Abstract
Previous studies reported a dysregulation of micro (mi)R-208b-5p expression level in various types of human cancer; however, the role of miR-208-5p in non-small cell lung cancer (NSCLC) remains unclear. Therefore, the present study aimed to determine whether miR-208b-5p could regulate NSCLC progression. A total of 62 pairs of primary tumor and adjacent normal tissues were collected from patients with NSCLC. miR-208b-5p expression level was determined by reverse transcription-quantitative polymerase chain reaction. Furthermore, miR-208b-5p mimics was transfected into NSCLC A549 and H1299 cells in order to upregulate miR-208b-5p expression. Dual-luciferase reporter assay was utilized to investigate the associations between miR-208b-5p and IL9 mRNA. The results demonstrated that miR-208b-5p expression decreased in NSCLC tissues and cell lines. Furthermore, miR-208b-5p overexpression inhibited A549 and H1299 cell proliferation and invasiveness. miR-208b-5p was demonstrated to bind directly to the 3′ untranslated region of interleukin-9 (IL-9) and therefore decreased its expression. In the NSCLC-derived cell lines, miR-208b-5p inactivated IL-9/signal transducer and activator of transcription 3 (STAT3) signaling pathway. Furthermore, enhanced IL-9 level decreased the miR-208b-5p-mediated suppression of epithelial-mesenchymal transition in NSCLC cells by inactivating the STAT3 signaling pathway. In conclusion, the findings from this study demonstrated that miR-208b-5p inhibited migration and invasion of NSCLC cells. The anti-tumor activity of miR-208b-5p may be mediated by IL-9 and STAT-3 pathway.
Keywords: non-small cell lung cancer, miR-208b-5p, invasion, proliferation, interleukin-9
Introduction
Lung cancer is the most prevalent human cancer and remains the main cause of cancer-associated mortality, with a 5-year survival rate of 15% (1,2). In addition, non-small cell lung cancer (NSCLC) subtype accounts for ~80% of lung cancer cases and comprises adenocarcinoma and squamous cell carcinoma. Patients with NSCLC have a 5-year survival rate of <15%, which is due to numerous factors, including difficulties in early diagnosis, frequent relapse and absence of effective treatments for advanced cases (3). It is therefore crucial to determine the underlying mechanisms of NSCLC onset and progression and develop novel therapeutic strategies.
Interleukin-9 (IL-9) is a T helper (Th) 2 cytokine that contributes to allergic diseases, including asthma and rhinitis (4). A previous study demonstrated that IL-9 is involved in tumor immunity mediated by regulatory T cells (Tregs) and mast cells (5). Increasing evidence indicates that IL-9 participates in the pathogenesis of different types of cancer, such as lung cancer, breast cancer and gastric cancer, predominantly acting as a cancer promoting factor, particularly in nonsolid tumors (6–8). Signal transducer and activator of transcription 3 (STAT3) is a latent cytoplasmic transcription factor, originally discovered as a transducer of signals from cell surface receptors to the nucleus (9). Numerous evidences suggest that STAT3 is constitutively activated in various types of cancer and serve a crucial role in tumor growth and metastasis (9–12). In addition, STAT3 regulates cellular proliferation, invasion, migration and angiogenesis, which are essential for cancer metastasis (13). Epithelial-to-mesenchymal-transition (EMT) is a fundamental biological process in which epithelial cells undergo biochemical shifts to acquire mesenchymal properties (14). It is known that during EMT, epithelial cells gain a mesenchymal phenotype, resulting in increased invasion and metastasis in cancer (15). Consequently, epithelial markers, such as E-cadherin, are downregulated, while mesenchymal markers, such as vimentin and N-cadherin are upregulated (16). Accumulating evidence has elucidated the essential role of EMT in the progression of NSCLC (11–13).
MicroRNAs (miRs) are evolutionary conserved non-coding RNA molecules composed of 20–24 ribonucleotides, which bind to mRNA 3′ untranslated regions (3′-UTRs), activating their degradation or impairing the translation process (17–19). Numerous studies reported that dysregulated expression of certain miRNAs is associated with the development of esophageal (20,21), hepatocellular (22) and breast (23) carcinomas in humans. The miR-208 family includes miR-208a, miR-208b and miR-449 (24–26). Deep miRNA sequencing demonstrated that numerous members of the miR-208 family are involved in the onset and progression of cardiac diseases, such as cardiac ischemia reperfusion injury (24) and acute myocardial infarction (25).
In particular, it was reported that the expression level of −3p and −5p isoforms of miR-208a and miR-208b is highly expressed in cardiac tissue and is dysregulated in various cardiovascular diseases (26–30). Inhibition of miR-208 improves cardiac function and patient survival during heart failure (30,31). However, the expression and function of miR-208 in different types of cancer, particularly in NSCLC remain unknown.
The present study aimed to determine the function and underlying molecular mechanisms of miR-208b-5p in the progression of NSCLC.
Materials and methods
Tissue specimens
A total of 62 tumor samples were isolated from patients with NSCLC who underwent surgical lung resection at the Zhejiang Provincial Zhongshan Hospital between January 2011 and December 2013. Patients had not been treated by radiotherapy or chemotherapy prior to the surgery. Tumor tissues were collected from the edge of NSCLC lesions. Adjacent normal tissues were also collected 5 cm from the lesion. NSCLC and adjacent non-tumor tissues were confirmed by at least two expert pathologists. All patients with NSCLC received checkup every 2–3 months during the first two years and every 3–6 months afterwards until the end of the follow-up period in January 2015 by two physicians who were blinded to the study. Samples were snap-frozen after resection and maintained at −80°C prior to use. This study was approved by the Ethics Committee of The First Hospital of Longyan city, Fujian Medical University. All patients provided written informed consent prior to the study. The clinicopathological characteristics of the patients included in the present study are presented in Table I.
Table I.
Association between miR-208b-5p expression level and the clinicopathological characteristic of patients with non-small cell lung cancer.
| miR-208b-5p expression | ||||
|---|---|---|---|---|
| Characteristic | Patient, n | High n=30 | Low n=32 | P-value |
| Sex | 0.075 | |||
| Male | 51 | 22 | 29 | |
| Female | 11 | 8 | 3 | |
| Age, years | 0.553 | |||
| <60 | 39 | 20 | 19 | |
| ≥60 | 23 | 10 | 13 | |
| Smoking status | 0.851 | |||
| Previous/Current | 40 | 19 | 21 | |
| Never | 22 | 11 | 11 | |
| Pathological type | 0.779 | |||
| Adenocarcinoma | 28 | 13 | 15 | |
| Squamous carcinoma | 34 | 17 | 17 | |
| Tumor differentiation | 0.286 | |||
| Well and moderate | 49 | 22 | 27 | |
| Poor | 13 | 8 | 5 | |
| Tumor size, cm | 0.830 | |||
| <3 | 51 | 25 | 26 | |
| ≥3 | 11 | 5 | 6 | |
| Lymphatic metastasis | 0.014 | |||
| Yes | 22 | 6 | 16 | |
| No | 40 | 24 | 16 | |
| TNM classification | 0.021 | |||
| I–II | 22 | 15 | 7 | |
| III–IV | 40 | 15 | 25 | |
miR, microRNA; TNM, tumor-node-metastasis.
Cell lines and transfection
The eight human NSCLC cell lines H460, PC9, H1299, A549, H1703, H1437, CALU-1 and H520, the 293T cell line and the normal HBEC Beas-2b cell line were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and placed at 37°C in a humidified incubator containing 5% CO2.
miR-208b-5p mimics (miR-208b-5p) and control mimics (control) were purchased from Sigma-Aldrich; Merck KGaA. A549 and H1299 cells (1×105) were co-transfected with IL-9 cDNA plasmid (without the 3′UTR; Shanghai GenePharma Co., Ltd.), an empty vector (pcDNA-NC; Shanghai GenePharma Co., Ltd.) was used as the control for IL-9 overexpression experiments and miR-208b-5p mimics for 72 h. The primer sequences were as follows: miR-208b-5p mimic forward, 5′-GUGCAUUAUGGUUGCAUCCCA-3′ and reverse, 5′-CAAUGCAACUACAAUGCACUU-3′; and NC mimic forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′. miR-208b-5p mimic transfections were performed using Lipofectamine™ 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
C188-9 is a small-molecule inhibitor of STAT3 that targets the phosphotyrosyl peptide binding site within the STAT3 Src homology 2 (SH2) domain, with Ki=136 nm (9). It does not inhibit upstream Jak or Src kinases (9). In order to define the role of miR-208b-5p inhibition NSCLC cells EMT through IL-9/STAT3 signaling pathway, A549 and H1299 cells were pre-treated with C188-9 (0.9 µM; Selleck Chemicals) for 1 h (9) and subsequently transfected with miR-208b-5p mimics or control mimics for 48 h. Cells without C188-9 treatment were used as the control.
Reverse transcription-quantitative (RT-qPCR)
Total RNA was extracted from NSCLC cell lines and 62 NSCLC tissues using TRIzol™ (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was generated with the Mir-X™ miRNA First-Strand Synthesis kit (Takara Bio, Inc.) for miRNA expression level analysis or using PrimeScript RT Reagent Kit (Takara Bio, Inc.) for mRNA expression level analysis according to the manufacturers' instructions. RT-qPCR was performed using SYBR Premix Ex Taq II kit (Takara Bio, Inc.) on a ViiA 7 RT-PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) as described by the manufacturers. RT-qPCR reactions were performed as follows: 30 cycles of denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec and extension at 72°C for 30 sec. U6 or β-actin were used as an internal controls. The primers were designed by Shanghai Sangong Pharmaceutical Co., Ltd., and are as follows: miR-208b-5p forward, 5′-GTCGTATCCAGTGCGTGTCGTC-3′ and reverse, 5′-CACACTCTTGATGTTCCAGGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; IL-9 forward, 5′-CTCTGTTTGGGCATTCCCTCT-3′ and reverse, 5′-GGGTATCTTGTTTGCATGGTGG-3′; β-actin forward, 5′-TGACGTGGACATCCGCAAAG-3′ and reverse, 5′-CTGGAAGGTGGACAGCGAGG-3′; E-cadherin forward, 5′-TACACTGCCCAGGAGCCAGA-3′ and reverse, 5′-TGGCACCAGTGTCCGGATTA-3′; N-cadherin forward, 5′-TCAGGCGTCTGTAGAGGCTT-3′ and reverse, 5′-ATGCACATCCTTCGATAAGACTG-3′; and vimentin forward, 5′-GACGCCATCAACACCGAGTT-3′ and reverse, 5′-CTTTGTCGTTGGTTAGCTGGT-3′. The relative expressions levels were normalized to endogenous controls and were expressed as 2−ΔΔCq (32).
In vitro cell migration and invasion
NSCLC cell migration and invasion were investigated via Transwell assays. For the migration assay, 2000 cells in FBS-free medium were seeded in the upper compartment of the Transwell (pore size, 8 µm; Corning Inc.). For the invasion assay, the chamber was precoated with Matrigel (Corning Inc.) for 48 h at 37°C prior to cell seeding (2,000 cells in FBS-free medium). The lower chamber was filled with medium supplemented with 20% FBS. After 24 h incubation, non-migratory cells were swabbed and removed. Cells that have migrated were fixed with 4% paraformaldehyde for 30 min at 25°C followed by crystal violet staining for 30 min at 25°C. The values for invasion or migration were obtained by counting 10 randomly selected fields per membrane using a light microscope (magnification, ×40; Olympus Corporation) and represented the average of 3 independent experiments.
Western blotting
Proteins were extracted from A549 and H1299 NSCLC cells on ice using RIPA buffer (Beyotime Institute of Biotechnology) supplemented with protease inhibitors (Cell Signaling Technology, Inc.) for 20 min. Protein concentration was measured using Bradford Protein Assay kit (Beyotime Institute of Biotechnology). Proteins (15 µg) were separated via SDS-PAGE on a 10% gel and transferred onto polyvinylidene fluoride membranes. Membranes were blocked with 5% non-fat milk dissolved in TBST for 1 h at room temperature and incubated with primary antibodies against E-cadherin (cat. no. ab194982; 1:1,000), N-cadherin (cat. no. ab202030; 1:1,000), vimentin (cat. no. ab193555; 1:1,000), IL-9 (cat. no. ab203386; 1:1,000), STAT3 (cat. no. ab68153; 1:1,000), phospho-STAT3 (cat. no. ab76315; 1:1,000) and β-actin (cat. no. ab179467; 1:5,000), which were all purchased from Abcam, overnight at 4°C. Subsequently, membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (cat. no. ab150077; 1:10,000) for 1 h at room temperature. Enhanced chemiluminescence reagent (Beyotime Institute of Biotechnology) was used to detect the signal on the membrane. The data were analyzed via densitometry using Bio-Rad Image Lab software (version 4.1; Bio-Rad Laboratories, Inc.) and normalized to the expression of the internal control, β-actin.
Luciferase reporter assay
To perform luciferase reporter assay, a pMIR-REPORT vector (Promega Corporation) was constructed. The 3′-UTR fragments of IL-9 mRNA, containing miR-208b-5p candidate binding sites synthesized by Shanghai Shandong Biology Engineering Technology Service, Ltd., was PCR-amplified and inserted into the vectors, referred to as the pMIR-IL-9-WT vectors (Promega Corporation). Furthermore, pMIR-IL-9-Mut vectors (Promega Corporation) were built with IL-9 subjected to site-directed mutation of the miR-208b-5p binding sequence via a Quik-Change Site-directed Mutagenesis Kit (Agilent Technologies, Inc.). Assays were performed in 96-well plates with 1.5×104 293T cells per well. Cells were incubated at 37°C for 24 h, followed by miR-208b-5p or negative control co-transfection using Effectene reagent (Qiagen China Co., Ltd.), with pMIR-target-WT or pMIR-target-Mut vectors for 48 h. Subsequently, luciferase activity was analyzed using the Dual-Luciferase Reporter Assay System (Promega Corporation)
IL-9 ELISA
Following A549 and H1299 NSCLC cell transfection with miR-208b-5p mimic or mimic NC for 48 h, supernatant was collected and IL-9 level was measured by using the Human IL-9 ELISA Kit (cat. no. ab46027; Abcam) according to the manufacturers' instructions (detection limit, 0.5 pg/ml).
Statistical analysis
The data were presented as the means ± standard deviation. Significant differences were determined using GraphPad software version 5.0 (GraphPad Software, Inc.). Comparison between NSCLC and adjacent normal tissues was performed using paired Student's t-test. Student's t-test was used to determine differences between two groups. One-way analysis of variance, followed by Tukey's post-hoc test was used to compare differences between three or more groups. χ2 test was used to determine the association between miR-208b-5p expression level and the clinicopathological characteristics of patients with NSCLC. Survival curves were plotted using Kaplan-Meier method and were compared via log-rank test. P<0.05 was considered to indicate a statistically significant difference. Each experiment was repeated three times.
Results
miR-208b-5p is downregulated in NSCLC tissues and inversely associated with prognosis
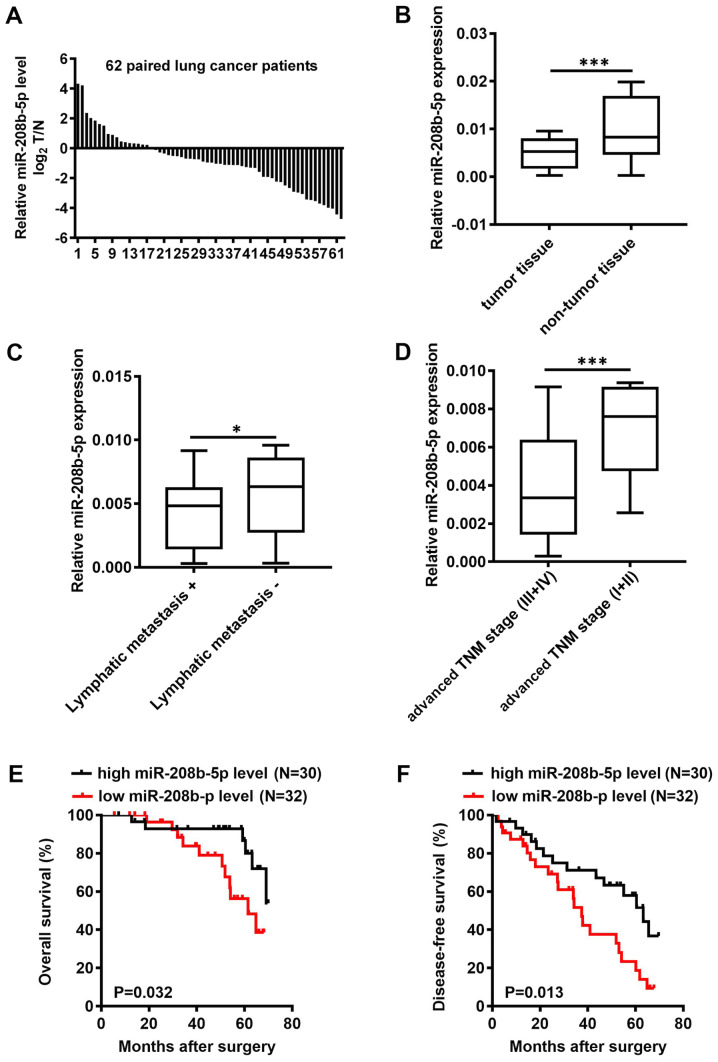
miR-208b-5p expression level was assessed by RT-qPCR in 62 pairs of NSCLC and adjacent normal tissues. The results demonstrated that miR-208b-5p expression level in tumor tissues was decreased in tumor tissues of 45 patients with NSCLC compared with adjacent normal tissues with 45 out of 62 patients have a lower miR-208b-5p level in NSCLC tumors compared to adjacent normal tissues (P<0.001; Fig. 1A and B). Based on the mean expression of miR-208b-5p in NSCLC tissues (cut-off value, 0.0057), patients were divided into a high miR-208b-5p expression group and a low miR-208b-5p expression group. In addition, miR-208b-5p expression level in NSCLC tissues was inversely associated with the presence of lymphatic metastasis and clinical TNM stage (Table I; P=0.014 and P=0.021, respectively). Patients with lymphatic metastasis or advanced TNM stage presented with decreased miR-208b-5p expression level (Fig. 1C and D; P<0.05 and P<0.0001, respectively). Furthermore, patients with low miR-208b-5p expression levels had significantly shorter overall survival (OS) and disease-free survival (DFS) compared with patients with high miR-208b-5p expression levels (Fig. 1E and F; P=0.032 and P=0.013, respectively). These findings suggested that miR-208b-5p downregulation in NSCLC tissues was inversely associated with lymphatic metastasis and clinical TNM stage. In addition, the results from OS and DFS analyses suggested that miR-208b-5p expression level may be considered as an independent prognostic factor in patients with NSCLC.
Figure 1.
miR-208b-5p expression level and significance in patients with NSCLC. (A) Relative miR-208b-5p expression level in tumor samples of 62 patients with NSCLC. (B) Relative miR-208b-5p expression level measured by reverse transcription-quantitative PCR in NSCLC and adjacent normal tissues. (C) Relative miR-208b-5p expression level in patients with and without lymphatic metastasis. (D) Relative miR-208b-5p expression level in patients with early or advanced stages. (E and F) 5-year overall survival and disease-free survival accessed via the Kaplan-Meier method in patients with NSCLS according to their expression level of miR-208b-5p. *P<0.05 and ***P<0.001. miR, microRNA; NSCLC, non-small cell lung cancer; TNM, Tumor-Node-Metastasis.
miR-208b-5p inhibits the migratory and invasive abilities and the EMT in NSCLC cells
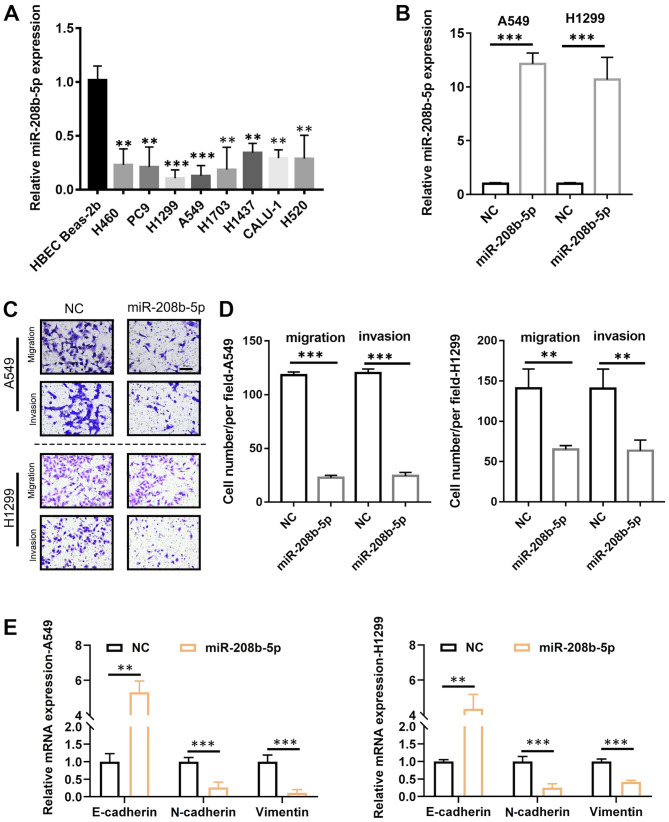
Following the determination of the association between miR-208b-5p expression level and metastasis in patients with NSCLC, the underlying mechanisms were evaluated. To do so, miR-208b-5p expression level in numerous NSCLC-derived cell lines was measured. The results demonstrated that miR-208b-5p expression level was significantly decreased in these cell lines compared with the normal Beas-2b bronchial cell line (Fig. 2A; P<0.05 and P<0.01). The cell lines A549 and H1299 were selected for subsequent analyses, since they were characterized by the lowest miR-208b-5p levels. To overexpress miR-208b-5p in these two cell lines, infection with a lentiviral vector was performed, and successful transfection was confirmed by RT-qPCR (Fig. 2B). The results from Transwell assays demonstrated that A549 and H1299 cells overexpressing miR-208b-5p had significantly decreased migratory and invasive abilities compared with control (Fig. 2C and D; P<0.001 for A549 cells and P<0.01 for H1299 cells). Furthermore, miR-208b-5p-overexpressing cells exhibited significantly increased mRNA expression of the epithelial marker E-cadherin and decreased mRNA expression of the mesenchymal markers N-cadherin and vimentin (P<0.01 and P<0.001; Fig. 2E). These findings suggested that miR-208b-5p may inhibit EMT and decrease NSCLC cell migratory and invasive abilities.
Figure 2.
miR-208b-5p inhibited NSCLC cell migratory and invasive abilities and the epithelial-mesenchymal transition. (A) miR-208b-5p expression level in normal human bronchial and NSCLC cell lines measured by RT-qPCR. (B) miR-208b-5p expression level in A549 and H1299 cells transfected with miR-208b-5p. (C and D) Representative images and quantification of the migratory and invasive abilities of A549 and H1299 cells overexpressing miR-208b-5p compared with control. (E) E-cadherin, N-cadherin, and vimentin mRNA levels in miR-208b-5p-transfected NSCLC cells determined by RT-qPCR. **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control; NSCLC, non-small cell lung cancer; RT-qPCR, reverse transcription-quantitative PCR.
miR-208b-5p targets IL-9 in NSCLC cells
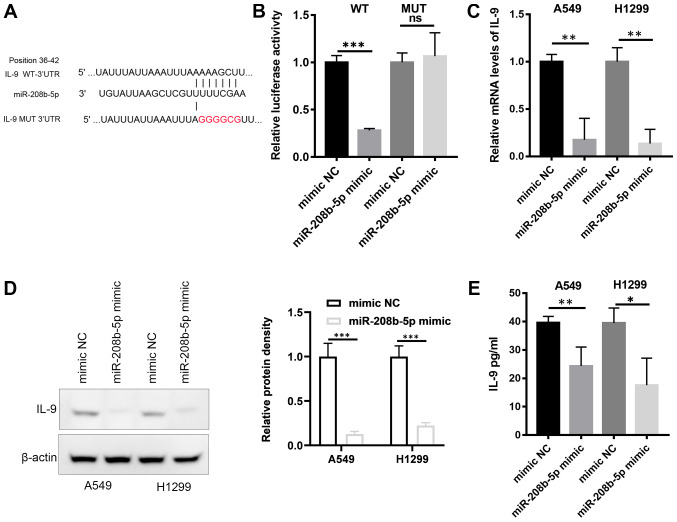
Analysis of the miRNA target database reported IL-9 as a potential target of miR-208b-5p (Fig. 3A). To analyze the interaction between miR-208b-5p and IL-9 mRNA, a wild type (WT) luciferase reporter was constructed including the 3′-UTR of IL-9. A mutant type (Mut) reporter with a mutated miR-208b-5p binding sequence was also generated (Fig. 3A). The constructs and miR-208b-5p were co-transfected into the 293T cell line. Compared with control cells (mimic NC), cells transfected with miR-208b-5p and the WT constructs displayed a significantly decreased luciferase activity (Fig. 3B; P<0.001). This effect was absent in cells transfected with the Mut reporter. These findings suggested a direct binding of miR-208b-5p to the 3′-UTR of IL-9. This result was confirmed by RT-qPCR, western blotting and ELISA assays, which demonstrated decrease in mRNA and protein levels of IL-9 in A549 and H1299 cells overexpressing miR-208b-5p (Fig. 3C-E, P<0.05). IL-9 mRNA may therefore be directly targeted by miR-208b-5p.
Figure 3.
miR-208b-5p directly targeted IL-9. (A) Illustration of the WT or Mut IL-9 3′-UTR vectors. (B) Luciferase reporter assay for the interaction between miR-208b-5p and IL-9. (C) Relative mRNA level of IL-9 in A549 and H1299 cells overexpressing miR-208b-5p measured by reverse transcription-quantitative PCR. (D) Western blotting analysis of IL-9 protein expression in A549 and H1299 cells overexpressing miR-208b-5p (left panel) and relative protein density (right panel). (E) Determination of IL-9 in culture medium of A549 and H1299 cells overexpressing miR-208b-5p determined by ELISA. *P<0.05, **P<0.01 and ***P<0.001. IL-9, interleukin 9; miR, microRNA; Mut, mutant; NC, negative control; WT, wilt type.
IL-9/STAT3 signaling pathway mediates the inhibition of EMT in NSCLC cells by miR-208b-5p
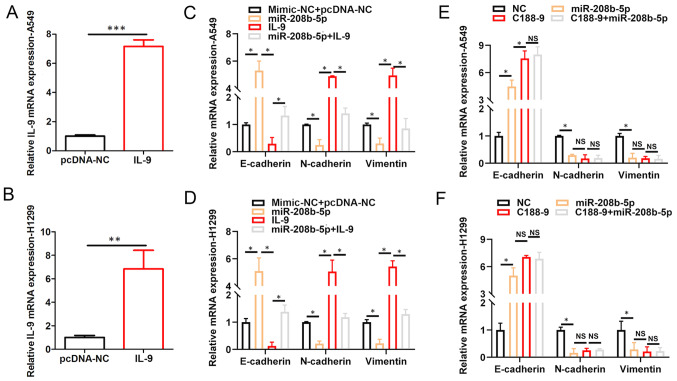
To further elucidate the interrelationship among miR-208b-5p expression level, IL-9 signaling and EMT, RT-qPCR analyses were performed. A549 and H1299 NSCLC cells were transfected with pcDNA.IL9, and the results demonstrated increased IL9 mRNA expression levels in these cells compared with the negative control (pcDNA.NC; P<0.05; Fig. 4A and B). Furthermore, miR-208b-5p overexpression increased E-cadherin mRNA expression, while decreasing N-Cadherin and vimentin mRNA expression levels in A549 and H1299 NSCLC cells (P<0.05; Fig. 4C and D). Conversely, increasing IL-9 levels decreased E-cadherin mRNA expression, while increasing N-Cadherin and vimentin mRNA expression levels in A549 and H1299 NSCLC cells (P<0.05; Fig. 4C and D). Furthermore, co-transfection with miR-208b-5p mimic + IL9 reversed the effects of miR-208b-5p overexpression on increasing E-Cadherin mRNA expression level, and decreasing N-Cadherin and vimentin mRNA expression levels in A549 and H1299 NSCLC cells (P<0.05; Fig. 4C and D). The STAT3 inhibitor, C188-9 attenuated the expression level of EMT-promoting proteins. In addition, miR-208b-5p overexpression had no further effect on the expression level of these proteins when combined with C188-9 treatment (Fig. 4E and F, P<0.05). These findings suggested that miR-208b-5p may inhibit EMT in NSCLC cells via STAT3-mediated decreased IL-9 expression.
Figure 4.
IL-9/STAT3 signaling pathway mediates the inhibition of EMT in NSCLC cells by miR-208b-5p. IL9 mRNA expression in (A) A549 and (B) H1299 cells transfected with IL9. Relative mRNA expression of E-cadherin, N-cadherin and vimentin assessed via RT-qPCR analysis in (C) A459 and (D) H1299 cells transfected with NC, miR-208b-5p, IL-9 or miR-208b-5p+IL-9. Relative mRNA expression of N-cadherin, E-cadherin and vimentin assessed by RT-qPCR in (E) A459 and (F) H1299 cells transfected or treated with NC, miR-208b-5p, C188-9 or C188-9+miR-208b-5p. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA, IL-9, interleukin 9; NC, negative control; NS, not significant; RT-qPCR, reverse transcription-quantitative PCR.
Discussion
Increasing evidence indicates that several types of miRNAs, such as miR-675-5p, miR-361-3p, miR-4286, miR-150-5p miR-625 and miR-4319 play crucial roles in NSCLC regulation (33–38). miR-208 is highly expressed in cardiac tissue and is dysregulated in several types of cardiovascular diseases (26–30), such as cardiac ischemia reperfusion injury (24) and acute myocardial infarction (25). Inhibition of miR-208 has been demonstrated to improve cardiac function and patient survival during heart failure (30,31). However, the expression and function of miR-208 in different types of cancer, particularly in NSCLC remain unknown. Thus, the present study aimed to investigate the potential role of miR-208b-5p in inhibiting NSCLC cell migration and invasion.
The present study demonstrated that miR-208b-5p expression level in NSCLC tissues was significantly decreased compared with adjacent normal tissues. Furthermore, miR-208b-5p expression level was associated with the presence of metastases, clinical TNM stage, OS and DFS. miR-208b-5p overexpression attenuated the migratory and invasive abilities of NSCLC cells and inhibited EMT. In addition, this study demonstrated that IL-9 mRNA may be a novel target for miR-208b-5p. Furthermore, miR-208b-5p ability to attenuate the STAT3 pathway was demonstrated. miR-208b-5p may therefore be considered as a cancer suppressor gene and may be used as a therapeutic target and prognostic marker in NSCLC.
The present study demonstrated that the mRNA coding IL-9 protein may be a novel direct target of miR-208b-5p. IL-9 belongs to the Th2-type of cytokines that have a pleiotropic effect on inflammatory responses in allergic diseases (4). A previous study reported the involvement of IL-9 in Treg and mast cells-mediated tumor immunity (5). Increasing evidence indicates that IL-9 participates in the pathogenesis of several types of cancer, predominantly acting as a cancer promoting factor, particularly in nonsolid tumors (6–8). IL-9 contributes to the signaling pathways of IL-2, IL-4, IL-7, IL-15 and IL-21, which involve heterodimeric receptors that interact with the common γ-chain members of the STATs family (39). STAT3 transduces signals from the cell membrane receptors to the nucleus, where it acts as a transcription factor. STAT3 is known to be constitutively activated in various types of cancer (9), such as cutaneous melanoma (10), squamous cell carcinoma (11) and oral squamous cell carcinoma (12). In addition to promoting angiogenesis, STAT3 regulates cell proliferation, migration and invasion of tumor cells, indicating its crucial role in the control of tumor growth and metastasis (10,40–42). The present study demonstrated that STAT3 inhibitor C188-9 attenuated the expression of EMT-promoting mRNA levels. Furthermore, miR-208b-5p overexpression did not further alter the mRNA levels of related-EMT genes in NSCLC cells treated with C188-9. Taken together, these findings suggested that miR-208b-5p may exert a tumor suppressor role in NSCLC cells by regulating IL-9/STAT3 pathway. Further investigation is required to confirm this hypothesis and determine the underlying mechanisms of miR-208-5p in NSCLC.
In conclusion, the present study demonstrated that miR-208b-5p was downregulated in NSCLC, and that its expression level was associated with metastasis and patient survival. These results suggested that miR-497-5p may act as a potential tumor suppressor in NSCLC. In addition, miR-208b-5p may prevent the activation of EMT, and decrease the migratory and invasive abilities of NSCLC cells by targeting IL-9/ STAT3 pathway.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- 3′-UTRs
3′ untranslated regions
- EMT
epithelial-mesenchymal transition
- IL-9
interleukin-9
- microRNA-208b-5p
miR-208b-5p
- NSCLC
non-small cell lung cancer
- TNM
Tumor-Node-Metastasis
Funding
This study was supported by the Special Fund for Scientific Research in Non-affiliated Hospitals of Fujian Medical University (grant. no. FZS13013Y) and the Youth Research Fund of Fujian Provincial Department of Health (grant. no. 2013-2-150).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JM and HT wrote the manuscript and contributed to the conception of the study. JL and FC performed the data analysis. CW, CC and JW contributed to data acquisition and analysis and revised the manuscript. SH performed the clinic patients' data analysis.. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of the First Hospital of Longyan city, Fujian Medical University (Longyan, China; approval no. 2018-LSKY-22. Signed informed consents were obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Heist RS, Engelman JA. SnapShot: Non-small cell lung cancer. Cancer Cell. 2012;21:448.e2. doi: 10.1016/j.ccr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tete S, Saggini A, Maccauro G, Rosati M, Conti F, Cianchetti E, Tripodi D, Toniato E, Fulcheri M, Salini V, et al. Interleukin-9 and mast cells. J Biol Regul Homeost Agents. 2012;26:319–326. [PubMed] [Google Scholar]
- 6.Knoops L, Renauld JC. IL-9 and its receptor: From signal transduction to tumorigenesis. Growth Factors. 2004;22:207–215. doi: 10.1080/08977190410001720879. [DOI] [PubMed] [Google Scholar]
- 7.Chen N, Lu K, Li P, Lv X, Wang X. Overexpression of IL-9 induced by STAT6 activation promotes the pathogenesis of chronic lymphocytic leukemia. Int J Clin Exp Pathol. 2014;7:2319–2323. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Wang WD, Geng QR, Wang L, Chen XQ, Liu CC, Lv Y. Serum levels of interleukin-9 correlate with negative prognostic factors in extranodal NK/T-cell lymphoma. PLoS One. 2014;9:e94637. doi: 10.1371/journal.pone.0094637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: Ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 2011;117:5701–5709. doi: 10.1182/blood-2010-04-280123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Yang Y, Xu J, Liao Y, Liu L, Deng L, Xiong X. IL22 drives cutaneous melanoma cell proliferation, migration and invasion through activation of miR-181/STAT3/AKT axis. J Cancer. 2020;11:2679–2687. doi: 10.7150/jca.40974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng S, Liu Q, Liu T, Yang L, Zhang Q, Shen T, Zhang X, Han X, Lu X. NME4 modulates PD-L1 expression via the STAT3 signaling pathway in squamous cell carcinoma. Biochem Biophys Res Commun. 2020;S0006-291X(30535-0) doi: 10.1016/j.bbrc.2020.03.055. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Qu Y, He Y, Yang Y, Li S, An W, Li Z, Wang X, Han Z, Qin L. ALDH3A1 acts as a prognostic biomarker and inhibits the epithelial mesenchymal transition of oral squamous cell carcinoma through IL-6/STAT3 signaling pathway. J Cancer. 2020;11:2621–2631. doi: 10.7150/jca.40171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fionda C, Malgarini G, Soriani A, Zingoni A, Cecere F, Iannitto ML, Ricciardi MR, Federico V, Petrucci MT, Santoni A, Cippitelli M. Inhibition of glycogen synthase kinase-3 increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: Role of STAT3. J Immunol. 2013;190:6662–6672. doi: 10.4049/jimmunol.1201426. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Jia G, Qu Y, Du Q, Liu B, Liu B. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. Med Sci Monit. 2017;23:3393–3403. doi: 10.12659/MSM.904892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M, Ang L, Huang J, Wang J. MicroRNAs regulate the epithelial-mesenchymal transition and influence breast cancer invasion and metastasis. Tumour Biol. 2017;39:1010428317691682. doi: 10.1177/1010428317691682. [DOI] [PubMed] [Google Scholar]
- 16.Banyard J, Bielenberg DR. The role of EMT and MET in cancer dissemination. Connect Tissue Res. 2015;56:403–413. doi: 10.3109/03008207.2015.1060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Chen X, Xin G, Li X. Chronic exposure to the ionic liquid [C8mim]Br induces inflammation in silver carp spleen: Involvement of oxidative stress-mediated p38MAPK/NF-κB signalling and microRNAs. Fish Shellfish Immunol. 2019;84:627–638. doi: 10.1016/j.fsi.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YW, Zhang H, Duan CJ, Gao Y, Cheng YD, He D, Li R, Zhang CF. miR-675-5p enhances tumorigenesis and metastasis of esophageal squamous cell carcinoma by targeting REPS2. Oncotarget. 2016;7:30730–30747. doi: 10.18632/oncotarget.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen J, Hu Y, Liu Q, Ling Y, Zhang S, Luo K, Xie X, Fu J, Yang H. miR-424 coordinates multilayered regulation of cell cycle progression to promote esophageal squamous cell carcinoma cell proliferation. EBioMedicine. 2018;37:110–124. doi: 10.1016/j.ebiom.2018.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang RM, Xiao S, Lei X, Yang H, Fang F, Yang LY. miRNA-487a promotes proliferation and metastasis in hepatocellular carcinoma. Clin Cancer Res. 2017;23:2593–2604. doi: 10.1158/1078-0432.CCR-16-0851. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Li D, Wu T, Xie D, Hua K, Hu J, Deng X, Ji C, Deng Y, Fang L. MicroRNA-301b promotes cell proliferation and apoptosis resistance in triple-negative breast cancer by targeting CYLD. BMB Rep. 2018;51:602–607. doi: 10.5483/BMBRep.2018.51.11.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Zheng H, Xie L, Zhang J. Decreased miR-208 induced ischemia myocardial and reperfusion injury by targeting p21. Pharmazie. 2016;71:719–723. doi: 10.1691/ph.2016.6740. [DOI] [PubMed] [Google Scholar]
- 25.Yan X, Liu J, Wu H, Liu Y, Zheng S, Zhang C, Yang C. Impact of miR-208 and its target gene nemo-like kinase on the protective effect of ginsenoside Rb1 in hypoxia/ischemia injuried cardiomyocytes. Cell Physiol Biochem. 2016;39:1187–1195. doi: 10.1159/000447825. [DOI] [PubMed] [Google Scholar]
- 26.Kakimoto Y, Tanaka M, Kamiguchi H, Hayashi H, Ochiai E, Osawa M. MicroRNA deep sequencing reveals chamber-specific miR-208 family expression patterns in the human heart. Int J Cardiol. 2016;211:43–48. doi: 10.1016/j.ijcard.2016.02.145. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C, Cui Q, Su G, Guo X, Liu X, Zhang J. MicroRNA-208b alleviates post-infarction myocardial fibrosis in a rat model by inhibiting GATA4. Med Sci Monit. 2016;22:1808–1816. doi: 10.12659/MSM.896428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Cai B, Pan Z, Lu Y. The roles of microRNAs in heart diseases: A novel important regulator. Curr Med Chem. 2010;17:407–411. doi: 10.2174/092986710790226129. [DOI] [PubMed] [Google Scholar]
- 29.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 30.Malizia AP, Wang DZ. MicroRNAs in cardiomyocyte development. Wiley Interdiscip Rev Syst Biol Med. 2011;3:183–190. doi: 10.1002/wsbm.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.He D, Wang J, Zhang C, Shan B, Deng X, Li B, Zhou Y, Chen W, Hong J, Gao Y, et al. Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol Cancer. 2015;14:73. doi: 10.1186/s12943-015-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Wang J, Liu S, Wang S, Cheng Y, Zhou W, Duan C, Zhang C. MicroRNA-361-3p suppresses tumor cell proliferation and metastasis by directly targeting SH2B1 in NSCLC. J Exp Clin Cancer Res. 2016;35:76. doi: 10.1186/s13046-016-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An X, Ge J, Guo H, Mi H, Zhou J, Liu Y, Weiyue, Wu Z. Overexpression of miR-4286 is an unfavorable prognostic marker in individuals with non-small cell lung cancer. J Cell Biochem. 2019;120:17573–17583. doi: 10.1002/jcb.29024. [DOI] [PubMed] [Google Scholar]
- 36.Dai FQ, Li CR, Fan XQ, Tan L, Wang RT, Jin H. miR-150-5p inhibits non-small-cell lung cancer metastasis and recurrence by targeting HMGA2 and β-catenin signaling. Mol Ther Nucleic Acids. 2019;16:675–685. doi: 10.1016/j.omtn.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan X, Jiang L, Wu X, Feng W, Lin Q. MicroRNA-625 inhibits the progression of non-small cell lung cancer by directly targeting HOXB5 and deactivating the Wnt/β-catenin pathway. Int J Mol Med. 2019;44:346–356. doi: 10.3892/ijmm.2019.4203. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Li H, Liu Y, Chi C, Ni J, Lin X. miR-4319 hinders YAP expression to restrain non-small cell lung cancer growth through regulation of LIN28-mediated RFX5 stability. Biomed Pharmacother. 2019;115:108956. doi: 10.1016/j.biopha.2019.108956. [DOI] [PubMed] [Google Scholar]
- 39.Hornakova T, Staerk J, Royer Y, Flex E, Tartaglia M, Constantinescu SN, Knoops L, Renauld JC. Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/STAT pathway via interleukin-9 receptor alpha homodimers. J Biol Chem. 2009;284:6773–6781. doi: 10.1074/jbc.M807531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N, Feng L, Qu H, Lu K, Li P, Lv X, Wang X. Overexpression of IL-9 induced by STAT3 phosphorylation is mediated by miR-155 and miR-21 in chronic lymphocytic leukemia. Oncol Rep. 2018;39:3064–3072. doi: 10.3892/or.2018.6367. [DOI] [PubMed] [Google Scholar]
- 41.Pérez C, Mondéjar R, García-Díaz N, Cereceda L, León A, Montes S, Durán Vian C, Pérez Paredes MG, González-Morán A, Alegre de Miguel V, et al. Advanced-stage mycosis fungoides. Role of the signal transducer and activator of transcription 3, nuclear factor-κB and nuclear factor of activated T cells pathways. Br J Dermatol. 2020;182:147–155. doi: 10.1111/bjd.18098. [DOI] [PubMed] [Google Scholar]
- 42.Qin JJ, Yan L, Zhang J, Zhang WD. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J Exp Clin Cancer Res. 2019;38:195. doi: 10.1186/s13046-019-1206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.