Abstract
Multiple myeloma (MM) represents an incurable hematologic malignancy. Despite significant advances over the past decade, with the advent of multiple new classes of anti-myeloma agents, including immunomodulatory drugs, proteasome inhibitors and monoclonal antibodies, patients ultimately relapse. Selinexor is a first-in-class exportin-1 inhibitor with activity in these multiply relapsed and refractory patients. Although the current Food and Drug Administration (FDA) approval is for the doublet of Selinexor in combination with dexamethasone, ongoing clinical trials are evaluating a number of combination regimens. These triplet and quadruplet, selinexor-based, regimens are showing significant activity in “triple-class” refractory patients. With appropriate combination drug choice, drug dosing, and supportive measures, patients with previously no viable options for therapy, now have multiple potential regimens to control their disease.
Keywords: myeloma, relapsed/refractory, selinexor, SINE
Introduction
Despite ongoing improvements in outcomes in the treatment of multiple myeloma (MM), the disease remains incurable. Most recent data from the Surveillance, Epidemiology, and End Results Program (SEER) database reveals a 5-year survival rate of 52.2% for patients diagnosed between 2009 and 2015.1 Current standards (both in the upfront and the relapsed/refractory settings) utilize combinations of immunomodulatory drugs (IMIDs), proteasome inhibitors (PIs), and monoclonal antibodies (MABs). Despite high-level responses, ultimately patients become refractory to these drugs and are termed “triple-class refractory”. Selinexor has emerged as a standard approach in this setting.
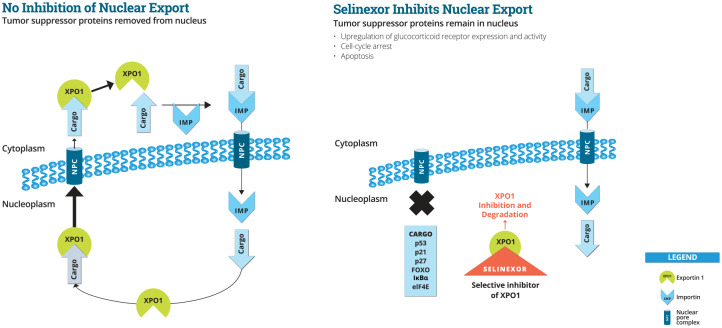
Selinexor is an oral, first-in-class, slowly reversible, potent selective inhibitor of nuclear export (SINE) compound that specifically blocks exportin 1 (XPO1) (see Figure 1). This mechanism of action is applicable across a variety of tumor types. Tumor reduction and increased survival was observed at doses of selinexor 15 through 60 mg/m2 (5 through 20 mg/kg) in mouse models of hematological and solid tumors. In addition, marked synergy was observed when selinexor was combined with a variety of chemotherapies and targeted therapies.2 On 3 July 2019, the United States Food and Drug Administration (FDA) granted accelerated approval to Selinexor in combination with dexamethasone for adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.3
Figure 1.
Selinexor and nuclear export.
IMP, importin; NPC, nuclear pore complex; XPO1, exportin 1.
Preclinical
Exportin-1 (XPO1) is overexpressed in a large variety of malignancies, including MM, as well as in a variety of solid and liquid tumors. XPO1 expression generally correlates with advanced disease, resistance to therapy, and poor survival. XPO1 exports tumor suppressor proteins and growth regulatory proteins out of the nucleus. XPO1 also regulates cytoplasmic localization, and, in turn, translation of key proto oncogenic (i.e., myc) that complex with the cargo protein, eukaryotic initiation factor 4E (eIF4E). In addition, XPO1 is involved in regulating nuclear levels of the glucocorticoid receptor (GR).
Inhibition of XPO1 by selinexor promotes apoptosis in malignant cells by blocking each of the above XPO1-mediated mechanisms. Normal cells undergo reversible cell cycle arrest following inhibition of XPO1, and recover when the block is removed. Selinexor is orally bioavailable, with a half-life (t½) of approximately 6–8 h. Following administration, pharmacokinetic (PK) and pharmacodynamic (PD) data revealed XPO1 mRNA induction and nuclear retention of tumor suppressor proteins such as p53, with a concomitant increase in apoptotic markers.
Selinexor in the treatment of relapsed myeloma: STORM
Selinexor’s first FDA approval was in combination with dexamethasone in heavily pretreated myeloma patients. This was based on the STORM (Selinexor in the Treatment of Relapsed Myeloma) trial.4 In this study, a total of 122 patients were included in the modified intention-to-treat population (primary analysis), and 123 were included in the safety population. The median age was 65 years, and the median number of previous regimens was 7; a total of 53% of the patients had high-risk cytogenetic abnormalities. A partial response or better was observed in 26% of patients, including two stringent complete responses. The clinical benefit rate (CBR; minimal response or better) was 39%. The median duration of response was 4.4 months, median progression-free survival (PFS) was 3.7 months, and median overall survival was 8.6 months. For patients achieving a molecular response (MR) or better, the medial overall survival was 15.6 months.
Fatigue, nausea, and decreased appetite were common, and were typically grade 1 or 2 (grade 3 events were noted in up to 25% of patients, and no grade 4 events were reported). Thrombocytopenia occurred in 73% of patients (grade 3 in 25% and grade 4 in 33%). Thrombocytopenia led to bleeding events of grade 3 or higher in six patients. In total, 18% of patients discontinued therapy due to Selinexor-related adverse events.
In this study, the dosing schema was 80 mg (orally) days 1 and 3 of each week, along with 20 mg of dexamethasone administered prior to each dose of Selinexor. In order to achieve clinically relevant responses (as well as response rates needed to ensure FDA approval) the doublet attempts to achieve maximal cell kill in this refractory patient population through twice weekly dosing. Of note, the population studied in STORM had not only extremely refractory disease but rapidly progressive disease as well, with an average 22% increase in measurable disease burden between screening and cycle 1/day 1 of dosing. In order to gain disease control in this kinetically aggressive state, an aggressive dosing strategy was needed. The twice weekly approach amounts to a cumulative dose of 160 mg per week. This is in contrast to the weekly dosing strategies in triplet combinations, which average 60–100 mg total per week. As a result of this approach, dose reductions were frequent. Dose reductions could be achieved either through dose, as well as schedule, modification. The first dose reduction could either be dropping from 80 mg to 60 mg on a twice-weekly schedule or switching to a weekly schedule at 100 mg. The FDA package insert ultimately reflects the latter approach.5 This stems from the early clinical experience of the improved tolerability at once weekly versus twice weekly dosing.
Weekly dosing provides a number of advantages. XPO1 inhibition can negatively affect thrombopoietin (TPO) pathways. As such, TPO-mimetics may be needed to manage platelet counts. Anecdotally, the weekly dosing schedule allows for better platelet control with TPO-mimetics. Furthermore, non-hematologic adverse events appear to be muted with weekly dosing. In turn, the combination trials with selinexor based triplet regimens utilized weekly dosing.
In general, therapies in myeloma are initially studied in the advanced disease setting and as single agents (+/– the addition of corticosteroids). Over time, earlier utilization, as well as rationale combinations, lead to enhanced response rates. The utilization of SINE-based doublet therapy in the advanced setting provides several distinct advantaged. Firstly, it provides an “all oral” therapy for patients who may have limited performance status. Secondly, advanced myeloma patients often have chronic toxicities stemming from prior treatments. As this may provide a relative contra-indication for re-retreatment with therapies of previously utilized mechanisms of action (i.e., immunomodulatory drugs and proteasome inhibitors), this class of agent is unique with a different toxicity profile. Of note, there was no significant neuropathic toxicity signal, which can affect many heavily pre-treated patients.
Bortezomib, selinexor, and dexamethasone in patients with multiple myeloma: BOSTON study
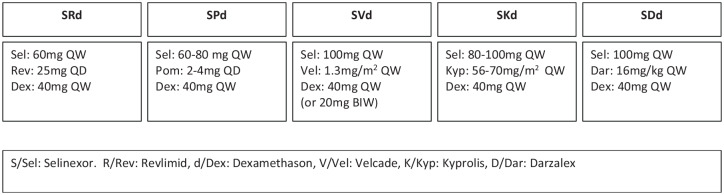
There are a number of ongoing clinical trials evaluating the efficacy and toxicity of combining Selinexor with other standard therapies in myeloma (Table 1). The BOSTON study is evaluating Bortezomib, Selinexor, and Dexamethasone in patients with MM. This ongoing phase III study is comparing the SVd regimen with Vd. Preclinical studies have shown synergistic antimyeloma activity between selinexor and proteasome Inhibitors through suppression of NF-κB signaling. This study utilizes a weekly dosing schedule of Selinexor (100 mg), bortezomib (1.3 mg/m2), and dexamethasone (40 mg). The results of this registration trial are integral in the full FDA approval of Selinexor.
Table 1.
Combination studies with Selinexor and standard therapies for Myeloma.
| Regimen | Selinexor Dosing | Dosing Frequency | Dexamethasone Dose | Concomitant Therapy Dose | Clinical Trial |
|---|---|---|---|---|---|
| Sd | 80 mg | twice weekly | 20 mg prior to each dose of Selinexor (weekly total: 40 mg) | n/a | STORM | NCT02336815 |
| SVd | 100 mg | once weekly | 40 mg weekly | Bortezomib 1.3 mg/m2 | BOSTON | NCT03110562 |
| SRd | 60 mg | once weekly | 40 mg weekly | Lenalidomide 25 mg | STOMP | NCT02343042 |
| SPd | 60 mg | once weekly | 40 mg weekly | Pomalidomide 4 mg | STOMP | NCT02343042 |
| SDd | 100 mg | once weekly | 40 mg weekly | Daratumumab 16 mg/kg | STOMP | NCT02343042 |
| SKd | 100 mg | once weekly | 40 mg (total weekly dose) | Carfilzomib 56 mg/m2 (weekly) | STOMP | NCT02343042 |
| SVDd | 100 mg | once weekly | 40 mg weekly | Bortezomib 1.3 mg/m2. | Daratumumab 16 mg/kg | SELIBORDARA | NCT03589222 |
| S-doxil-d | varied | once/twice weekly | 40 mg weekly | Liposomal Doxorubicin: dose escalation | NCT02186834 |
| SId | varied | twice weekly | 40 mg weekly | Ixazomib: dose escalation | NCT02831686 |
S= Selinexor, V: Bortezomib, R: Lenalidomide, P: Pomalidomide, D: Daratumumab, d: dexamethasone. I: Ixazomib
The dosing schema for the BOSTON study was derived from previous work by Bahlis et al.6 In this dose-escalation study, 42 patients were enrolled; half of which were refractory to a PI. The overall response rate (ORR) for the entire population was 63%: 84% for PI nonrefractory and 43% for PI-refractory patients. The median PFS for all patients was 9.0 months; 17.8 months for PI nonrefractory, and 6.1 months for PI refractory. For patients treated at the recommended phase II dose (RP2D), the ORR was 58% with a CBR: ⩾MR of 79%. These results are quite impressive, understanding that the median number of prior lines was 3 (1–11). Most adverse events were grade 1/2, and, overall, the regimen was well tolerated. Furthermore, the weekly dosing schedule was functionally well tolerated for patients and avoided the need for multiple physician office visits in a given week. Of note, 86% of the patients were previously exposed to bortezomib; however, the overall rate of peripheral neuropathy was minimal, with only 5% of the patients experiencing grade 2 neuropathy and no grade 3 reported.
The combination of Selinexor with Bortezomib is a clinically appealing regimen in the relapsed and refractory patient. Although many patients are treated with bortezomib in the upfront setting, they are often not treated until toxicity or progression. Transplant-eligible patients move onto stem cell transplant while still being sensitive to the drug. Furthermore, transplant ineligible patients are frequently treated either with lenalidomide and dexamethasone alone or treated with an RVD-lite strategy,7 and then transitioned to long-term lenalidomide maintenance. In these scenarios, the patient remains not only PI-sensitive but bortezomib sensitive. In an early relapse setting, this can be exploited given naiveté to the SINE in combination with sensitivity to bortezomib. Later relapsed patients are frequently treated with other agents, including carfilzomib, pomalidomide, and daratumumab. A significant number of these patients do not receive bortezomib-based therapy in the earlier relapses. As there are some differences (albeit with significant overlap) between the mechanisms of resistance between bortezomib and carfilzomib,8 there has been anecdotal experience of clinical responses with bortezomib-based therapies in carfilzomib-exposed and refractory patients. This allows for further potential benefit of the SVd combination in this PI-refractory group. Furthermore, there is laboratory evidence of XPO1 inhibition overcoming PI resistance through inactivation of the NFκB pathway by IκBα.9 This was seen in patients refractory to both bortezomib as well as carfilzomib.
In March of 2020, topline data was released from the ongoing BOSTON study. The study met its primary endpoint of a statistically significant increase in PFS with median durations of 13.93 months in the SVd arm compared with 9.46 months in the Vd arm. This translates to a 4.47 month (47%) increase in median PFS (hazard ratio = 0.70; p = 0.0066).10 Further follow up is expected later in the year to be presented at the larger hematology and oncology annual meetings.
Selinexor and backbone treatments of MM patients: STOMP study
Selinexor’s initial approval is congruent with the typical pathway for new myeloma drugs. In general, new therapies for myeloma are initially approved (in combination with steroids) in patients with no standard-of-care options. Following this initial approval, anti-myeloma therapies are studied in combination trials. These studies typically utilize the novel therapy in three and four drug combinations, along with previously approved therapies. In turn, the STOMP trial is a multi-arm study evaluating three-drug regimens involving Selinexor in combination with IMIDs, PIs, and MAbs (Figure 2).
Figure 2.
Sel and backbone treatments of MM patients: study schema.
D/Dar, Darzalex; d/Dex, dexamethasone; K/Kyp, Kyprolis; MM, multiple myeloma; S/Sel, Selinexor; R/Rev, Revlimid; V/Vel, Velcade..
Extensive pre-clinical studies have been performed to evaluate the synergy between XPO1 inhibition and already established anti-myeloma therapies (Table 2). KPT-185, an XPO1-inhibitor demonstrated synergistically induced cytotoxicity in myeloma cell lines when combined with IMIDs, PIs, corticosteroids as well as melphalan.11
Table 2.
Activity of selinexor in combination with established myeloma therapies in cell lines.
| Cell line(s) | Drug(s) added | Model | Effect | Reference |
|---|---|---|---|---|
| MM1.S | Dexamethasone | in vitro, in vivo | Synergistic | Argueta et al.1 |
| MM1.S | Dexamethasone | in vitro | Additive/Synergistic | Chen et al.2 |
| MM1.S | Dexamethasone | in vivo | Synergistic | Chen et al.3 |
| MM1.S | Dexamethasone | in vitro | Synergistic | Landesman et al.4 |
| MM1.S | Lenalidomide | in vivo | Synergistic | Carlson et al.5 |
| MM1.S | Bortezomib | in vivo | Additive | Muz etal.6 |
| H929, RPMI-8226, U266B1 | Bortezomib, Carfilzomib | in vitro | Synergistic | Turner et al.7 |
| U266, RPMI-8226 | Bortezomib, Carfilzomib | in vitro, in vivo | Synergistic | Turner et al.8 |
| Primary bone marrow mononuclear cells | Carfilzomib | in vitro | Synergistic | Rosebeck et al.9 |
| H929, RPMI-8226 | Doxorubicin, Bortezomib, Carfilzomib | in vitro, ex vivo | Synergistic | Turner et al.10 |
| U266, RPMI-8226, Patient MM cells | Ixazomib | in vitro, in vivo | Additive/Synergistic | Turner et al.11 |
| MM1.S, H929 | Panobinostat | in vitro | Synergistic | Elloul et al.12 |
| RPMI-8226, U266, H929, patient MM cells | Melphalan | in vitro, ex vivo | Synergistic | Cui et al.13 |
| H929, RPMI-8226, U266 | Melphalan | in vitro, ex vivo | Synergistic | Turner et al.14 |
| H929, RPMI-8226, U266 | Liposomal Doxorubicin | in vitro, in vivo | Synergistic | Turner et al.15 |
1Argueta C, Kashyap T, Klebanov B, et al. Selinexor synergizes with dexamethasone to repress mTORC1 signaling and induce multiple myeloma cell death. Oncotarget 2018; 9: 25529–25544.
2Chen C, Gutierrez M, Brown P, et al. Anti-tumor activity of selinexor (KPT-330), an oral selective inhibitor of nuclear export (SINE), +/– dexamethasone in multiple myeloma preclinical models & translation in patents with multiple myeloma. Presented at the European Hematology Association 2014 Annual Meeting, Lombardy, Italy. Abstract P953.
3Chen C, Gutierrez M, Siegel DS, et al. Selinexor demonstrates marked synergy with dexamethasone (Sel-Dex) in preclinical models and in patients with heavily pretreated refractory multiple myeloma (MM). Blood 2014; 124: 4773.
4Landesman Y, Kashyap T, Klebanov B, et al. Selective inhibitor of nuclear export (SINE) compounds show synergistic anti-tumor activity in combination with dexamethasone in multiple myeloma. Presented at the AACR 2015 Annual Meeting, Philadelphia, PA. Abstract 2074.
5Carlson R, et al. In Vitro and in vivo anti-multiple myeloma activity of selinexor (KPT-330), an oral selective inhibitor of nuclear export (Sine™) compound, is enhanced through combination with standard anti–myeloma agents. Presented at the European School of Hematology: 2nd International Conference on Multiple Myeloma, 7–9 November 2014, Athens, Greece. Abstract 2461.
6Muz B, Azab F, de la Puente P, et al. Selinexor overcomes hypoxia-induced drug resistance in multiple myeloma. Transl Oncol 2017; 10: 632–640.
7Turner JG, Dawson JL, Cubitt CL, et al. Combination therapy of human multiple myeloma using proteosome and CRM1 inhibitors. Presented at the AACR 2013 Annual Meeting, Washington, DC. Abstract 2066.
8Turner JG, Kashyap T, Dawson JL, et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IκBα and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget 2016; 7: 78896–78909.
9Rosebeck S, Alonge MM, Kandarpa M, et al. Synergistic myeloma cell death via novel intracellular activation of caspase-10-dependent apoptosis by carfilzomib and selinexor. Mol Cancer Ther 2016; 15: 60–71.
10Turner JG, Dawson J, Emmons MF, et al. CRM1 inhibition sensitizes drug resistant human myeloma cells to topoisomerase II and proteasome inhibitors both in vitro and ex vivo. J Cancer 2013; 4: 614–625.
11Turner JG, Dawson J, Bauer A, et al. Ixazomib combined with the nuclear export inhibitors selinexor or eltanexor for the treatment of multiple myeloma. Presented at the AACR 2019 Annual Meeting, Atlanta, GA. Abstracts 285.
12Elloul S, Chang H, Klebanov B, et al. Synergistic antitumor effect of selinexor, a selective inhibitor of nuclear export (SINE) compound and panobinostat in a mouse model of multiple myeloma. Presented at the AACR 2016 Annual Meeting, New Orleans, LA. Abstract 4720.
13Cui Y, Turner JG, Dawson JL, et al. The Synergistic Effect of Melphalan and XPO1 Inhibition in Pre-Clinical Models of Multiple Myeloma. Blood 2016; 128: 5662
14Turner JG, Dawson JL, Grant S, et al. Melphalan and XPO1 inhibitor combination therapy for the treatment of multiple myeloma. Blood 2014; 124: 2084.
15Turner JG, Dawson JL, Grant S, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J Hematol Oncol 2016; 9: 73.
The combination of SINE and carfilzomib has been studied extensively, in both pre-clinical as well as clinical settings. The pre-clinical work has shown three distinct findings in myeloma cell lines when these agents are given in concert with one another.12
Reduced Bcl-2 expression and cleavage and inactivation of Akt, two pro-survival regulators of apoptosis and autophagy.
Intracellular membrane-associated aggregation of active caspases, which depend on caspase-10 protease activity.
Novel association of caspase-10 and autophagy-associated proteins p62 and LC3 II, which may prime activation of the caspase cascade.
The combination of Selinexor, Carfilzomib, and dexamethasone (SKd) is one of the regimens being studied in the multi-arm STOMP trial. The data from a separate phase I trial has been published previously. The RP2D of twice-weekly SKd was selinexor 60 mg, carfilzomib 20/27 mg/m2, and dexamethasone 20 mg. This was a heavily pretreated group of patients with a median of four lines of prior therapy (range 2–10). Most patients were dual-class refractory/quad-exposed (81%). Across the entire cohort of patients, the median PFS was 3.7 months, and overall survival was 22.4 months. The CBR was 71% with an acceptable toxicity profile. The majority of the adverse events were grade 1/2 and mostly hematologic, with fatigue, nausea, and dyspnea comprising the most common non-hematologic issues.13
Following the results of the A.R.R.O.W. study,14 many combination regimens have explored the use of weekly carfilzomib dosing. In turn, the STOMP study is evaluating this approach in combination with Selinexor. The RP2D of this approach is weekly Selinexor at 80 mg, with weekly carfilzomib dosed at 56 mg/m2. Data from this SKd group was presented at the 2019 European Hematology Association (EHA) meeting. The results presented showed an ORR of 78%, with a 78% CBR in patients with a median of 4 prior lines of therapy (range 2–8).15 Overall, this approach was well tolerated, and resulted in rapid reductions in disease burden. Longer follow up will elucidate the broader applicability of weekly SKd.
Given the favorable efficacy and toxicity profile of daratumumab, this agent has been studied as an adjunct to numerous standard of care regimens. The combination of Daratumumab, Selinexor, Dexamethasone (SDd) is one of the arms in the STOMP trial, and the data available shows this to be an extremely promising option. Gasparetto et al. presented data at the 2019 EHA meeting showing the results from the first 34 patients. These patients had a median number of 3 prior therapies (2–10). Some patients had already received daratumumab-based therapy. The ORR for the entire population was 69%, with a 73% ORR in the daratumumab naïve group. The CBR was 81% and 87%, respectively. At the time of this data-cut, the median duration of response (DOR) and PFS had not yet been reached. The RP2D is Selinexor 100 mg weekly, Daratumumab 16 mg/kg, and dexamethasone 40 mg weekly.16 The regimen was well tolerated, with expected hematologic toxicities and manageable constitutional and gastrointestinal adverse events.
As Selinexor is an oral chemotherapeutic, combinations with other oral anti-myeloma therapies is highly coveted by both physicians and patients. IMIDs (thalidomide, lenalidomide, and pomalidomide) have been the backbone of many therapies in myeloma. Recent data with the combination of Selinexor, pomalidomide, and dexamethasone (SPd) presented at the 2019 American Society of Hematology (ASH) meeting was quite promising. SPd was associated with an ORR of 58% and a median PFS (mPFS) of 12.2 months in a population with a median of 4 prior lines (range 2–13). From a toxicity standpoint, there was ⩽2% Grade 3/4 nausea, vomiting, diarrhea, weight decrease, and decreased appetite.17 Although cross-trial comparisons are inherently flawed, the ORR of ~60% is on par with previously published trials with pomalidomide-based triplet regimens. The ability, however, to provide an all oral triplet, is of distinct advantage.
Future directions
Modern-day approvals for new therapies in MM have typically been in combination with dexamethasone, and in the more advanced relapsed/refractory setting. Over time, further studies evaluate the role of these new therapies in multi-drug combinations as well as in earlier disease settings. Triplet regimens have become a standard approach in both the upfront and relapsed settings. Recent data has shown a role for quadruplets in this setting with the Cassiopeia, Alcyone, and Griffin trials.18–20 The intra-patient clonal heterogeneity of myeloma is one explanation for the potential advantage of four-drug regimens above the standard two- and three-drug approach. Furthermore, heavily relapsed patients may require this degree of therapy simply to control their biologically aggressive and resistant disease. The aforementioned trials have demonstrated not only efficacy but an acceptable toxicity profile of adding Daratumumab to a standard triplet regimen. To this end, the SELBORDARA trial is seeking to take the BOSTON regimen and augmenting it with daratumumab. At the time of this publication, there is no available data for subjects treated with this approach. However, given the non-overlapping toxicities of these agents, in concert with the previous trials showing benefit from the addition of daratumumab; the results of this study are anxiously being awaited.
High-dose melphalan with autologous stem cell transplantation remains a standard of care option for younger and fitter patients. Throughout the years, a number of studies have sought to improve outcomes with the addition of other agents to melphalan. To date, there has been insufficient data to support any of these approaches outside of clinical trials; as opposed to single-agent melphalan. An ongoing trial is seeking to evaluate the potential benefit of combining selinexor with melphalan for patients undergoing stem cell transplant [ClinicalTrials.gov identifier: NCT02780609]. As one of the main toxicities of selinexor is hematologic, this concept allows for cytopenia mitigation through subsequent stem cell infusion. Data from the first 12 patients was presented at the 2019 ASH meeting. The combination with selinexor 80 mg po with high-dose melphalan at 100 mg/m2 on days −3 and −2 (RP2D) was well tolerated and engraftment kinetics were not altered. Neutrophil engraftment occurred within a median of 11 days, and a platelet engraftment with a median of 15 days.21 The phase II portion of the study is ongoing.
To date there is no data (or active clinical trials) evaluating the role of selinexor in newly diagnosed multiple myeloma. Furthermore, there is no data on any potential impact on stem cells and stem cell collection. That being said, this agent has demonstrated significant activity in relapsed patients. This is likely to be not only further enhanced in a less refractory patient, but also potentially better tolerated given the lack of prior treatment-related toxicities. In addition, there continues to be a subset of patients for whom IMIDs are relatively or absolutely contra-indicated. In this population, there may be a potential to augment PIs and MAbs to achieve deep remissions, and to restore marrow health prior to stem cell collection and transplantation.
Chimeric antigen receptor T-cell (CAR-T) therapy is currently FDA-approved for certain types of lymphoma and leukemia. Ongoing studies are evaluating this modality in myeloma; with hopes of approval within the coming year. Although studies to date have been extremely encouraging in regard to ORR and PFS in heavily pre-treated patients, unfortunately most patients treated with CAR-T in this setting ultimately relapse. Although mechanisms of relapse and resistance to CAR-T therapy are under active investigation, appropriate clinical management of a post-CAR-T relapse in MM is unknown. That being said, two patients in the STORM study had undergone prior CAR-T therapy. Both these patients achieved a PR with Selinexor plus dexamethasone. This represents the only published data of active therapy beyond CAR-T. As commercially available BCMA-targeted CAR-T are likely to entire clinical practice for MM within the next year, an unfortunate consequence is the creation of not only penta-refractory patients, but “hexa-refractory” patients as well. These patients may be able to be salvaged with Selinexor based regimens.
Optimizing supportive care
Clinical success with selinexor, as with many chemotherapeutic agents, lies in the appropriate dosing, dose adjustment, and supportive care details. To this end, the clinical trial and commercial-use to date have provided a clear framework for known potential toxicities and how to manage them.
Dosing of Selinexor as a doublet (with dexamethasone) was initiated at 80 mg on a twice weekly basis. This was done to maximize response in a patient group with highly refractory and rapidly worsening disease burden. In patients who are treated with this doublet, the recommended approach is to start out at the 80 mg twice weekly dose, if/when significant toxicities are encountered, hold the drug and dose reduce to 100 mg weekly. For triplet combinations that already utilize a weekly dosing schedule, the dose reductions for toxicity are outlines in the package insert.
Hematologic toxicity/management is highly important. There is a tropism for platelets and thrombocytopenia is the main hematologic adverse event. In patients with baseline treatment and disease related cytopenias, aggressive usage of TPO-mimetics may be needed to allow continuation of effective dosing of drug. As the drug itself my interact with TPO pathways, there is anecdotal evidence that TPO-mimetics are more effective with weekly as opposed to twice weekly dosing. This, in part, drives some of the clinical decision tree on dose reduction to 100 mg weekly as opposed to 60 mg twice weekly. In general, our clinical experience has shown better efficacy of TPO-mimetic dosing at higher levels such as 10 mcg/kg of romiplostim and 50–100 mg of eltrombopag. Utilization of erythropoietin stimulating agents (ESAs) to correct anemia may help to both offset anemia-induced fatigue and prevent the need for red blood cell transfusion. Filgrastim and peg-filgrastim may be given concomitantly with Selinexor as needed to correct/prevent neutropenia and febrile neutropenia.
Nausea was one of the most common non-hematologic toxicities associated with Selinexor. Most patients will require multiple anti-emetic agents to properly manage these issues. This typically includes prophylactic use of steroids and 5-HT3 receptor antagonists. For those patients felt to be at particularly high risk of chemotherapy induced nausea and vomiting the use of olanzapine and NK1 receptor antagonists (such as rolapitant) are recommended. For patients who experience lower GI symptoms with diarrhea, loperamide has been found to be quite effective.
Early trials with Selinexor revealed hyponatremia a common electrolyte disturbance. The overwhelming majority of this was found to be clinically mild. Hydration status should be monitored to be sure the patient is not becoming both salt and water deplete. Sodium tablets and/or intravenous saline may be needed in more severe cases.
Patients with advanced myeloma may suffer from both treatment- and disease-related asthenia. This is often multifactorial. General supportive measures such as exercise (when applicable), minimizing anemia (with ESAs and transfusions), and sleep hygiene can be highly effective. For those patients with more recalcitrant asthenia, the use of stimulants such as methylphenidate (dosed at 10 mg daily) may be needed.
Conclusion
Selinexor represents a first-in-class SINE/anti-myeloma agent. Any time a new mechanism of action becomes clinically available, the number of new therapeutic options for our patients increases substantially. This results from the ability to combine with other classes of agents, and, in turn, yields numerous new options in the relapsed and refractory setting. The approval of the doublet with dexamethasone is simply the beginning of the potential of this drug. Ongoing clinical studies of triplet and quadruplet combinations with Selinexor are extremely encouraging, especially in heavily pretreated patients. The results of the STOMP trial will help to elucidate which combinations are optimal in specific clinical scenarios.
Optimization of dosing, dosing schedule, and supportive measures are key to maximizing response and tolerance, and, in turn, outcomes with selinexor-based therapy. The key dose limiting toxicities of the drug are cytopenias (most notably thrombocytopenia), gastrointestinal issues (nausea and anorexia), and asthenia. Patients with pre-existing marrow compromise and high risk for nausea may benefit from aggressive prophylactic measures with growth factors and anti-emetics respectively. As the drug clearly has activity in heavily pretreated patients, it represents one of the few agents that may recapture relapsing disease in multi-class resistant myeloma.
Footnotes
Conflict of interest statement: Speakers Bureau: Celgene, Janssen Advisory Board/Consulting: Karyopharm, Celgene, BMS, Janssen, Antengene, Oncopeptides, Adaptive Biotechnologies, X4 Pharmaceuticals.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Joshua Richter  https://orcid.org/0000-0002-0274-0585
https://orcid.org/0000-0002-0274-0585
Contributor Information
Joshua Richter, Icahn School of Medicine at Mount Sinai Tisch Cancer Institute Ringgold Standard Institution – Hematology/Oncology, 1 Gustave L. Levy Pl., New York, NY 10029, USA.
Deepu Madduri, Icahn School of Medicine at Mount Sinai Tisch Cancer Institute Ringgold Standard Institution – Hematology/Oncology, New York, USA.
Shambavi Richard, Icahn School of Medicine at Mount Sinai Tisch Cancer Institute Ringgold Standard Institution – Hematology/Oncology, New York, USA.
Ajai Chari, Icahn School of Medicine at Mount Sinai Tisch Cancer Institute Ringgold Standard Institution – Hematology/Oncology, New York, USA.
References
- 1. National Center Institute: Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma, https://seer.cancer.gov/statfacts/html/mulmy.html (accessed 23 December 2019).
- 2. Allegra A, Innao V, Allegra AG, et al. Selective inhibitors of nuclear export in the treatment of hematologic malignancies. Clin Lymphoma Myeloma Leuk 2019; 19: 689–698. [DOI] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration. FDA grants accelerated approval to selinexor for multiple myeloma, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-selinexor-multiple-myeloma (accessed 23 December 2019).
- 4. Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 2019; 381: 727–738. [DOI] [PubMed] [Google Scholar]
- 5. XPOVIO™ (selinexor). Karyopharm Therapeutics Inc, Newton, MA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212306s000lbl.pdf (accessed 23 December 2019). [Google Scholar]
- 6. Bahlis NJ, Sutherland H, White D, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood 2018; 132: 2546–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol 2018; 182: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huber EM, Heinemeyer W, Groll M. Bortezomib-resistant mutant proteasomes: structural and biochemical evaluation with carfilzomib and ONX 0914. Structure 2015; 23: 407–417. [DOI] [PubMed] [Google Scholar]
- 9. Turner JG, Kashyap T, Dawson JL, et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IκBα and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget 2016; 7: 78896–78909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karyopharm Therapeutics Inc. Karyopharm announces phase 3 BOSTON study meets primary endpoint with significant increase in progression-free survival in patients with multiple myeloma following one to three prior lines of therapy, https://www.globenewswire.com/news-release/2020/03/02/1993339/0/en/Karyopharm-Announces-Phase-3-BOSTON-Study-Meets-Primary-Endpoint-with-Significant-Increase-in-Progression-Free-Survival-in-Patients-with-Multiple-Myeloma-Following-One-to-Three-Pri.html (accessed 23 March 2020).
- 11. Kong SY, Landesman Y, Jakubikova J, et al. Blockade of nuclear export protein CRM1 (chromosomal region maintenance 1, XPO1) by a novel, potent and selective CRM1 inhibitor KPT-185 induces significant antitumor activity against human multiple myeloma. Blood 2011; 118: 2913. [Google Scholar]
- 12. Rosebeck S, Alonge MM, Kandarpa M, et al. Synergistic myeloma cell death via novel intracellular activation of caspase-10-dependent apoptosis by carfilzomib and selinexor. Mol Cancer Ther 2016; 15: 60–71. [DOI] [PubMed] [Google Scholar]
- 13. Jakubowiak AJ, Jasielec JK, Rosenbaum CA, et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br J Haematol 2019; 186: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreau P, Mateos MV, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol 2018; 19: 953–964. [DOI] [PubMed] [Google Scholar]
- 15. Gasparetto C, Schillar GJ, Callander NS, et al. A phase 1b/2 study of selinexor, carfilzomib, and dexamethasone (SKD) in relapsed/refractory multiple myeloma (RRMM). Presented at the EHA 2019 Annual Meeting, Amsterdam, Netherlands, 15 June 2019. Abstract 3423. [Google Scholar]
- 16. Gasparetto C, Lentzsch S, Schiller G, et al. Safety and efficacy of combination of selinexor, daratumumab, and dexamethasone (SDd) in patients with multiple myeloma (MM) previously exposed to proteasome inhibitors and immunomodulatory drugs. Presented at the EHA 2019 Annual Meeting, Amsterdam, Netherlands, 16 June 2019. Abstracts S1606. [Google Scholar]
- 17. Chen C, Gasparetto C, White D, et al. Selinexor, pomalidomide, and dexamethasone (SPD) in patients with relapsed or refractory multiple myeloma. Blood 2019; 134(Suppl. 1): Abstract 141. [Google Scholar]
- 18. Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378: 518–528. [DOI] [PubMed] [Google Scholar]
- 19. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019; 394: 29–38. [DOI] [PubMed] [Google Scholar]
- 20. Voorhees PM, Kaufman JL, Laubach JP, et al. Depth of response to daratumumab (DARA), lenalidomide, bortezomib, and dexamethasone (RVd) improves over time in patients (pts) with transplant-eligible newly diagnosed multiple myeloma (NDMM): Griffin study update. Blood 2019; 134(Suppl. 1): Abstract 691. [Google Scholar]
- 21. Nishihori T, Alsina M, Ochoa J, et al. The result of a phase 1 study of selinexor in combination with high-dose melphalan and autologous hematopoietic cell transplantation for multiple myeloma. Blood 2019; 134(Suppl. 1): Abstract 3314. [Google Scholar]