Abstract
Four peptides with cytotoxic activity against BRIN-BD11 rat clonal β-cells were purified from the venom of the black-necked spitting cobra Naja nigricollis using reversed-phase HPLC. The peptides were identified as members of the three-finger superfamily of snake toxins by ESI-MS/MS sequencing of tryptic peptides. The most potent peptide (cytotoxin-1N) showed strong cytotoxic activity against three human tumor-derived cell lines (LC50 = 0.8 ± 0.2 μM for A549 non-small cell lung adenocarcinoma cells; LC50 = 7 ± 1 μM for MDA-MB-231 breast adenocarcinoma cells; and LC50 = 9 ± 1 μM for HT-29 colorectal adenocarcinoma cells). However, all the peptides were to varying degrees cytotoxic against HUVEC human umbilical vein endothelial cells (LC50 in the range 2–22 μM) and cytotoxin-2N was moderately hemolytic (LC50 = 45 ± 3 μM against mouse erythrocytes). The lack of differential activity against cells derived from non-neoplastic tissue limits their potential for development into anti-cancer agents. In addition, two proteins in the venom, identified as isoforms of phospholipase A2, effectively stimulated insulin release from BRIN-BD11 cells (an approximately 6-fold increase in rate compared with 5.6 mM glucose alone) at a concentration (1 μM) that was not cytotoxic to the cells suggesting possible application in therapy for Type 2 diabetes.
Keywords: Naja, Cytotoxicity, Insulinotropic activity, Three-finger toxins, Phospholipase A2
Graphical abstract
Highlights
-
•
Four members of the three-finger superfamily of toxins were isolated from N. nigricollis venom.
-
•
All peptides were cytotoxic to human tumor-derived cells.
-
•
The peptides were also cytotoxic to non-neoplastic HUVEC cells.
-
•
Two isoforms of phospholipase A2 effectively stimulated insulin release from rat clonal β-cells.
Abbreviations
- KRB
Krebs-Ringer bicarbonate
- LDH
Lactate dehydrogenase
- PLA2
Phospholipase A2
- T2DM
Type 2 Diabetes mellitus
- TFA
Trifluoroacetic acid
- 3FTx
Three-finger toxin
1. Introduction
The toxic peptides and proteins present in snake venoms undoubtedly arose by natural selection in order that the organism could subjugate prey and as a component of its defence strategy against predators. At first sight, it appears paradoxical that snake venoms, estimated to be responsible for between 81,000 and 138,000 fatalities and around three times as many amputations and other permanent disabilities each year (W.H.O. Fact Sheet, 2019), should represent an important source of compounds with therapeutic potential. These include peptides and proteins with broad-spectrum antibacterial (Charvat et al., 2018), antifungal (Cavalcante et al., 2017), antiparasitic (Allane et al., 2018) and antiviral (Chen et al., 2017) activities, as well as components whose anti-inflammatory (Sartim et al., 2018), anticoagulant (Khan et al., 2018), wound healing (Thakur et al., 2019), anti-hypertensive (Almeida et al., 2017) and analgesic (Brzezicki and Zakowicz, 2018) properties have potential clinical relevance. Such compounds may show greater potency and increased stability compared with peptides derived from mammals. Recent advances in methodology, permitting purification and structural characterization of these components from small quantities of material (Calvete, 2018) has meant that snake venoms are becoming increasingly important as a source of natural products with the potential for development into therapeutically valuable drugs (Estevão-Costa et al., 2018, Lazarovici, 2020).
There is a constant need for new types of anti-cancer agents particularly in cases where the tumor is not responsive to conventional pharmaceutical intervention due to the development of drug resistance (Lord and Ashworth, 2013). The presence in venoms of peptides and proteins with potent in vitro cytotoxic activity against tumor cells has been described for a wide range of snake species, particularly those belonging to the Viperidae and Elapidae families [reviewed in (Vyas et al., 2013, Uzair et al., 2018, Zainal Abidin et al., 2019)]. However, to describe such peptides as “anti-cancer” or even “anti-tumor” is premature as no snake venom peptide has been shown to produce reduced tumor mass in clinical trials involving human subjects and there have been relatively few in vivo studies in animal models of cancer. In addition, although a particular peptide may show some selectivity for tumor-derived cells compared with non-neoplastic cells, the difference in cytotoxic potency is often insufficient to suggest the possibility of therapeutic application.
The prevalence of type 2 diabetes mellitus (T2DM) is increasing worldwide and there is clearly a need for new effective agents that improve glucose tolerance and address the associated complications of the disease (Bailey, 2018). Long-standing T2DM is associated with a combination of insulin resistance and defective β-cell function and several snake venom-derived components have demonstrated in vitro insulinotropic activities. Examples include cardiotoxin-I from Naja kaouthia venom (Nguyen et al., 2014), a [Lys49]phospholipase A2 isoform from Bothrops jararacussu venom (Fagundes et al., 2011) and phospholipases A2 and disintegrins from the venoms of Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis (Moore et al., 2015).
The black-necked spitting cobra Naja nigricollis (Hallowell, 1857) (Elapidae) is widely distributed and common in sub-Saharan Africa. The species usually inhabits savanna and semi-desert regions at altitudes up to 1800 m but has demonstrated an ability to adapt to life in farmland regions. Although the mortality rate in untreated cases of snakebite is relatively low (5–10%), the species can project venom, which is a powerful irritant to the eyes, with remarkable accuracy. The resulting ophthalmia can result in permanent blindness (Goldman and Seefeld, 2010).
The aim of the present study was to analyse venom samples from N. nigricollis collected in Nigeria for the presence of components with potential for development into anticancer agents by determining their cytotoxic activities against A549 human non-small cell lung adenocarcinoma cells, MDA-MB-231 human breast adenocarcinoma cells, and HT-29 human colorectal adenocarcinoma cells. Their activities against tumor cells was compared with cytotoxic activity against HUVEC human umbilical vein endothelial cells. In addition, the presence of components in the venom with potential for development in agents for use in T2DM therapy was investigated by determining their abilities to stimulate the release of insulin in vitro using BRIN-BD11 rat clonal β-cells.
2. Materials and methods
2.1. Cytotoxicity assays
The presence of cytotoxic peptides in chromatographic effluent was monitored by incubation of lyophilized aliquots (20 μL) with BRIN-BD11 clonal β-cells (McClenaghan et al., 1996) for 20 min at 37 °C in Krebs-Ringer bicarbonate (KRB) buffer supplemented with 5.6 mM glucose. The rate of lactate dehydrogenase (LDH) release was determined using a CytoTox 96 non-radioactive cytotoxicity assay kit (Promega, Southampton, UK) according to the manufacturer's instructions as previously described (Owolabi et al., 2016).
A549 human non-small cell lung adenocarcinoma cells were maintained at 37 °C in RPMI 1640 medium containing 2 mM L-glutamine and supplemented with 10% fetal calf serum (FCS, Biowest, Nouaille, France), and antibiotics (penicillin 50 U/mL; streptomycin 50 μg/mL) (Attoub et al., 2013). MDA-MB-231 human breast adenocarcinoma cells and HT-29 human colorectal adenocarcinoma cells were maintained in Dulbecco's Modified Eagle's Medium supplemented with antibiotics (penicillin 50U/mL; streptomycin 50 μg/mL) and 10% FCS (Attoub et al., 2013). EndoGRO human umbilical vein endothelial cells (HUVECs) were maintained in EndoGRO MV-VEGF Complete Media Kit (Millipore, Temecula, CA, USA) (Conlon et al., 2013). In all experiments, cell viability was higher than 99% using trypan blue dye exclusion. Cells were seeded in 96-well plates at a density of 5 × 103 cells/well. After 24 h incubation, cells were treated for 24 h with increasing concentrations of the purified toxins (0.3–30 μM) in triplicate. The effect of the peptides on cell viability was determined by measurement of ATP concentrations using a CellTiter-Glo Luminescent Cell Viability assay (Promega Corporation, Madison, WI, USA). Luminescent signals were measured using a GLOMAX Luminometer system. The LC50 value, calculated by non-linear regression analysis using commercially available software (GraphPad Prism version 5), was taken as the mean concentration of peptide producing 50% cell death in three independent experiments.
In order to determine hemolytic activity, peptides in the concentration range 11–90 μM were incubated in triplicate with washed erythrocytes (2 × 107 cells) from NIH Swiss mice in KRB buffer pH 7.4 (100 μL) for 1 h at 37 °C. After centrifugation (12,000×g for 15 s), the absorbance at 450 nm of the supernatant was measured. A parallel incubation in the presence of 1% v/v Triton-X100 was carried out to determine the absorbance associated with 100% hemolysis. The LC50 value was taken as the mean concentration of peptide producing 50% hemolysis in two independent experiments.
2.2. In vitro insulin release studies using BRIN-BD11 cells
The procedure for studying the effects of peptides on the release of insulin from BRIN-BD11 rat clonal β-cells has been described in detail previously (Owolabi et al., 2016). Incubations with fractions of chromatographic effluent and with N. nigricollis phospholipase A2 (PLA2) isoforms (1 μM; n = 4) were carried out for 20 min at 37 °C using KRB buffer supplemented with 5.6 mM glucose. After incubation, aliquots of cell supernatant were removed for insulin radioimmunoassay (Flatt and Bailey, 1981). Incubations (n = 4) of BRIN-BD11 cells with the established insulin secretagogue, alanine (10 mM) were carried out in parallel.
2.3. Purification of the cytotoxic peptides
A pooled sample of venom was prepared from adult specimens of N. nigricollis from Nigeria that were housed at the Liverpool School of Tropical Medicine. In the first chromatography, the lyophilized venom sample (550 μg) was redissolved in 0.1% (v/v) trifluoroacetic acid (TFA)/water (2 mL) and injected onto a (1.0 cm × 25 cm) Vydac 218TP510 (C-18) reversed-phase HPLC column (Grace, Deerfield, IL, USA) equilibrated with 0.1% (v/v) TFA/water at a flow rate of 2.0 mL/min. The concentration of acetonitrile in the eluting solvent was raised to 21% (v/v) over 10 min and to 63% (v/v) over 60 min using linear gradients. Absorbance was monitored at 214 nm and fractions (1 min) were collected. Freeze-dried aliquots (20 μL) of the fractions were reconstituted in KRB buffer (100 μL) and their abilities to produce cytolysis of BRIN-BD11 cells were determined as described in the cytotoxicity assays section. Fractions containing peptides with cytotoxic activity were successively chromatographed on a (1 cm × 25 cm) Vydac 214TP510 (C-4) column and a (1 cm × 25 cm) Vydac 208TP510 (C-8) column at a flow rate of 2.0 mL/min. The concentration of acetonitrile in the eluting solvent was raised from 21% to 56% over 50 min using a linear gradient. The purified peptides were analysed by MALDI-ToF mass spectrometry using a Bruker UltraFlextreme instrument as previously described (Conlon et al., 2018b).
In the second preparative chromatography, the venom sample (20 mg) was redissolved in 0.1% (v/v) TFA/water (4 mL) and the total amount injected onto a (2.2 cm × 25 cm) Vydac 218TP1022 (C-18) reversed-phase HPLC column equilibrated with 0.1% (v/v) TFA/water at a flow rate of 6.0 mL/min. The concentration of acetonitrile in the eluting solvent was raised to 21% (v/v) over 10 min and to 63% (v/v) over 60 min using linear gradients. The major peaks in the chromatogram were collected by hand and peptides/proteins were purified to near homogeneity (>98% purity) on semi-preparative Vydac C-4 and C-8 columns as described above.
2.4. Proteomic analysis of the purified toxins
The molecular masses of the purified components were determined by nano-Acquity Ultra Performance LC (Waters Corporation, Milford, MA, USA) using a BEH130 C-18 (100 μm × 100 mm, 1.7 μm particle size) column in-line with a Waters SYNAPT G2 High Definition Mass Spectrometry System. The flow rate was set to 0.6 μL/min and the column was developed with a linear gradient of 0.1% formic acid in water (solution A) and 0.1% formic acid in acetonitrile (solution B), isocratically 1% B for 1 min, followed by 1–12% B for 1min, 12–40% B for 15min, 40–85% B for 2 min. Isotope-averaged molecular masses were calculated by manual deconvolution of the isotope-resolved multiply-charged MS1 mass spectra.
The purified cytotoxic peptides were initially analysed by SDS-PAGE on 15% polyacrylamide gels under reducing and non-reducing conditions and the protein bands were excised from Coomassie Brilliant Blue-stained gels and subjected to automated reduction, alkylation, and in-gel digestion with sequencing grade porcine pancreatic trypsin using a Progest™ digestor (Genomic Solutions, Ann Arbor, MI, USA). Tryptic digests were dried in a SpeedVac vacuum centrifuge, redissolved in 14 μL of 5% acetonitrile containing 0.1% formic acid and submitted to LC-MS/MS. Tryptic peptides were separated by nano-Acquity Ultra Performance LC as described above. Doubly and triply charged ions were selected for collision-induce dissociation (CID)-MS/MS. Fragmentation spectra were interpreted (a) manually (de novo sequencing), (b) using the on-line form of the MASCOT Server (version 2.6) at http://www.matrixscience.com against the last update (Release 234 of October 15th, 2019) of the NCBI non-redundant database, and (c) processed in the Waters Corporation's ProteinLynx Global SERVER 2013 version 2.5.2. (with Expression version 2.0). The following search parameters were used: Taxonomy: bony vertebrates; Enzyme: trypsin (two missed cleavage allowed); MS/MS mass tolerance was set to ± 0.6 Da; carbamidomethyl cysteine and oxidation of methionine were selected as fixed and variable modifications, respectively. All matched MS/MS data were manually checked. Peptide sequences assigned by de novo MS/MS were matched to homologous proteins available in the NCBI non-redundant protein sequences database using the online BLASTP program at https://blast.ncbi.nlm.nih.gov/Blast.cgi.
3. Results
3.1. Purification of the cytotoxic and insulin-releasing peptides
The elution profile on a semi-preparative Vydac C-18 column of a small amount (550 μg) of the venom from N. nigricollis is shown in Fig. 1A. Under the conditions of assay, the peaks designated 1, 4, and 7 contained components that displayed strong cytotoxic activity (>98% cell death) against BRIN-BD11 cells during a 20 min incubation and against A549 cells during a 24 h incubation. The peaks designated 1–5 and 7 contained components that stimulated the rate of insulin release from BRIN-BD11 cells. The peptide present in peak 6 displayed weak cytotoxic activity against BRIN-BD11 and A549 cells and did not stimulate insulin release from BRIN-BD11 cells.
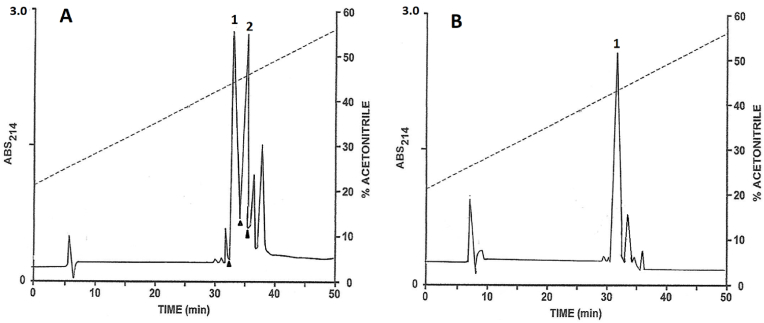
Fig. 1.
Reversed-phase HPLC on (A) a semi-preparative Vydac C-18 column of venom (550 μg dry weight) and (B) a preparative Vydac C-18 column of venom (20 mg dry weight) from N. nigricollis. In panel A, the peaks designated 1 and 4–7 contained components that displayed strong cytotoxic activity against BRIN-BD11 clonal β-cells. The peaks designated 1–5 and 7 contained components that stimulated the rate of insulin release from BRIN-BD11 cells. In panel B, material in peaks 1, 2 + 3, 4 + 5, 6 and 7 were purified to near homogeneity by further chromatography on Vydac C-4 and C-8 columns. The dashed line shows the concentration of acetonitrile in the eluting solvent.
The fractions were analysed by MALDI-ToF mass spectrometry and the molecular masses of the proteins present in peaks 8 and 9 were >20,000 Da and these components did not display cytotoxicity against A549 cells and BRIN-BD11 cells and did not stimulate insulin release. A comparison of the HPLC chromatogram shown in Fig. 1A with those from a range of species belonging to the genus Naja (Petras et al., 2011) suggests that these proteins belong to the snake venom metalloproteinase (SVMP) family. Purification to near homogeneity, as assessed by a symmetrical peak shape and mass spectrometry, of the components in peaks 1–4, 6 and 7 was accomplished by chromatography on a semi-preparative Vydac C-4 and C-8 columns. The material in peak 5 was too heterogenous to permit isolation of individual components.
In order to obtain sufficient pure material for assessment of biological activity, a larger quantity (20.0 mg) of venom was chromatographed on a preparative Vydac C-18 column (Fig. 1B). The peak designated 2 + 3 contained both phospholipase A2 -1N and phospholipase A2-2N and peak designate 4 + 5 contained cytotoxin-2N and several uncharacterized components. The components were purified to near homogeneity on Vydac C-4 and C-8 columns as previously described. The methodology is illustrated by the separation of the two isoforms of phospholipase A2 on a semipreparative Vydac C-4 column (Fig. 2A) and purification to near homogeneity, as assessed by symmetrical peak shape and mass spectrometry, of phospholipase A2-1N on a semipreparative Vydac C-8 column (Fig. 2B). The yields of the purified components, determined by dry weight using an ultramicrobalance, were peak 1 peptide (subsequently shown to be cytotoxin-1N) 1020 μg, peak 2 protein (phospholipase A2-1N) 480 μg, peak 3 protein (phospholipase A2-2N) 680 μg, peak 4 peptide (cytotoxin-2N) 525 μg, peak 6 peptide (cytotoxin-3N) 270 μg, and peak 7 peptide (cytotoxin-4N) 1635 μg. The molecular masses of the purified peptides determined by electrospray ionization (ESI) mass spectrometry are shown in Supplementary Figs. 1A–F.
Fig. 2.
Partial separation of the phospholipase A2-1 N (peak 1) and phospholipase A2-2 N (peak 2) (derived from peak 2 + 3 in Fig. 1B) on a semipreparative Vydac C-4 column (Panel A), and purification to near homogeneity of phospholipase A2-1 N (peak 1) on a semipreparative Vydac C-8 column (Panel B). The arrowheads show where peak collection began and ended. The dashed line shows the concentration of acetonitrile in the eluting solvent.
3.2. Identification of the cytotoxic peptides
The identities of the peptides were determined by CID-MS/MS mass spectrometric analysis of fragments generated by in-gel tryptic digestion. The primary structures of these fragments are shown aligned to the best database hit in Fig. 3. Peak 1 peptide (Fig. 1A), designated cytotoxin-1N (average native molecular mass [M]av = 6743.6 Da) was identified as a three-finger toxin (3FTx) by sequence similarity with cytotoxin-4 (also known as cardiotoxin V(II)4) from the Mozambique spitting cobra Naja mossambica (UniProtKB: P01452). Peak 4 peptide (Fig. 1A), designated cytotoxin-2N, ([M]av = 6887.3 Da) was identified as a 3FTx by sequence similarity with the anticoagulant peptide naniproin (PODSN1) from N. nigricollis. Peak 7 peptide (Fig. 1A) ([M]av=6819.1 Da) was identified as a 3FTx toxin by sequence similarity with cytotoxin-1 (also known as cardiotoxin IIB) from N. mossambica (P01467). The average molecular mass of cytotoxin-3N was 6686.6 Da. A peptide with this molecular mass and similar retention time on HPLC was previously detected in N. nigricollis venom collected in Nigeria and was identified as a 3FTx family member on the basis of N-terminal sequence similarity (Petras et al., 2011). ESI-MS/MS sequencing of two tryptic peptides confirmed the assignment of cytotoxin-3N to the ortholog 3FTx of N. mossambica cytotoxin-5 (P25517) (Fig. 3).
Fig. 3.
Identification of the cytotoxins from N. nigricollis venom by comparison of the primary structures of their tryptic peptides (shown in red) with the structures of corresponding regions of known toxins. The sequences of cytotoxins-1, -4, and -5 and PLA2-2 N are from N. mossambica and the sequences of naniproin and PLA2-1 N are from N. nigricollis.
3.3. Identification of the insulin-releasing peptides
The protein in peak 2 (Fig. 1A), designated PLA2-1N ([M]av = 13,221.6 Da) was identified as belonging to the [Asp]49phospholipase A2 family by sequence similarity with phospholipase A2 isozyme III from N. nigricollis (P00605). The protein in peak 3 (Fig. 1A), designated PLA2-2N ([M]av = 13,289.0 Da) was identified as belonging to the [Asp]49 PLA2 family by sequence similarity with basic PLA2 CM-II from N. mossambica (P00603) (Fig. 3).
3.4. Cytotoxic activities of the peptides
The effects of increasing concentrations of the purified three-finger toxins on the viability of A549 human non-small cell lung adenocarcinoma cells, MDA-MB-231 breast adenocarcinoma cells, HT-29 colorectal adenocarcinoma cells, and HUVEC human umbilical vein endothelial cells are shown in Fig. 4. The LC50 values are shown in Table 1. Consistent with previous data relating to 3FTxs from the Eastern green mamba Dendroaspis angusticeps (Elapidae) (Conlon et al., 2014), A549 cells were the most sensitive to the cytotoxic action of the peptides and HT-29 cells were the most resistant. Cytotoxin-2N was the only peptide tested with appreciable hemolytic activity against mouse erythrocytes (LC50 = 45 ± 3 μM) (Table 1).
Fig. 4.
Effects of cytotoxin-1N, cytotoxin-2N, cytotoxin-3N and cytotoxin-4N from N. nigricollis venom on the viability of A549 non-small cell lung adenocarcinoma cells; MDA-MB-231 breast adenocarcinoma cells; HT-29 colorectal adenocarcinoma cells; and HUVEC umbilical vein endothelial cells All experiments were repeated at least three times. Columns: mean; bars: SEM.
Table 1.
Cytotoxicities of cytotoxins from N. nigricollis venom against human non-small cell lung adenocarcinoma A549 cells, breast adenocarcinoma MDA-MB-231 cells, colorectal adenocarcinoma HT-29 cells, human umbilical vein endothelial HUVEC cells and mouse red blood cells (RBC).
| Peptide | A549 | MDA-MB-231 | HT-29 | HUVEC | RBC |
|---|---|---|---|---|---|
| Cytotoxin-1N | 0.8 ± 0.2 | 7 ± 1 | 9 ± 1 | 7 ± 1 | >90 (20) |
| Cytotoxin-2N | 1.4 ± 0.2 | 6 ± 1 | 8 ± 1 | 7 ± 1 | 45 ± 3 |
| Cytotoxin-3N | 7 ± 1 | >30 | >30 | 22 ± 2 | ND |
| Cytotoxin-4N | 0.9 ± 0.2 | 8 ± 1 | 25 ± 3 | 2 ± 0.2 | >90 (12) |
Data show LC50 values (μM) ± S.E.M. ND: not determined. The values in parentheses show the % hemolysis at 90 μM.
3.5. Insulin-releasing activity of the peptides
Under the conditions of assay, chromatographic fractions containing cytotoxins-1, -2, and -4 stimulated insulin release from BRIN-BD11 clonal β-cells. However, at the same concentration, the toxins also markedly stimulated release of the cytosolic enzyme LDH indicating that the integrity of the plasma membrane had been compromised (data not shown). In contrast, fractions containing PLA2-1N and PLA2-2N stimulated insulin release from BRIN-BD11 cells at concentrations that did not result in increased release of LDH. There was insufficient pure material to investigate concentration-response effects in detail but incubation with PLA2-1N(1 μM) with BRIN-BD11 cells produced an increase in the rate of insulin-release from 1.1 ± 0.1 ng/106 cells/20 min in the presence of 5.6 mM glucose alone to 6.9 ± 0.4 ng/106 cells/20 min. The corresponding rate of insulin-release produced by incubation with PLA2-2N (1 μM) was 6.5 ± 0.4 ng/106 cells/20 min. At this concentration, there was no significant increase in the rate of LDH release. Parallel incubations with 10 mM alanine produced an increase in rate of insulin release to 6.6 ± 0.5 ng/106 cells/20 min.
4. Discussion
The venom of the spitting cobra N. nigricollis is a repository of several structurally well characterized peptides and proteins whose diverse biological activities have been investigated in detail. These include the membrane-damaging cardiotoxin, toxin γ (Kao et al., 2009), toxin α, a neurotoxin that specifically blocks the activity of the nicotinic acetylcholine receptor (Zinn-Justin et al., 1992), nawaprin, a peptide that is structurally, but not functionally, related to the human leukocyte elastase-specific inhibitor, elafin (Torres et al., 2003), the basic and strongly anticoagulant phospholipase A2 CM-IV, and the weakly anticoagulant phospholipases A2 CM-1 and CM-II (Kini, 2005). The aim of the present study was to examine N. nigricollis venom for the presence of components with therapeutic potential for development of anti-cancer and anti-diabetic agents.
The study has led to the purification of four peptides in whose partial structures, obtained by MS/MS analysis of tryptic fragments, identify them as members of the widely distributed 3FTx superfamily (Kini and Doley, 2010, Utkin, 2013). Peptides in this family are characterized by three β-stranded loops emerging from a globular core that are stabilized by four or five disulfide bridges. 3FTx peptides are not confined to the venoms of elapids (mambas, cobras, and kraits) but have also been identified the venoms of colubrids, hydrophiids and vipers and more than 500 members have been described (Utkin, 2013). Despite the overall similarity in conformation, the biological properties of 3FTx peptides vary greatly. They may function as postsynaptic neurotoxins targeting the nicotinic and muscarinic acetylcholine receptors, β-blockers targeting β1-and β2-adrenergic receptors, antagonists of α1A and α2A adrenergic receptors, blockers of L-type calcium channels, as well as cardiotoxins targeting phospholipid membranes and anticoagulants targeting various coagulation complexes [reviewed in (Girish et al., 2012)].
The 3FTx peptides cytotoxin-1N, -2N, and --4N isolated from N. nigricollis venom display potent and concentration-dependent cytotoxic activity against three diverse human tumor-derived cell lines but their potential for development into therapeutically valuable anti-cancer agents is low because of their strong cytotoxicity against a cell line derived from non-neoplastic tissue. In addition, cytotoxin-2N was appreciably hemolytic. These peptides also stimulated insulin release from a rat clonal β-cell line but only a concentration that also stimulated release of the cytosolic enzyme LDH. It is concluded, therefore, the insulinotropic activity of these peptides is, at least in part, a non-specific consequence of cell necrosis.
Like 3FTx peptides, members of the PLA2 (EC 3.1.1.4) enzyme family are widely distributed in venoms from Elapidae, Hydrophidae, and Viperidae species and exhibit a diverse range of biological activities. With very few exceptions, PLA2 proteins in the venom of Naja spp. contain an Asp residue at position 49 and, as well as catalyzing the hydrolysis of the sn-2 ester bond in a variety of different phospholipids, have been shown to exhibit antimicrobial, neurotoxic, myotoxic, anticoagulant, prostaglandin-mediated hypotensive, inflammatory, and antiangiogenic properties [reviewed in (Xiao et al., 2017, Trento et al., 2019)]. The venoms of species of the Viperidae family may also contain multiple molecular forms of [Lys49]PLA2 that are enzymatically inactive and [Ser49]PLA2 that are weakly esterolytic. These components frequently display high cytotoxic potency against a range of mammalian cell types, including tumor cells (Sobrinho et al., 2016) and make an important contribution to the local tissue necrosis and damage to the vasculature observed at the site of the snakebite (Conlon et al., 2013, Gutiérrez et al., 2018).
Both isoforms of [Asp49]PLA2 isolated from the venom of N. nigricollis effectively stimulate insulin release from BRIN-BD11 rat clonal β-cells (an approximately 6-fold increase in rate at a concentration of 1 μM). At this concentration, the proteins did not stimulate LDH release indicating that they are not cytotoxic to the cells. The early stages of T2DM may be associated with hypersecretion of insulin to overcome insulin resistance but, as the disease progresses, impaired β-cell secretion becomes apparent. Several cytotoxic peptides present in norepinephrine-stimulated frog skin secretions that were first identified on the basis of their antimicrobial actions have been shown to stimulate insulin release in vitro from BRIN-BD11 cells and isolated mouse islets and in animal models of T2DM [reviewed in (Conlon et al., 2018a)]. In addition, certain frog skin peptides belonging to the esculentin-1a (Musale et al., 2018a) and temporin (Musale et al., 2018b) families protect BRIN-BD11 cells against cytokine-induced apoptosis and augment proliferation of the cells. The strong insulinotropic activity of PLA2-1 N and PLA2-2 N suggests that they may represent templates for development in agents with a role in the treatment of patients with T2DM. With the availability of more purified material, future studies will investigate whether the proteins may also function as β-cell proliferative and protective agents in vitro and as antihyperglycaemic agents in a rodent model of T2DM such as mice with diet-induced obesity, glucose-intolerance, and insulin resistance (O'Harte et al., 2016).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
All procedures involving animals were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63EU for animal experiments and approved by Ulster University Animal Ethics Review Committee.
CRediT authorship contribution statement
J.M. Conlon: Conceptualization, Investigation, Methodology, Formal analysis, Project administration, Writing - original draft, Writing - review & editing. Samir Attoub: Investigation, Methodology, Data curation, Formal analysis. Vishal Musale: Investigation, Data curation, Formal analysis. Jérôme Leprince: Investigation, Data curation, Formal analysis. Nicholas R. Casewell: Conceptualization, Resources. Libia Sanz: Investigation, Data curation, Formal analysis. Juan J. Calvete: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Acknowledgements
This work was partly supported by the Ministerio de Ciencia, Innovación y Universidades, Madrid, Spain (grant number BFU 2017-89103-P) to JJC. The authors thank Kholoud Arafat, U.A.E. University and Gervonne Barran, University of the West Indies for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2020.100030.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Allane D., Oussedik-Oumehdi H., Harrat Z., Seve M., Laraba-Djebari F. Isolation and characterization of an anti-leishmanial disintegrin from Cerastes cerastes venom. J. Biochem. Mol. Toxicol. 2018;32 doi: 10.1002/jbt.22018. https://doi: 10.1002/jbt.22018 [DOI] [PubMed] [Google Scholar]
- Almeida J.R., Resende L.M., Watanabe R.K., Carregari V.C., Huancahuire-Vega S., da S Caldeira C.A., Coutinho-Neto A., Soares A.M., Vale N., de C Gomes P.A., Marangoni S., de A Calderon L., Da Silva S.L. Snake venom peptides and low mass proteins: molecular tools and therapeutic agents. Curr. Med. Chem. 2017;24:3254–3282. doi: 10.2174/0929867323666161028155611. https://doi: 10.2174/0929867323666161028155611 [DOI] [PubMed] [Google Scholar]
- Attoub S., Arafat H., Mechkarska M., Conlon J.M. Anti-tumor activities of the host-defense peptide hymenochirin-1B. Regul. Pept. 2013;187:51–56. doi: 10.1016/j.regpep.2013.10.006. https://doi: 10.1016/j.regpep.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Bailey C.J. Glucose-lowering therapies in type 2 diabetes: opportunities and challenges for peptides. Peptides. 2018;100:9–17. doi: 10.1016/j.peptides.2017.11.012. https://doi: 10.1016/j.peptides.2017.11.012 [DOI] [PubMed] [Google Scholar]
- Brzezicki M.A., Zakowicz P.T. Mambalgins, the venom-origin peptides as a potentially novel group of analgesics: mini review. CNS Neurol. Disord. - Drug Targets. 2018;17:87–97. doi: 10.2174/1871527317666171221110419. https://doi: 10.2174/1871527317666171221110419 [DOI] [PubMed] [Google Scholar]
- Calvete J.J. Snake venomics - from low-resolution toxin-pattern recognition to toxin-resolved venom proteomes with absolute quantification. Expert Rev. Proteomics. 2018;15:555–568. doi: 10.1080/14789450.2018.1500904. https://doi: 10.1080/14789450.2018.1500904 [DOI] [PubMed] [Google Scholar]
- Cavalcante C.S., Falcão C.B., Fontenelle R.O., Andreu D., Rádis-Baptista G. Anti-fungal activity of Ctn[15-34], the C-terminal peptide fragment of crotalicidin, a rattlesnake venom gland cathelicidin. J. Antibiot. (Tokyo) 2017;70:231–237. doi: 10.1038/ja.2016.135. https://doi: 10.1038/ja.2016.135 [DOI] [PubMed] [Google Scholar]
- Charvat R.A., Strobel R.M., Pasternak M.A., Klass S.M., Rheubert J.L. Analysis of snake venom composition and antimicrobial activity. Toxicon. 2018;50:151–167. doi: 10.1016/j.toxicon.2018.05.016. https://doi: 10.1016/j.toxicon.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Chen M., Aoki-Utsubo C., Kameoka M., Deng L., Terada Y., Kamitani W., Sato K., Koyanagi Y., Hijikata M., Shindo K., Noda T., Kohara M., Hotta H. Broad-spectrum antiviral agents: secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16130-w. https://doi: 10.1038/s41598-017-16130-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J.M., Attoub S., Arafat H., Mechkarska M., Casewell N.R., Harrison R.A., Calvete J.J. Cytotoxic activities of [Ser⁴⁹]phospholipase A₂ from the venom of the saw-scaled vipers Echis ocellatus, Echis pyramidum leakeyi, Echis carinatus sochureki, and Echis coloratus. Toxicon. 2013;71:96–104. doi: 10.1016/j.toxicon.2013.05.017. https://doi: 10.1016/j.toxicon.2013.05.017 [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Prajeep M., Mechkarska M., Arafat K., Attoub S., Adem A., Pla D., Calvete J.J. Peptides with in vitro anti-tumor activity from the venom of the Eastern green mamba, Dendroaspis angusticeps (Elapidae) J. Venom Res. 2014;5:16–21. PMID: 25035794. [PMC free article] [PubMed] [Google Scholar]
- Conlon J.M., Mechkarska M., Abdel-Wahab Y.H., Flatt P.R. Peptides from frog skin with potential for development into agents for Type 2 diabetes therapy. Peptides. 2018;100:275–281. doi: 10.1016/j.peptides.2017.09.001. https://doi: 10.1016/j.peptides.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Conlon J.M., Moffett R.C., Leprince J., Flatt P.R. Identification of components in frog skin secretions with therapeutic potential as antidiabetic agents. Methods Mol. Biol. 2018;1719:319–333. doi: 10.1007/978-1-4939-7537-2_21. https://doi: 10.1007/978-1-4939-7537-2_21 [DOI] [PubMed] [Google Scholar]
- Estevão-Costa M.I., Sanz-Soler R., Johanningmeier B., Eble J.A. Snake venom components in medicine: from the symbolic rod of Asclepius to tangible medical research and application. Int. J. Biochem. Cell Biol. 2018;104:94–113. doi: 10.1016/j.biocel.2018.09.011. https://doi: 10.1016/j.biocel.2018.09.011 [DOI] [PubMed] [Google Scholar]
- Fagundes F.H., Aparício R., dos Santos M.L., Diz Filho E.B., Oliveira S.C., Toyama D.O., Toyama M.H. A catalytically inactive Lys49 PLA2 isoform from Bothrops jararacussu venom that stimulates insulin secretion in pancreatic beta cells. Protein Pept. Lett. 2011;18:1133–1139. doi: 10.2174/092986611797200940. https://doi: 10.2174/092986611797200940 [DOI] [PubMed] [Google Scholar]
- Flatt P.R., Bailey C.J. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabete. 1981;20:573–577. doi: 10.1007/BF00252768. https://doi: 10.1007/bf00252768 [DOI] [PubMed] [Google Scholar]
- Girish V.M., Kumar S., Joseph L., Jobichen C., Kini R.M., Sivaraman J. Identification and structural characterization of a new three-finger toxin hemachatoxin from Hemachatus haemachatus venom. PloS One. 2012;7 doi: 10.1371/journal.pone.0048112. https://doi: 10.1371/journal.pone.0048112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D.R., Seefeld A.W. Ocular toxicity associated with indirect exposure to African spitting cobra venom. Wilderness Environ. Med. 2010;21:134–136. doi: 10.1016/j.wem.2009.12.007. https://doi: 10.1016/j.wem.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Escalante T., Hernández R., Gastaldello S., Saravia-Otten P., Rucavado A. Why is skeletal muscle regeneration impaired after myonecrosis induced by viperid snake venoms? Toxins. 2018;10 doi: 10.3390/toxins10050182. https://doi: 10.3390/toxins10050182 pii: E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P.H., Wu M.J., Chang L.S. Membrane-bound conformation of Naja nigricollis toxin gamma affects its membrane-damaging activity. Toxicon. 2009;53:342–348. doi: 10.1016/j.toxicon.2008.12.003. https://doi: 10.1016/j.toxicon.2008.12.003 [DOI] [PubMed] [Google Scholar]
- Khan S., Gul A., Noreen R., Ahmed S., Ashraf M., Awan M.S.B., Niazi Z.R., Khan N. Potential applications of venom peptides as anti-thrombotic agents for management of arterial and deep-vein thrombosis. Protein Pept. Lett. 2018;25:677–687. doi: 10.2174/0929866524666180614100101. https://doi: 10.2174/0929866524666180614100101 [DOI] [PubMed] [Google Scholar]
- Kini R.M. Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon. 2005;45:1147–1161. doi: 10.1016/j.toxicon.2005.02.018. https://doi: 10.1016/j.toxicon.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Kini R.M., Doley R. Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon. 2010;56:855–867. doi: 10.1016/j.toxicon.2010.07.010. https://doi: 10.1016/j.toxicon.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Lazarovici P. Snake- and spider-venom-derived toxins as lead compounds for drug development. Methods Mol. Biol. 2020;2068:3–26. doi: 10.1007/978-1-4939-9845-6_1. https://doi: 10.1007/978-1-4939-9845-6_1 [DOI] [PubMed] [Google Scholar]
- Lord C.J., Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 2013;9:1381–13888. doi: 10.1038/nm.3369. https://doi: 10.1038/nm.3369 [DOI] [PubMed] [Google Scholar]
- McClenaghan N.H., Barnett C.R., Ah-Sing E., Abdel-Wahab Y.H., O'Harte F.P., Yoon T.W., Swanston-Flatt S.K., Flatt P.R. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- Moore S.W., Bhat V.K., Flatt P.R., Gault V.A., McClean S. Isolation and characterisation of insulin-releasing compounds from Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis venom. Toxicon. 2015;101:48–54. doi: 10.1016/j.toxicon.2015.05.002. https://doi: 10.1016/j.toxicon.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Musale V., Abdel-Wahab Y.H.A., Flatt P.R., Conlon J.M., Mangoni M.L. Insulinotropic, glucose-lowering, and beta-cell anti-apoptotic actions of peptides related to esculentin-1a(1-21).NH2. Amino Acids. 2018;50:723–734. doi: 10.1007/s00726-018-2551-5. https://doi: 10.1007/s00726-018-2551-5 [DOI] [PubMed] [Google Scholar]
- Musale V., Casciaro B., Mangoni M.L., Abdel-Wahab Y.H.A., Flatt P.R., Conlon J.M. Assessment of the potential of temporin peptides from the frog Rana temporaria (Ranidae) as anti-diabetic agents. J. Pept. Sci. 2018;24 doi: 10.1002/psc.3065. https://doi: 10.1002/psc.3065 [DOI] [PubMed] [Google Scholar]
- Nguyen T.T., Folch B., Létourneau M., Truong N.H., Doucet N., Fournier A., Chatenet D. Design of a truncated cardiotoxin-I analogue with potent insulinotropic activity. J. Med. Chem. 2014;57:2623–2633. doi: 10.1021/jm401904q. https://doi: 10.1021/jm401904q [DOI] [PubMed] [Google Scholar]
- O'Harte F.P.M., Ng M.T., Lynch A.M., Conlon J.M., Flatt P.R. Dogfish glucagon analogues counter hyperglycaemia and enhance both insulin secretion and action in diet-induced obese diabetic mice. Diabetes Obes. Metabol. 2016;18:1013–1024. doi: 10.1111/dom.12713. https://doi: 10.1111/dom.12713 [DOI] [PubMed] [Google Scholar]
- Owolabi B.O., Ojo O.O., Srinivasan D.K., Conlon J.M., Flatt P.R., Abdel-Wahab Y.H. In vitro and in vivo insulinotropic properties of the multifunctional frog skin peptide hymenochirin-1B: a structure-activity study. Amino Acids. 2016;48:535–547. doi: 10.1007/s00726-015-2107-x. https://doi: 10.1007/s00726-015-2107-x [DOI] [PubMed] [Google Scholar]
- Petras D., Sanz L., Segura A., Herrera M., Villalta M., Solano D., Vargas M., León G., Warrell D.A., Theakston R.D., Harrison R.A., Durfa N., Nasidi A., Gutiérrez J.M., Calvete J.J. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011;10:1266–1280. doi: 10.1021/pr101040f. https://doi: 10.1021/pr101040f [DOI] [PubMed] [Google Scholar]
- Sartim M.A., Menaldo D.L., Sampaio S.V. Immunotherapeutic potential of Crotoxin: anti-inflammatory and immunosuppressive properties. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018;17(24):39. doi: 10.1186/s40409-018-0178-3. https://doi: 10.1186/s40409-018-0178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho J.C., Simões-Silva R., Holanda R.J., Alfonso J., Gomez A.F., Zanchi F.B., Moreira-Dill L.S., Grabner A.N., Zuliani J.P., Calderon L.A., Soares A.M. Antitumoral potential of snake venom phospholipases A2 and synthetic peptides. Curr. Pharmaceut. Biotechnol. 2016;7:1201–1212. doi: 10.2174/1389201017666160808154250. https://doi: 10.2174/1389201017666160808154250 [DOI] [PubMed] [Google Scholar]
- Thakur R., Chattopadhyay P., Mukherjee A.K. The wound healing potential of a pro-angiogenic peptide purified from Indian Russell's viper (Daboia russelii) venom. Toxicon. 2019;165:78–82. doi: 10.1016/j.toxicon.2019.04.009. https://doi: 10.1016/j.toxicon.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Torres A.M., Wong H.Y., Desai M., Moochhala S., Kuchel P.W., Kini R.M. Identification of a novel family of proteins in snake venoms. Purification and structural characterization of nawaprin from Naja nigricollis snake venom. J. Biol. Chem. 2003;278:40097–400104. doi: 10.1074/jbc.M305322200. https://doi: 10.1074/jbc.M305322200 [DOI] [PubMed] [Google Scholar]
- Trento M.V.C., Sales T.A., de Abreu T.S., Braga M.A., Cesar P.H.S., Marques T.R., Marcussi S. Exploring the structural and functional aspects of the phospholipase A2 from Naja spp. Int. J. Biol. Macromol. 2019;140:49–58. doi: 10.1016/j.ijbiomac.2019.08.125. https://doi: 10.1016/j.ijbiomac.2019.08.125 [DOI] [PubMed] [Google Scholar]
- Utkin Y.N. Three-finger toxins, a deadly weapon of elapid venom--milestones of discovery. Toxicon. 2013;62:50–55. doi: 10.1016/j.toxicon.2012.09.007. https://doi: 10.1016/j.toxicon.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Uzair B., Atlas N., Malik S.B., Jamil N., Ojuolape S.T., Rehman M.U., Khan B.A. Snake venom as an effective tool against colorectal cancer. Protein Pept. Lett. 2018;25:626–632. doi: 10.2174/0929866525666180614112935. https://doi: 10.2174/0929866525666180614112935 [DOI] [PubMed] [Google Scholar]
- Vyas V.K., Brahmbhatt K., Bhatt H., Parmar U. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pac. J. Trop. Biomed. 2013;3:156–162. doi: 10.1016/S2221-1691(13)60042-8. https://doi: 10.1016/S2221-1691(13)60042-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation Fact Sheet 2019 Snakebite Envenoming. Accessible at https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming.
- Xiao H., Pan H., Liao K., Yang M., Huang C. Snake venom PLA2, a promising target for broad-spectrum antivenom drug development. BioMed Res. Int. 2017;2017:6592820. doi: 10.1155/2017/6592820. https://doi: 10.1155/2017/6592820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal Abidin S.A., Lee Y.Q., Othman I., Naidu R. Malaysian cobra venom: a potential source of anti-cancer therapeutic agents. Toxins. 2019;11 doi: 10.3390/toxins11020075. https://doi: 10.3390/toxins11020075 pii: E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn-Justin S., Roumestand C., Gilquin B., Bontems F., Ménez A., Toma F. Three-dimensional solution structure of a curaremimetic toxin from Naja nigricollis venom: a proton NMR and molecular modelling study. Biochemistry. 1992;31:11335–11347. doi: 10.1021/bi00161a011. https://doi: 10.1021/bi00161a011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.