Abstract
A large outbreak of liver toxicity in dairy cows that were consuming swede (rutabaga, Brassica napus ssp. napobrassica) crops in Southland and Otago, New Zealand in 2014 prompted the search for the toxin(s) responsible for brassica-associated liver disease (BALD). Analysis of swede plant material showed that the ultra-dominant glucosinolate was progoitrin. The two nitrile derivatives of progoitrin, 1-cyano-2-hydroxy-3-butene (CHB, also known as crambene) and 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB), were custom-synthesised. In this pilot trial, individual progoitrin nitriles were administered by gavage to rats in order to establish a “subtoxic” dose, i.e. the dose where apparently clinically normal rats show liver injury based on altered serum biochemical indicators and histological lesions. We found that consecutive daily doses of 1 mmol/kg CHB produced severe pancreatic and mild liver histological lesions in the absence of notable biochemical changes in clinically normal rats. No evidence of a cumulative effect was seen. Single doses of 1 mmol/kg of CHEB caused elevated concentrations of serum creatinine and distinctive renal and stomach histological lesions in apparently clinically normal rats. Consecutive daily 1 mmol/kg doses of CHEB had a considerable cumulative effect and proved severely hepato- and nephrotoxic with creatinine concentrations peaking after three daily doses. Three other commercially available nitriles (3-butenenitrile, 4-pentenenitrile and 5-hexenenitrile) derived from minor glucosinolates in the swedes were also investigated in this pilot trial. Single combined 1 mmol/kg doses of both progoitrin nitriles as well as these two nitriles plus small doses of the other three failed to demonstrate any synergism, however, the characteristic and apparently dominant effects of CHEB were consistently demonstrated. The results of this pilot study confirmed the previously reported pancreatotoxicity of CHB and nephrotoxicity of CHEB. CHEB also caused intraepithelial pustules, submucosal oedema, erosions and ulcers in the squamous portion of the stomach. These stomach lesions, as well as the renal lesions, appear identical to those caused by another epithionitrile, 1-cyano-3,4-epithiobutane, derived from gluconapin, which was a minor glucosinolate in the swedes. Because of the fact that cyanide can be released with the metabolism of some nitriles, we analysed cyanide in the livers of treated rats. The liver of a rat dosed with 1 mmol/kg of 3-butenenitrile contained 0.5 μg/g of cyanide. The hypothesis that BALD is due to nitrile toxicity requires further testing.
Keywords: Brassica-associated liver disease; Progoitrin; 1-Cyano-2-hydroxy-3-butene; 1-Cyano-2-hydroxy-3,4-epithiobutane; Toxicity in rats
Highlights
-
•
Cause of Brassica-associated liver disease (BALD) in cattle unknown.
-
•
Progoitrin is the glucosinolate in highest concentration in implicated Brassica spp.
-
•
Pilot trial to find subtoxic dose of two progoitrin nitrile derivatives in rats.
-
•
Single 1 mmol/kg 1-cyano-2-hydroxy-3-butene pancreatotoxic, consecutive doses not cumulative, higher doses also hepatotoxic.
-
•
Single 1 mmol/kg 1-cyano-2-hydroxy-3,4-epithiobutane nephrotoxic, consecutive doses cumulative, higher doses also hepatotoxic.
1. Introduction
In the winter and spring of 2014 there was a large, unprecedented and widely publicised outbreak of liver toxicity in pregnant or newly lactating dairy cows given access to swede (rutabaga, Brassica napus ssp. napobrassica) crops in Southland and Otago, New Zealand (NZ). The outbreak was of considerable regional economic significance with >400 cows dead or euthanised, and some individual farmers losing 40 or more cows. Commonly reported health problems included inappetence, lethargy, ill-thrift, photosensitisation and/or jaundice, elevated liver enzyme activities, abortion, periparturient disease(s), and increased mortality (Bryan et al., 2015, Collett et al., 2015). Histological findings in the livers of affected cattle included ductopenia, cholestasis, subtle small bile duct lesions, and occasional but variable parenchymal necrosis (Collett et al., 2015).
Fast growing forage brassica crops are a valuable component of many NZ pasture-grazing systems, especially over winter in the lower South Island, and are considered “safe” crops during late summer and autumn in the North Island when facial eczema risks are high. Performance of animals grazing Brassica spp. is known to be inconsistent (Westwood and Mulcock, 2012) and the feeding of such crops requires careful management to avoid ruminal acidosis, and other occasional health conditions such as goitre, red water, bloat, and nitrate toxicity. In addition, photosensitisation can be common and was considered by an Australian survey to be the most prevalent sign of disease in cattle grazing Brassica spp. (Morton and Campbell, 1997).
Liver disease and photosensitisation in cattle grazing brassica forage crops, especially turnips (B. rapa ssp. rapa), has been described (Collett and Matthews, 2014). The disease associated with swede consumption appears to be the same as that caused by turnips, hence we refer to the condition as brassica-associated liver disease (BALD). However, to date, no compounds from any brassica have been shown to be hepatotoxic to farm animals or humans. Thus, finding exactly what is causing hepatotoxicity will have important implications for both animal and human health.
Brassica spp. are known to contain a large variety of secondary compounds, with the principal ones being the sulphur-containing glucosinolates (GSLs) (Collett and Matthews, 2014). Nearly 130 unique GSLs have been discovered so far. Each brassica variety contains 20 or more different GSLs in varying ratios and concentrations. In addition, there is marked variation in the occurrence of specific GSLs and their concentrations in different parts of the same plant. GSL concentrations tend to be higher in the upper leaves, stems, flowers and seeds (Dalley et al., 2015). GSLs, in themselves, are not toxic. However, when plants are crushed or chewed, the endogenous myrosinase enzyme causes the GSLs to immediately break down into several different derivatives. One class of derivatives, formed in the presence of unique specifier proteins, and under certain pH conditions, include the nitriles and epithionitriles, about which not a lot is known. All parent GSLs can produce unique daughter nitrile derivatives but only certain ones produce the epithio variants as well (Collett et al., 2014).
Because it had already been hypothesised that conditions in the rumen of hungry, pregnant or lactating cows favour the formation of daughter nitriles and epithionitriles from GSLs and that these may be the cause of liver disease (Collett et al., 2014), the swede crops implicated in the 2014 outbreak were sampled for the analysis of 28 different GSLs. The results of this investigation showed that the concentration of progoitrin (also known as glucorapiferin, or 2(R)-hydroxy-3-butenyl GSL) was 10–50 times greater than the other GSLs (Dalley et al., 2015). The highest concentration of progoitrin (approximately 42 mmol/kg dry weight) was in the upper stems, upper leaves and flowers of swede plants (Dalley et al., 2015). The GSL with the second highest concentration was gluconapin at 1.7 mmol/kg. The concentrations of the other GSLs were much lower, for example glucobrassicanapin and sinigrin had concentrations of 0.3 and 0.1 mmol/kg, respectively (Dalley et al., 2015).
Progoitrin is well known as a precursor of goitrin, an oxazolidine-2-thione, which is a cause of goitre. Goitre, however, was not a notable feature in cows, nor in calves born to cows that fed on swedes while pregnant during the outbreak. Other derivatives of progoitrin are 1-cyano-2-hydroxy-3-butene (CHB, also known as crambene) and 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) (Collett et al., 2014).
Previously reported research has shown that certain nitriles and epithionitriles can be hepatotoxic, nephrotoxic and pancreatotoxic in rats and mice. A comprehensive review of the nitrile and epithionitrile toxicity studies in rats and mice has been given before (Collett et al., 2014). Most of the studies have focused on the effects of nitriles formed from epi-progoitrin (2(S)-hydroxy-3-butenyl GSL), the dominant GSL in Crambe abyssinica. The epi-progoitrin molecule is a mirror image (optical isomer) of progoitrin, hence epi-progoitrin is S and progoitrin is R.
In this paper, we report the results of a pilot study in which we investigated the toxicity of the two progoitrin nitriles, CHB and CHEB in rats. As part of this investigation, we also tested the readily commercially available nitriles from three other alkenyl GSLs that were in very small concentrations in the swedes (Dalley et al., 2015), namely sinigrin, gluconapin and glucobrassicanapin, in rats. These nitriles were 3-butenenitrile (3-BN, also known as allyl cyanide or allyl nitrile), 4-pentenenitrile (4-PN), and 5-hexenenitrile (5-HN) (Collett et al., 2014), respectively. The aim was to establish a “subtoxic” dose, where biochemical and histological evidence of liver damage could be induced in apparently clinically normal rats. Because of the fact that cyanide can be released with the metabolism of some nitriles (Silver et al., 1982, Tanii and Hashimoto, 1984, Willhite and Smith, 1981), we also analysed cyanide in the livers of treated rats.
2. Materials and methods
2.1. Animals
Entire male Sprague-Dawley rats, weighing 286–613 g, were obtained from the Small Animal Production Unit (SAPU), Massey University, Palmerston North. Animals were housed in a controlled environment with a 12/12 h photo period and a 1 h dawn/dusk phase, 22 °C, and relative humidity of 30–70%. The rats were provided with Massey University feed mill “Diet 86” pellets (comprising mainly wheat and barley with no Brassica spp. nor glucosinolate ingredients) and water ad libitum. The weight of feed consumed each day was measured. Animals were habituated for 2 weeks before treatments, which included daily handling and restraint, and provision of treats.
This was a terminal study, with all control and treated rats euthanised using carbon dioxide asphyxiation. All studies were approved by the Massey University Animal Ethics Committee (protocol nos. 16/80, 16/120 and 17/21).
2.2. Chemicals
Both CHB and CHEB were custom-synthesised by BDG Synthesis, Wellington, NZ. Both of these chemicals are likely to be 50:50 racemic mixtures of the two optical isomer forms, R and S. CHB is stable (Bohus et al., 2009) but CHEB has a propensity for polymerisation, so it was provided as a 20% solution in acetone (Gould et al., 1980, Gould et al., 1985). 3-BN, 4-PN and 5-HN were purchased from Sigma-Aldrich Chemicals Co. (St Louis, MO, USA). The parent glucosinolates, and derivatives with synonyms are shown in Table 1.
Table 1.
Glucosinolates and daughter nitriles with the treatment compounds (in bold type) used in this study.
| Glucosinolate name, synonym(s), formula and structure | Nitrile name, synonym(s), formula and structure | Epithionitrile name, synonym(s), formula and structure |
|---|---|---|
| Progoitrin (Glucorapiferin, 2(R)-hydroxy-3-butenyl GSL) C11H19NO10S2 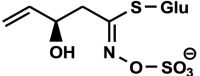
|
1-cyano-2-hydroxy-3-butene (CHB, 3-hydroxy-4-pentenenitrile, crambene) C5H7NO 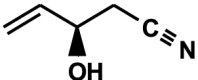
|
(2R)-1-cyano-2-hydroxy-3,4-epithiobutane (CHEB, β-hydroxy-thiiranepropanenitrile, (2R)-3-hydroxy-3-(thiiran-2-yl)-propanenitrile) C5H7NOS [diastereoisomers] 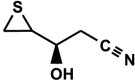
|
| Sinigrin (2-propenyl GSL, allyl GSL) C10H17NO9S 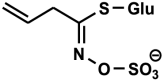
|
3-butenenitrile, (3-BN, allyl cyanide, allyl nitrile) C4H5N 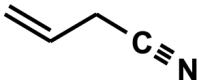
|
1-cyano-2,3-epithiopropane (3,4-epithiobutanenitrile, thiiraneacetonitrile) C4H5NS (3(R,S)-isomers) 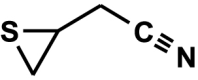
|
| Gluconapin (3-butenyl GSL) C11H18NO9S2 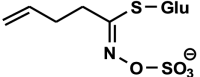
|
4-pentenenitrile, (4-PN, 1-cyano-3-butene) C5H7N 
|
1-cyano-3,4-epithiobutane (CEB, thiiranepropanenitrile, 4,5-epithiovaleronitrile) C5H7NS (4(R,S)-isomers) 
|
| Glucobrassicanapin (4-pentenyl GSL) C12H20NO9S2 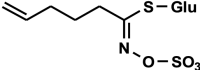
|
5-hexenenitrile, (5-HN, 1-cyano-4-pentene) C6H9N 
|
1-cyano-4,5-epithiopentane (thiiranebutanenitrile, 5,6-epithiohexylnitrile) C6H9NS (5(R,S)-isomers) 
|
2.3. Treatments
To begin with, we calculated the potential dose of progoitrin nitriles that a hungry, pregnant or lactating, 500 kg bodyweight (BW) cow, would consume in a day. We estimated that the cows had a daily intake (DI) of 15 kg swedes (dry matter, DM), comprising mostly upper stems, leaves and flowers. Laboratory analysis showed that progoitrin accounted for 75–80% of total GSL (molar concentration) in the more toxic plant material, and that there was a mean of approximately 42 mmol/kg (DM) of progoitrin (progoitrin concentration, PC) in the upper stems, leaves and flowers (Dalley et al., 2015). Provided conditions were right, we estimated that up to 90% (i.e. a proportion, Pr, of 0.9) of progoitrin would be converted to CHB (molar mass, MM, 97.12 g/mol) and/or CHEB (MM 129.2 g/mol). Hence, we used the following formula to calculate the daily dose in mmol/kg:
Daily dose in mmol/kg = (DI/BW) x PC x Pr = (15 kg/500 kg) x 42 mmol/kg x 0.9 = 1.13 mmol/kg, which equates to 110 mg/kg CHB and 146 mg/kg CHEB.
From this calculation, we decided on an “index” or starting dose of 1 mmol/kg.
Our desired outcome was the establishment of a subtoxic dose as shown by the presence of liver toxicity with elevated serum liver enzyme activities and/or histological liver lesions in rats that appeared clinically normal prior to euthanasia. Signs of illness were graded using a humane endpoint score (see below). Provided no, or minimal clinical signs (humane endpoint score <6) developed, individual rats were euthanised at set times post-dosing (see below). In all cases a single rat was used per treatment.
For the first part of the trial, we used the index dose of 1 mmol/kg for each compound. Once we had established that this dose induced no clinical signs of toxicity, we adapted the “Up-and-Down Procedure” (OECD Guidelines for the Testing of Chemicals) and increased the dose of each compound to 3 mmol/kg for two rats to be terminated at 24 and 48 h, respectively. When this dose proved highly toxic for both compounds, we dropped the dose to 2 mmol/kg for a further two rats per compound.
Regarding CHB and CHEB, five different treatment schedules were utilised:
-
(1)
Single doses of 1 mmol/kg per compound to six rats, with individual animals euthanised at different time points post-dosing (24, 48, 72 or 96 h, and 7 or 14 days);
-
(2)
Two or more consecutive daily doses of 1 mmol/kg per compound to five rats (to mimic natural exposure of animals grazing brassica crops) with individual animals euthanised at 48, 72 or 96 h, and 7 or 14 days, respectively;
-
(3)
Single doses of 1 mmol/kg of each compound, combined, given to six rats with termination at 24, 48, 72 or 96 h, and 7 or 14 days, respectively;
-
(4)
Single doses of 3 mmol/kg per compound to two rats with termination at 24 and 48 h, respectively; and
-
(5)
Single doses of 2 mmol/kg per compound to two rats with termination at 24 and 48 h, respectively.
For 3-BN, a single rat was dosed with 1 mmol/kg and, since this proved highly toxic, the proposed dosing of a second was abandoned. For the remaining two compounds, 4-PN and 5-HN, single doses of 1 mmol/kg were administered with the scheduled euthanasia times being 24 h and 48 h. The final treatment schedule involved six rats dosed with 1 mmol/kg each of CHB and CHEB plus 0.2 mmol/kg of each of the other three nitriles, and with euthanasia times being 24, 48, 72, 96 h, and 7 and 14 days.
Six rats were used as controls. Two received no treatment, two were dosed with the emulsion only, and two were dosed with the emulsion and acetone only. The reason for using acetone was that CHEB was supplied dissolved in acetone to prevent polymerisation. It was proposed to euthanise one of each pair of rats at 24 h and the other at 48 h post-dosing. See Table 2 for the full proposed treatment regimen.
Table 2.
Proposed treatment schedule.
| Rat No. | Compound | Dose | Planned euthanasia |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 7 days | 14 days | ||||
| 1 | CHB single dose | 1 mmol/kg | Dose | Kill | ||||||
| 2 | 1 mmol/kg | Dose | Kill | |||||||
| 3 | 1 mmol/kg | Dose | Kill | |||||||
| 4 | 1 mmol/kg | Dose | Kill | |||||||
| 5 | 1 mmol/kg | Dose | Kill | |||||||
| 6 | 1 mmol/kg | Dose | Kill | |||||||
| 7 | 2 mmol/kg | Dose | Kill | |||||||
| 8 | 2 mmol/kg | Dose | Kill | |||||||
| 9 | 3 mmol/kg | Dose | Kill | |||||||
| 10 | 3 mmol/kg | Dose | Kill | |||||||
| 11 | CHB daily consecutive dose | 1 mmol/kg x 2 | Dose | Dose | Kill | |||||
| 12 | 1 mmol/kg x 3 | Dose | Dose | Dose | Kill | |||||
| 13 | 1 mmol/kg x 4 | Dose | Dose | Dose | Dose | Kill | ||||
| 14 | 1 mmol/kg x 5 | Dose | Dose | Dose | Dose | Dose | Kill | |||
| 15 | 1 mmol/kg x 5 | Dose | Dose | Dose | Dose | Dose | Kill | |||
| 16 | CHEB single dose | 1 mmol/kg | Dose | Kill | ||||||
| 17 | 1 mmol/kg | Dose | Kill | |||||||
| 18 | 1 mmol/kg | Dose | Kill | |||||||
| 19 | 1 mmol/kg | Dose | Kill | |||||||
| 20 | 1 mmol/kg | Dose | Kill | |||||||
| 21 | 1 mmol/kg | Dose | Kill | |||||||
| 22 | 2 mmol/kg | Dose | Kill | |||||||
| 23 | 2 mmol/kg | Dose | Kill | |||||||
| 24 | 3 mmol/kg | Dose | Kill | |||||||
| 25 | 3 mmol/kg | Dose | Kill | |||||||
| 26 | CHEB daily consecutive dose | 1 mmol/kg x 2 | Dose | Dose | Kill | |||||
| 27 | 1 mmol/kg x 3 | Dose | Dose | Dose | Kill | |||||
| 28 | 1 mmol/kg x 4 | Dose | Dose | Dose | Dose | Kill | ||||
| 29 | 1 mmol/kg x 5 | Dose | Dose | Dose | Dose | Dose | Kill | |||
| 30 | 1 mmol/kg x 5 | Dose | Dose | Dose | Dose | Dose | Kill | |||
| 31 | Combined dose - CHB and CHEB | 1 mmol/kg CHB +1 mmol/kg CHEB | Dose | Kill | ||||||
| 32 | Dose | Kill | ||||||||
| 33 | Dose | Kill | ||||||||
| 34 | Dose | Kill | ||||||||
| 35 | Dose | Kill | ||||||||
| 36 | Dose | Kill | ||||||||
| 37 | 3-BN | 1 mmol/kg | Dose | Kill | ||||||
| 38 | 4-PN | 1 mmol/kg | Dose | Kill | ||||||
| 39 | 1 mmol/kg | Dose | Kill | |||||||
| 40 | 5-HN | 1 mmol/kg | Dose | Kill | ||||||
| 41 | 1 mmol/kg | Dose | Kill | |||||||
| 42 | Combined dose – All nitriles | 1 mmol/kg CEB +1 mmol/kg CHEB +0.2 mmol/kg each of 3-BN, 4-PN, and 5-HN | Dose | Kill | ||||||
| 43 | Dose | Kill | ||||||||
| 44 | Dose | Kill | ||||||||
| 45 | Dose | Kill | ||||||||
| 46 | Dose | Kill | ||||||||
| 47 | Dose | Kill | ||||||||
| 48 | Controls | No treatment | Dose | kill | ||||||
| 49 | No treatment | Dose | kill | |||||||
| 50 | Emulsion only | Dose | kill | |||||||
| 51 | Emulsion only | Dose | kill | |||||||
| 52 | Emulsion + Acetone | Dose | kill | |||||||
| 53 | Emulsion + Acetone | Dose | kill | |||||||
2.4. Dosing procedure
On Day 0, each rat was weighed, and the required dose of each test compound was calculated. This dose was dissolved in 1 mℓ of an emulsion made up of one third 10% lecithin in water, one third 20% sucrose in water, and one third soya oil. Emulsions were ultrasonicated for 1 min in 5 s increments, to minimise heating of the solution.
A 15 G sterile flexible plastic feeding needle (15 G x 3.1”, 2.8 mm, PTFE; Cadence Science, Inc, Japan) was used for all orogastric administrations. The length of the tube to be inserted was determined prior to placement, by measuring from the tip of the nose to the last rib.
2.5. Monitoring and humane endpoints
Following gavage, the rats were placed on a table to monitor for any acute adverse signs. After 1 min, if none were noted, the animal was placed back into its cage. Rats were then checked for signs of illness every 3 h for the first 24, and then three times daily.
A grading system for allocating a humane endpoint score (HES) (Table 3) was used during all checks to record and track any changes in clinical signs. Rats showing obvious toxicity, with a HES of 6 or more, were euthanised immediately via CO2 asphyxiation.
Table 3.
Humane endpoint score (HES) grading system.
| Assigned score | |
|---|---|
| Daily body weight changes: | |
| No change | 0 |
| <5% loss | 1 |
| 5–10% loss | 2 |
| >10% daily loss, or >20% over the course of trial | 6 |
| Clinical observations: | |
| Normal | 0 |
| Rough coat (lack of grooming) | 2 |
| Excessive salivation | 2 |
| Nasal and/or ocular discharges (porphyrin staining) | 2 |
| Hunched posture | 2 |
| Decreased activity or inactivity | 2 |
| Rapid/shallow/laboured respiration | 2 |
| Dehydration | 2 |
| Diarrhoea | 2 |
| Stumbling/falling | 2 |
| Tremors | 2 |
| Cumulative score: |
2.6. Blood sampling
Immediately following euthanasia or as soon as possible after death, all rats were blood sampled via cardiac puncture into a vacutainer (BD vacutainers, Franklin Lakes, NJ, USA) containing a clot activator (silicone-coated plastic for serum separation). Serum was processed for biochemistry at the IDEXX-New Zealand Veterinary Pathology laboratory, Palmerston North, NZ, for the following biomarkers: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyltransferase (GGT), glutamate dehydrogenase (GDH), bilirubin, total bile acids, creatinine, amylase, and lipase. Rats were sampled once only, at death.
2.7. Pathology
Rats were necropsied immediately following cardiac puncture. Tissue samples from the liver, kidney, bladder, pancreas, adrenal gland, spleen, oesophagus, stomach, small intestine, caecum, large intestine, lung, heart, thyroid, and brain were placed in 10% buffered formalin for histological examination. Formalin-fixed samples were processed routinely, sectioned at 3 μm, and stained with haematoxylin and eosin (H&E).
2.8. Cyanide analysis
In addition, samples of liver from each rat treated with a single dose of an individual nitrile were placed in liquid nitrogen for free cyanide analysis by gas chromatography at Manaaki Whenua Landcare Research, Lincoln, NZ. The method limit for cyanide detection was 0.01 μg/g.
3. Results
3.1. Humane endpoint scores and serum biochemistry
3.1.1. Single 1 mmol/kg doses of individual nitriles
No clinical signs of toxicity were seen in any of the rats following a single dose of 1 mmol/kg of either CHB or CHEB, and all rats reached their scheduled euthanasia times of 24, 48, 72, 96 h, 7 or 14 days. Rats treated with 1 mmol/kg 4-PN or 5-HN did not show any evidence of toxicity and were euthanised at their scheduled times of 24 or 48 h. Only one rat was dosed with 1 mmol/kg 3-BN and it was euthanised at 18.5 h because it had a HES of 6 (inactivity, rapid respiration and tremors). The only rats to show elevated serum biomarkers were those dosed with CHEB; the creatinine concentration had risen by 48 h and was still elevated (122 [reference range 15–31] μmol/ℓ) at 96 h. The CHEB-dosed rat euthanised at 14 d had a normal serum creatinine concentration. There were no abnormalities in liver or pancreatic biomarkers in all of the dosed rats compared to the controls.
3.1.2. Single 2 and 3 mmol/kg doses of the progoitrin nitriles (CHB and CHEB)
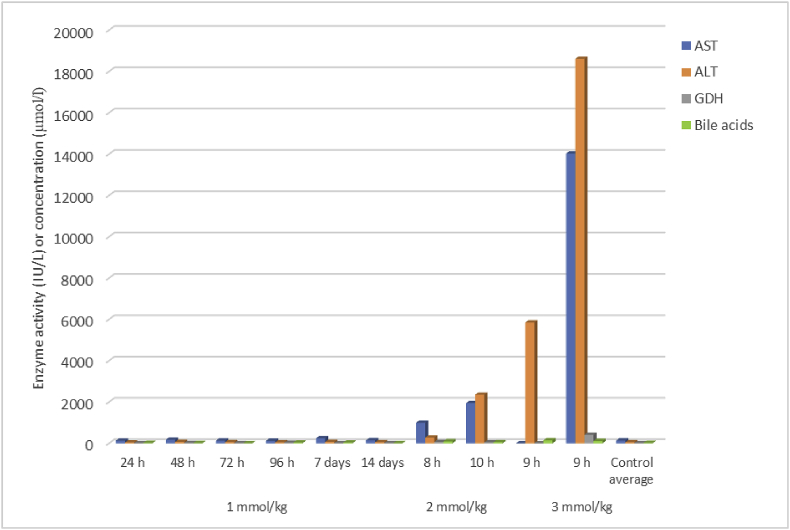
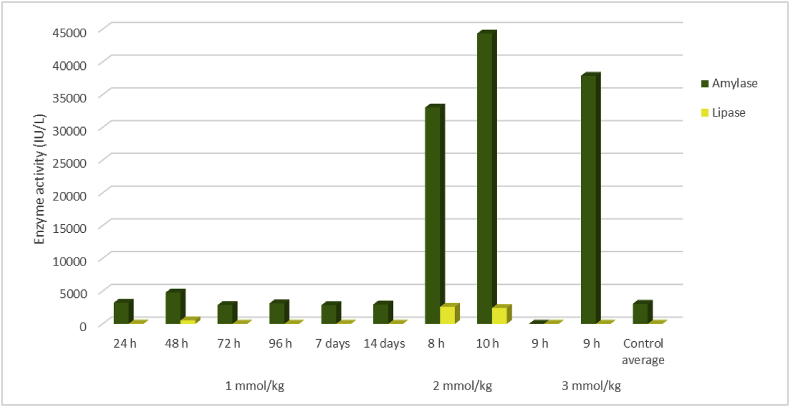
Both CHB and CHEB were found to be severely toxic when the dose was tripled to 3 mmol/kg, as well as when the dose was dropped to 2 mmol/kg. For CHB, at these doses, the rats had to be euthanised earlier than scheduled, at 9 and 9 h, and 8 and 10 h, after dosing, respectively, due to HES >6 (hyperpnoea, salivation, mild to moderate diarrhoea, and inactivity). Marked elevations in the activities of ALT and/or AST (Fig. 1) were seen in the rats that received 3 mmol/kg CHB (one rat had an ALT of 18,606 IU/ℓ [reference range 26–37 IU/ℓ], an AST of 14,026 IU/ℓ [40–53 IU/ℓ], and a GDH of 409 [<20 IU/ℓ]), while elevations were less dramatic in those that received 2 mmol/kg CHB. Pancreatic biomarkers, notably amylase, showed dramatically elevated activities within 8–10 h following doses of 2 or 3 mmol/kg; one rat had an amylase activity of 44,322 IU/ℓ and lipase of 2,421, compared to <3500 IU/ℓ and <10 IU/ℓ in the control rats, respectively (Fig. 2).
Fig. 1.
Liver enzyme activities and total bile acids concentration in serum harvested immediately after death in rats given a single oral dose of 1-cyano-2-hydroxy-3-butene (CHB) at 1, 2, or 3 mmol/kg compared to control rats given the emulsion only (AST = aspartate aminotransferase, ALT = alanine aminotransferase, GDH = glutamate dehydrogenase).
Fig. 2.
Pancreatic enzyme activities in serum harvested immediately after death in rats given a single oral dose of 1-cyano-2-hydroxy-3-butene (CHB) at 1, 2, or 3 mmol/kg compared to control rats given the emulsion only.
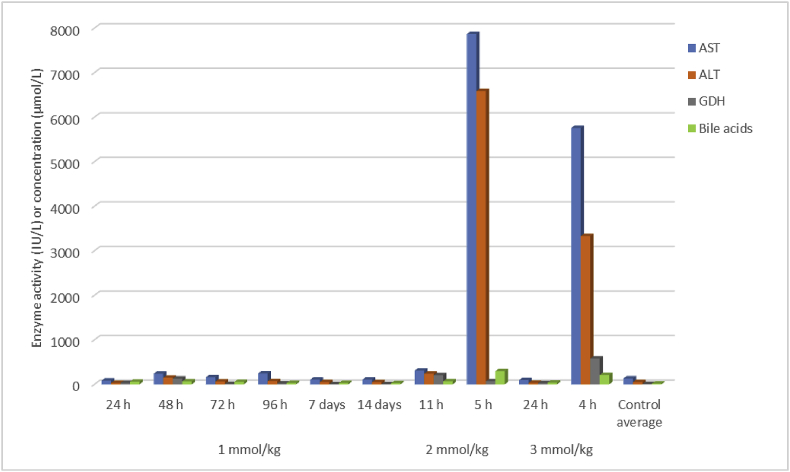
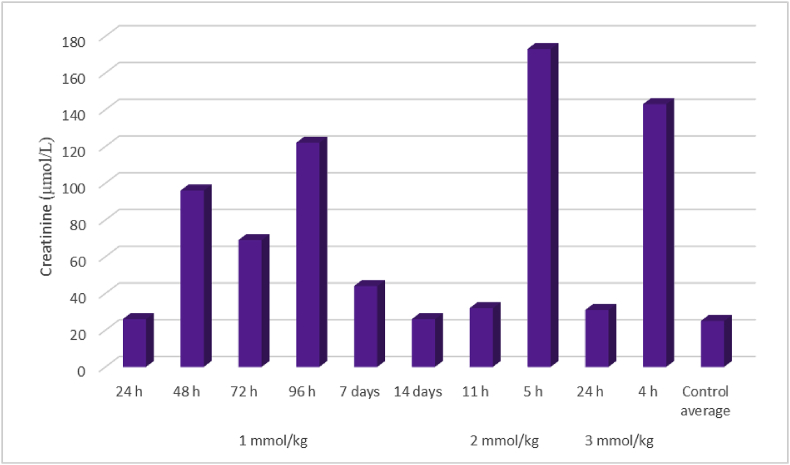
For CHEB, one of the rats dosed with 3 mmol/kg suddenly deteriorated and died before it could be euthanised 4 h after dosing (HES 8, inactive, weeping eyes, rapid respiration and diarrhoea). The enzyme activities of AST, ALT and GDH were all greatly elevated (5756 [40–53 IU/ℓ], 3328 [26–37 IU/ℓ] and 579 [<10] IU/ℓ, respectively). The creatinine concentration was raised (140 [15–31] μmol/ℓ). A rat dosed with 2 mmol/kg was euthanised 5 h after dosing (HES 8, salivation, weeping eyes, diarrhoea, and decreased activity). The activities of AST and ALT in this animal were also greatly elevated (7865 and 6584 IU/ℓ, respectively), and the creatinine concentration was elevated at 165 μmol/ℓ. Both of these rats also had elevated total bile acids concentrations (209 and 294 μmol/ℓ, respectively, compared to a mean of 11 μmol/ℓ for control rats given the emulsion plus acetone only). The other rats treated with 3 and 2 mmol/kg were euthanised at 24 h and 11 h, respectively; both had an HES of 2 (inactivity). The main liver biomarkers showing changes as well as the creatinine concentrations are shown in Fig. 3, Fig. 4, respectively.
Fig. 3.
Liver enzyme activities and total bile acids concentration in serum harvested immediately after death in rats given a single oral dose of 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) at 1, 2, or 3 mmol/kg compared to control rats given the emulsion and acetone only (AST = aspartate aminotransferase, ALT = alanine aminotransferase, GDH = glutamate dehydrogenase).
Fig. 4.
Creatinine concentrations in serum harvested immediately after death in rats given a single oral dose of 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) at 1, 2, or 3 mmol/kg compared to control rats given the emulsion and acetone only (mean 22 μmol/ℓ).
3.1.3. Consecutive daily doses of 1 mmol/kg of either of the progoitrin nitriles (CHB and CHEB)
For CEB at 1 mmol/kg, all rats received their planned number of doses (2, 3, 4, or 5 doses) and were euthanised at their scheduled times with an HES = 0 for each. No changes were observed in any of the serum biomarkers.
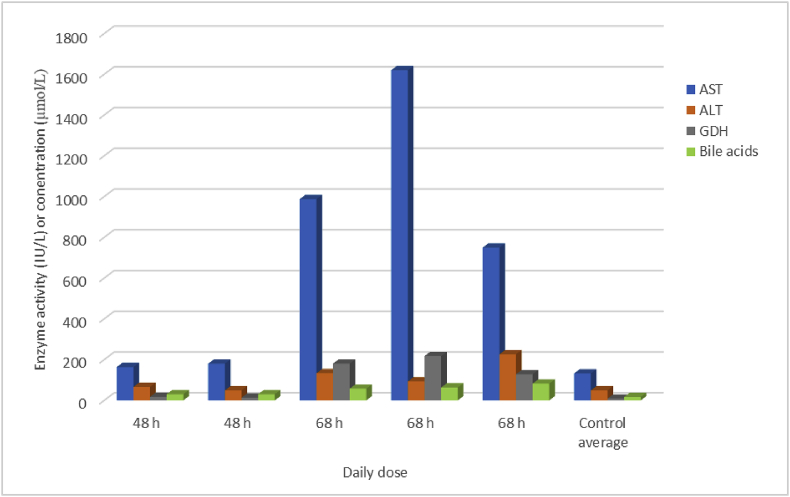
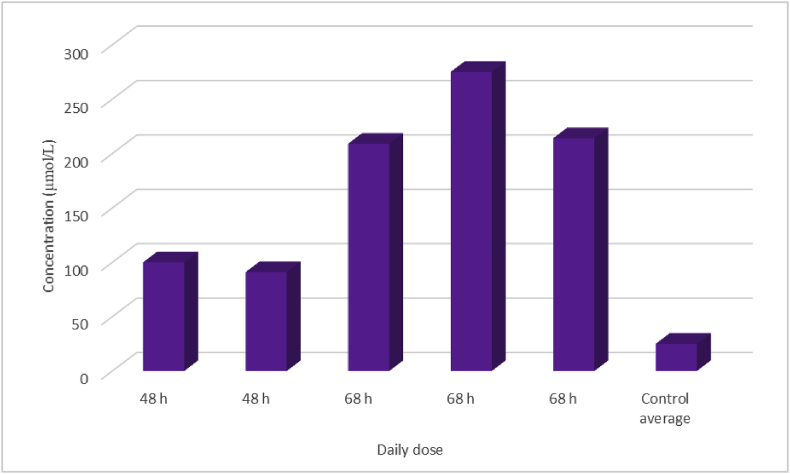
For CHEB, consecutive daily doses of 1 mmol/kg proved highly toxic. Two rats needed to be euthanised after two doses at 48 h. One had a HES of 10 (rough coat, staining of the nose with porphyrin, loss of >10% body weight) and the other had a HES of 8 (rough coat, loss of >10% body weight). The remaining three rats had to be euthanised after three daily doses at 68 h with HESs of 6 (rough coat, hunched posture, decreased activity). Rats given three doses showed considerable elevations in the serum activities of AST, ALT and GDH and bile acids, while those that received two doses were less spectacular (Fig. 5). All of the rats given consecutive daily doses of 1 mmol/kg CHEB had elevated concentrations of serum creatinine, with those given three doses having the highest (Fig. 6).
Fig. 5.
Liver enzyme activities and total bile acids concentration in serum harvested immediately after death in rats given consecutive daily (2 or 3 X) oral doses of 1 mmol/kg of 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) compared to control rats given the emulsion and acetone only (AST = aspartate aminotransferase, ALT = alanine aminotransferase, GDH = glutamate dehydrogenase).
Fig. 6.
Creatinine concentrations in serum harvested immediately after death in five rats given consecutive daily oral 1 mmol/kg doses of 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) compared to control rats given the emulsion and acetone only (mean 22 μmol/ℓ). Two rats received two daily doses and three received three. All five rats had to be euthanised because of high HESs.
3.1.4. Single combined doses of 1 mmol/kg of each of CHB and CHEB, and single combined doses of CHB and CHEB plus 0.2 mmol/kg of each of the other three nitriles
All rats treated with either 1 mmol/kg solutions of each of CHB and CHEB combined, or 1 mmol/kg CHB and 1 mmol/kg CEB, plus 0.2 mmol/kg of each of 3-BN, 4-PN and 5-HN, reached their scheduled euthanasia times, with HES = 0. No behavioural changes were seen in any of the rats dosed with either of the combined solutions. Concerning serum biochemistry, the only notable feature was that the rat that received 1 mmol/kg of each of CHB and CHEB and that was euthanised at 7 days had a creatinine concentration of 207 (reference range 15–31) μmol/ℓ.
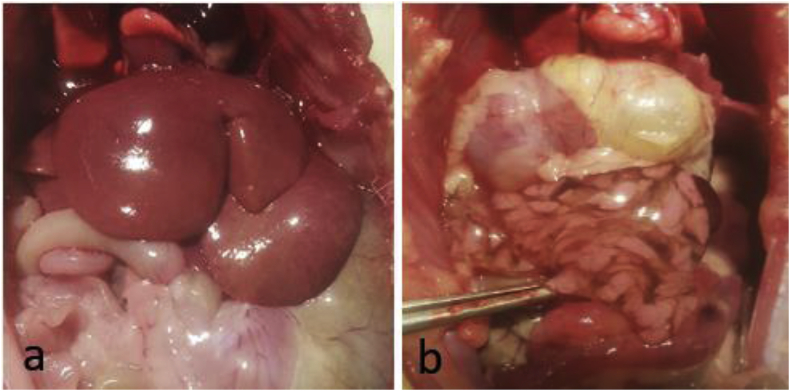
3.2. Gross pathology
At the higher doses of 2 mmol/kg and 3 mmol/kg, both CHB and CHEB caused swelling and pallor of the liver (Fig. 7a). CHB also caused severe oedema of the pancreas (Fig. 7b). Rats that received two or three doses of 1 mmol/kg of CHEB had kidneys that were paler brown than normal, and one animal that received three doses had a blood clot in the stomach.
Fig. 7.
Gross pathology of rats. a) Enlarged liver in a rat dosed with 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) at 3 mmol/kg b) Severe oedema of the pancreas in a rat dosed with 3 mmol/kg of 1-cyano-2-hydroxy-3-butene (CHB).
3.3. Histopathology
3.3.1. 1-Cyano-2-hydroxy-3-butene (CHB)
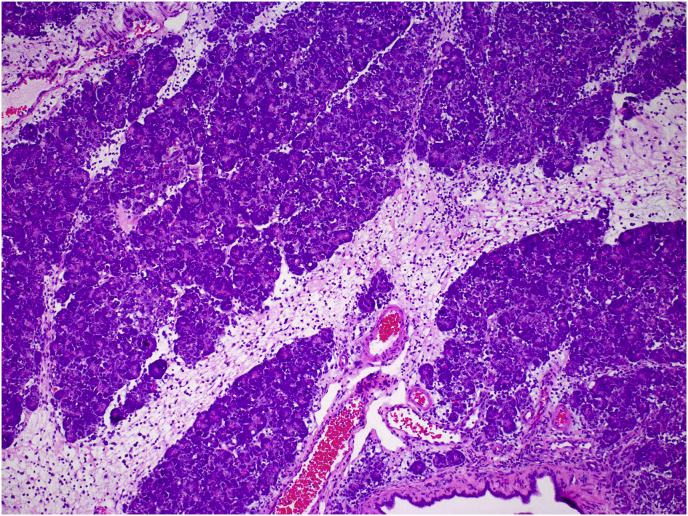
In the rats given a single dose of 1 mmol/kg CHB (crambene), lesions occurred in the pancreas only. At 24 h post-dosing, a slight reduction in cytoplasmic zymogen in acinar cells and an increase in macrophage numbers were noted. At 48 h, the pancreas showed severe, diffuse acinar cell apoptosis, severe depletion of zymogen (post-apoptotic atrophy), interlobular oedema and an increase in macrophage numbers in the oedema fluid and in the interstitium (Fig. 8). At 96 h after dosing, acinar cell mitoses were plentiful and cytoplasmic zymogen was returning to normal. Daily doses (up to five) of CHB at 1 mmol/kg caused a prolongation of the pancreatic apoptosis that quickly regenerated and appeared within normal limits by Day 14. In the livers of these animals receiving daily doses, hepatocytes tended to have variably sized nuclei (anisokaryosis).
Fig. 8.
Photomicrograph of pancreas of a rat 48 h after a single dose of 1 mmol/kg 1-cyano-2-hydroxy-3-butene (CHB) showing acinar cell apoptosis, depletion of cytoplasmic zymogen, interlobular oedema with macrophages in the interstitial oedema fluid. H&E x10.
All rats that were dosed with either 2 mmol/kg or 3 mmol/kg CHB died or were euthanised within 10 h of dosing. All had a massively oedematous pancreas with a few apoptotic acinar cells and infiltrated macrophages. In their livers, scattered single hepatocytes showed lysis or paraptosis (pyknotic nucleus with vesicular cytoplasm), and there were also randomly scattered foci of coagulation necrosis or hepatocytes with hypereosinophilic cytoplasm and pyknotic nuclei. In the portal areas, especially surrounding bile ducts, scattered apoptotic fragments and occasional karyorrhectic cells were present. Mild focal duodenal erosions (necrosis of villus tips) or karyorrhexis of crypt epithelial cells in the ileum were also noted in the rats dosed with 3 mmol/kg CHB.
3.3.2. 1-Cyano-2-hydroxy-3,4-epithiobutane (CHEB)
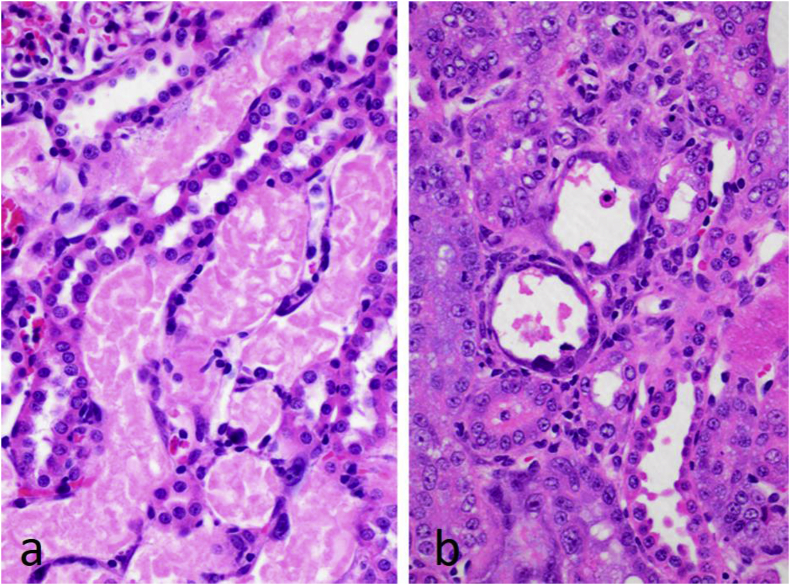
In the case of CHEB, lesions were most pronounced in the kidney, squamous portion of the stomach and, to a lesser extent, the liver. A single dose of 1 mmol/kg of CHEB caused necrosis of occasional epithelial cells in the renal proximal tubules at 24 h which became more extensive involving the straight portions of the loop of Henle by 48 h. By 96 h, the tubular necrosis had reached its zenith and healing by regeneration was well underway by Day 7 (Fig. 9) with almost complete healing by Day 14.
Fig. 9.
Photomicrographs of kidneys after a single dose of 1 mmol/kg 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) showing a) necrosis of tubules of the pars recta of the proximal tubules at 96 h, and b) regeneration of tubular epithelium at 7 days. H&E x40.
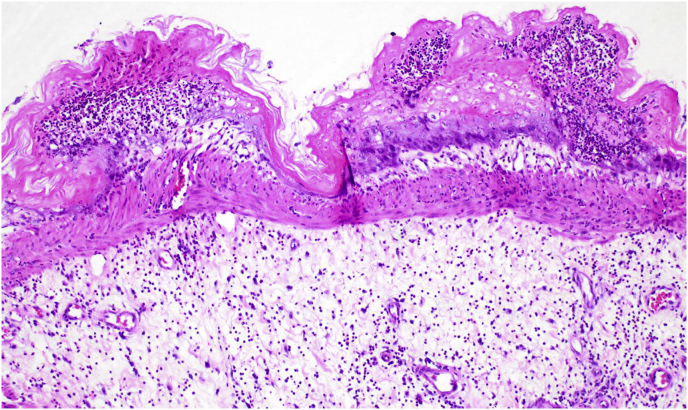
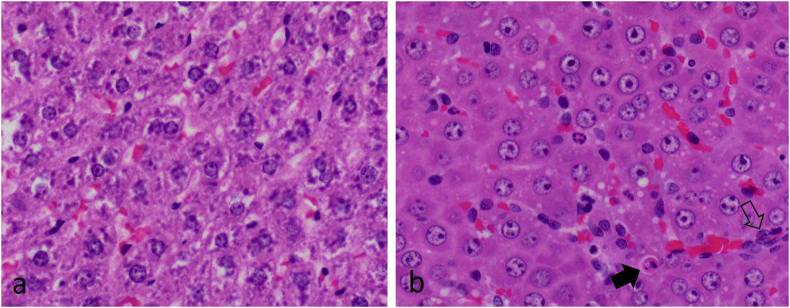
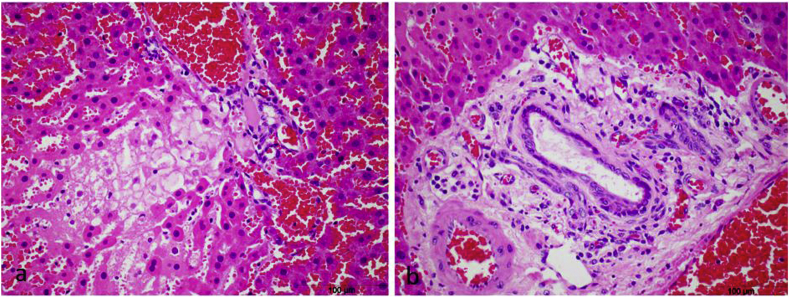
In rats given consecutive daily doses of 1 mmol/kg CHEB, renal tubular necrosis was so severe that rats died or were euthanised after only two or three doses. In the margo plicatus of the stomach, submucosal oedema was prominent and eosinophils, which are normally plentiful, were frequently conspicuously pyknotic or apoptotic. Elsewhere in the gastric pars oesophagea, localised groups of submucosal blood vessels were dilated and/or the epithelium contained one or more pustules while the submucosa was severely oedematous (Fig. 10). In rats given single or consecutive daily doses of 1 mmol/kg, these pars oesophagea lesions were first seen at 24 h and developed into small ulcers by Day 7. In rats given two or three consecutive daily 1 mmol/kg doses, hepatocytes had enlarged, vesicular nuclei (megalocytosis) with one or multiple prominent nucleoli, as well as occasional apoptotic or necrotic hepatocytes (Fig. 11). In one rat that was given a dose of 2 mmol/kg, and that was euthanised 5 h later, multiple, often coalescing, foci of lytic necrosis frequently adjacent to portal triads were conspicuous. In the portal areas of this animal, occasional bile duct epithelial cells were necrotic and scattered residual bodies were noted in the portal interstitium (Fig. 12). Another rat, given 3 mmol/kg CHEB died at 4 h. The liver had several randomly distributed foci of coagulative necrosis, the fundic stomach had a few small erosions, and karyorrhexis was conspicuous in the cells of the lamina propria at the tips of small intestinal villi. In the rats that received high doses of CHEB, isolated apoptotic acinar cells were present in pancreata and haemorrhages were present in the adrenal cortices.
Fig. 10.
Photomicrograph of the pars oesophagea of the stomach of a rat 24 h after a single dose of 1 mmol/kg 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) showing intraepithelial pustules and severe submucosal oedema. H&E x10.
Fig. 11.
Photomicrographs at the same magnification of a) the liver of a control rat that shows cytoplasmic glycogen within hepatocytes, and b) the liver of a rat euthanised after three consecutive daily doses of 1 mmol/kg 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) showing enlarged vesicular hepatocyte nuclei (approximately double the area of those in the control rat, i.e. megalocytosis) with one or more prominent nucleoli. An apoptotic hepatocyte (solid arrow) lies adjacent to a bile ductule (open arrow). H&E x40.
Fig. 12.
Photomicrographs of the liver of a rat euthanised 5 h after a single dose of 2 mmol/kg 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) showing a) a focus of lytic necrosis adjacent to a portal triad, and b) periportal oedema, necrosis of occasional bile duct epithelial cells with residual bodies in the portal interstitium. H&E x40.
3.3.3. Combined doses
In rats that received single combined doses of 1 mmol/kg of each of CHB and CHEB, and single combined doses of CHB and CHEB plus 0.2 mmol/kg of each of the other three nitriles, and that were euthanised at 24, 48, 72 and 96 h, and 7 and 14 days, no lesions were detected in pancreata. The characteristic kidney lesions due to CHEB, described above, were present and reached their zenith by 7 days (CHB and CHEB only) or by 72 h (all nitriles). In both groups of rats, the characteristic submucosal oedema and inflammation as well as intra-epithelial pustules in the squamous portion of the stomach were present by 48 h and progressed to small ulcers by 7 (both compounds) or 14 days (all nitriles). In both groups, liver lesions that comprised anisokaryosis with occasional apoptotic or necrotic hepatocytes were evident by 48 h.
3.3.4. Minor nitriles
No lesions were detected in the rats dosed with 1 mmol/kg of either 4-PN or 5-HN. The lung of the rat dosed with 1 mmol/kg of 3-BN was congested with some alveolar emphysema while other organs and tissues were normal.
A summary of the serum biochemistry and histological lesions associated with single, consecutive daily or combined doses of CHB and CHEB in this pilot trial in rats is provided in Table 4.
Table 4.
Summary of the serum biochemistry and histological lesions that resulted from orogastric doses of 1-cyano-2-hydroxy-3-butene (CHB) and 1-cyano-2-hydroxy-3,4-epithiobutane (CHEB) in rats in this pilot trial.
| Compound | Dose (mmol/kg); no. of daily doses; time to death or euthanasia | Pancreas enzymes | Pancreas lesions | Liver enzymes | Liver lesions | Serum creatinine | Kidney lesions | Stomach lesions | Small intestine lesions |
|---|---|---|---|---|---|---|---|---|---|
| CHB | 1; 1; 24 h–14 days | – | ++++ | – | – | – | – | – | – |
| “ | 1; 2–5; 48 h–14 days | – | ++++ | – | + | – | – | – | – |
| “ | 2; 1; 8–10 h | ++++ | ++ | ++ | + | – | – | – | – |
| “ | 3; 1; 9 h | +++ | ++ | ++++ | ++ | – | – | – | + |
| CHEB | 1; 1; 24 h–14 days | – | – | – | – | ++ | +++ | + | – |
| “ | 1; 2–3; 48–68 h | – | – | ++ | ++ | ++ | ++++ | ++ | – |
| “ | 2; 1; 5 h | – | + | +++ | ++ | +++ | + | – | – |
| “ | 3; 1; 4 h | – | + | ++ | + | +++ | + | + | + |
| CHB + CHEB | 1; 1–5; 24 h–14 days | – | – | – | + | + | ++ | ++ | – |
|
CHB + CHEB + minor nitriles |
1; 1–5; 24 h–14 days | – | – | – | + | – | ++ | ++ | – |
“-” = no lesions or serum biochemical changes.
3.4. Cyanide concentrations in the livers of rats that received single doses of each nitrile
The concentration of free cyanide in the liver of the rat dosed with 1 mmol/kg 3-BN was 0.5 μg/g. The only other rat with a raised concentration (0.2 μg/g) was one animal dosed with 3 mmol/kg CHB and euthanised 9 h later. The results of the liver cyanide analyses are shown in Table 5.
Table 5.
Free cyanide concentrations in livers from rats dosed with varying concentrations of nitriles. Samples were analysed using gas chromatography, with a method limit of detection (MDL) of 0.01 μg/g.
| Rat # | Treatment | Final humane endpoint score | Time interval between dose and death/euthanasia (h) | Cyanide (μg/g) |
|---|---|---|---|---|
| 1 | CHB 1 mmol/kg | 0 | 24 | <MDL |
| 2 | CHB 1 mmol/kg | 0 | 48 | <MDL |
| 7 | CHB 2 mmol/kg | 4 | 8a | 0.01 |
| 8 | CHB 2 mmol/kg | 4 | 10 | 0.01 |
| 9 | CHB 3 mmol/kg | 4 | 9 | 0.01 |
| 10 | CHB 3 mmol/kg | 4 | 9a | 0.02 |
| 16 | CHEB 1 mmol/kg | 0 | 24 | <MDL |
| 17 | CHEB 1 mmol/kg | 0 | 48 | <MDL |
| 22 | CHEB 2 mmol/kg | 2 | 11 | <MDL |
| 23 | CHEB 2 mmol/kg | 8 | 5 | <MDL |
| 24 | CHEB 3 mmol/kg | 2 | 24 | <MDL |
| 25 | CHEB 3 mmol/kg | 8 | 4a | <MDL |
| 37 | 3-BN 1 mmol/kg | 6 | 18.5 | 0.5 |
| 38 | 4-PN 1 mmol/kg | 0 | 24 | 0.01 |
| 39 | 4-PN 1 mmol/kg | 2 | 48 | 0.01 |
| 40 | 5-HN 1 mmol/kg | 0 | 24 | <MDL |
| 41 | 5-HN 1 mmol/kg | 0 | 48 | <MDL |
| 48 | Control - no treatment | 0 | 24 | <MDL |
| 49 | Control - no treatment | 0 | 48 | <MDL |
| 50 | Control - emulsion | 0 | 24 | <MDL |
| 51 | Control - emulsion | 0 | 48 | <MDL |
Rats were found dead, or in extremis in the case of Rat 25, at the set monitoring time. The reported final humane endpoint score is the score given to the rat at the last monitoring check, 3 h prior.
4. Discussion
The intention of this work was to induce biochemical and histological changes in rats dosed with two progoitrin nitriles and three other readily available nitriles from GSLs present in smaller concentrations in swedes that were implicated in the 2014 outbreak of BALD in cattle. The goal was to induce these changes in apparently clinically normal rats, to establish a subtoxic dose.
The design of our pilot study dosing regimen was loosely based on that of a study that found that histological changes were most pronounced 24 h after dosing alpha-naphthylisothiocyanate (ANIT) with increased GDH activity and concentrations of bile acids at 48 h and bilirubin at 72 h (Cullen et al., 2016).
Our results (Table 4) show that consecutive daily doses of 1 mmol/kg CHB produced severe pancreatic and mild liver histological lesions in the absence of notable biochemical changes, nor any evidence of a cumulative effect, in clinically normal rats. On the other hand, single doses of 1 mmol/kg of CHEB caused elevated concentrations of serum creatinine and characteristic renal and stomach histological lesions in apparently clinically normal rats, while there was a considerable cumulative effect with consecutive daily 1 mmol/kg doses proving severely hepato- and nephrotoxic and with creatinine concentrations reaching a peak after three daily doses (Fig. 6). Although single combined 1 mmol/kg doses of the progoitrin nitriles, as well as these nitriles plus small doses of the other three failed to demonstrate any synergism, the distinctive and apparently dominant effects of CHEB were consistently demonstrated.
The lesions in the exocrine pancreas in the rats dosed with CHB in our study were consistent with those described previously (Bohus et al., 2009, Kelly et al., 1999, Wallig et al., 1988a, Wallig and Jeffery, 1990). CHB is a selective pancreatotoxin in rats (Wallig et al., 1988a). We found that severe pancreatic lesions could be induced in rats given 1 mmol/kg, and that doses of 2 mmol/kg were extremely toxic with massive increases in serum amylase activity, pancreatic lesions, plus elevated liver enzyme activities and liver lesions. This is in contrast to previously published work that demonstrated that daily doses of 200 mg (2.1 mmol)/kg for up to 4 days was required to elicit consistent pancreatic lesions without any liver damage in rats that were clinically normal when killed (Wallig et al., 1988a, Wallig and Jeffery, 1990). It had previously been shown that the LD50 for CHB is in the region of 200 mg (2.1 mmol/kg)/kg (Nishie and Daxenbichler, 1980).
In a metabonomics study, rats given single subcutaneous injections of 150 mg (1.54 mmol)/kg of CHB produced histological lesions consistent with the known effects in the exocrine pancreas (and statistically non-significant elevations in serum amylase), however the authors considered an animal with severe liver and kidney lesions an outlier (Bohus et al., 2009). CHB also caused a prolonged elevation of hepatic and pancreatic glutathione and induced glutathione S-transferases when rats were given either 100 mg (1.03 mmol)/kg or 50 mg (0.51 mmol)/kg daily for 7 days (March et al., 1998).
In our pilot study where only one or two rats were used per treatment, some variability in response was seen between animals treated with the same compound and dose. For example, one of the two rats dosed with CHEB at 3 mmol/kg had a hyperacute response (HES = 8) at 4 h post-dosing necessitating euthanasia, while the other rat only had a HES of 2, and no serum biomarker changes, at the 24 h termination. We consider the non-response of the latter rat as an outlier.
Results for CHEB (Table 4) in our pilot study revealed the characteristic necrosis of the pars recta of the proximal renal tubules (Fig. 9) with associated increases in serum creatinine concentrations following single, consecutive daily, or combined dosing with CHB at 1 mmol/kg, as well as at higher doses. Distinctive lesions were also seen in the squamous portion of the stomach (Fig. 10) following the same dosing regimens. Liver lesions and increased activities of liver enzymes were seen with consecutive daily doses of 1 mmol/kg or single doses of 2 or 3 mmol/kg. Previous reports in rats that were fed the two S diastereoisomers of CHEB (derived from Crambe abyssinica) described the development of liver (bile duct hyperplasia, fibrosis, megalocytosis and zonal necrosis) and kidney lesions (degeneration and necrosis of the pars recta of the proximal tubules in acute toxicity and tubular epithelial karyomegaly in chronic toxicity), and these were correlated with serum biochemical alterations, including increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin and creatinine (Gould et al., 1980, Gould et al., 1985, VanEtten et al., 1969). In rats receiving 50 mg (0.39 mmol) or 100 mg (0.78 mmol)/kg/day CHEB via gavage, the typical lesions in the pars recta of the proximal tubules in the kidney were present by 48 h (Gould et al., 1985).
Regarding the characteristic renal lesions involving necrosis of the pars recta of the proximal tubules, it is interesting that identical lesions have been described in rats given 1.1 mmol/kg doses of 1-cyano-3,4-epithiobutane (CEB), the epithionitrile derived from gluconapin (4-PN is the other nitrile derivative) (VanSteenhouse et al., 1989, Wallig et al., 1988b). Rats given single doses of 1.6 mmol/kg of CEB can die within 24 h (Dietz et al., 1991). As with CHEB, histological changes were the first indication of toxicity with lesions preceding biomarker alterations by 24 h (Wallig et al., 1988b). At 1.1 mmol/kg, CEB is not hepatotoxic (VanSteenhouse et al., 1991), but at very high doses (>5 mmol/kg), it causes centrilobular necrosis in rats (Dietz et al., 1991). It has been suggested that the epithio group plays a role in the nephrotoxicity of epithionitriles (Wallig et al., 1988b). Conjugation with glutathione is a significant pathway in CEB metabolism (VanSteenhouse et al., 1989, VanSteenhouse et al., 1999). Epithionitriles bind covalently with proteins and to DNA (Brocker et al., 1984).
In addition, the lesions that we found in the pars oesophagea of the stomach of rats dosed with the progoitrin epithionitrile, CHEB, are almost identical to those caused by the gluconapin epithionitrile, CEB (Wallig et al., 1988b). Conceivably, these gastric lesions are due to direct contact of the epithionitrile with the forestomach epithelium (Wallig et al., 1988b). It seems likely that the distinctive stomach and mild intestinal lesions were responsible for the diarrhoea seen in some of the poisoned rats.
In its pure form, CHEB is unstable and tends to polymerise unless it is stored in an acetone solution (Gould et al., 1980, Gould et al., 1985). Administration of the polymerised form of CEB (from gluconapin) has proved non-toxic (Dietz et al., 1991).
The acute effect of a dose of 1 mmol/kg of 3-BN, the lack of serum liver biochemistry activity changes, and the presence of free cyanide in the liver lead us to believe that the death of Rat 37 was due to cyanide poisoning. Previous studies have shown that metabolism of 3-BN in the body results in the release of cyanide (Ahmed and Farooqui, 1982, Silver et al., 1982, Tanii, 2017, Tanii and Hashimoto, 1984). Other changes induced by 3-BN in rodents include behavioural abnormalities, ototoxicity (Gagnaire et al., 2001) and vestibulotoxicity, that are believed to be caused by another metabolite, 3,4-epoxybutyronitrile (Tanii, 2017, Tanii et al., 2004). It is not surprising that the rats dosed with 1 mmol/kg 4-PN (derivative of gluconapin) showed no toxicity; doses of more than 7 mmol/kg have previously been recorded as non-toxic (Dietz et al., 1991).
In conclusion, this pilot study showed that single doses of either CHB or CHEB at 1 mmol/kg failed to elicit clinical toxicity; however, histological lesions of pancreatic damage with CHB, and kidney and stomach lesions with CHEB were present. With regard to CHB, consecutive daily doses at 1 mmol/kg failed to demonstrate a cumulative effect, but this was not the case with CHEB, where consecutive daily dosing proved highly hepato- and nephrotoxic. Such a cumulative effect may be of relevance to the situation in cows grazing Brassica forage crops on a daily basis. Higher doses of the progoitrin nitriles (2 and 3 mmol/kg) resulted in severe clinical toxicity, both being hepatotoxic, with pancreatic toxicity (CHB) and nephrotoxicity (CHEB) also prominent, and manifesting within a few hours. Further investigation into the hypothesis of BALD being due to nitrile toxicity is warranted.
Author contributions
ZM performed the majority of the animal manipulations, collection of samples, and the clinical biochemistry graphs, as well as the design of the trial and a portion of the text. KP assisted with the animal manipulations, collection of samples and a portion of the text. MC performed the histopathological examinations and contributed to the design of the trial and most of the text.
Ethical statement
All studies were approved by the Massey University Animal Ethics Committee (protocol nos. 16/80, 16/120 and 17/21).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Brian Tapper for his help and leadership regarding the sourcing of CHB and CHEB, as well as his knowledge of glucosinolate chemistry. We are grateful to PGG Wrightson (Seeds) for financing this project, and to Charlotte Westwood and Andy Dumbleton for their enthusiastic encouragement. We thank Matthew Wallig for sharing his experience regarding the pathology of crambene and the epithio in rats. Thanks too to Evelyn Lupton and Saritha Gils for their histology technical skills. Thanks to SAPU and especially Aimee Hamlin for her support, remarkable animal handling skills during gavaging, and help with monitoring of the rats when required.
References
- Ahmed A.E., Farooqui M.Y.H. Comparative toxicities of aliphatic nitriles. Toxicol. Lett. 1982;12:157–163. doi: 10.1016/0378-4274(82)90179-5. [DOI] [PubMed] [Google Scholar]
- Bohus E., Rácz A., Noszál B., Coen M., Beckonert O., Keun H.C., Ebbels T.M.D., Cantor G.H., Wijsman J.A., Holmes E., Lindon J.C., Nicholson J.K. Metabonomic investigations into the global biochemical sequelae of exposure to the pancreatic toxin 1-cyano-2-hydroxy-3-butene in the rat. Magn. Reson. Chem. 2009;47:S26–S35. doi: 10.1002/mrc.2485. [DOI] [PubMed] [Google Scholar]
- Brocker E.R., Benn M.H., Lüthy J., Von Däniken A. Metabolism and distribution of 3,4-epithiobutanenitrile in the rat. Food Chem. Toxicol. 1984;22:227–232. doi: 10.1016/0278-6915(84)90132-7. [DOI] [PubMed] [Google Scholar]
- Bryan M., Hea S.-Y., Wilkinson S. Proceedings of the Society of Dairy Cattle Veterinarians of the NZVA. New Zealand Veterinary Association; Queenstown, New Zealand: 2015. A clinical and epidemiological analysis of swede-related deaths in Southland and Otago over winter 2014; pp. 269–285. [Google Scholar]
- Collett M.G., Matthews Z.M. Photosensitivity in cattle grazing Brassica crops. Int. J. Poisonous Plant Res. 2014;3:7–22. [Google Scholar]
- Collett M.G., Stegelmeier B.L., Tapper B.A. Could nitrile derivatives of turnip (Brassica rapa) glucosinolates be hepato- or cholangiotoxic in cattle? J. Agric. Food Chem. 2014;62:7370–7375. doi: 10.1021/jf500526u. [DOI] [PubMed] [Google Scholar]
- Collett M.G., Westwood C.T., Gill J. Proceedings of the Society of Dairy Cattle Veterinarians of the NZVA. New Zealand Veterinary Association; Queenstown, New Zealand: 2015. Clinical biochemistry and histopathology of brassica liver disease; pp. 255–268. [Google Scholar]
- Cullen J.M., Faiola B., Melich D.H., Peterson R.A., Jordan H.L., Kimbrough C.L., Miller R.T. Acute alpha-naphthylisothiocyanate-induced liver toxicity in germfree and conventional male rats. Toxicol. Pathol. 2016;44:987–997. doi: 10.1177/0192623316662360. [DOI] [PubMed] [Google Scholar]
- Dalley D., Verkerk G., Kyte R., McBeth C., Petch S., Kuhn-Sherlock B., Leach C., Irwin A., Harding N., Morley C., Ryan T. 2015. Swede Associated Toxicity in Dairy Cattle during Winter 2014; pp. 1–84.https://www.dairynz.co.nz/media/3343448/swede-associated-toxicity-in-dairy-cattle-during-winter-2014.pdf DairyNZ. [Google Scholar]
- Dietz H.M., Panigrahi S., Harris R.V. Toxicity of hydrolysis products from 3-butenyl glucosinolate in rats. J. Agric. Food Chem. 1991;39:311–315. [Google Scholar]
- Gagnaire F., Marignac B., Ban M., Langlais C. The ototoxic effects induced in rats by treatment for 12 weeks with 2-butenenitrile, 3-butenenitrile and cis-2-pentenenitrile. Pharmacol. Toxicol. 2001;88:126–134. doi: 10.1034/j.1600-0773.2001.d01-93.x. [DOI] [PubMed] [Google Scholar]
- Gould D.H., Fettman M.J., Daxenbichler M.E., Bartuska B.M. Functional and structural alterations of the rat kidney induced by the naturally occurring organonitrile 2S-1-cyano-2-hydroxy-3,4-epithiobutane. Toxicol. Appl. Pharmacol. 1985;78:190–201. doi: 10.1016/0041-008x(85)90283-2. [DOI] [PubMed] [Google Scholar]
- Gould D.H., Gumbmann M.R., Daxenbichler M.E. Pathological changes in rats fed the crambe meal-glucosinolate hydrolytic products, 2S-1-cyano-2-hydroxy-3,4-epithiobutanes (erythro and threo) for 90 days. Food Cosmet. Toxicol. 1980;18:619–625. doi: 10.1016/s0015-6264(80)80010-1. [DOI] [PubMed] [Google Scholar]
- Kelly L., Reid L., Walker N.I. Massive acinar cell apoptosis with secondary necrosis, origin of ducts in atrophic lobules and failure to regenerate in cyanohydroxybutene pancreatopathy in rats. Int. J. Exp. Pathol. 1999;80:217–226. doi: 10.1046/j.1365-2613.1999.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March T.H., Jeffery E.H., Wallig M.A. The cruciferous nitrile, crambene, induces rat hepatic and pancreatic glutathione S-transferases. Toxicol. Sci. 1998;42:82–90. doi: 10.1006/toxs.1998.2428. [DOI] [PubMed] [Google Scholar]
- Morton J.M., Campbell P.H. Disease signs reported in south-eastern Australian dairy cattle while grazing Brassica species. Aust. Vet. J. 1997;75:109–113. doi: 10.1111/j.1751-0813.1997.tb14169.x. [DOI] [PubMed] [Google Scholar]
- Nishie K., Daxenbichler M.E. Toxicology of glucosinolates, related compounds (nitriles, R-goitrin, isothiocyanates) and vitamin U found in cruciferae. Food Cosmet. Toxicol. 1980;18:159–172. doi: 10.1016/0015-6264(80)90070-x. [DOI] [PubMed] [Google Scholar]
- Silver E.H., Kuttab S.H., Hasan T., Hassan M. Structural considerations in the metabolism of nitriles to cyanide in vivo. Drug Metab. Dispos. 1982;10:495–498. [PubMed] [Google Scholar]
- Tanii H. Allyl nitrile: toxicity and health effects. J. Occup. Health. 2017;59:104–111. doi: 10.1539/joh.16-0147-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanii H., Hashimoto K. Studies on the mechanism of acute toxicity of nitriles in mice. Arch. Toxicol. 1984;55:47–54. doi: 10.1007/BF00316585. [DOI] [PubMed] [Google Scholar]
- Tanii H., Takayasu T., Higashi T., Leng S., Saijoh K. Allylnitrile: generation from cruciferous vegetables and behavioral effects on mice of repeated exposure. Food Chem. Toxicol. 2004;42:453–458. doi: 10.1016/j.fct.2003.10.007. [DOI] [PubMed] [Google Scholar]
- VanEtten C.H., Gagne W.E., Robbins D.J., Booth A.N., Daxenbichler M.E., Wolff I.A. Biological evaluation of crambe seed meals and derived products by rat feeding. Cereal Chem. 1969;46:145–155. [Google Scholar]
- VanSteenhouse J.L., Fettman M.J., Gould D.H. Sequential changes in hepatic and renal glutathione and development of renal karyomegaly in 1-cyano-3,4-epithiobutane toxicity in rats. Food Chem. Toxicol. 1989;27:731–739. doi: 10.1016/0278-6915(89)90078-1. [DOI] [PubMed] [Google Scholar]
- VanSteenhouse J.L., Fettman M.J., Gould D.H. The effect of glutathione depletion by buthionine sulphoximine on 1-cyano-3,4-epithiobutane toxicity. Food Chem. Toxicol. 1991;29:153–157. doi: 10.1016/0278-6915(91)90032-3. [DOI] [PubMed] [Google Scholar]
- VanSteenhouse J.L., Prescott J.S., Swenson D.H. Protection from 1-cyano-3,4-epithiobutane nephrotoxicity by aminooxyacetic acid and effect on xenobiotic-metabolizing enzymes in male Fischer 344 rats. J. Appl. Toxicol. 1999;19:237–249. doi: 10.1002/(sici)1099-1263(199907/08)19:4<237::aid-jat569>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wallig M.A., Gould D.H., Fettman M.J. Selective pancreatotoxicity in the rat induced by the naturally-occurring plant nitrile 1-cyano-2-hydroxy-3-butene. Food Chem. Toxicol. 1988;26:137–147. doi: 10.1016/0278-6915(88)90110-x. [DOI] [PubMed] [Google Scholar]
- Wallig M.A., Gould D.H., Fettman M.J., Willhite C.C. Comparative toxicities of the naturally occurring nitrile 1-cyano-3,4-epithiobutane and the synthetic nitrile n-valeronitrile in rats: differences in target organs, metabolism and toxic mechanisms. Food Chem. Toxicol. 1988;26:149–157. doi: 10.1016/0278-6915(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Wallig M.A., Jeffery E.H. Enhancement of pancreatic and hepatic glutathione levels in rats during cyanohydroxybutene intoxication. Fundam. Appl. Toxicol. 1990;14:144–159. doi: 10.1016/0272-0590(90)90240-k. [DOI] [PubMed] [Google Scholar]
- Westwood C.T., Mulcock H. Nutritional evaluation of five species of forage brassica. Proc. N. Z. Grassl. Assoc. 2012;74:31–38. [Google Scholar]
- Willhite C.C., Smith R.P. The role of cyanide liberation in the acute toxicity of aliphatic nitriles. Toxicol. Appl. Pharmacol. 1981;59:589–602. doi: 10.1016/0041-008x(81)90314-8. [DOI] [PubMed] [Google Scholar]