Abstract
Targeted therapies are efficient in the context of oncogenic driver mutations. Epidermal growth factor receptor (EGFR)-mutant lung cancers represent a distinct subset of non-small-cell lung cancer (NSCLC) with marked sensitivity to EGFR tyrosine kinase inhibitors (TKIs). Despite the high response rate to EGFR TKIs in EGFR-mutant lung cancer, resistance and tumor recurrence are unavoidable. Therapeutic options are restricted in patients after exhaustion of targeted therapies. Immune checkpoint inhibitors (ICIs) represent a novel therapeutic option for advanced NSCLC with significant overall survival benefit in registration trials. No superiority in terms of long-term survival was observed in the EGFR mutation subgroup when ICIs were given as monotherapy in second-line treatment in earlier studies. Thus, the appropriate application of ICIs to patients harboring EGFR mutations remains an important field of ongoing research. Here, we discuss different immune checkpoint blockade strategies, including ICIs alone and in combination with TKIs, chemotherapy, radiation, and antiangiogenic agents in EGFR-mutant NSCLC as first-line and subsequent treatments. We also summarize the evidence concerning the heterogeneous molecular features and immune signatures of EGFR mutations and their associations with ICI therapy outcomes. This study was performed to improve our understanding of the optimal mode of immune-based treatment approaches in EGFR-mutant NSCLC.
Keywords: epidermal growth factor receptor, immune checkpoint inhibitor, non-small-cell lung cancer, tumor microenvironment
Introduction
Treatment strategies for advanced non-small-cell lung cancer (NSCLC) have undergone significant transitions over the past decade. Gene and molecule-based targeted therapy provides considerable benefits for patients with specific aberrations.
Epidermal growth factor receptor (EGFR) gene alteration, with a frequency of 15% in Caucasian patients and 40% in Asian patients, can be targeted therapeutically with tyrosine kinase inhibitors (TKIs). Improved overall response rate (ORR) and prolonged progression-free survival (PFS) has been substantially observed in EGFR-mutant NSCLCs when treated with EGFR-sensitizing TKIs.1 However, resistance inevitably occurs regardless of generations of EGFR-TKIs. Third-generation EGFR-TKI osimertinib is currently used in the first-line setting, independently of T790M mutation due to the clinical benefits of both PFS and overall survival (OS) observed in the FLAURA trial.2 Of note, therapeutic options are limited after exhaustion of targeted therapies and concerns occur especially when patients progress after third-generation EGFR-TKIs.
Immune checkpoint inhibitors (ICIs) that block programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) represent a novel standard of care for advanced NSCLC without driver mutations.3–5 Unfortunately, no superiority in terms of long-term survival has consistently been observed in the EGFR-mutant subgroup when ICIs were given as monotherapy as second-line treatment in previous trials.6–9
Therefore, the appropriate application of ICIs to patients harboring EGFR mutations remains an important field of ongoing research. This article presents a review of the treatment strategies with ICIs in EGFR-mutant NSCLC as first-line and later treatments, and summarizes the evidence concerning the heterogeneous molecular features of EGFR mutations and their associations with the outcomes of ICI therapy.
Immunotherapy strategies for EGFR-mutant NSCLCs
Immune monotherapy in the first-line setting
Immunotherapy in advanced NSCLC without driver gene aberration has become the new standard of care. However, questions remain regarding the efficacy of ICIs in EGFR-mutant subgroups (Table 1). The KEYNOTE-010 phase I trial demonstrated possible superiority of outcome in TKI-naive NSCLC in patients (n = 4) harboring EGFR mutations compared to those previously treated with TKIs (n = 26) when receiving pembrolizumab (ORR, 50% versus 4%, respectively; median PFS, 157.5 versus 56 days, respectively; median OS, 559 versus 120 days, respectively).10 To verify the reliability of this result, a subsequent phase II trial was conducted. This trial enrolled 11 EGFR-mutant, PD-L1-positive, and TKI-naive patients, of whom 64% had sensitizing EGFR alterations, and 73% had a PD-L1 tumor proportional score (TPS) ⩾50%.11 Unfortunately, none achieved partial response, and thus the enrollment was terminated after treating 11 out of 25 planned patients. Similarly, CheckMate 012, a phase I multicohort trial to evaluate the efficacy of first-line nivolumab alone or combined with standard therapies in advanced NSCLC, showed inferior response rate and lower survival benefits in the EGFR-mutant subgroup in the monotherapy cohort.12 A rather poor outcome was reported in the EGFR-positive group compared to the EGFR wild-type group (ORR, 14% versus 30%, respectively; median PFS, 1.8 versus 6.6 months, respectively).
Table 1.
Efficacy of immune check-point inhibitors in EGFR-mutant NSCLC.
| Study | Subgroup | Treatment | Outcome |
|---|---|---|---|
| 1L immune strategy | |||
| 1L ICI monotherapy | |||
| KEYNOTE-010 phase I | EGFR+, TKI-naive (n = 4) EGFR+, TKI-pretreated (n = 26) |
Pembrolizumab Pembrolizumab |
ORR: 50%; mPFS: 157.5d; mOS: 559d; ORR: 4%; mPFS: 56d; mOS:120d |
| KEYNOTE-010 phase II | EGFR+, PD-L1+, TKI-naive (n = 10) | Pembrolizumab |
ORR: 0 |
| CheckMate 012 | EGFR+ (n = 7) EGFR– (n = 30) |
Nivolumab Nivolumab |
ORR: 14%; DCR: 29%; 24-wk PFS rate: 14%; mPFS: 1.8mo; mOS: 18.8mo; 18-mo OS: NC; ORR: 30%; DCR: 50%; 24-wk PFS rate: 51%; mPFS: 6.6mo; mOS: NR; 18-mo OS: 67% |
| 1L ICI+chemotherapy | |||
| CheckMate 012 | EGFR+ (n = 6) EGFR– (n = 30) |
Nivolumab+chemotherapy Nivolumab+chemotherapy |
ORR: 17%; DCR: 83%; mPFS: 4.8mo; 24-wk PFS rate: 40%; mOS: 20.5mo; 2-yr OS: 17%; ORR: 47%; DCR: 87%; mPFS: 7.5mo; 24-wk PFS rate: 60%; mOS: 24.5mo; 2-yr OS: 52% |
| 1L ICI+TKI | |||
| NCT02088112 | EGFR+, TKI-naive (n = 30) EGFR+, gefitinib-pretreated (n = 10) |
Durvalumab+gefitinib Durvalumab+gefitinib |
ORR: 63.3%; DoR:9.2mo; mPFS: 10.1mo; ORR: 70.0%; DoR:12.6mo; mPFS: 12.0mo |
| 1L ICI+ICI | |||
| CheckMate 012 | EGFR+ (n = 8) EGFR– (n = 54) |
Nivolumab+ipilimumab Nivolumab+ipilimumab |
ORR: 50%; ORR: 41% |
| 2+L immune strategy | |||
| 2+L ICI monotherapy | |||
| CheckMate 057 | EGFR+ (n = 82) EGFR– (n = 340) EGFR not report (n = 160) |
Nivolumab versus docetaxel Nivolumab versus docetaxel Nivolumab versus docetaxel |
HR 1.18 (favours docetaxel) HR 0.66 (favours nivolumab) HR 0.74 (favours nivolumab) |
| OAK | EGFR+ (n = 85) EGFR– (n = 628) |
Atezolizumab versus docetaxel Atezolizumab versus docetaxel |
HR 1.24 (favours docetaxel) HR 0.69 (favours atezolizumab) |
| KEYNOTE-010 | EGFR+, PD-L1 ⩾1% (n = 86) EGFR–, PD-L1 ⩾1% (n = 875) |
Pembrolizumab versus docetaxel Pembrolizumab versus docetaxel |
HR 0.88 (favours pembrolizumab) HR 0.66 (favours pembrolizumab) |
| ATLANTIC | EGFR+/ALK+, PD-L1 ⩾25% (n = 86) EGFR–/ALK–, PD-L1 ⩾25% (n = 875) |
Durvalumab Durvalumab |
ORR: 12%; TTR: 1.8mo; DOR: 7.4mo; mPFS: 1.9mo; mOS: 13.3mo; 1-yr OS: 54.8%; ORR: 16%; TTR: 1.9mo; DOR: 12.3mo; mPFS: 3.3mo; mOS: 10.9mo; 1-yr OS: 47.7% |
| 2+L ICI+chemotherapy | |||
| KEYNOTE-789 | EGFR+ EGFR+ |
Pembrolizumab+chemotherapy Placebo+chemotherapy |
Ongoing |
| CheckMate 722 | EGFR+ EGFR+ |
Nivolumab+chemotherapy Placebo+chemotherapy |
Ongoing |
| NCT03513666 | EGFR+, EGFR-TKI pretreated (n = 40) | Toripalimab+chemotherapy | ORR: 54.8%; DCR: 93.5%; mPFS: 7.6mo |
| 2+L ICI+ICI | |||
| KEYNOTE-021 cohorts D and H |
EGFR+, TKI-pretreated (n = 10) | Pembrolizumab+ ipilimumab | ORR: 10% |
| 2+L ICI+TKI | |||
| CheckMate 012 | EGFR+ (n = 21, 20 erlotinib-pretreated+1 TKI-naïve) | nivolumab+erlotinib | ORR: 19%; 24-wk PFS rate: 51% |
| TATTON | EGFR+, EGFR-TKI pretreated (n = 23) EGFR+, EGFR-TKI naive (n = 11) |
Osimertinib+durvalumab | ORR: 43% (9/21); incidence of interstitial lung disease:26% (6/23); ORR: 70% (7/10); incidence of interstitial lung disease:64% (7/11) |
| 2+L ICI+anti-angiogenetic agent+chemotherapy | |||
| IMpower150 | EGFR+, EGFR-TKI pretreated (n = 45) EGFR+, EGFR-TKI pretreated (n = 34) EGFR+, EGFR-TKI pretreated (n = 45) |
Atezolizumabb+Carboplatinc+Paclitaxeld Atezolizumabb+Carboplatinc+Paclitaxeld +Bevacizumab Carboplatinc+Paclitaxeld+Bevacizumab |
ORR: 36%; DOR: 5.6mo; mPFS: 6.9mo; mOS: 21.4mo; ORR: 71%; DOR: 11.1mo; mPFS: 10.2mo; mOS: NE; ORR: 42%; DOR: 4.7mo; mPFS: 6.9mo; mOS: 18.7mo |
ALK, anaplastic lymphoma kinase; d, day; DCR, disease control rate; DoR, duration of response; EGFR, epidermal growth factor receptor; HR, hazard ratio; ICI, immune checkpoint inhibitor; mo, month; mOS, median overall survival; mPFS, median progression-free survival; NC, not calculated; NE, not estimable; NR, not reached; ORR, overall response rate; PD-L1, programmed cell death ligand-1; TKI, tyrosine kinase inhibitor; TTR, time to response; wk, week; yr, year.
Immune combined therapy in the first-line setting
The first-line application of single-agent therapy got into a dilemma in EGFR-mutant NSCLC with unsatisfactory efficacy, but combined therapy brought some insights. In the CheckMate 012 trial, nivolumab in combination with platinum-based doublet chemotherapy showed reduced benefits in the EGFR-mutant subgroup compared with the EGFR wild-type group (ORR, 17% versus 47%, respectively; median PFS, 4.8 versus 7.5 months, respectively; 2-year OS, 17% versus 52%, respectively).13 In another phase I study assessing the combination of TKIs and ICIs at first-line in locally advanced/metastatic NSCLC patients with EGFR mutation, arm 1+1a received gefitinib plus durvalumab, and arm 2 first received gefitinib for 4 weeks followed by combined gefitinib and durvalumab. Two arms showed comparable outcomes (arm 1+1a versus arm 2: ORR, 63.3% versus 70%; PFS, 10.1 versus 12 months). Of importance, the incidence of pneumonitis was as high as 38%.14 Similarly, pembrolizumab plus gefitinib was not feasible due to severe liver toxicity.15 Emerging data showed that PD-L1 blockade followed by osimertinib may be associated with severe immunotherapy-related adverse events (irAEs), which were most frequent among patients who had recently (less than 1 year) received PD-L1 blockade. This association appears to be specific to osimertinib.16 In contrast, pembrolizumab plus erlotinib did not improve the objective response rate compared with previous monotherapy studies despite tolerable toxicity profile.15 On the other hand, when given a combination of the PD-1 inhibitor nivolumab plus CTLA4 inhibitor ipilimumab, four of eight EGFR-mutant individuals showed a partial response, suggesting that the use of double immune agents may be feasible in NSCLC with EGFR alterations. However, further studies are required to confirm these observations.17
The first-line application of immune monotherapy in EGFR mutants showed poor outcomes, with low ORR or even complete lack of efficacy regardless of ICI agents, although we realize that there are differences in activity among ICIs. Application of immune agent doublets or the combination of an immune agent plus TKI represented a potentially beneficial strategy, with ORR of 50–63.3%,14,17 but large cohort studies and cautious safety analyses are needed to verify the efficacy and evaluate toxicity.
Immune monotherapy in second-line setting and beyond
Outcomes were still poor when ICIs were given as monotherapy to EGFR-mutant individuals in later rounds of treatment. A series of double-blind randomized controlled clinical trials (RCTs) showed negative outcomes with regard to potential survival benefits in EGFR-mutant NSCLC treated with a single ICI agent as second-line therapy.6–9 However, the ATLANTIC trial, a multicenter phase II study, reported the opposite trend.18 In advanced NSCLC with EGFR/anaplastic lymphoma kinase (ALK) aberrations in which ⩾25% of tumor cells were positive for expression of PD-L1, application of durvalumab monotherapy as a third-line or later treatment demonstrated an ORR of 12.2% and a longer OS of 13.3 months compared to the wild-type group (ORR, 16%; OS, 10.9 months). However, the ATLANTIC trial had a number of limitations: the single-arm design made the results less convincing, and the testing platform for PD-L1 was Ventana SP263 in the ATLANTIC study but Ventana SP142, Dako 22C3, and Dako 28-8 in previous studies. Different platforms with different antibodies and different cut-off values made the direct comparison among trials impossible.
Immune combined therapy in second-line setting and beyond
Consistent with first-line treatment, later treatment with ICIs in the context of other standards of care showed improved outcomes. The combination of ICIs plus chemotherapy is a potentially beneficial strategy that is under investigation in several ongoing trials for the subgroup of patients harboring EGFR mutations. The KEYNOTE-789, CheckMate 722, and WJOG8515L trials enrolled patients with advanced non-squamous NSCLC with EGFR mutations who progressed after prior TKI therapies, and patients were assigned to receive chemotherapy alone or combined with immune agent; the results of these trials are awaiting further disclosure.19–21 Very recently, data from a phase II trial of toripalimab plus chemotherapy reported a confirmed ORR of 54.8%, disease control rate (DCR) of 93.5%, and median PFS of 7.6 months.22 Additional survival data are still expected. By contrast, the KEYNOTE-021 trial cohorts D (dose-finding cohort) and H (dose expansion cohort) explored the benefits of pembrolizumab plus ipilimumab at later rounds of treatment in advanced NSCLC.23 In contrast to the observations from first-line nivolumab plus ipilimumab, the immune doublets showed limited response, and one of 10 EGFR-mutant patients archived partial response (PR). On the other hand, the CheckMate 012 trial assessed the efficacy of nivolumab plus erlotinib in an EGFR-mutant group consisting of 20 patients pretreated with erlotinib and one naive to TKIs, resulting in an ORR of 19% and 24-week PFS rate of 51%.24 However, in the TATTON study, when patients in the EGFR subgroup were given osimertinib plus durvalumab, the high incidence of interstitial lung disease led to permanent suspension of enrollment, although the ORR was as high as 43% in TKI-pretreated patients and 70% in TKI-naive patients.25 The IMpower150 study tested the hypothesis that chemotherapy combined with an antiangiogenic agent and/or ICI is more efficient for advanced NSCLC, including in patients with EGFR mutation.26 In the EGFR-mutant subgroup after resistance to TKIs, the addition of atezolizumab to bevacizumab plus chemotherapy improved ORR and prolonged PFS and OS compared to the bevacizumab plus chemotherapy arm (ORR, 71% versus 42%; median PFS, 10.2 versus 6.9 months, hazard ratio (HR), 0.61; median OS, not reached versus 18.7 months, HR, 0.61). With these encouraging outcomes, the novel combination of ICIs plus antiangiogenic agents plus chemotherapy is a promising strategy for second-line treatment of advanced NSCLC harboring EGFR mutations.
Generally, the efficacy of immune single agents as later treatment remains limited. The optimal combined therapy involves ICIs combined with chemotherapy, especially quartet therapy consisting of atezolizumab plus bevacizumab plus carboplatin plus paclitaxel. Particular caution is needed in the application of combinations of targeted therapy and immunotherapy because of the high rate of adverse events.
Efficacy of ICIs in NSCLC with different EGFR mutations
Beyond different combined strategies and rounds of treatment, heterogeneity of EGFR subtypes also results in variations in therapeutic efficacy. A multicenter retrospective study analyzed clinical data of 171 EGFR-mutant lung cancers treated with PD-(L)1 blocker alone or in combination with CTLA4 inhibitor.27 The ORR of patients with EGFR 19 deletion showed inferior responses compared to those with L858R mutation, and both subtypes showed less benefit from immune treatment than the EGFR wild-type group (ORR, 7% in EGFR 19 deletion subgroup versus 16% in L858R subgroup versus 22% in wild-type group). Considering atypical aberrations, the response was best in patients with G719 and poorest in patients with L861Q mutations. Consistent with another retrospective study,28 it was concluded that the survival benefit from immunotherapy was more significant in cases with EGFR mutations in exon 21, less so with exon 19, and least in T790M. However, this study did not further compare the subgroups of immune monotherapy and immune doublet therapy, which may mask different outcomes between treatment subsets. Therefore, screening of mutant subtypes of EGFR alterations is vital for prognostic analysis. Further studies in larger cohorts are needed for verification.
Immune signatures for immunotherapy in NSCLC with EGFR mutation
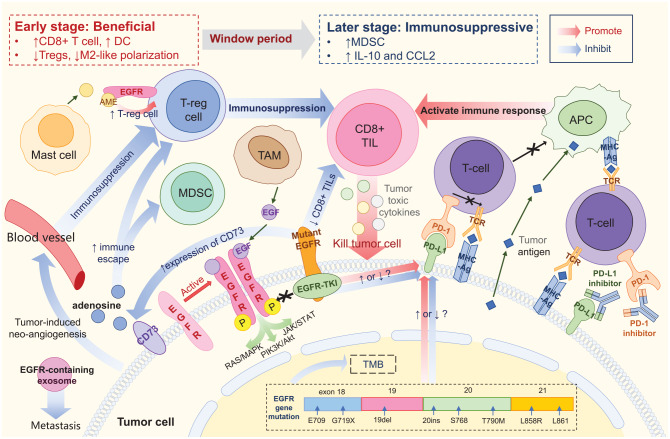
The data outlined above indicated unsatisfactory therapeutic efficacy of ICIs in EGFR-mutant NSCLC patients; the reasons for this outcome remain unclear. It has been suggested that the changes in tumor mutation burden (TMB) and PD-L1 expression as well as the unique tumor microenvironment (TME) of EGFR mutants all contribute to the lack of efficacy of immunotherapy (Figure 1).
Figure 1.
Tumor microenvironment of EGFR-mutant NSCLC. EGFR protein expressed on the tumor cell surface could be activated by homodimerization, and may then activate downstream pathways. Mutant EGFR protein could bind to EGFR-TKI, thus blocking signal transduction. PD-L1 binding to PD-1 prevents T cells from recognizing tumor cells, thereby causing immune escape. PD-(L)1 inhibitor blocks PD-1 or PD-L1, promoting the binding of TCR on T cells to MHC molecules on tumor cells and reinitiating the immune response. Tumor antigens can be directly taken up by APC and presented to T cells. CD8+ TIL are major effector cells that produce an immune-mediated tumor-killing effect by secreting tumor toxic cytokines. EGFR mutation is associated with reduced density and function of CD8+ TIL, resulting in a poor response to ICIs. Treg is the predominant immunosuppressive cell type. AME released by mast cells could bind to EGFR expressed on Treg, promoting the function of Treg and indirectly promoting immunosuppression. CD73 expressed on tumor cells may induce the formation of adenosine, further promoting Treg and MDSC and hindering the immune response. Mutant EGFR is associated with upregulation of CD73. EGFR-containing exosomes are secreted by tumor cells and may drive metastasis. Tumor-induced neoangiogenesis leads to immunosuppression by promoting immunosuppressive cells and inhibiting effector T cells. Meanwhile, EGFR mutation is associated with reduced TMB, but its effect on PD-L1 expression is still controversial. Application of EGFR-TKI is suspected to cause dynamic changes in PD-L1 expression, but the specific effects remain unclear. The TME of EGFR-aberrant NSCLC represents a dynamic transition from beneficial in the early stages to immunosuppressive in later stages during EGFR-TKI treatment. Ag, antigen; AME, amphiregulin; APC, antigen presenting cell; CCL2, C–C motif chemokine ligand 2; DC, dendritic cell; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; IL-10, interleukin-10; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; NSCLC, non-small-cell lung cancer; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor; TIL, tumor-infiltrating lymphocyte; TKI, tyrosine kinase inhibitor; TAM, tumor-associated macrophage; TMB, tumor mutation burden; TME, tumor microenvironment; Treg, regulatory T cell
Tumor mutation burden
TMB was defined as the total number of somatic, coding, base substitution, and indel mutations per megabase of genome.29 But there are differences in the definition between tissue-based TMB (tTMB) and blood-based TMB (bTMB). The former includes both single-nucleotide variants (SNVs) and insertions and deletions (indels) at an allele frequency of ⩾5%, whereas the latter only includes SNVs but at an allele frequency of ⩾0.5%.30 The definite value of TMB is calculated via whole exon sequencing. Alternatively, TMB determined by foundationOne CDx (F1CDx) large panel was also approved by the US Food and Drug Administration (FDA) in 2017 for cancer clinical diagnosis. TMB was the first biomarker used for immunotherapy in patients with advanced melanoma.31 The CheckMate 026 and CheckMate 227 trials showed that administration of nivolumab led to a higher response rate and improved PFS in patients with high TMB than in those with medium or low TMB.32 TMB is expected to become a useful biomarker for predicting the efficacy of immunotherapy. TMB is relatively low in lung cancer harboring known driver mutations.33 EGFR+ NSCLC patients carry ~3–6 mutations/Mb, patients with ALK fusion ~2–4 mutations/Mb, ROS1 ~4.0 mutations/Mb, and RET ~4.8 mutations/Mb.27,34–36 The low TMB in EGFR-mutant patients is related to the fact that the EGFR mutation is more common in never smokers, who have a lower mutational rate. Meanwhile, TMB differs through different EGFR mutation subtypes. In EGFR19 del tumors, TMB is comparable to those in EGFR20Ins and EGFRL861Q, but significantly lower than that in EGFRL858R (TMB in this study was calculated as the total number of non-synonymous mutations divided by the coding region captured for each individual platform).27 TMB in the EGFRG719 group is also higher than that in EGFR19del.27 Thus, TMB may be one potential explanation for the distinct responses to ICIs in different subtypes of EGFR mutants.
Low TMB levels and subsequent neoantigens result in limited efficacy of ICIs in EGFR mutants, and combined therapy aims to alter TMB to increase the potency of ICIs. Given the paucity of data from NSCLC, evidence from ovarian cancer showed that post-treatment samples harbored more somatic mutations than did pretreatment samples and exhibited evidence of chemotherapy-associated mutations.37 Moreover, chemotherapy can contribute to a minor, but detectable and specific, increase in neoantigen levels.38 On the other hand, focal radiation therapy may upregulate neoantigen release by the abscopal effect and increase ICI efficacy.39,40 Therefore, it is reasonable to speculate that elevated TMB induced by chemotherapy or radiotherapy may be responsible for the enhanced responsiveness to combined treatment strategies.
However, the methodological shortcomings for TMB calculation should be noted. Although associated, the absolute values of TMB estimated by whole-exome sequencing differ from that by gene panels, and the degree of difference is greater for estimations using different gene panels. Consequently, universal cut-off values for high, medium, and low levels of TMB are lacking. If these questions remain unanswered, TMB is unlikely to be utilized in clinical practice. Further investigations are required to draw valid conclusions.
Programmed death-ligand 1
Another crucial biomarker for immunotherapy efficacy is PD-L1 expression on tumor cells as a biomarker independent of TMB. Early retrospective studies exhibited higher levels of cell-surface expression of PD-L1 in NSCLC cell lines with EGFR mutations compared to wild-type cells.41,42 It was suggested that EGFR mutations were involved in the upregulation of PD-L1 expression, possibly via the IL-6/JAK/STAT3 signaling pathway and p-ERK1/2/p-c-Jun pathway.43,44 However, negative relationships of EGFR mutation and high PD-L1 expression level with unfavorable treatment responses were more strongly supported in recent real-world data, large cohort studies, and meta-analyses.27,45–50 It is unclear whether EGFR mutations upregulate or downregulate PD-L1 expression. Notably, differences in antibody choice and TPS cut-off values used in PD-L1 evaluation affect the results. In addition to expression, stability of PD-L1 is also a contributing factor. It was observed in breast cancer that activation of EGFR stabilized PD-L1 via GSK3b inactivation, and in mouse models that gefitinib could destabilize PD-L1, enhance antitumor T-cell immunity and therapeutic efficacy of PD-1 inhibitor.51 But these associations require further validation in lung cancer.
To date, EGFR-TKIs remain the first-line choice for treatment of EGFR mutants, which is supported by strong evidence.52 Changes in PD-L1 expression were observed in EGFR+ NSCLC after treatment with TKIs. TKI treatment can induce PD-L1 upregulation, inhibit proliferation, and slightly promote T cell apoptosis, followed by immune escape and the development of TKI resistance.53–55 Technically, ICIs are offered as later treatment following TKIs. T790M-negative patients with EGFR+ NSCLC were reported to be more likely to benefit from nivolumab after EGFR-TKI treatment, possibly as a result of elevated PD-L1 expression.56,57 Interestingly, conflicting results have also been reported. Experimental studies showed that activation of the EGFR pathway upregulated PD-L1 through p-ERK1/2/p-c-Jun, resulting in immunosuppression.44,58 Application of EGFR-TKIs may enhance antitumor immunity through the downregulation of PD-L1.44,58,59 Also, anti-CTLA4 antibody can induce PD-L1 expression, which is mediated by CTLA4 and the EGFR pathway involving phosphorylation of MEK and ERK.60 In turn, the adverse effects of tumor PD-L1 expression on EGFR-TKI efficacy were observed, especially in NSCLC patients with de novo resistance.61 However, two emerging studies showed that PD-L1 expression did not affect the efficacy of EGFR-TKIs in EGFR-mutated NSCLC.62,63 In addition, there was a significant increase in PD-L1+ T cells after one week of EGFR-TKI in patients whose disease progressed compared to patients without disease progression.64 The questions remain whether TKI upregulates or downregulates PD-L1 expression and whether a dual-phase regulation occurs, that is, downregulation of PD-L1 at an early phase together with potent antitumor capability of TKIs, followed by upregulation with the development of TKI resistance. If the latter is the case, dynamic monitoring of PD-L1 expression during TKI treatment may capture the optimal timing for the switch from TKIs to an immune-based strategy. Of note, dynamic monitoring requires repeated tissue biopsies thus the feasibility is limited. Also, the appropriate interval between PD-L1 evaluations is an issue to be determined. Exploration of substitute biomarker in peripheral blood sample may be one of the directions of future efforts.
Due to the extremely poor response of EGFR+ patients with low PD-L1 expression to ICIs, the question of how to upregulate PD-L1 expression to maximize the efficacy of ICIs has become a critical issue. As discussed previously, early clinical trials demonstrated encouraging therapeutic efficacy of combined treatment in EGFR+ NSCLC.13,26,65 Cohort studies in specific EGFR populations are lacking. However, we may gain some insight from evidence in non-EGFR-selected patients. Chemotherapy can increase PD-L1 expression in 30–40% of NSCLC patients,66–69 especially with platinum-based neoadjuvant chemotherapy.68,69 Radiotherapy plus ICIs demonstrated meaningful clinical benefits, leading to synergistic enhancement of the antitumor effect.65,70,71 Radiotherapy may activate non-inflamed NSCLC towards a more inflamed tumor microenvironment and may induce PD-L1 expression.65,72–74 The effects of antiangiogenesis therapy on PD-L1 expression are currently unknown.
Tumor microenvironment
The TME, the internal environment for tumor survival and growth, involves an immune regulatory network that includes effector T cells, macrophages, regulatory T cells (Tregs), dendritic cells (DCs), mast cells, natural killer (NK) cells, myeloid-derived suppressor cells (MDSCs), and cytokines. Tregs, MDSCs, and partial cytokines usually show immunosuppressive effects by formation of an immunosuppressive environment. Importantly, EGFR-mutant NSCLC has a unique TME.75 EGFR mutation and application of EGFR-TKI may have an impact on TME and thus influence the efficacy of immunotherapy.
Tumor-infiltrating lymphocytes (TILs) are a group of lymphocytes that infiltrate tumor nests and stroma. CD8+ TIL exerts major tumor-killing effects, and its high infiltration was shown to be associated with beneficial outcomes in NSCLCs receiving immunotherapy.76 However, EGFR mutants present with both significantly reduced levels of CD8+ TIL45,56,77 and depleted CD8+ TIL function,78 leading to impaired cytotoxicity and resultant poor response to ICIs. Meanwhile, a higher ratio of PD-L1− to TIL− cells was observed in EGFR-mutant patients compared to the EGFR wild-type group.45 PD-L1−/TIL− TME lacks an immune reaction, leading to a poor prognosis.79 The combination of anti-CTLA-4 agents and anti-PD-1 agents may be a potential strategy to bring T cells into the tumor and avoid their being turned off.79
Tregs, a major group of active immunosuppressive cells, secrete transforming growth factor (TGF)-β, interleukin (IL)-10, and IL-35 to form an immunosuppressive TME, downregulating NK cells and CD4+, CD8+ T cells.75 Amphiregulin (AME) is an epidermal growth factor (EGF)-like growth factor released by mast cells that is often associated with a poor prognosis in cancer patients.80,81 AME may interact with EGFR expressed on the Treg surface, resulting in overactivation of Treg via the EGFR/GSK-3β/Foxp3 axis in NSCLC, leading to increased immune escape in EGFR mutants.82,83
Other immunosuppressive cells also exert an impact on TME. MDSCs are associated with suppression of the immune response, inducing angiogenesis, and promoting metastasis in tumor growth.84 Tumor-associated macrophages (TAMs) could be stimulated by colony stimulating factor 1 (CSF1) and produce EGF, thus activating the EGFR pathway and promoting cell growth and survival.85,86
CD73 is a negative immunomodulatory molecule expressed on the tumor cell membrane that induces the formation of adenosine, an immunosuppressive mediator, which favors the development of Tregs and MDSCs, hindering the process of antigen presentation and tumor-killing effects.62 The CD73–adenosine axis is involved in tumor immune escape, and high levels of CD73 expression are associated with poor outcomes in NSCLC patients.62,63 Importantly, studies in NSCLC patients have found an association between EGFR mutation and elevated expression of CD73, and studies in NSCLC cell lines revealed dose-dependent inhibition of CD73 expression associated with EGFR-TKI treatment.63,87 These findings may support the combination of an anti-CD73 agent with an anti-PD-1/PD-L1 agent as a potential strategy for treatment of EGFR-mutant NSCLC.62,87
Exosomes are small vesicles that are secreted by tumor cells and carry molecules that affect the microenvironment.75 EGFR-containing exosomes derived from cancer cells can be delivered into the liver and develop a microenvironment promoting liver-specific metastasis.88 However, the role of this process in immune efficacy in the treatment of NSCLC must be explored further.
The TME of EGFR-aberrant NSCLC is not a static immune background. A recent study in a mouse model demonstrated a dynamic impact of EGFR-TKI on TME from beneficial in the early stages to immunosuppressive in later stages,89 consistent with the hypothesis discussed above in the PD-L1 section. In early treatment, EGFR-TKI induced a short-term tumor inhibitory TME by increasing CD8+ T cells and DCs, inhibiting Tregs and M2-like polarization. However, TME gradually transformed into an immunosuppressive microenvironment with upregulation of MDSCs and increased secretion of IL-10 and C–C motif chemokine ligand 2 (CCL2). The latter two molecules further mediated the activation of MDSCs via the STAT3 pathway and eventually induced immunosuppression. The authors therefore proposed the concept of a window period, the optimal timing for administration of immune agents in EGFR mutants when TME is the most immune-promoting under the use of EGFR-TKI. In advanced melanoma, patients with TME of PD-L1+/TILs+ showed greater benefit from a single anti-PD(L)-1 blocker.79 Whether the TKI-ICI-favored window and benefit of immunoinflammatory TME can be reproduced in NSCLC remains unknown, and further validation in clinical trials is urgently required.
Neoangiogenesis induced by tumor cells in the TME will promote a hypoxic and acid milieu, contributing to immunosuppression by activating Tregs, inhibiting effector T cells, and recruiting TAMs.86,90,91 In a mouse lung cancer model, low-dose apatinib, an antiangiogenesis TKI, reversed the immunosuppressive TME, and its combination with ICIs retarded tumor growth and prolonged survival.92 Therefore, EGFR-mutant patients may benefit from the addition of an antiangiogenic agent to immunotherapy due to the modulatory effect on the TME.86 Clinically, the IMpower150 trial in line has drawn additional survival benefit in EGFR-mutant patients when treated by immunotherapy, chemotherapy plus antiangiogenic agent.26
EGFR aberrations and immune checkpoint inhibitor-associated hyperprogressive disease
A novel pattern of hyperprogressive disease (HPD) was recognized as significantly accelerated tumor growth rate (TGR) after anti-PD-1/PD-L1 therapy. The evaluation of HPD is usually based on time to treatment failure (TTF), the increase in tumor burden compared with pre-immunotherapy imaging, and TGR.93 The reported prevalence of HPD in NSCLC after ICI monotherapy varies from 6% to 25% due to various definitions of HPD, but is inconclusive in the EGFR-mutant subgroup.93–95 Older age, MDM2 family amplification, and EGFR alterations were correlated with poorer outcomes and markedly increased TGR after single-agent immunotherapy.93,96 In patients with metastatic cancer, regardless of the tumor type, 20% of patients with EGFR mutations exhibited a pattern of hyperprogression.93 In NSCLC patients receiving a single ICI agent, a higher rate of EGFR mutation was observed in the overgrowth subgroup compared to the non-hyperproliferative subgroup.97
NSCLC patient-derived xenograft (PDX) with EGFR L858R mutation in severe combined immunodeficiency disease (SCID) mice exhibited HPD after nivolumab treatment, with tumor-associated macrophages playing a pivotal role.95 Our group recently presented a case harboring EGFR 20 exon insertion and MYC amplification and suffering from HPD after treatment with nivolumab, leading rapidly to death within 2 months.98 The rebound effect induced by the sudden quit from TKI treatment might well be one of the causes of the hyperprogression. However, conflicting results have also been reported in NSCLC. None of 16 patients with mutant EGFR status showed HPD, and there was no difference in the rate of EGFR mutation between the HPD and non-HPD groups (25.8% versus 19.8%, respectively; p = 0.439) when treated with ICIs.94,99 The association between EGFR mutation and HPD is controversial, as is the potential mechanism underlying disease deterioration. Further in-depth studies of the mechanisms of HPD are required, along with the development of screening methods to identify specific subgroups in whom immune treatment would be harmful.
Conclusions and perspectives
TKI remains the preferred first-line therapy for EGFR-mutant NSCLC, with strong evidence. Although recommended by the National Comprehensive Cancer Network (NCCN) guidelines, ICI monotherapy at later rounds of treatment should be applied with caution in EGFR-aberrant NSCLC, given the limited efficacy and the risk of HPD. ICIs plus chemotherapy with/without anti-angiogenesis agent is a potentially effective strategy for NSCLC with EGFR aberrations after exhaustion of targeted therapies.
Future directions to improve the efficacy and safety of immunotherapy in EGFR aberrant subgroup includes: first, test of different combination of agents on PD-1/PD-L1 pathway with other classic approaches, such as chemotherapy, radiotherapy, anti-angiogenesis drug and even TKIs. For example, checkMate 722 (NCT02864251) and KEYNOTE-789 (NCT03515837) evaluates PD-1 inhibitor plus chemotherapy in EGFR-mutated advanced NSCLCs either selected by T790M or regardless of T790M status.19,20 Trial ORIENT-31 (NCT03802240) assessed the therapeutic effect of sintilimab plus IBI305 (an anti-vascular endothelial growth factor (VEGF) monoclonal antibody) plus pemetrexed-cisplatin in EGFR-mutant advanced NSCLCs who have failed with EGFR-TKIs.100 The results of these ongoing studies will provide stronger evidence for combined back-line immune therapy in EGFR-mutated NSCLCs.
Second, application of novel immune agents other than PD-1/PD-L1 inhibitors in terms of the TME characteristics of EGFR-mutant lung cancer; NSCLC with EGFR mutation presents unique immune characteristics. A reduced level and depleted function of TIL has been reported in EGFR mutants. Persistent antigen-stimulation in cancer induces LAG3 expression, promoting T cell exhaustion.101 Thus, agents such as eftilagimod alpha (IMP321), a recombinant LAG-3Ig fusion protein, is expecting to be functional. Pilot results showed that IMP321 combined with pembrolizumab achieved an ORR of 47% in NSCLC at first-line.102 In addition, activation of EGFR is associated with overactivation of Tregs. CD36, as a metabolic modulator, made Tregs more adaptable to TME.103 Genetic ablation of CD36 in Tregs suppressed tumor growth and targeting CD36 enhanced antitumor efficacy of PD-L1 therapy.103 CD73 was also involved in immune escape in EGFR-mutant NSCLC. Study of TJ004309, an anti-CD73 drug, in combination with atezolizumab in patients with advanced or metastatic cancer is in progress (NCT03835949).104 Collectively, these findings uncovered the therapeutic potential of targeting EGFR-related pathway in the future.
Third, identification of benefit population within EGFR-mutant patients by existing and emerging biomarkers; EGFR mutation subtypes, PD-L1 expression and TMB based on tissue sample helps screening the responding patients to immunotherapy. Immune microenvironment detection via multiplex immunohistochemistry/immunofluorescence presented improved performance in evaluating the efficacy of anti-PD-(L)1 treatment.105 Moreover, circulating tumor DNA (ctDNA)106 and exosomal PD-L1 expression107 is capable of identifying clinical responders to PD-1 inhibitor, serving as potential biomarkers. In addition, TCR diversity and clonality in peripheral blood PD-1+ CD8+ T cells is the emerging predicting biomarker in NSCLC.108 More potential biomarkers in tissue and, importantly, blood, remain for further discovery. Utilization of these novel biomarkers would be of great help to screen out the benefit population among EGFR mutants.
The optimal ICI-based strategy for the EGFR-mutant population remains to be determined. Further studies are required to determine the mechanisms underlying the poor response of EGFR-mutant patients to ICIs. Individualized and precise strategies are required to modify or reverse resistance and render the lesions sensitive to ICIs.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [81870022] and the Zhejiang Provincial Natural Science Foundation [LY20H010004].
Conflict of interest: The authors declare that there is no conflict of interest.
ORCID iD: Yang Xia  https://orcid.org/0000-0003-2487-2244
https://orcid.org/0000-0003-2487-2244
Contributor Information
Rui Jin, Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Jing Zhao, Department of Medical Oncology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Lexin Xia, Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Qin Li, Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Wen Li, Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Ling Peng, Department of Radiotherapy, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310000, China.
Yang Xia, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, 88 Jiefang Road, Hangzhou, Zhejiang 310009, China.
References
- 1. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29: 2866–2874. [DOI] [PubMed] [Google Scholar]
- 2. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 3. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 4. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 5. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 8. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garon EB, Wolf B, Lisberg A, et al. Prior TKI therapy in NSCLC EGFR mutant patients associates with lack of response to anti-PD-1 treatment. J Thorac Oncol 2015; 10: S269. [Google Scholar]
- 11. Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol 2018; 13: 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Creelan BC, Yeh T, Kim SW, et al. Phase I study of gefitinib (G) plus durvalumab (D) for locally advanced/metastatic non-small cell lung cancer (NSCLC) harbouring epidermal growth factor receptor (EGFR) sensitising mutations. Ann Oncol 2019; 30 (Suppl. 2): ii31–ii37. [Google Scholar]
- 15. Yang JCH, Gadgeel SM, Sequist LV, et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol 2019; 14: 553–559. [DOI] [PubMed] [Google Scholar]
- 16. Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 2019; 30: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riely G, Hui R, Carbone D, et al. Phase 3 study of pemetrexed-platinum with or without pembrolizumab for TKI-resistant/EGFR-mutated advanced NSCLC: KEYNOTE-789. J Thorac Oncol 2018; 13: S494. [Google Scholar]
- 20. Nakagawa K, Yang JCH, Park K, et al. Checkmate 722: a phase 3 trial of nivolumab with chemotherapy or ipilimumab vs chemotherapy in epidermal growth factor receptor (EGFR)-mutation, T790M-negative stage IV or recurrent non-small cell lung cancer (NSCLC) after EGFR tyrosine kinase inhibitor (TKI) therapy. Ann Oncol 2016; 27 (Suppl. 9): ix139–ix156. [Google Scholar]
- 21. Hayashi H, Chiba Y, Sakai K, et al. A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for patients with EGFR mutation-positive nonsquamous non-small-cell lung cancer who acquire resistance to tyrosine kinase inhibitors not due to a secondary T790M mutation: rationale and protocol design for the WJOG8515L study. Clin Lung Cancer 2017; 18: 719–723. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Zhou C, Zhao Y, et al. A PII study of toripalimab, a PD-1 mAb, in combination with chemotherapy in EGFR+ advanced NSCLC patients failed to prior EGFR TKI therapies. J Thorac Oncol 2019; 14: S292. [Google Scholar]
- 23. Gubens MA, Sequist LV, Stevenson JP, et al. Pembrolizumab in combination with ipilimumab as second-line or later therapy for advanced non-small-cell lung cancer: KEYNOTE-021 cohorts D and H. Lung Cancer 2019; 130: 59–66. [DOI] [PubMed] [Google Scholar]
- 24. Gettinger S, Rizvi N, Chow LQ, et al. 1054PDNivolumab (ANTI-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) or Erlotinib (ERL) in advanced non-small cell lung cancer (NSCLC). Ann Oncol 2014; 25 (Suppl. 4): iv363. [Google Scholar]
- 25. Ahn MJ, Yang J, Yu H, et al. Osimertinib combined with durvalumab in EGFR-mutant non-small-cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol 2016; 11: S115. [Google Scholar]
- 26. Mok TSK, Socinski MA, Reck M, et al. IMpower150: an exploratory analysis of efficacy outcomes in patients with EGFR mutations. Ann Oncol 2018; 29 (Suppl. 9): ix173–ix178. [Google Scholar]
- 27. Hastings K, Yu H, Wei W, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol 2019; 30: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019; 30: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018; 24: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 31. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016; 34: 9017. [Google Scholar]
- 34. Offin M, Rizvi H, Tenet M, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res 2019; 25: 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorotsky H, Cabanero M, Moskovitz M, et al. The prognostic effect of tumor mutation burden and smoking history in resected EGFR mutant non-small-cell lung cancer. J Thorac Oncol 2018; 13: S921. [Google Scholar]
- 36. Singal G, Miller PG, Agarwala V, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small-cell lung cancer using a clinicogenomic database. JAMA 2019; 321: 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015; 521: 489–494. [DOI] [PubMed] [Google Scholar]
- 38. O’Donnell T, Christie EL, Ahuja A, et al. Chemotherapy weakly contributes to predicted neoantigen expression in ovarian cancer. BMC Cancer 2018; 18: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018; 24: 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkins A, McDonald F, Harrington K, et al. Radiotherapy enhances responses of lung cancer to CTLA-4 blockade. J Immunother Cancer 2019; 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small-cell lung cancer. Ann Oncol 2014; 25: 1935–1940. [DOI] [PubMed] [Google Scholar]
- 42. D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015; 112: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small-cell lung cancer. Int J Oncol 2016; 49: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 44. Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 2015; 10: 910–923. [DOI] [PubMed] [Google Scholar]
- 45. Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small-cell lung cancer. Oncoimmunology 2017; 6: e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep 2017; 7: 10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dietel M, Savelov N, Salanova R, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer 2019; 134: 174–179. [DOI] [PubMed] [Google Scholar]
- 48. Li L, Guo C, Guo L, et al. PD-L1 expression in primary lung adenocarcinoma and its relation with EGFR/KRAS mutation and clinicopathological features. J Thorac Oncol 2018; 13: S761. [Google Scholar]
- 49. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small-cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22: 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol 2016; 11: 1879–1890. [DOI] [PubMed] [Google Scholar]
- 51. Li CW, Lim SO, Xia W, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun 2016; 7: 12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non-Small Cell Lung Cancer, Version 4.2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed 20 May 2020).
- 53. Omori S, Kenmotsu H, Abe M, et al. Changes in programmed death ligand 1 expression in non-small-cell lung cancer patients who received anticancer treatments. Int J Clin Oncol 2018; 23: 1052–1059. [DOI] [PubMed] [Google Scholar]
- 54. Jiang L, Guo F, Liu X, et al. Continuous targeted kinase inhibitors treatment induces upregulation of PD-L1 in resistant NSCLC. Sci Rep 2019; 9: 3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fumagalli C, Guerini-Rocco E, Vacirca D, et al. The immune profile of EGFR-mutated non-small-cell lung cancer at disease onset and progression after tyrosine kinase inhibitors therapy. Immunotherapy 2018; 10: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 56. Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol 2017; 28: 1532–1539. [DOI] [PubMed] [Google Scholar]
- 57. Hata A, Katakami N, Nanjo S, et al. Programmed death-ligand 1 expression and T790M status in EGFR-mutant non-small-cell lung cancer. Lung Cancer 2017; 111: 182–189. [DOI] [PubMed] [Google Scholar]
- 58. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013; 3: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin K, Cheng J, Yang T, et al. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun 2015; 463: 95–101. [DOI] [PubMed] [Google Scholar]
- 60. Zhang H, Dutta P, Liu J, et al. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small-cell lung cancer. J Cell Mol Med 2019; 23: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Su S, Dong ZY, Xie Z, et al. Strong programmed death ligand 1 expression predicts poor response and De Novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol 2018; 13: 1668–1675. [DOI] [PubMed] [Google Scholar]
- 62. Brown H, Vansteenkiste J, Nakagawa K, et al. Programmed cell death ligand 1 expression in untreated EGFR mutated advanced NSCLC and response to osimertinib versus comparator in FLAURA. J Thorac Oncol 2020; 15: 138–143. [DOI] [PubMed] [Google Scholar]
- 63. Hsu PC, Wang CW, Kuo SCH, et al. The co-expression of programmed death-ligand 1 (PD-L1) in untreated EGFR-mutated metastatic lung adenocarcinoma. Biomedicines 2020; 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meniawy TM, Lake RA, McDonnell AM, et al. PD-L1 on peripheral blood T lymphocytes is prognostic in patients with non-small-cell lung cancer (NSCLC) treated with EGFR inhibitors. Lung Cancer 2016; 93: 9–16. [DOI] [PubMed] [Google Scholar]
- 65. Gong X, Li X, Jiang T, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small-cell lung cancer. J Thorac Oncol 2017; 12: 1085–1097. [DOI] [PubMed] [Google Scholar]
- 66. Sakai H, Takeda M, Sakai K, et al. Impact of cytotoxic chemotherapy on PD-L1 expression in patients with non-small-cell lung cancer negative for EGFR mutation and ALK fusion. Lung Cancer 2019; 127: 59–65. [DOI] [PubMed] [Google Scholar]
- 67. Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small-cell lung cancer. Sci Rep 2016; 6: 20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shin J, Chung JH, Kim SH, et al. Effect of platinum-based chemotherapy on PD-L1 expression on tumor cells in non-small-cell lung cancer. Cancer Res Treat 2019; 51: 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lacour M, Hiltbrunner S, Lee SY, et al. Adjuvant chemotherapy increases programmed death-ligand 1 (PD-L1) expression in non-small-cell lung cancer recurrence. Clin Lung Cancer 2019; 20: 391–396. [DOI] [PubMed] [Google Scholar]
- 70. Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small-cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019; 5: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen H, Zhao L, Fu K, et al. Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 2019; 9: 7948–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adams DL, Adams DK, He J, et al. Sequential tracking of PD-L1 expression and RAD50 induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin Cancer Res 2017; 23: 5948–5958. [DOI] [PubMed] [Google Scholar]
- 73. Takamori S, Toyokawa G, Takada K, et al. Combination therapy of radiotherapy and Anti-PD-1/PD-L1 treatment in non-small-cell lung cancer: a mini-review. Clin Lung Cancer 2018; 19: 12–16. [DOI] [PubMed] [Google Scholar]
- 74. Yoneda K, Kuwata T, Mori M, et al. Alteration in tumor immune microenvironment after chemo-radiotherapy for locally advanced non-small cell lung cancer. J Clin Oncol 2019; 37 (Suppl. 15): 8530. [Google Scholar]
- 75. Lin A, Wei T, Meng H, et al. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer 2019; 18: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bremnes RM, Busund LT, Kilvaer TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol 2016; 11: 789–800. [DOI] [PubMed] [Google Scholar]
- 77. Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8+ tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res 2018; 24: 407–419. [DOI] [PubMed] [Google Scholar]
- 78. Toki MI, Mani N, Smithy JW, et al. Immune marker profiling and programmed death ligand 1 expression across NSCLC mutations. J Thorac Oncol 2018; 13: 1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Teng MWL, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75: 2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Higginbotham JN, Beckler MD, Gephart JD, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol 2011; 21: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zaiss DM, Yang L, Shah PR, et al. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science 2006; 314: 1746. [DOI] [PubMed] [Google Scholar]
- 82. Wang S, Zhang Y, Wang Y, et al. Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3β/Foxp3 axis. J Biol Chem 2016; 291: 21085–21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zaiss DMW, van Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013; 38: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Poggio M, Hu T, Pai CC, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019; 177: 414–427; e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004; 64: 7022–7029. [DOI] [PubMed] [Google Scholar]
- 86. Santaniello A, Napolitano F, Servetto A, et al. Tumour microenvironment and immune evasion in EGFR addicted NSCLC: hurdles and possibilities. Cancers (Basel) 2019; 11: 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Park LC, Rhee K, Kim WB, et al. Immunologic and clinical implications of CD73 expression in non-small cell lung cancer (NSCLC). J Clin Oncol 2018; 36 (Suppl. 15): 12050. [Google Scholar]
- 88. Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun 2017; 8: 15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jia Y, Li X, Jiang T, et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: implications for combination therapies. Int J Cancer 2019; 145: 1432–1444. [DOI] [PubMed] [Google Scholar]
- 90. Fukurnura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15: 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Westendorf AM, Skibbe K, Adamczyk A, et al. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting treg activity. Cell Physiol Biochem 2017; 41: 1271–1284. [DOI] [PubMed] [Google Scholar]
- 92. Zhao S, Ren S, Jiang T, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res 2019; 7: 630–643. [DOI] [PubMed] [Google Scholar]
- 93. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018; 4: 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res 2019; 25: 989–999. [DOI] [PubMed] [Google Scholar]
- 96. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 97. Kim S, Kim J, Hong S, et al. Hyperprogression and pseudoprogression in patients with non-small cell lung cancer on checkpoint blocking immunotherapy. J Thorac Oncol 2018; 13: S888–S889. [Google Scholar]
- 98. Huang X, Xia L, Lan F, et al. Treatment of nivolumab results in hyperprogressive disease in a patient harboring EGFR exon 20 insertion and MYC amplification. J Thorac Oncol 2019; 14: e189–e191. [DOI] [PubMed] [Google Scholar]
- 99. Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019; 30: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 100. ClinicalTrials.gov. Sintilimab ± IBI305 plus chemotherapy (Pemetrexed + Cisplatin) for EGFRm + locally advanced or metastasis non-squamous NSCLC patients After EGFR-TKI treatment failure. https://clinicaltrials.gov/ct2/show/NCT03802240. (accessed 22 April 2020).
- 101. Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12: 492–499. [DOI] [PubMed] [Google Scholar]
- 102. Immutep reports positive TACTI-002 data. https://seekingalpha.com/pr/17783262-immutep-reports-positive-tactiminus-002-data (2020) (accessed 22 April 2020).
- 103. Wang H, Franco F, Tsui YC, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol 2020; 21: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. ClinicalTrials.gov. Study of TJ004309 in combination with atezolizumab (Tecentriq) in patients with advanced or metastatic cancer. https://clinicaltrials.gov/ct2/show/NCT03835949 (accessed 22 April 2020).
- 105. Lu S, Stein JE, Rimm DL, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol 2019; 5: 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Anagnostou V, Forde PM, White JR, et al. Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res 2019; 79: 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018; 560: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Han J, Duan J, Bai H, et al. TCR repertoire diversity of peripheral PD-1+ CD8+ T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res 2020; 8: 146–154. [DOI] [PubMed] [Google Scholar]