Highlights
-
•
Expression is restricted to epithelial cells.
-
•
Gene knockout promotes increased risk of cancer.
-
•
Is associated with fibrosis and multiple cancers.
-
•
Mutations in humans have been associated with alopecia, amelogenesis imperfecta, periodontitis and emphysema, matching murine data.
Abbreviations: LAP, Latency Associated Peptide; TGFβ1, Transforming growth factor β1; OSCC, oral squamous cell carcinoma cells; STAT3, signal transducer and activator transcription 3; HDAC, Histone deacetylase; CREB, cAMP response element; HAT, histone acetyltransferase; UTR, Untranslated region
Keyword: Integrin, Cancer, Fibrosis
Abstract
Integrin αvβ6 is a membrane-spanning heterodimeric glycoprotein involved in wound healing and the pathogenesis of diseases including fibrosis and cancer. Therefore, it is of great clinical interest for us to understand the molecular mechanisms of its biology. As the limiting binding partner in the heterodimer, the β6 subunit controls αvβ6 expression and availability. Here we describe our understanding of the ITGB6 gene encoding the β6 subunit, including its structure, transcriptional and post-transcriptional regulation, the biological effects observed in ITGB6 deficient mice and clinical cases of ITGB6 mutations.
Introduction
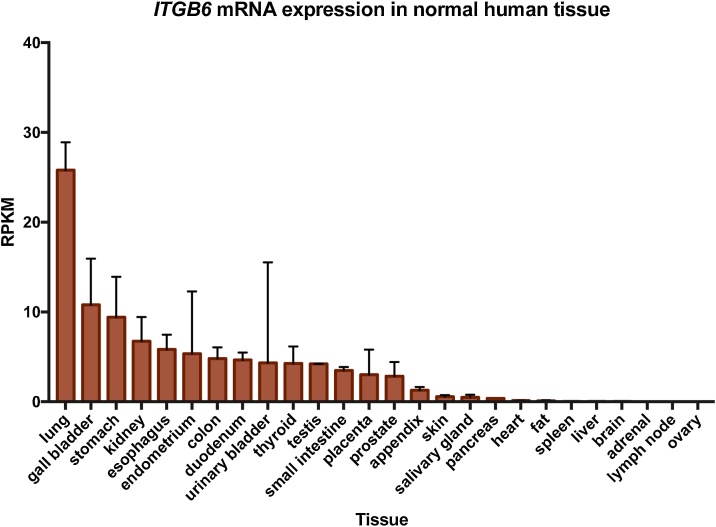
Integrins are heterodimeric cell surface proteins that facilitate signalling between the intracellular and extracellular environments. Each integrin consists of one α and one β subunit. Together, they are capable of forming 24 unique integrins in humans (reviewed by Hynes, 2002). αvβ6 is expressed exclusively on epithelial cells but is absent or expressed at low levels in normal healthy adult tissue (Breuss et al., 1993). In contrast, αvβ6 expression is increased during development (Breuss et al., 1995), wound healing (Haapasalmi et al., 1996), cancer (reviewed by Bandyopadhyay and Raghavan, 2009) and fibrosis (Munger et al., 1999), all of which are processes requiring tissue remodelling (Fig. 1).
Fig. 1.
ITGB6 RNA expression in human normal tissue plotted as rates per kilobase million (RPKM). Data were obtained from Human Protein Atlas Dataset available from proteinatlas.org.
<https://www.proteinatlas.org/ENSG00000115221-ITGB6/tissue#gene_information>
αvβ6 binds to proteins containing the RGD (Arg-Gly-Asp) motif (Busk et al., 1992). Well characterised αvβ6 ligands include fibronectin (Busk et al., 1992) and the Transforming Growth Factor Beta (TGF-β) pro-peptide, Latency Associated Peptide (LAP) (Munger et al., 1999; Annes et al., 2002). LAP is post-translationally cleaved from TGF-β1 but remains directly associated with the cytokine, anchored as a homodimer to the extracellular matrix (ECM) via latent TGFβ binding protein 1 (Annes et al., 2004). This whole complex remains inert and is referred to as latent TGF-β1. Binding of αvβ6 to LAP physically moves LAP and exposes TGF-β1 to its receptors on adjacent cells, permitting an acto-myosin dependent activation of TGF-β1 (Munger et al., 1999, Annes et al., 2004). Binding domains are also present on the cytoplasmic tail of integrin β6, which recruits signalling and structural partners including HAX-1, Fyn, ERK2, and Kindlin-1 (Ahmed et al., 2002; Li et al., 2003; Ramsay et al., 2007; Moser et al., 2008). Extracellular ligand binding to αvβ6 is able to effect a conformational change in the cytoplasmic tail and thus initiate downstream signalling (outside-in signalling), while cytoplasmic tail binding may also affect extracellular ligand binding (inside-out signalling).
Through these and other signalling pathways, αvβ6 regulates many basic functions of the cell, such as proliferation (Agrez et al., 1994), migration (Huang et al., 1998), and ECM degradation (Thomas et al., 2001; Morgan et al., 2004). Expression of αvβ6 is found in up to one third of all solid tumours (Saha et al., 2010) and is associated with a poorer prognosis and increased invasiveness (Niu and Li, 2017). αvβ6 expression is also associated with the progression of pulmonary fibrosis through its activation of TGF-β1 (Goodwin and Jenkins, 2009).
The β6 subunit was first isolated from guinea pig epithelial cells in 1990 (Sheppard et al., 1990). At this time, only three β subunit structures had been identified in mammalian cells, with emerging evidence for several other subunits (Cheresh et al., 1989). Sheppard and colleagues, observing that β1, β2 and β3 had large consensus regions in their sequences, designed primers against these conserved regions and successfully amplified β6. Further screening in human cell lines led to the identification of the β6 nucleotide sequence from the pancreatic cell line FG-2. It was later determined that ITGB6 was located on chromosome 2 (Krissansen et al., 1992), specifically 2q24.2 (Fernandez-Ruiz and Sanchez-Madrid, 1994).
Transcriptional regulation
As the exclusive binding partner of αv, β6 availability determines the cellular expression of αvβ6. Breakthrough work characterising the structure and transcriptional regulation of ITGB6 was performed by Xu et al., 2015. They identified both the transcriptional start site (TSS), 204 bp upstream of the translation initiation site, and the core promoter of ITGB6 using oral squamous cell carcinoma cells (OSCCs). A functional TATA box was located -24bp to -18bp upstream of the TSS, and binding sites for transcription factors C/EBPα (CCAAT-enhancer-binding protein α) and signal transducer and activator transcription 3 (STAT3) between -289bp and -150bp. Further binding sequences for Ets-1 and Smad proteins were also identified, however the basal rate of ITGB6 expression in OSCC cells was primarily defined by STAT3 and C/EBPα binding (Xu et al., 2015).
This was not the first time STAT3 had been implicated in the possible regulation of ITGB6. Previous work showed that constitutive STAT3 activation led to an increase in cell migration and motility in prostate cancer cell lines (Azare et al., 2007). This was attributed to an increase in ITGB6 mRNA expression within 4 hours of STAT3 simulation, whereas inhibition of STAT3 by shRNA resulted in downregulation of ITGB6 mRNA.
Work performed in colon carcinoma cell lines by Bates and colleagues in 2005 also revealed a binding domain for Ets-1 in the ITGB6 promoter region. As an initiating factor of the epithelial-mesenchymal transition (EMT), it was hypothesised that Ets-1 could possibly play a role in ITGB6 expression. They demonstrated that Ets-1 was able to bind to the ITGB6 promoter region and transactivate the promoter, resulting in increased ITGB6 transcription (Bates et al., 2005).
In 1992, it was discovered that TGF-β1 works as part of a positive feedback loop promoting expression of αvβ6 (Sheppard et al., 1992). In an effort to determine the mechanisms by which TGF-β1 regulates ITGB6 expression using a pulmonary fibrosis model, Tatler and colleagues identified the location of a suppressor region in the ITGB6 gene (Tatler et al., 2016a,b). They found that deletion of the region -818bp to -738bp leads to a significant increase in ITGB6 expression. This confirmed work by Xu and colleagues, who also showed that deletion from -909bp to -421bp leads to maximum transcriptional activity (Xu et al., 2015).
Another transcription factor of the Ets family was also shown to have a role in regulating ITGB6 (Tatler et al., 2016a,b). Following in silico analysis, it was found that the ETS domain-containing protein Elk1 was able to bind to a site in the apparent suppressor region and reduce ITGB6 expression. Loss of Elk1 in vivo led to an increased basal expression ITGB6 mRNA (Tatler et al., 2016a,b).
Smad transcription factors mediate classical TGF-β1 signalling (Derynck et al., 1998). Disruption of a Smad binding site within the same suppressor region of ITGB6 (-818bp to -738bp) led to abrogation of ITGB6 transcription induced by TGF-β1 (Tatler et al., 2016a,b). Inhibition of Smad3 led to a decrease in ITGB6 transcription and, moreover, Smad3-/- mice treated with TGF-β1 had reduced expression of αvβ6. TGF-β1-induced ITGB6 expression regulated by Smad proteins has been extensively documented, including in bile duct epithelial cells (BDECs) where Smad3 inhibition reduced TGF-β1 induced ITGB6 mRNA (Verrecchia and Mauviel, 2002; Schiffer et al., 2000). Conversely, Xu and colleagues (2015) concluded that Smad binding domain activity was not essential for basal expression in OSCCs.
Regulation of ITGB6 by TGF-β1 was further explored by Xu and colleagues (2017). They identified that the AP1 subunit JunB binds directly to the ITBG6 promoter region. Furthermore, they identified a role for histone H3 and H4 acetylation following treatment with TGF-β. Inhibition of Histone Deacetylase (HDAC) resulted in increased acetylation of H3 and H4, leading to an increased expression of ITGB6. Histone acetylation was initiated by recruitment of the cAMP response element binding protein (CBP), which acts as both a histone acetyltransferase (HAT) and a scaffold that promotes the recruitment of further components required for gene transcription (Korzus et al., 2004).
Given that transcription of ITGB6 is almost certainly a mechanism determining its availability at the cell surface, understanding its regulation could identify potential therapeutic targets in diseases where αvβ6 is overexpressed. Although recent work has greatly improved our understanding of the structure of the promoter region, and binding sites of transcription factors, extensive work still remains to fully characterise how these transcription factors regulate ITGB6 transcription (Fig. 2).
Fig. 2.
ITGB6 Gene Promoter Region Structure. ITGB6 contains a TATA box -18 bp upstream of the TSS (Transcriptional Start Site). An area found to promote ITGB6 transcription is located at -289bp to 150 bp, including binding domains for C/EBPα (CCAAT-enhancer-binding protein α), AP-1 (Activator Protein 1) and STAT3 (Signal transducer and activator of transcription 3). A suppressor region can be found -738bp to -818bp with a binding region for Elk1 ETS Like-1 protein). Smad3 (Smad Family Member 3) binding domain is also found in this region, but results in ITGB6 promotion. Translation Initiation Site (TIS) is found +204 bp downstream of the TSS. (Xu et al., 2015; Tatler et al., 2016a,b.
Post-Transcriptional Regulation
Following gene transcription, protein expression may also be regulated by multiple post-transcriptional processes. One of the most significant points of post-transcriptional regulation happens at the initiation of mRNA translation. Eukaryotic translation initiation factor 4 (eIF4E) binds to the 5’ end of mRNA, initiating protein translation (Rhoads, 1988). eIF4E is known to selectively increase the expression of proteins with ‘weak’ mRNA (Graff and Zimmer, 2003). This refers to proteins with a long and/or complex 5’ UTR (untranslated region), usually proteins expressed at low levels in healthy tissue, as opposed to ‘strong’ mRNAs, which have a short and simple 5’UTR and are usually housekeeping genes. eIF4E expression is known to be dysregulated in malignancy (, Siddiqui and Sonenberg, 2015). De Benedetti and Graff (2004) reviewed the effect of eIF4E in malignancy, describing its ability to selectively upregulate translation of some pro-tumorigenic proteins (e.g. Vascular Endothelial Growth Factor (VEGF)), which tend to have a more complex 5’UTR and require eIF4E for translation.
ITGB6 mRNA has a long 5’-UTR and is GC rich, both of which are characteristics of ‘weak’ mRNA. Combined expression of eIF4E and ITGB6 can predict a poorer prognosis in colon cancer (Niu et al., 2014). Furthermore, siRNA-induced depletion of eIF4E significantly reduced protein expression of β6 (Enyu et al., 2015). Further investigation in to eIF4E and αvβ6 expression in other models has yet to be performed. Thus, while we now know a significant number of the transcriptional regulators of ITGB6, we have not yet linked these data to how ITGB6 is upregulated in disease, especially cancer.
Mouse Models
Itgb6 knockout mice have been used to study the downstream effects of αvβ6 and improve our understanding of its biology. The first characterisation of Itgb6 deficient mice was published in 1996, with surprising results (Huang et al., 1996). Given the role αvβ6 plays in wound healing in human tissue (Haapasalmi et al., 1996), it was hypothesised that Itgb6 knockout mice would exhibit wound healing abnormalities. The authors also postulated that Itgb6 knockout mice would display developmental abnormalities due to the involvement of αvβ6 in cell migration and proliferation (Agrez et al., 1994; Huang et al., 1998). However, the Itgb6 knockout mice developed normally, with no difference in their wound healing ability. Rather, they exhibited moderate but significant inflammation in the lungs and skin. In the same study, Itgb6 knockout mice did not develop fibrosis in response to bleomycin. This phenotype is remarkably similar to TGF-β1 deficient mice, and is attributable to TGF-β1 activation by αvβ6 (Munger et al., 1999). Interestingly, inflammation was only observed in the skin and airways of Itgb6 knockout mice, despite the potential for expression of αvβ6 in other epithelial tissues. The authors offered this was perhaps due to the housing conditions of the mice causing irritation in the skin and lungs. Neonatal Itgb6 knockout mice also developed a temporary baldness. Whether this was due to the subsequent discovery that αvβ6 plays a role in hair follicle regeneration remains to be determined (Xie et al., 2012).
The fibrosis phenotype of Itgb6 knockout mice was further explored in Itgb6/Thrombospondin 1 (Tsp-1) double-null mice (Ludlow et al., 2005). Thrombospondin-1 also activates latent-TGF-β1 (Crawford et al., 1998) and in the double knockout model there was a higher incidence of inflammation and pneumonia, which more closely resembles the phenotype of TGF-β1 null mice (Shull et al., 1992). Whilst no significant differences in tumour incidence were observed between the Itgb6-/- and the Itgb6-/; Tsp-1-/- mice, the authors found that Itgb6-/- mice developed both benign and malignant tumours at a significantly higher rate than the Tsp-/- mice.
Another characteristic of Itgb6-/- mice is their eventual development of emphysema (Morris et al., 2003). In the alveolar macrophages of Itgb6-/- mice, MMP12, an enzyme strongly associated with the pathogenesis of emphysema, is expressed 200-fold higher than in wild-type counterparts (Houghton, 2015). Although at birth the size of the alveoli is normal, by six months of age the alveolae of Itgb6-/- mice are significantly larger and exhibit mild emphysema, which increases in severity with age (Houghton, 2015).
Mice deficient in Itgb6 develop chronic periodontal disease (periodontitis) (Ghannad et al., 2008), an advanced stage of gingivitis in which the gums pull away from the teeth, leaving space or ‘pockets’ between the teeth and gums that become infected. The consequent immune response leads to degradation of the connective tissue between the teeth and gums. An important interface in the development of periodontal disease is the junctional epithelium (JE), which expresses αvβ6 and is involved in adhesion of the gingiva to the teeth. By 12 months of age, Itgb6-/- mice develop abnormal JE, exhibit periodontal pockets and show evidence of inflammation and bone degradation (Ghannad et al., 2006; Bi et al., 2017).
Itgb6-/- mice also develop amelogenesis imperfecta, a disorder affecting the development of teeth (Mohazab et al., 2013). Amelogenesis imperfecta causes a range of symptoms, including abnormally small teeth prone to breakage and discolouration. Cases of amelogenesis imperfecta have also been observed in clinical studies in patients with ITGB6 mutations (Wang et al., 2014; Poulter et al., 2014).
Clinical Cases of ITGB6 mutations
The first clinical case associated with an ITGB6 mutation was documented in 2013, in which a 7-year-old girl presented with enamel malformations but was otherwise asymptomatic (Wang et al., 2014). Following whole genome sequencing, it was revealed that she had inherited 2 distinct missense mutations in ITGB6. As part of the same study, a second child displaying symptoms of amelogenesis imperfecta was examined and he too had a bi-allelic ITGB6 mutation. Polymorphism Phenotyping (Polyphen) analysis indicated that both mutations were of high probability of affecting β6 protein structure and causing disease. These and other cases of ITGB6 mutations have been documented across a number of conditions (Table 1).
Table 1.
Summary of reported clinical cases where ITBG6 is mutated or deleted.
| Patient | Mutation | Location | Protein Change | Clinical Presentation | Publication |
|---|---|---|---|---|---|
| 7 year old female | 2 distinct missense mutations: c.427 G > A c.825 T > A | Exon 4 Exon 6 | A143T H275Q | Amelogenesis Imperfecta | Wang et al., 2014 |
| 8 year old male | c.1846C > T | Exon 11 | R616X | Amelogenesis Imperfecta | Wang et al., 2014 |
| 3 sisters; aged 7,9 and unknown | c.586C > A | Exon 4 | P196T | Amelogenesis Imperfecta | Poulter et al., 2014 |
| Newborn female (15 min) | Deletion | 2q22q31 | n/a | Multiple congenital abnormalities. Deceased. | McConnell et al. 1980 |
| 20 year old female | Deletion | 2q23q31 | n/a | Cognitive deficit and dysmorphic features. Deceased. | Shabtai et al.1982 |
| 2 year old male | Deletion | 2q23q24.3 | n/a | Dysmorphic features, congenital heart defects. Deceased. | Maas et al. 2000a,b |
| 3 year old female | Deletion | 2q24.1q31.1 | n/a | Dysmorphic features, developmental delay and seizures. Deceased. | Langer et al. 2006 |
| 9 year old male | Deletion | 2q23q31 | n/a | Cognitive deficit, seizures | Grosso et al. 2008 |
| 2 month old female | Deletion | 2q24.2q24.3 | n/a | Severe pulmonary emphysema, seizures, delayed growth and dysmorphic features | Takatsuki et al. 2010 |
| 3 siblings; Male aged 40; Females aged 39 and 33 | c.898 G > A | Exon 3 | E300K | Alopecia and Cognitive deficit, Amelogenesis Imperfecta | Ansar et al. 2015 |
These cases were the first reported examples of mutations in the ITGB6 gene causing amelogenesis imperfecta. Interestingly, they reported no other clinical symptoms, such as pulmonary inflammation as reported in mouse models. However, it is unknown whether the authors performed a detailed clinical examination of the children. Similarly, further reported cases of amelogenesis imperfecta with ITGB6 mutations failed to disclose other possible clinical symptoms, or whether these were investigated (Poulter et al., 2014).
There is a collection of clinical cases where a large deletion in the 2q24 region encompassing the ITGB6 gene has resulted in respiratory complications in the patient (Takatsuki et al., 2010). These authors described a case of a 2-month-old girl who, among other symptoms, developed severe pulmonary emphysema. After confirming ITGB6 was deleted, they hypothesised that there was a relationship between this deletion and the respiratory complications experienced by the child. However, it is estimated that a total of 34 genes were affected by this deletion. Thus, her emphysema may not have been caused by the ITGB6 deletion alone, despite the similarities in phenotype with the Itgb6-/- mice. A further three cases (Shabtai et al., 1982; Moller et al., 1984 and Langer et al., 2006) document patients with large deletions in the 2q24 region that included ITGB6, two of which resulted in fatalities caused by respiratory complications.
A novel homozygous missense mutation in ITGB6 was found in three members of a Pakistani family presenting with alopecia, intellectual disabilities, and symptoms consistent with amelogenesis imperfecta (Ansar et al., 2016). It is perhaps worth noting that cognitive disabilities have not been noted in experimental mouse studies of Itgb6. The mutation was found in a highly conserved region of ITGB6 which was predicted by in silico analysis to cause significant change to β6, which may its function. Thus, humans with loss of function ITGB6 mutations exhibit some of the phenotypes observed in Itgb6-/- mice (alopecia, amelogenesis imperfecta, periodontitis, and emphysema) but this seems to vary between individuals, presumably due to the clinical severity of the different mutations. Further clinical exploration will result in a clearer picture of how specific mutations manifest.
Conclusions
Although a substantial amount of work has been performed to determine the structure of the ITGB6 gene, there are many unanswered questions as to how β6 protein expression is regulated. Of note, limited work has been performed to further our understanding of post-transcriptional regulation of ITGB6, which likely plays a significant role in αvβ6 expression and biology. Furthermore, investigations of clinical cases involving ITGB6 mutations have not been comprehensive and it is possible we have missed some understanding of the human phenotype that would help us fully appreciate the role of ITGB6 in humans.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Author Contributions Statement
Both authors contributed equally to the conception and writing of the manuscript.
References
- Agrez M., Chen A., Cone R.I., Pytela R., Sheppard D. The alpha v beta 6 integrin promotes proliferation of colon carcinoma cells through a unique region of the beta 6 cytoplasmic domain. J Cell Biol. 1994;127(2):547–556. doi: 10.1083/jcb.127.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N., Niu J., Dorahy D.J., Gu X., Andrews S., Meldrum C.J., Scott R.J., Baker M.S., Macreadie I.G., Agrez M.V. Direct integrin αvβ6-ERK binding: implications for tumour growth. Oncogene. 2002;21(9):1370. doi: 10.1038/sj.onc.1205286. [DOI] [PubMed] [Google Scholar]
- Annes J.P., Rifkin D.B., Munger J.S. The integrin αVβ6 binds and activates latent TGFβ3. FEBS letters. 2002;511(1-3):65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- Annes J.P., Chen Y., Munger J.S., Rifkin D.B. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165(5):723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar M., Jan A., Santos-Cortez R.L., Wang X., Suliman M., Acharya A., Habib R., Abbe I., Ali G., Lee K., Smith J.D., Nickerson D.A., Shendure J., Bamshad M.J., Ahmad W., Leal S.M. Expansion of the spectrum of ITGB6-related disorders to adolescent alopecia, dentogingival abnormalities and intellectual disability. Eur J Hum Genet. 2016;24(8):1223–1227. doi: 10.1038/ejhg.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azare J., Leslie K., Al-Ahmadie H., Gerald W., Weinreb P.H., Violette S.M., Bromberg J. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin beta 6. Mol Cell Biol. 2007;27(12):4444–4453. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R.C., Bellovin D.I., Brown C., Maynard E., Wu B., Kawakatsu H., Sheppard D., Oettgen P., Mercurio A.M. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115(2):339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A.D., Graff J.R. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23(18):3189. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- Bi J., Koivisto L., Pang A., Li M., Jiang G., Aurora S., Wang Z., Owen G.R., Dai J., Shen Y., Grenier D., Haapasalo M., Häkkinen L., Larjava H. Suppression of αvβ6 Integrin Expression by Polymicrobial Oral Biofilms in Gingival Epithelial Cells. Scientific Reports. 2017;7(1):4411. doi: 10.1038/s41598-017-03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuss J.M., Gallo J., DeLisser H.M., Klimanskaya I.V., Folkesson H.G., Pittet J.F., Nishimura S.L., Aldape K., Landers D.V., Carpenter W. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Breuss J.M., Gillett N., Lu L., Sheppard D., Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. Journal of Histochemistry & Cytochemistry. 1993;41(10):1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- Busk M., Pytela R., Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992;267(9):5790–5796. [PubMed] [Google Scholar]
- Cheresh D.A., Smith J.W., Cooper H.M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Crawford S.E., Stellmach V., Murphy-Ullrich J.E., Ribeiro S.M., Lawler J., Hynes R.O., Boivin G.P., Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Derynck R., Zhang Y., Feng X.H. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Enyu L., Zhengchuan N., Jiayong W., Benjia L., Qi S., Ruixi Q., Cheng P., Khan A.Q., Wei S., Jun N. Integrin beta6 can be translationally regulated by eukaryotic initiation factor 4E: Contributing to colonic tumor malignancy. Tumour Biol. 2015;36(8):6541–6550. doi: 10.1007/s13277-015-3348-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz E., Sanchez-Madrid F. Regional localization of the human integrin beta 6 gene (ITGB6) to chromosome 2q24-q31. Genomics. 1994;21(3):638–640. doi: 10.1006/geno.1994.1325. [DOI] [PubMed] [Google Scholar]
- Goodwin A., Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37(Pt 4):849–854. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- Graff J.R., Zimmer S.G. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clinical & experimental metastasis. 2003;20(3):265–273. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- Grosso S., Pucci L., Curatolo P., Coppola G., Bartalini G., Di Bartolo R., Scarinci R., Renieri A., Balestri P. Epilepsy and electroencephalographic anomalies in chromosome 2 aberrations. A review. Epilepsy Res. 2008;79(1):63–70. doi: 10.1016/j.eplepsyres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Haapasalmi K., Zhang K., Tonnesen M., Olerud J., Sheppard D., Salo T., Kramer R., Clark R.A., Uitto V.J., Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol. 1996;106(1):42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- Houghton A.M. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44-46:167–174. doi: 10.1016/j.matbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Huang X., Wu J., Spong S., Sheppard D. The integrin alphavbeta6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111(Pt 15):2189–2195. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- Huang X.Z., Wu J.F., Cass D., Erle D.J., Corry D., Young S.G., Farese R.V., Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133(4):921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. Integrins: bidirectional. allosteric signaling machines. cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Korzus E., Rosenfeld M.G., Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissansen G.W., Yuan Q., Jenkins D., Jiang W.M., Rooke L., Spurr N.K., Eccles M., Leung E., Watson J.D. Chromosomal locations of the genes coding for the integrin beta 6 and beta 7 subunits. Immunogenetics. 1992;35(1):58–61. doi: 10.1007/BF00216629. [DOI] [PubMed] [Google Scholar]
- Langer S., Geigl J.B., Wagenstaller J., Lederer G., Hempel M., Daumer-Haas C., Leifheit H.J., Speicher M.R. Delineation of a 2q deletion in a girl with dysmorphic features and epilepsy. Am J Med Genet A. 2006;140(7):764–768. doi: 10.1002/ajmg.a.31141. [DOI] [PubMed] [Google Scholar]
- Li X., Yang Y., Hu Y., Dang D., Regezi J., Schmidt B.L., Atakilit A., Chen B., Ellis D., Ramos D.M. Alphavbeta6-Fyn signaling promotes oral cancer progression. J Biol Chem. 2003;278(43):41646–41653. doi: 10.1074/jbc.M306274200. [DOI] [PubMed] [Google Scholar]
- Ludlow A., Yee K.O., Lipman R., Bronson R., Weinreb P., Huang X., Sheppard D., Lawler J. Characterization of integrin beta6 and thrombospondin-1 double-null mice. J Cell Mol Med. 2005;9(2):421–437. doi: 10.1111/j.1582-4934.2005.tb00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S.M., Hoovers J.M., van Seggelen M.E., Menzel D.M., Hennekam R.C. Interstitial deletion of the long arm of chromosome 2: a clinically recognizable microdeletion syndrome? Clin Dysmorphol. 2000;9(1):47–53. doi: 10.1097/00019605-200009010-00010. [DOI] [PubMed] [Google Scholar]
- Maas S.M., Hoovers J.M., van Seggelen M.E., Menzel D.M., Hennekam R.C. Interstitial deletion of the long arm of chromosome 2: a clinically recognizable microdeletion syndrome? Clin Dysmorphol. 2000;9(1):47–53. doi: 10.1097/00019605-200009010-00010. [DOI] [PubMed] [Google Scholar]
- McConnell T.S., Kornfeld M., McClellan G., Aase J. Partial deletion of chromosome 2 mimicking a phenotype of trisomy 18: case report with autopsy. Hum Pathol. 1980;11(2):202–205. doi: 10.1016/s0046-8177(80)80146-8. [DOI] [PubMed] [Google Scholar]
- Mohazab L., Koivisto L., Jiang G., Kytomaki L., Haapasalo M., Owen G.R., Wiebe C., Xie Y., Heikinheimo K., Yoshida T., Smith C.E., Heino J., Hakkinen L., McKee M.D., Larjava H. Critical role for alphavbeta6 integrin in enamel biomineralization. J Cell Sci. 2013;126(Pt 3):732–744. doi: 10.1242/jcs.112599. [DOI] [PubMed] [Google Scholar]
- Morris D.G., Huang X., Kaminski N., Wang Y., Shapiro S.D., Dolganov G., Glick A., Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422(6928):169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- Moser M., Nieswandt B., Ussar S., Pozgajova M., Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- Munger J.S., Huang X., Kawakatsu H., Griffiths M.J., Dalton S.L., Wu J., Pittet J.F., Kaminski N., Garat C., Matthay M.A., Rifkin D.B., Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Niu J., Li Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett. 2017;403:128–137. doi: 10.1016/j.canlet.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Niu Z., Wang J., Muhammad S., Niu W., Liu E., Peng C., Liang B., Sun Q., Obo S., He Z., Liu S. Protein expression of eIF4E and integrin αvβ6 in colon cancer can predict clinical significance, reveal their correlation and imply possible mechanism of interaction. Cell & bioscience. 2014;4(1):23. doi: 10.1186/2045-3701-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter J.A., Brookes S.J., Shore R.C., Smith C.E., Abi Farraj L., Kirkham J., Inglehearn C.F., Mighell A.J. A missense mutation in ITGB6 causes pitted hypomineralized amelogenesis imperfecta. Hum Mol Genet. 2014;23(8):2189–2197. doi: 10.1093/hmg/ddt616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay A.G., Keppler M.D., Jazayeri M., Thomas G.J., Parsons M., Violette S., Weinreb P., Hart I.R., Marshall J.F. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin αvβ6. Cancer research. 2007;67(11):5275–5284. doi: 10.1158/0008-5472.CAN-07-0318. [DOI] [PubMed] [Google Scholar]
- Rhoads R.E. Cap recognition and the entry of mRNA into the protein synthesis initiation cycle. Trends in biochemical sciences. 1988;13(2):52–56. doi: 10.1016/0968-0004(88)90028-x. [DOI] [PubMed] [Google Scholar]
- Saha A., Ellison D., Thomas G.J., Vallath S., Mather S.J., Hart I.R., Marshall J.F. High‐resolution in vivo imaging of breast cancer by targeting the pro‐invasive integrin αvβ6. The Journal of pathology. 2010;222(1):52–63. doi: 10.1002/path.2745. [DOI] [PubMed] [Google Scholar]
- Schiffer M., von Gersdorff G., Bitzer M., Susztak K., Bottinger E.P. Smad proteins and transforming growth factor-beta signaling. Kidney Int Suppl. 2000;77:S45–S52. doi: 10.1046/j.1523-1755.2000.07708.x. [DOI] [PubMed] [Google Scholar]
- Shabtai F., Klar D., Halbrecht I. Partial monosomy of chromosome 2. Delineable syndrome of deletion 2 (q23-q31) Ann Genet. 1982;25(3):156–158. [PubMed] [Google Scholar]
- Sheppard D., Cohen D.S., Wang A., Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992;267(24):17409–17414. [PubMed] [Google Scholar]
- Sheppard D., Rozzo C., Starr L., Quaranta V., Erle D.J., Pytela R. Complete amino acid sequence of a novel integrin beta subunit (beta 6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem. 1990;265(20):11502–11507. [PubMed] [Google Scholar]
- Shull M.M., Ormsby I., Kier A.B., Pawlowski S., Diebold R.J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui N., Sonenberg N. Signalling to eIF4E in cancer. Biochem Soc Trans. 2015;43(5):763–772. doi: 10.1042/BST20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki S., Nakamura R., Haga Y., Mitsui K., Hashimoto T., Shimojima K., Saji T., Yamamoto T. Severe pulmonary emphysema in a girl with interstitial deletion of 2q24.2q24.3 including ITGB6. Am J Med Genet A. 2010;152a(4):1020–1025. doi: 10.1002/ajmg.a.33362. [DOI] [PubMed] [Google Scholar]
- Tatler A.L., Goodwin A.T., Gbolahan O., Saini G., Porte J., John A.E., Clifford R.L., Violette S.M., Weinreb P.H., Parfrey H., Wolters P.J., Gauldie J., Kolb M., Jenkins G. Amplification of TGFbeta Induced ITGB6 Gene Transcription May Promote Pulmonary Fibrosis. PLoS One. 2016;11(8):e0158047. doi: 10.1371/journal.pone.0158047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatler A.L., Habgood A., Porte J., John A.E., Stavrou A., Hodge E., Kerama-Likoko C., Violette S.M., Weinreb P.H., Knox A.J., Laurent G., Parfrey H., Wolters P.J., Wallace W., Alberti S., Nordheim A., Jenkins G. Reduced Ets Domain-containing Protein Elk1 Promotes Pulmonary Fibrosis via Increased Integrin alphavbeta6 Expression. J Biol Chem. 2016;291(18):9540–9553. doi: 10.1074/jbc.M115.692368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G.J., Lewis M.P., Hart I.R., Marshall J.F., Speight P.M. αvβ6integrin promotes invasion of squamous carcinoma cells through up‐regulation of matrix metalloproteinase‐9. International journal of cancer. 2001;92(5):641–650. doi: 10.1002/1097-0215(20010601)92:5<641::aid-ijc1243>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Verrecchia F., Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118(2):211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Wang S.K., Choi M., Richardson A.S., Reid B.M., Lin B.P., Wang S.J., Kim J.W., Simmer J.P., Hu J.C. ITGB6 loss-of-function mutations cause autosomal recessive amelogenesis imperfecta. Hum Mol Genet. 2014;23(8):2157–2163. doi: 10.1093/hmg/ddt611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., McElwee K.J., Owen G.R., Hakkinen L., Larjava H.S. Integrin beta6-deficient mice show enhanced keratinocyte proliferation and retarded hair follicle regression after depilation. J Invest Dermatol. 2012;132(3 Pt 1):547–555. doi: 10.1038/jid.2011.381. [DOI] [PubMed] [Google Scholar]
- Xu M., Chen X., Yin H., Yin L., Liu F., Fu Y., Yao J., Deng X. Cloning and characterization of the human integrin beta6 gene promoter. PLoS One. 2015;10(3):e0121439. doi: 10.1371/journal.pone.0121439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Araya J., Cambier S., Markovics J.A., Wolters P., Jablons D., Hill A., Finkbeiner W., Jones K., Broaddus V.C., Sheppard D., Barzcak A., Xiao Y., Erle D.J., Nishimura S.L. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117(11):3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor D.I., Cheruku H.R., Nice E.C., Baker M.S. Integrin alphavbeta6 sets the stage for colorectal cancer metastasis. Cancer Metastasis Rev. 2015;34(4):715–734. doi: 10.1007/s10555-015-9591-z. [DOI] [PubMed] [Google Scholar]
- Chen C., Sheppard D. Identification and molecular characterization of multiple phenotypes in integrin knockout mice. Methods Enzymol. 2007;426:291–305. doi: 10.1016/S0076-6879(07)26013-6. [DOI] [PubMed] [Google Scholar]
- Chung J., Kim T.H. Integrin-dependent translational control: Implication in cancer progression. Microsc Res Tech. 2008;71(5):380–386. doi: 10.1002/jemt.20566. [DOI] [PubMed] [Google Scholar]
- De Franceschi N., Arjonen A., Elkhatib N., Denessiouk K., Wrobel A.G., Wilson T.A., Pouwels J., Montagnac G., Owen D.J., Ivaska J. Selective integrin endocytosis is driven by interactions between the integrin α-chain and AP2. Nature structural & molecular biology. 2016;23(2):172. doi: 10.1038/nsmb.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducceschi M., Clifton L.G., Stimpson S.A., Billin A.N. Post-transcriptional regulation of ITGB6 protein levels in damaged skeletal muscle. J Mol Histol. 2014;45(3):329–336. doi: 10.1007/s10735-014-9567-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyu L., Na W., Chuanzong Z., Ben W., Xiaojuan W., Yan W., Zequn L., Jianguo H., Jiayong W., Benjia L., Cheng P. The clinical significance and underlying correlation of pStat-3 and integrin αvβ6 expression in gallbladder cancer. Oncotarget. 2017;8(12):19467. doi: 10.18632/oncotarget.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm K., Lukashev M.E., Luo Y., Yang W.J., Dolinski B.M., Weinreb P.H., Simon K.J., Chun Wang L., Leone D.R., Lobb R.R., McCrann D.J., Allaire N.E., Horan G.S., Fogo A., Kalluri R., Shield C.F., 3rd, Sheppard D., Gardner H.A., Violette S.M. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170(1):110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkinen L., Koivisto L., Gardner H., Saarialho-Kere U., Carroll J.M., Lakso M., Rauvala H., Laato M., Heino J., Larjava H. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164(1):229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J.N., Steffensen B., Hakkinen L., Krogfelt K.A., Larjava H.S. Skin wound healing in diabetic beta6 integrin-deficient mice. Apmis. 2010;118(10):753–764. doi: 10.1111/j.1600-0463.2010.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P.A., Brown J.K., Wright S.H., Thornton E.M., Pate J.A., Miller H.R. Aberrant mucosal mast cell protease expression in the enteric epithelium of nematode-infected mice lacking the integrin alphavbeta6, a transforming growth factor-beta1 activator. Am J Pathol. 2007;171(4):1237–1248. doi: 10.2353/ajpath.2007.061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Kudo M., Sundaram A., Ren X., Huang K., Bernstein X., Wang Y., Raymond W.W., Erle D.J., Abrink M., Caughey G.H., Huang X., Sheppard D. The alphavbeta6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest. 2012;122(2):748–758. doi: 10.1172/JCI58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.P., Kassel K.M., Manley S., Baker A.K., Luyendyk J.P. Regulation of transforming growth factor-beta1-dependent integrin beta6 expression by p38 mitogen-activated protein kinase in bile duct epithelial cells. J Pharmacol Exp Ther. 2011;337(2):471–478. doi: 10.1124/jpet.110.177337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F., Sanli K. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352):eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- Wu W., Hutcheon A.E.K., Sriram S., Tran J.A., Zieske J.D. Initiation of fibrosis in the integrin Alphavbeta6 knockout mice. Exp Eye Res. 2018;180:23–28. doi: 10.1016/j.exer.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Yin L., Cai Y., Hu Q., Huang J., Ji Q., Hu Y., Huang W., Liu F., Shi S., Deng X. Epigenetic regulation of integrin beta6 transcription induced by TGF-beta1 in human oral squamous cell carcinoma cells. J Cell Biochem. 2018;119(5):4193–4204. doi: 10.1002/jcb.26642. [DOI] [PubMed] [Google Scholar]
- Yang Z., Mu Z., Dabovic B., Jurukovski V., Yu D., Sung J., Xiong X., Munger J.S. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176(6):787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M., Tang Y., Liu B., Li Q., Zhou X., Yu S., Fu S., Cai Y., Yuan X. Genetic variants in the ITGB6 gene is associated with the risk of radiation pneumonitis in lung cancer patients treated with thoracic radiation therapy. Tumour Biol. 2016;37(3):3469–3477. doi: 10.1007/s13277-015-4171-y. [DOI] [PubMed] [Google Scholar]
- Zhao P., Mao B., Cai X., Jiang J., Liu Z., Lin J., He X. 2q24 deletion in a 9-month old girl with anal atresia, hearing impairment, and hypotonia. Int J Pediatr Otorhinolaryngol. 2018;109:96–100. doi: 10.1016/j.ijporl.2018.03.031. [DOI] [PubMed] [Google Scholar]