Abstract
With rising incidence rates, endometrial cancer is one of the most common gynecologic malignancies in the United States. While surgery provides significant survival benefit to early-stage patients, those with advanced or recurrent metastatic disease have a dismal prognosis. Limited treatment options include chemotherapy and radiation therapy. Hence there is a compelling need for developing molecularly targeted therapy. Here we show that the polycomb ring finger protein BMI1, also known as a stem cell factor, is significantly overexpressed in endometrial cancer cell lines, endometrial cancer patient tissues as well as in non-endometrioid histologies and associated with poor overall survival. PTC-028, a second generation inhibitor of BMI1 function decreases invasion of endometrial cancer cells and potentiates caspase dependent apoptosis while normal cells with minimal expression of BMI1 remain unaffected. In an aggressive uterine carcinosarcoma xenograft model, single agent PTC-028 significantly delayed tumor growth and increased tumor doubling time compared to the standard carboplatin/paclitaxel therapy. Therefore, anti-BMI1 strategies may represent a promising targeted approach in patients with advanced or recurrent endometrial cancer, a population where treatment options are limited.
Keywords: Endometrial Cancer, BMI1, apoptosis
INTRODUCTION:
Endometrial cancer is expected to be diagnosed in approximately 61,000 new patients this year in the United States, making it the most common gynecologic malignancy [1, 2]. While Type 1 is characterized by low grade, has endometrioid histology, and is hormonally driven, Type 2 cancers have a higher grade, are aneuploid and have a high frequency of TP53 mutations. These consist of histologies such as serous, carcinosarcoma and clear cell as well as some high grade endometrioid tumors [2, 3]. Five-year overall survival for type 1 endometrial cancers is approximately 85% while type 2 cancers ranges from 50–60% demonstrating an urgent need to improve treatment options for the more aggressive subtypes of endometrial cancer [4]. Research focused on genetic abnormalities in endometrial cancer has led to the development of clinical trials exploiting targeted alterations in the PI3K/AKT and mTOR pathways [2]. However, despite our increasing knowledge there are currently no approved molecularly targeted therapies. Therefore research into potential targets for therapeutic treatment of endometrial cancer is of utmost importance.
BMI1, a member of the Polycomb Repressor Complex 1 (PRC1), mediates gene silencing by regulating chromatin structure and is frequently upregulated in major types of cancer where its expression correlates with poor prognosis [5–10]. However, there has been limited research into the role of BMI1 in endometrial cancer to date. A previous study reported that in addition to breast, ovarian and cervical cancer BMI1 was also significantly overexpressed in endometrial cancer [11]. Inhibiting BMI1 levels by micro-RNA 194, reverted highly invasive endometrial cancer cell lines from a mesenchymal to a more epithelial phenotype [12]. The presence of micro-RNA 194, which is known to inhibit BMI1 levels, was inversely related to tumor stage in Type 1 endometrial cancer samples [13]. Realizing the pathological significance, chemical inhibitors against BMI1 were developed very recently. The first generation BMI1 inhibitor, PTC-209 was tested in colorectal and ovarian cancer models showing promising results, however, intra-tumoral administration was required [10, 14]. Here, we demonstrate that BMI1 levels were significantly elevated in endometrial cancer cell lines as well as in endometrial cancer patient tumors. PTC-028 [15], a second generation inhibitor of BMI1 function with optimized pharmaceutical properties, depleted BMI1 levels that led to decreased cellular invasion and reduced cellular viability by potentiating caspase-dependent apoptosis. In a mouse model of endometrial cancer, treatment with PTC-028 significantly increased the tumor doubling time and delayed tumor growth compared to the standard-of-care carboplatin and paclitaxel. Therefore, targeting BMI1 may serve as a promising therapeutic approach in endometrial cancer.
MATERIALS AND METHODS:
Cell culture and chemicals:
AN3CA [16], HEC1A and HEC1B cells [17] authenticated by the American Type Culture Collection (ATCC) were purchased by our laboratory and used within 6–8 months. RL95–2 [18] originally from ATCC, was kindly provided by Dr. Danny Dhanasekaran, OUHSC and authenticated by short tandem repeat (STR) profiling. D6B is a primary culture of the human endometrium that was established and kindly provided by Dr. Doris Benbrook, OUHSC [19]. CS99 was established from a uterine carcinosarcoma by Dr. H. J. Schulten et. al. [20] and further characterized and provided to us by Dr. Jason Somarelli, Duke University [21]. Ishikawa cells [22] were authenticated by the European Collection of Authenticated Cell Cultures (ECACC) was purchased by our laboratory. MFE296 [23] originally from ECACC was provided by Dr. Jie Wu, OUHSC and authenticated by STR profiling. AN3CA cells were cultured in EMEM + 10% FBS. HEC1A was cultured in McCoy’s Modified Media + 10% FBS and HEC1B was cultured in EMEM + 10% FBS. RL95–2 was cultured in DMEM and F12 (1:1) + 10% FBS. D6B and CS99 cells were cultured in DMEM + 10% FBS. Ishikawa and MFE296 cells were cultured in RPMI + 10% FBS. All the cells were cultured with 1X penicillin-streptomycin in a 5% CO2 humidified atmosphere. PTC-028 was provided by PTC Therapeutics.
Cell Lysis, Cell fractionation, SDS-PAGE, and Western blotting:
Total cellular lysate was prepared in RIPA (Boston Bioproducts) and protein concentration was measured using a BCA assay kit (Pierce, 23225). A standard protocol was used for immunoblotting. The cell lysates were separated on 10% or 15% glycine SDS-PAGE gel and transferred to PVDF membrane. Membranes were blocked in 5% BSA in TBS with 0.1% TWEEN-20 (TBST) for 1 h at room temperature. Primary antibodies were prepared in TBST with 5% BSA and membranes were incubated in primary antibody at 4°C for overnight. Antibodies were purchased from the following venders; BMI1 from Invitrogen (37–5400) Bethyl Laboratories (IHC-00606); beta-Actin (4970), PARP (9542), Cleaved Caspase-3 (9664), Cleaved, Cleaved Caspase-9 (7237) from Cell Signaling Technology and secondary antibodies conjugated with horseradish peroxidase IgG Rabbit (A6154) and Mouse (A4416) from Sigma. Primary antibodies were used in dilutions recommended by the manufacturer. Secondary antibodies were used at a concentration of 1:10,000.
Immunohistochemistry and Tissue Microarray:
A tissue microarray (TMA) containing 203 patients samples consisting of all endometrial cancer histologies was used for detection of BMI1 expression by immunohistochemistry (IHC). The patient samples were collected at the University of Oklahoma Health Science Center. Written informed consent was obtained from all women enrolled into the study and Institutional Review Board approval was provided by OUHSC. IHC was performed using BMI1 (1:200) antibody and based on immunoreactivity and intensity, staining was graded as 1 (low), 2 (intermediate) and 3 (high). IHC of BMI1 was scored using the standardized H-Score, which takes into account both intensity and percentage of cells stained. The difference in H-score between groups were assessed by Wilcoxon rank-sum or Kruskal-Wallis test. For ease of interpretation, the H-score, range 0–240, was analyzed as a binary variable (sample median, 40 as the cutoff) and a 3-level categorical variable (sample tertiles as cutoffs), when assessing its association with clinical factors (stage, grade, histology, LVSI, DOI) using Chi-square test and survival outcomes (PFS and OS). Kaplan-Meier method and log-rank test were used to examine the unadjusted association between H-score and survival outcomes. Adjusted analyses were also performed using the Cox regression, controlling for histology, age at diagnosis, and stage. Statistical significance was defined as a two-sided p-value of < 0.05. SAS v. 9.3 was used for all the analyses.
Determination cell viability and of apoptosis:
We used the ApoTox-Glo™ triplex assay kit (G6321) from Promega to determine apoptosis in various endometrial cancer cell lines as well as normal endometrial cells. Cells were treated with PTC-028 at various concentrations. The cells were incubated simultaneously to measure two protease activities; one is a marker of cell viability, and the other is a marker of cell death. The live and dead-cell proteases produced different products, AFC and R110, which had different excitation and emission spectra, allowing them to be detected concurrently. The second part of the assay utilized a luminogenic Caspase-3-Caspase-7 substrate (the tetrapeptide sequence DEVD), in a reagent optimized for caspase activity, luciferase activity and cell lysis. Luminescence was proportional to the amount of caspase activity present. In addition, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) positivity was assessed in PTC-028 treated and untreated CS99 and Ishikawa cells. We utilized the TUNEL apoptosis detection kit (DeadEnd Fluorometric TUNEL system, Promega) according to the supplier’s instructions. They were then analyzed by fluorescence microscopy. The number of TUNEL positive nuclei were counted from ~300 cells per treatment group. For caspase-rescue, CS99 and Ishikawa cells were treated with the pan caspase inhibitor z-VAD-fmk (10µM) (Selleckchem, Houston, TX, USA) for 3h with or without PTC-028 (50 nM) for 48h and analyzed cell viability using the MTS assay.
Gelatin Degradation Assay:
Acid-washed coverslips were first coated with 50 ug/mL poly-L-lysine for 20 min at room temperature, and then fixed with 0.5% glutaraldehyde for 15 min. Gelatin matrix was prepared by mixing 0.2% gelatin and Oregon Green ® 488 Gelatin Conjugate (Life Technologies, Rockford, IL, USA) at an 8:1 ratio. After coating for 10 min, coverslips were washed with PBS and self-fluorescence was quenched with 5mg/ml sodium borohydride for 15 min followed by washing with PBS. For degradation assay, 25,000 cells for CS99 and 30,000 cells for Ishikawa were seeded in each well of a 12 –well plate containing 488 Gelatin Conjugate cover slips. 12 hours after plating, cells were treated with PTC-028 (20nM) or vehicle for 24 hours and 36 hours for CS99 and Ishikawa respectively. Next, cells were fixed in 4% paraformaldehyde (PFA) and stained with Alexa Flour ® 555 Phalloidin (Life Technologies, Rockford, IL, USA) for 15 min at room temperature. The cells were washed with PBS and mounted with VECTASHIELD ® mounting medium containing DAPI (Vector Laboratories). Images were acquired at 40X (with 1.6X Optovar) using the Zeiss Axio-Observer Z1 (Göttingen, German). Cells that degraded the ECM at focal adhesions (FA) sites were scored positive and ~100 random cells were quantified. The percentage of cells showing degradation was plotted.
Mouse Xenograft Models:
Female athymic nude mice (NCr-nu; 6 to 8 wks old) were purchased from the Harlan Laboratory (Indianapolis, IN). All mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and NIH. All studies were approved and supervised by the University of Oklahoma Animal Facility under the guidance of the IACUC #16-004-SSHIC. CS99 cells (1 × 106/100 μl) were injected subcutaneously into the flanks of female athymic nude mice and randomized when the tumor reached the volume of ~100mm3. Mice were randomized to three different groups receiving vehicle, PTC-028, or carboplatin (Hospira, Inc., Lake Forest, IL, USA) and paclitaxel (Actavis Pharma, Inc., Parsippany, NJ, USA) (C+T) which is the standard of care for advanced or recurrent endometrial cancer. PTC-028 was administered orally at 15mg/kg twice weekly. Carboplatin and paclitaxel were administered weekly by intraperitoneal (IP) route at 50mg/kg and 15mg/kg respectively. After 2 cycles of treatment, mice were followed for tumor growth and euthanized when their tumor volume exceeded 1500 mm3 as per the IACUC limit. Tumor Doubling Time (DT) was calculated according to Mehrara et al. [24] using the equation DT=LN(2)/SGR, where SGR (specific growth rate) = ln(V2 / V1) / (t2 − t1). In this experiment, we have utilized 7 mice in the vehicle treated group, 8 mice in PTC-028 group and 7 mice in C+T group respectively.
Data analysis and Statistics:
All the experiments were repeated independently 3 times unless otherwise noted. Data are expressed as means ± standard deviation (SD) unless otherwise noted. One-way ANOVA was performed to compare the mean among three or more groups, and Student’s t test was performed to compare the mean between two groups. Survival analysis was performed by the Kaplan-Meirer method and log-rank analysis. Statistical significance was set at P < 0.05, using GraphPad Prism 6 software. For the Tissue Microarray: The descriptive statistics (mean, standard deviation, count and percentage) were reported. The difference in H-score between groups were assessed by Wilcoxon rank-sum or Kruskal-Wallis test. For ease of interpretation, the H-score was analyzed as a binary variable (sample median as the cutoff), when assessing its association with survival outcomes (PFS and OS). Kaplan-Meier method and log-rank test were used to examine the unadjusted association between H-score and survival outcomes. Adjusted analyses were also performed using the Cox regression, controlling for histology, age at diagnosis, and stage. Statistical significance was defined as a two-sided p-value of < 0.05. SAS v. 9.3 was used for all analyses.
RESULTS:
BMI1 is elevated in endometrial cancer.
The expression levels of BMI1 were determined in the immortalized normal endometrial cells (D6B), as well as in both Type 1 and Type 2 endometrial cancer cell lines. Type 1 was represented by endometrioid adenocarcinoma cell lines i.e., HEC1A, HEC1B, ISHIKAWA, MFE296, and RL95–2. Type 2 was represented by AN3CA (dedifferentiated adenocarcinoma), and CS99 (carcinosarcoma). Compared to normal endometrial cells, BMI1 levels were elevated in all the cancer cell lines (Fig. 1A). To evaluate if this finding translated to endometrial patient samples, a tissue microarray (TMA), representing 203 patients consisting of all endometrial cancer histologies was developed from previously archived patient samples at the University of Oklahoma Health Science Center. Immunohistochemistry (IHC) for BMI1 was performed and scored using the standardized H-Score, which takes into account both intensity and percentage of cells stained [25, 26]. When present, predominant nuclear staining for BMI1 was observed. Representative high, intermediate and low staining tissues are shown in Fig. 1B. Utilizing the median H-score as a cutoff we categorized the samples into low or high staining cohorts (Table 1). The median age of the cohort was 61 (range 35–90). The majority of the patients had disease confined to the uterus, defined as stage IA and 1B (n=174, 86%) and endometrioid histology (n= 158, 78%). There was no association between age, depth of invasion, lymphovascular space invasion (LVSI) and BMI1 level. There was a statistically significant association between non-endometrioid histology and high BMI1 (p=0.03) as well as a trend towards an increased recurrence rate in patients with high BMI1 (20%) versus low BMI1 (12%). Both progression-free (Fig. 1C) and overall (Fig. 1D) survival were compared between these two cohorts. Patients with high BMI1 expression had worse overall survival (p=0.002) (Fig. 1D) and a trend towards worse progression free survival (PFS) (p=0.080) (Fig. 1C). Based on these results, a multivariate Cox proportional hazards model was designed controlling for age, stage, and histology, which are all known to impact prognosis in endometrial cancer [27–29]. As with univariate analysis, the hazard ratio (HR) for PFS was not significant (p=0.40). However, there was a significant association of BMI1 with overall survival (HR: 2.4, 95% CI 1.1–5, p=0.021). Together these results indicate that BMI1 is elevated in endometrial cancer cell lines and patient tissues and suggests that high BMI1 levels may be an independent prognostic marker for overall survival in patients with endometrial cancer.
Figure 1:
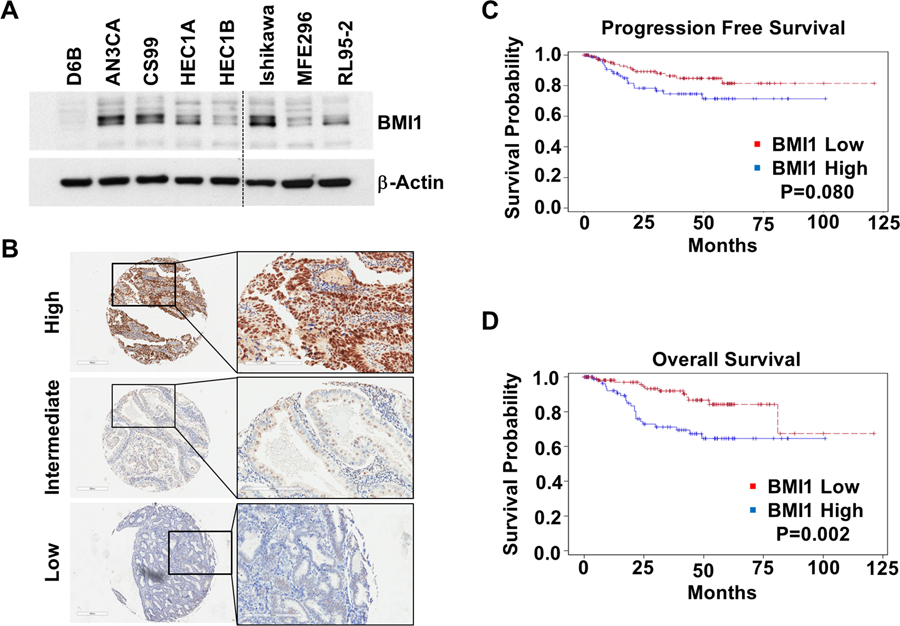
BMI1 levels are elevated in endometrial cancer. (A) Expression of BMI1 in normal and malignant endometrial cells. (B) Immunohistochemical staining of endometrial cancer patient TMA. Representative images at 4X magnification are shown of (i) high, (ii) intermediate and (iii) low staining tissues. Inset shows magnified areas at 20X (C) Kaplan–Meier analysis of progression free survival and (D) overall survival based on high and low BMI1 expression levels utilizing the median H-score. The proportion survival is plotted versus time since diagnosis in months. Kaplan-Meier curves with a log-rank test where P value<0.05 was considered significant.
Table 1.
Demographic Variables and Association with BMI1
| All Patients | BMI1 Low | BMI1 High | P Value | |
|---|---|---|---|---|
| (n =203) | (n = 114) | (n=89) | ||
| Median age at diagnosis (range) | 61 ( 35–90) | 61 (38–88) | 62 (35–90) | 0.81 |
| FIGO Stage | ||||
| IA | 129 (64%) | 71 (62%) | 58 (65%) | |
| IB | 45 (22%) | 26 (23%) | 19 (21%) | |
| II-IV | 29 (14%) | 17 (15%) | 12 (14%) | 0.91 |
| Histology | ||||
| Endometrioid | 158 (78%) | 95 (83%) | 63 (71%) | |
| Non-Endometrioid | 45 (22%) | 19 (17%) | 26 (29%) | 0.03 |
| Depth of Invasion | ||||
| None | 48 (24%) | 24 (21%) | 24 (27%) | |
| <50% | 90 (44%) | 51 (45%) | 39 (44%) | |
| >50% | 63 (31%) | 38 (33%) | 25 (28%) | |
| Unknown | 2 (1%) | 1 (1%) | 1 (1%) | 0.55 |
| LVSI | ||||
| Yes | 38 (19%) | 21 (18%) | 17 (19%) | |
| No | 156 (77%) | 88 (78%) | 68 (76%) | |
| Unknown | 9 (4%) | 5 (4%) | 4 (5%) | 0.90 |
| Recurrence | ||||
| Yes | 32 (16%) | 14 (12%) | 18 (20%) | |
| No | 171 (84%) | 100 (88%) | 71 (80%) | 0.12 |
PTC-028 attenuates endometrial cancer cell viability and invasion.
Among the endometrial cancer cells tested, significant expression of BMI1 was observed in Ishikawa (type1), CS99 and AN3CA (type2) lines (Fig 1A). To determine the effect of BMI1 depletion by PTC-028, normal and malignant endometrial cells were treated with increasing concentrations of PTC-028 for 48 h followed by the ApoTox-Glo Triplex assay that simultaneously determines cell viability, cytotoxic cell death and Caspase 3/7 activity by utilizing two different protease markers and a luminogenic caspase substrate respectively [14]. PTC-028 did not affect viability, cytotoxic cell death or caspase activity in normal endometrial cells (Fig. 2A), that have minimal BMI1 expression (Fig. 1A) but dose dependently inhibited cell viability of Ishikawa, AN3CA and CS99 (Fig. 2B–D). The IC50 value of PTC-028 in Ishikawa, AN3CA and CS99 lines was ∼50nM (Fig. 2B–D). In the low BMI1 expressing HEC1A, IC50 was ∼100nM and in the least BMI1 expressing HEC1B, IC50 could not be reached up to a concentration of ∼ 100nM (Fig. S1). While a dose-dependent increase in Caspase-3/7 activity was observed, there was no effect on cytotoxic cell death (Fig. 2B–D). We previously established that a cytotoxic cell death signal is generated in this assay only by a disruption of the cellular membrane (Triton-X) but not by apoptotic stimuli such as cisplatin [14]. Importantly treatment with PTC-028 at 50nM for 48 h significantly depleted BMI1 levels in AN3CA, Ishikawa and CS99 cells (Fig 2E). These results suggest that inhibition of BMI1 by PTC-028 significantly attenuates endometrial cancer cell viability by triggering the apoptotic mode of cell death.
Figure 2:
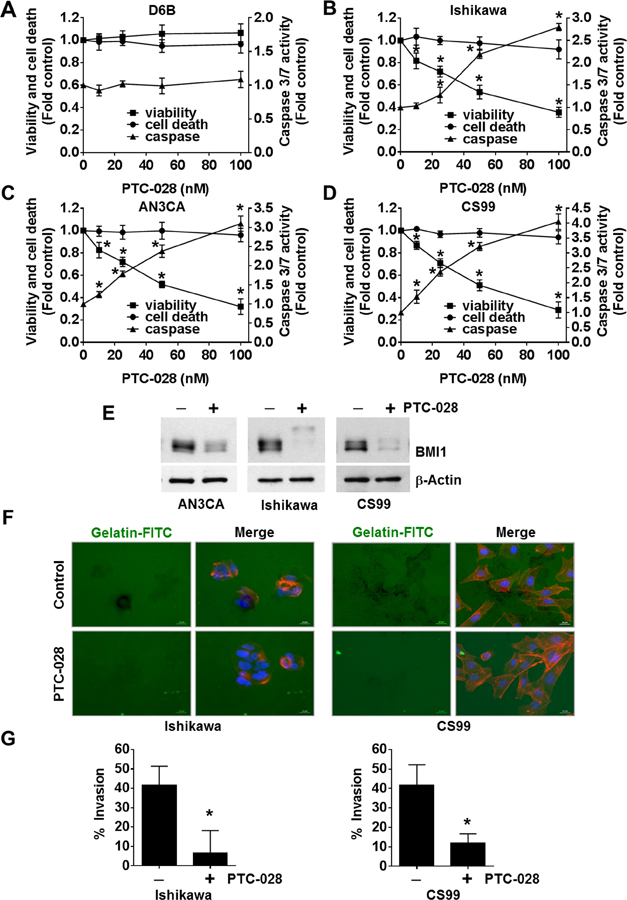
PTC-028 inhibits endometrial cancer cell viability and invasion. (A) D6B, (B) Ishikawa, (C) AN3CA and (D) CS99 cells were treated with increasing concentrations of PTC-028 for 48h and cell viability, cytotoxic cell death and caspase 3/7 activity was evaluated using the ApoTox-Glo Triplex assay. Data are mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective vehicle treated control by two-way ANOVA. (E) AN3CA, Ishikawa and CS99 cells were treated with 50 nM PTC-028 for 48h. Expression of BMI1 was determined by immunoblotting. (F) Representative images of Ishikawa and CS99 cells plated on Oregon Green® 488 Gelatin coated coverslips and treated with 20 nM PTC-028 for 24h (CS99) or 36h (Ishikawa). The cells were fixed and stained with Alexa Fluor® 555 Phalloidin and mounted in Vectashield mounting medium containing DAPI. (G) Quantification of invaded cells were counted from ~100 cells per treatment group as represented in Fig. 2F. Data are mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective vehicle treated control by Student’s t-test.
Since, the depth of myometrial and cervical invasion of the endometrium is recognized as a prognostic factor for lymph node metastasis and overall survival [30–32], the phenotypic effects of PTC-028 treatment on cellular invasion was investigated. A representative endometrial cancer cell line each of type 1 (Ishikawa) and type 2 (CS99) served as models. The degradation of extracellular matrix components by matrix metalloproteases is a hallmark of cellular invasion, therefore the FITC-gelatin degradation assay was performed [33]. Interestingly, treatment with PTC-028 at sub-IC50 concentrations, significantly reduced the number of cells invading the matrix by 6.2 fold and 3.5 fold in Ishikawa and CS99 respectively compared to control (Fig. 2F–G). Together these results suggest that inhibition of BMI1 by PTC-028 decreases viability and invasive potential of endometrial cancer cells.
PTC-028 induces endometrial cancer cell death via caspase dependent apoptosis.
Results from the Apotox-Triplex assay (Fig. 2B–D) suggested that the activation of caspase mediated apoptosis may be responsible for decreased cellular viability. To confirm the apoptotic mode of cell death, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) positivity was evaluated in the endometrial cancer cells that were treated with 50nM PTC-028 for 48h. Compared to the untreated control, significant TUNEL positivity was observed in the PTC-028 treated Ishikawa (~48%), and CS99 (~42%) cells respectively (Fig. 3A). To further corroborate these results, both the cell lines were treated with PTC-028 and BMI1 levels and apoptotic markers evaluated by immunoblotting. A significant decrease in levels of BMI1 and increase in cleavage of poly ADP-ribose polymerase (PARP), Caspase 3 and Caspase 9 was observed in the PTC-028 treated endometrial cancer cells (Fig. 3B). These results indicate that depletion of BMI1 by PTC-028 induces caspase-mediated apoptotic cell death. To further confirm that PTC-028 mediated decrease in cell viability was due to apoptosis, we treated the cells with the pan-caspase inhibitor z-VAD-fmk (10µM) for 3h with or without PTC-028 (50 nM) for 48h and analyzed cell viability using the MTS assay. Compared to PTC-028 only, dual treatment with z-VAD-fmk significantly rescued cell viability; by ~38% in Ishikawa and ~23% in CS99 cells respectively (Fig. 3C). These results confirm that PTC-028 mediated depletion of BMI1 induces caspase-dependent apoptosis in endometrial cancer cells.
Figure 3:
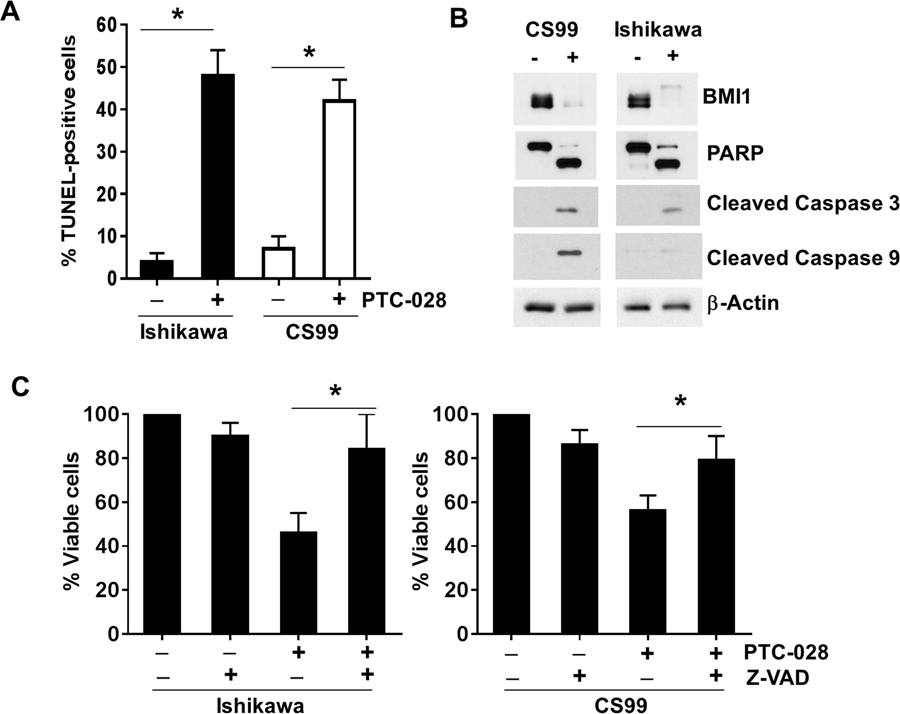
PTC-028 induces caspase dependent apoptosis in endometrial cancer cells. (A) Ishikawa and CS99 cells were treated with 50 nM PTC-028 for 48h, cells were subjected to the TUNEL assay and analyzed by fluorescence microscopy. The number of TUNEL positive nuclei were counted from ~400 cells per treatment group. Data represent the mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by Student’s t-test. (B) CS99 and Ishikawa cells were treated with 50 nM PTC-028 for 48h. Expression of BMI1, PARP, cleaved caspase 3, cleaved caspase 9, and beta actin was determined by immunoblotting. (C) Ishikawa and CS99 cells were pre-treated with or without the pan caspase inhibitor z-VAD-fmk (z-VAD) at 10µM for 3h followed by PTC-028 at 50 nm for 48h. Cell viability was assessed by the MTS assay. Vehicle treated control cells were set to 100%. Data are mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by a one-way ANOVA.
PTC-028 delays in-vivo tumor growth.
In order to evaluate the efficacy of PTC-028 in-vivo, a mouse xenograft model was developed using CS99 cells [34]. When tumor volume reached ~100 mm3 mice were randomized into three groups. The first group received PTC-028 at 15 mg/kg twice weekly by oral administration. The second group received intraperitoneal injections of carboplatin and paclitaxel at 50 mg/kg and 15 mg/kg weekly for two cycles [35]. The third group received vehicle only. After two cycles of treatment, mice were followed for tumor growth and euthanized when their tumor burden reached IACUC approval limits. Due to tumor burden, all animals in the vehicle group were euthanized by ~ day 8 while those in the carboplatin/paclitaxel group were euthanized by ~ day18 and those in the PTC-028 group were euthanized by ~ day 24 after initiation of treatment. ~24 days (Fig 4A). To compare tumor growth rates over the entire experimental period, tumor doubling time was calculated according to Mehrara et.al. [24] (Fig. 4B). Compared to the vehicle (~1.9 days) and carboplatin/paclitaxel (~3.3 days) treated group, the tumor doubling time was significantly delayed in the PTC-028 (~4.9 days) treated group (Fig 4B). These results indicate that single agent PTC-028 provides significantly delayed tumor growth compared to the standard-of-care chemotherapy in an endometrial cancer mouse model.
Figure 4:
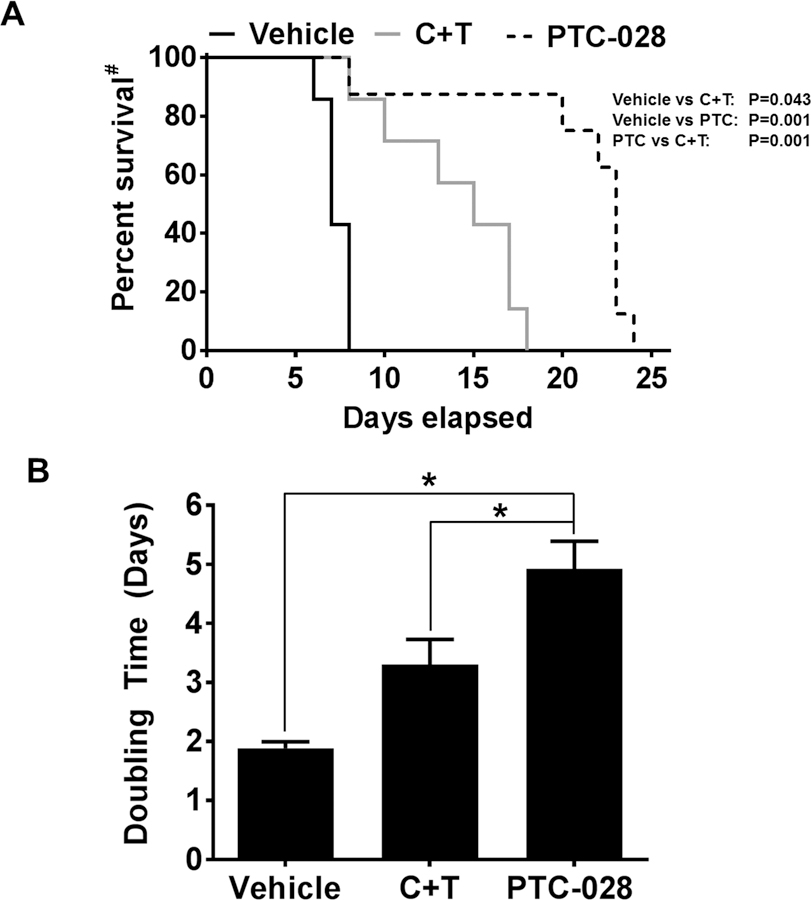
Treatment with PTC-028 decreased tumor doubling time and delayed tumor growth in mouse xenograft model. (A) CS99 cells were injected into 6-week old female athymic nude mice. Mice were randomized into 3 groups of 7–10 each and treatment initiated when tumor volume exceeded ~100 mm3. PTC-028 was administered orally at 15 mg/kg twice weekly. Carboplatin at a dose of 50 mg/kg/weekly and paclitaxel at 15 mg/kg/weekly (C+T) were administered by intraperitoneal injections and vehicle control given orally and intraperitoneally. Treatment continued for two cycles (14 days) and mice were followed for survival. #Percent survival was calculated by Kaplan-Meier method and P values determined by Log Rank Test based on the number of days the animals survived before the euthanization as per IACUC limits. B) Tumor doubling time was calculated for each treatment groups according to Mehrara et.al. [24]. Data are mean ± S.D and *P<0.05 when comparing with between indicated groups by one-way ANOVA.
DISCUSSION:
While, majority of endometrial cancers are effectively treated with surgery, those patients with advanced or recurrent disease have limited treatment options and a high rate of chemoresistance. To improve response rates, strategies for development of more effective targeted therapy are needed in this patient population.
While the expression of BMI1 has been linked with poor prognosis in other cancers [5, 6, 9–11, 36, 37], there have been limited studies in endometrial cancer. It was previously reported that inhibiting BMI1 levels by micro-RNA 194 reverted highly invasive endometrial cancer cell lines from a mesenchymal to a more epithelial phenotype [12]. We find that BMI1 levels were significantly elevated in both type 1 and type 2 models of endometrial cancer cell lines. These observations were corroborated by the TMA where 91% of the endometrial cancer patient tumor tissues expressed BMI1 compared to 9% that did not. Within the TMA, expression of BMI1 significantly correlated with type 2 histologies. However, a significant number of type 1 endometrial cancers also had elevated BMI1 levels (40%). In addition, elevation of BMI1 led to worse overall survival independent of histology. This suggests that elevated BMI1 may be an independent prognostic marker and could be utilized moving forward to help potentially identify patients in both types of endometrial cancer that have worse survival and will benefit from adjuvant treatment, potentially targeted at BMI1 elevation.
The first generation BMI1 inhibitor, PTC-209 inhibited self-renewal of cancer-initiating cells causing irreversible impairment in primary colorectal tumor growth [10]. However, to inhibit sub-cutaneous xenograft tumors, intra-tumoral administration of PTC-209 at 60 mg/kg/day for 10 days was required [10] which is not feasible from a therapy perspective. Here, we utilized the second generation inhibitor of BMI1; PTC-028 that with superior pharmacologic properties [15] potentiates tumor inhibition upon oral administration. In vitro, PTC-028 decreased expression of BMI1 and dose dependently decreased viability of endometrial cancer cells. Importantly, PTC-028 had no effect on normal endometrial cells (D6B) that have minimal BMI1 levels; demonstrating specificity of the inhibitor against BMI1. Furthermore at sub-IC50 concentrations PTC-028 inhibited invasion consistent with reversal of EMT and invasion by BMI1-shRNA in endometrial cancer cells [12]. We further confirmed that PTC-028 led to a caspase-dependent apoptotic mode of cell death in endometrial cancer cells.
Importantly, carcinosarcoma represents one of the most aggressive subsets of endometrial cancer with a 5 year survival of less than 10% in patients presenting with advanced stage disease [38]. Platinum-based chemotherapy is the standard for both local and advanced disease; however, response rates of only ~50% necessitate the development of other treatment approaches [39]. Interestingly, in a carcinosarcoma xenograft model, orally administered PTC-028 demonstrated impressive single agent activity compared to intra-peritoneally delivered carboplatin and paclitaxel. This was exemplified by a significant increase in tumor doubling time and delayed tumor growth of mice bearing CS99 xenograft tumors. Taken together, these results indicate that anti-BMI1 strategies might provide an opportunity to improve outcome in women with advanced or recurrent endometrial cancer and also provide a rationale for further studies to determine its therapeutic benefit along with combination therapy in a clinical setting. Several proteins that are clinically relevant are directly or indirectly linked with expression of BMI1 for example, c-Myc, MDR1, PTEN and BID [40–43]. Therefore, the BMI1 and associated protein scores can be stratified based on their expression from tumor tissues versus adjacent non-tumor tissue. It may be expected then that patients with a higher BMI1 score would respond favorably to anti-BMI1 therapy. However, the efficacy of this strategy can only be appreciated in the context of clinical outcome that is linked to the BMI1 score in pre versus post treated tumor tissues.
Supplementary Material
ACKNOWLEDGEMENTS:
This study was supported by the National Institutes of Health (NIH) CA 157481 and by a Presbyterian Health Foundation Seed Grant to R. Bhattacharya. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank an Institutional Development Award (IDeA) grant (P20 GM103639) from the National Institute of General Medical Sciences of the National Institutes of Health supporting the use of the Histology and Immunohistochemistry Core.
FINANCIAL SUPPORT: This study was supported by the National Institutes of Health (NIH) CA 157481 and by a Presbyterian Health Foundation Seed Grant to R. Bhattacharya.
Footnotes
CONFLICTS OF INTEREST: The corresponding author has no financial interest to declare but PTC-028 was developed and provided by PTC therapeutics, South Plainfield, New Jersey. No potential conflicts of interest were disclosed.
REFERENCES:
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A: Colorectal cancer statistics, 2017. CA Cancer J Clin 2017, 67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E: Endometrial cancer. Lancet 2016, 387(10023):1094–1108. [DOI] [PubMed] [Google Scholar]
- 3.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC et al. : Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz APM, Ngan HYS, Pecorelli S: Carcinoma of the Corpus Uteri. International Journal of Gynecology & Obstetrics 2006, 95:S105–S143. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya R, Mustafi SB, Street M, Dey A, Dwivedi SK: Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis 2015, 2(3):225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, Calin GA, Mukherjee P: MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res 2009, 69(23):9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glinsky GV, Berezovska O, Glinskii AB: Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 2005, 115(6):1503–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayry V, Tynninen O, Haapasalo HK, Wolfer J, Paulus W, Hasselblatt M, Sariola H, Paetau A, Sarna S, Niemela M et al. : Stem cell protein BMI-1 is an independent marker for poor prognosis in oligodendroglial tumours. Neuropathol Appl Neurobiol 2008, 34(5):555–563. [DOI] [PubMed] [Google Scholar]
- 9.Hoenerhoff MJ, Chu I, Barkan D, Liu ZY, Datta S, Dimri GP, Green JE: BMI1 cooperates with H-RAS to induce an aggressive breast cancer phenotype with brain metastases. Oncogene 2009, 28(34):3022–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W, Sydorenko N et al. : Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014, 20(1):29–36. [DOI] [PubMed] [Google Scholar]
- 11.Honig A, Weidler C, Hausler S, Krockenberger M, Buchholz S, Koster F, Segerer SE, Dietl J, Engel JB: Overexpression of polycomb protein BMI-1 in human specimens of breast, ovarian, endometrial and cervical cancer. Anticancer Res 2010, 30(5):1559–1564. [PubMed] [Google Scholar]
- 12.Dong P, Kaneuchi M, Watari H, Hamada J, Sudo S, Ju J, Sakuragi N: MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol Cancer 2011, 10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhai H, Karaayvaz M, Dong P, Sakuragi N, Ju J: Prognostic significance of miR-194 in endometrial cancer. Biomark Res 2013, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey A, Mustafi SB, Saha S, Kumar Dhar Dwivedi S, Mukherjee P, Bhattacharya R: Inhibition of BMI1 induces autophagy-mediated necroptosis. Autophagy 2016, 12(4):659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey A, Xiong X, Crim A, Dwivedi SKD, Mustafi SB, Mukherjee P, Cao L, Sydorenko N, Baiazitov R, Moon YC et al. : Evaluating the Mechanism and Therapeutic Potential of PTC-028, a Novel Inhibitor of BMI-1 Function in Ovarian Cancer. Mol Cancer Ther 2018, 17(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawe CJ, Banfield WG, Morgan WD, Slatick MS, Curth HO: Growth in Continuous Culture, and in Hamsters, of Cells from a Neoplasm Associated with Acanthosis Nigricans. J Natl Cancer Inst 1964, 33:441–456. [PubMed] [Google Scholar]
- 17.Kuramoto H: Studies of the growth and cytogenetic properties of human endometrial adenocarcinoma in culture and its development into an established line. Acta Obstet Gynaecol Jpn 1972, 19(1):47–58. [PubMed] [Google Scholar]
- 18.Way DL, Grosso DS, Davis JR, Surwit EA, Christian CD: Characterization of a new human endometrial carcinoma (RL95–2) established in tissue culture. In Vitro 1983, 19(3 Pt 1):147–158. [DOI] [PubMed] [Google Scholar]
- 19.Kamelle S, Sienko A, Benbrook DM: Retinoids and steroids regulate menstrual phase histological features in human endometrial organotypic cultures. Fertil Steril 2002, 78(3):596–602. [DOI] [PubMed] [Google Scholar]
- 20.Schulten HJ, Wolf-Salgo J, Grundker C, Gunawan B, Fuzesi L: Characterization of a newly established uterine carcinosarcoma cell line featuring the sarcomatous phenotype of the tumor in vitro. Int J Gynecol Cancer 2008, 18(2):339–344. [DOI] [PubMed] [Google Scholar]
- 21.Somarelli JA, Schaeffer D, Marengo MS, Bepler T, Rouse D, Ware KE, Hish AJ, Zhao Y, Buckley AF, Epstein JI et al. : Distinct routes to metastasis: plasticity-dependent and plasticity-independent pathways. Oncogene 2016, 35(33):4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lessey BA, Ilesanmi AO, Castelbaum AJ, Yuan L, Somkuti SG, Chwalisz K, Satyaswaroop PG: Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the alpha1 integrin. J Steroid Biochem Mol Biol 1996, 59(1):31–39. [DOI] [PubMed] [Google Scholar]
- 23.Hackenberg R, Beck S, Filmer A, Hushmand Nia A, Kunzmann R, Koch M, Slater EP, Schulz KD: Androgen responsiveness of the new human endometrial cancer cell line MFE-296. Int J Cancer 1994, 57(1):117–122. [DOI] [PubMed] [Google Scholar]
- 24.Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P: Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res 2007, 67(8):3970–3975. [DOI] [PubMed] [Google Scholar]
- 25.Detre S, Saclani Jotti G, Dowsett M: A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995, 48(9):876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John T, Liu G, Tsao MS: Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene 2009, 28 Suppl 1:S14–23. [DOI] [PubMed] [Google Scholar]
- 27.Bosse T, Peters EE, Creutzberg CL, Jurgenliemk-Schulz IM, Jobsen JJ, Mens JW, Lutgens LC, van der Steen-Banasik EM, Smit VT, Nout RA: Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer 2015, 51(13):1742–1750. [DOI] [PubMed] [Google Scholar]
- 28.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC: Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol 2000, 182(6):1506–1519. [DOI] [PubMed] [Google Scholar]
- 29.Jolly S, Vargas CE, Kumar T, Weiner SA, Brabbins DS, Chen PY, Floyd W, Martinez AA: The impact of age on long-term outcome in patients with endometrial cancer treated with postoperative radiation. Gynecol Oncol 2006, 103(1):87–93. [DOI] [PubMed] [Google Scholar]
- 30.Hasumi K, Matsuzawa M, Chen HF, Takahashi M, Sakura M: Computed tomography in the evaluation and treatment of endometrial carcinoma. Cancer 1982, 50(5):904–908. [DOI] [PubMed] [Google Scholar]
- 31.Goff BA, Rice LW: Assessment of depth of myometrial invasion in endometrial adenocarcinoma. Gynecol Oncol 1990, 38(1):46–48. [DOI] [PubMed] [Google Scholar]
- 32.Alektiar KM, McKee A, Lin O, Venkatraman E, Zelefsky MJ, Mychalczak BR, McKee B, Hoskins WJ, Barakat RR: The significance of the amount of myometrial invasion in patients with Stage IB endometrial carcinoma. Cancer 2002, 95(2):316–321. [DOI] [PubMed] [Google Scholar]
- 33.Cao H, Eppinga RD, Razidlo GL, Krueger EW, Chen J, Qiang L, McNiven MA: Stromal fibroblasts facilitate cancer cell invasion by a novel invadopodia-independent matrix degradation process. Oncogene 2016, 35(9):1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mi S, Lin M, Brouwer-Visser J, Heim J, Smotkin D, Hebert T, Gunter MJ, Goldberg GL, Zheng D, Huang GS: RNA-seq Identification of RACGAP1 as a Metastatic Driver in Uterine Carcinosarcoma. Clin Cancer Res 2016, 22(18):4676–4686. [DOI] [PubMed] [Google Scholar]
- 35.Miller DSFG, Mannel R, Cohn D, Matsumoto T, K Tewari, DiSilvestro P, Peral M, Zaino R: Randomized phase III noninferiority trial of first-line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study In: Society of Gynecology Oncology: 2012; Austin, TX; 2012. [Google Scholar]
- 36.Mayr C, Wagner A, Loeffelberger M, Bruckner D, Jakab M, Berr F, Di Fazio P, Ocker M, Neureiter D, Pichler M et al. : The BMI1 inhibitor PTC-209 is a potential compound to halt cellular growth in biliary tract cancer cells. Oncotarget 2016, 7(1):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bansal N, Bartucci M, Yusuff S, Davis S, Flaherty K, Huselid E, Patrizii M, Jones D, Cao L, Sydorenko N et al. : BMI-1 Targeting Interferes with Patient-Derived Tumor-Initiating Cell Survival and Tumor Growth in Prostate Cancer. Clin Cancer Res 2016, 22(24):6176–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, Keeney GL: The impact of multi-modal therapy on survival for uterine carcinosarcomas. Gynecol Oncol 2010, 116(3):419–423. [DOI] [PubMed] [Google Scholar]
- 39.Powell MA, Filiaci VL, Rose PG, Mannel RS, Hanjani P, Degeest K, Miller BE, Susumu N, Ueland FR: Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. J Clin Oncol 2010, 28(16):2727–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M: Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev 1999, 13(20):2678–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee Mustafi S, Chakraborty PK, Naz S, Dwivedi SK, Street M, Basak R, Yang D, Ding K, Mukherjee P, Bhattacharya R: MDR1 mediated chemoresistance: BMI1 and TIP60 in action. Biochim Biophys Acta 2016, 1859(8):983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, Kong QL, Xu LH, Zhang X, Liu WL et al. : The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest 2009, 119(12):3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Z, Min L, Chen D, Hao D, Duan Y, Qiu G, Wang Y: Overexpression of BMI-1 promotes cell growth and resistance to cisplatin treatment in osteosarcoma. PLoS One 2011, 6(2):e14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


