mo M, Xu W, Cai X, Chen X, Yuan J, Wu X. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany NY) 2020;12:4558–4572. doi: 10.18632/aging.102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe N, Wada M, Irukayama-Tomobe Y, Ogata Y, Tsujino N, Suzuki M, Furutani N, Sakurai T, Yamamoto M. A single nucleotide polymorphism of the neuropeptide B/W receptor-1 gene influences the evaluation of facial expressions. PLoS One. 2012;7:e35390. doi: 10.1371/journal.pone.0035390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Kim WH, Byeon SJ, Lee BL, Kim MA. Epigenetic downregulation and growth inhibition of IGFBP7 in gastric cancer. Asian Pac J Cancer Prev. 2018;19:667–675. doi: 10.22034/APJCP.2018.19.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Yoo BK, Santhekadur PK, Gredler R, Bhutia SK, Das SK, Fuller C, Su ZZ, Fisher PB, Sarkar D. Insulin-like growth factor-binding protein-7 functions as a potential tumor suppressor in hepatocellular carcinoma. Clin Cancer Res. 2011;17:6693–6701. doi: 10.1158/1078-0432.CCR-10-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 41.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Huang X, Wang W, Xie H, Li J, Hu Z, Zheng Z, Li H, Teng L. LncRNA CDKN2BAS predicts poor prognosis in patients with hepatocellular carcinoma and promotes metastasis via the miR-153-5p/ARHGAP18 signaling axis. Aging (Albany NY) 2018;10:3371–3381. doi: 10.18632/aging.101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Feng F, Gao X, Wang C, Sun H, Zhang C, Zeng Z, Lu Y, An L, Qu J, et al. MiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cells. Curr Cancer Drug Targets. 2015;15:176–187. doi: 10.2174/1568009615666150225122635. [DOI] [PubMed] [Google Scholar]
- 44.Niu G, Li B, Sun L, An C. MicroRNA-153 inhibits osteosarcoma cells proliferation and invasion by targeting TGF-β2. PLoS One. 2015;10:e0119225. doi: 10.1371/journal.pone.0119225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Zhou J, Xie M, Shi Y, Luo B, Gong G, Li J, Wang J, Zhao W, Zi Y, Wu X, Wen J. MicroRNA-153 functions as a tumor suppressor by targeting SET7 and ZEB2 in ovarian cancer cells. Oncol Rep. 2015;34:111–120. doi: 10.3892/or.2015.3952. [DOI] [PubMed] [Google Scholar]
- 46.Rane JK, Scaravilli M, Ylipää A, Pellacani D, Mann VM, Simms MS, Nykter M, Collins AT, Visakorpi T, Maitland NJ. MicroRNA expression profile of primary prostate cancer stem cells as a source of biomarkers and therapeutic targets. Eur Urol. 2015;67:7–10. doi: 10.1016/j.eururo.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Chang H, Kim N, Park JH, Nam RH, Choi YJ, Lee HS, Yoon H, Shin CM, Park YS, Kim JM, Lee DH. Different microRNA expression levels in gastric cancer depending on Helicobacter pylori infection. Gut Liver. 2015;9:188–196. doi: 10.5009/gnl13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR, et al. Oslo Breast Cancer Consortium (OSBREAC): The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni XF, Zhao LH, Li G, Hou M, Su M, Zou CL, Deng X. MicroRNA-548-3p and MicroRNA-576-5p enhance the migration and invasion of esophageal squamous cell carcinoma cells via NRIP1 down-regulation. Neoplasma. 2018;65:881–887. doi: 10.4149/neo_2018_171206N803. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Qiu M, Wu Y, Hai L. MiR-548-3p functions as an anti-oncogenic regulator in breast cancer. Biomed Pharmacother. 2015;75:111–116. doi: 10.1016/j.biopha.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Lu J, Zhang M, Yang X, Cui T, Dai J. MicroRNA-548c-3p inhibits T98G glioma cell proliferation and migration by downregulating c-Myb. Oncol Lett. 2017;13:3866–3872. doi: 10.3892/ol.2017.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Z, Jian S, Peng X, Liu Y, Wang J, Zheng L, Ou C, Wang Y, Zeng W, Zhou M. Loss of MiR-664 expression enhances cutaneous malignant melanoma proliferation by upregulating PLP2. Medicine (Baltimore) 2015;94:e1327. doi: 10.1097/MD.0000000000001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, Cho ME, Li TW, Peng H, Ko KS, Mato JM, Lu SC. MicroRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. J Clin Invest. 2013;123:285–298. doi: 10.1172/JCI63861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao Y, Chen B, Wu Q, Hu K, Xi X, Zhu W, Zhong X, Chen J. Overexpression of miR-664 is associated with enhanced osteosarcoma cell migration and invasion ability via targeting SOX7. Clin Exp Med. 2017;17:51–58. doi: 10.1007/s10238-015-0398-6. [DOI] [PubMed] [Google Scholar]
- 55.Sahin Y, Altan Z, Arman K, Bozgeyik E, Koruk Ozer M, Arslan A. Inhibition of miR-664a interferes with the migration of osteosarcoma cells via modulation of MEG3. Biochem Biophys Res Commun. 2017;490:1100–1105. doi: 10.1016/j.bbrc.2017.06.174. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, He W, Gao J, Luo J, Huang X, Gao C. Computational prediction and experimental validation of a novel synthesized pan-PIM inhibitor PI003 and its apoptosis-inducing mechanisms in cervical cancer. Oncotarget. 2015;6:8019–8035. doi: 10.18632/oncotarget.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Chen SH, Li YM. Differential expression profiles of genes and miRNAs in alcoholic hepatitis. Zhonghua gan zang bing za zhi. 2012;20:883–887. doi: 10.3760/cma.j.issn.1007-3418.2012.12.002. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 58.Venkatesan N, Deepa PR, Khetan V, Krishnakumar S. Computational and in vitro investigation of miRNA-Gene regulations in retinoblastoma pathogenesis: miRNA mimics strategy. Bioinform Biol Insights. 2015;9:89–101. doi: 10.4137/BBI.S21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu H, Wang G, Zhou X, Song X, Gao H, Ma C, Chang H, Li H, Liu FF, Lu J, Ma J. miR-1299 suppresses cell proliferation of hepatocellular carcinoma (HCC) by targeting CDK6. Biomed Pharmacother. 2016;83:792–797. doi: 10.1016/j.biopha.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 60.Cai Q, Zhang B, Sung H, Low SK, Kweon SS, Lu W, Shi J, Long J, Wen W, Choi JY, et al. Genome-wide association analysis in East Asians identifies breast cancer susceptibility loci at 1q32.1, 5q14.3 and 15q26.1. Nat Genet. 2014;46:886–890. doi: 10.1038/ng.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin M, Chen W, Huang J, Gao H, Ye Y, Song Z, Shen X. MicroRNA expression profiles in human colorectal cancers with liver metastases. Oncol Rep. 2011;25:739–747. doi: 10.3892/or.2010.1112. [DOI] [PubMed] [Google Scholar]
- 62.Medimegh I, Troudi W, Stambouli N, Khodjet-El-Khil H, Baroudi O, Ayari H, Omrane I, Uhrhammer N, Privat M, Mezlini A, et al. Wild-type genotypes of BRCA1 gene SNPs combined with micro-RNA over-expression in mammary tissue leading to familial breast cancer with an increased risk of distant metastases' occurrence. Med Oncol. 2014;31:255. doi: 10.1007/s12032-014-0255-6. [DOI] [PubMed] [Google Scholar]
- 63.Xu X, Cai N, Zhi T, Bao Z, Wang D, Liu Y, Jiang K, Fan L, Ji J, Liu N. MicroRNA-1179 inhibits glioblastoma cell proliferation and cell cycle progression via directly targeting E2F transcription factor 5. Am J Cancer Res. 2017;7:1680–1692. [PMC free article] [PubMed] [Google Scholar]
- 64.Mancikova V, Castelblanco E, Pineiro-Yanez E, Perales-Paton J, de Cubas AA, Inglada-Perez L, Matias-Guiu X, Capel I, Bella M, Lerma E, et al. MicroRNA deep-sequencing reveals master regulators of follicular and papillary thyroid tumors. Mod Pathol. 2015;28:748–757. doi: 10.1038/modpathol.2015.44. [DOI] [PubMed] [Google Scholar]
- 65.Lin C, Hu Z, Yuan G, Su H, Zeng Y, Guo Z, Zhong F, Jiang K, He S. MicroRNA-1179 inhibits the proliferation, migration and invasion of human pancreatic cancer cells by targeting E2F5. Chem Biol Interact. 2018;291:65–71. doi: 10.1016/j.cbi.2018.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.