Abstract
Background:
We investigated the structural changes associated with Alzheimer’s disease, dementia with Lewy bodies and Parkinson disease dementia by means of cortical thickness analysis.
Methods:
Two hundred and forty-five participants: 76 Alzheimer’s disease, 65 dementia with Lewy bodies, 29 Parkinson disease dementia and 76 cognitively normal controls underwent 3-T T1-weighted magnetic resonance imaging and clinical and cognitive assessments. We implemented FreeSurfer to obtain cortical thickness estimates to contrast patterns of cortical thinning across groups and their clinical correlates.
Results:
In Alzheimer’s disease and dementia with Lewy bodies, a largely similar pattern of regional cortical thinning was observed relative to controls apart from a more severe loss within the entorhinal and parahippocampal structures in Alzheimer’s disease. In Parkinson disease dementia, regional cortical thickness was indistinguishable from controls and dementia with Lewy bodies, suggesting an ‘intermediate’ pattern of regional cortical change. In terms of global cortical thickness, group profiles were controls > Parkinson disease dementia > dementia with Lewy bodies > Alzheimer’s disease (F3, 241 ⩽ 123.2, p < 0.001), where percentage wise, the average difference compared to controls were −1.8%, −5.5% and −6.4%, respectively. In these samples, cortical thinning was also associated with cognitive decline in dementia with Lewy bodies but not in Parkinson disease dementia and Alzheimer’s disease.
Conclusion:
In a large and well-characterised cohort of people with dementia, regional cortical thinning in dementia with Lewy bodies was broadly similar to Alzheimer’s disease. There was preservation of the medial temporal lobe structures in dementia with Lewy bodies compared with Alzheimer’s disease, supporting its inclusion as a supportive biomarker in the revised clinical criteria for dementia with Lewy bodies. However, there was less global cortical thinning in Parkinson disease dementia, with no significant regional difference between Parkinson disease dementia and controls. These findings highlight the overlap across the Alzheimer’s disease/Parkinson disease dementia spectrum and the potential for differing mechanisms underlying neurodegeneration and cognition in dementia with Lewy bodies and Parkinson disease dementia.
Keywords: Parkinson disease dementia, dementia with Lewy bodies, Alzheimer’s disease, cortical thickness, magnetic resonance imaging
Introduction
Dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD) are common forms of dementia in older age, while in Parkinson’s disease (PD), development of dementia (Parkinson disease dementia [PDD]) occurs in up to 80% of people aged 15–20 years after PD diagnosis. Although the neuropsychiatric and cognitive presentation in PDD is broadly similar to DLB (Aarsland et al., 2003), there are differences including timing of the cognitive, neuropsychiatric and motor symptoms as well as treatment responses and underlying neuropathology (Goldman et al., 2014).
Structural imaging changes are well characterised in AD with temporoparietal atrophy shown to be a relatively specific disease biomarker (Whitwell et al., 2018). In Lewy body disease, magnetic resonance imaging (MRI) studies have reported greater cortical atrophy in DLB than in PDD (Beyer et al., 2007; Sanchez-Castaneda et al., 2009), while relative preservation of the medial temporal lobes compared to AD is a consistent finding across both conditions (Burton et al., 2009; Jellinger, 2018; Tam et al., 2005), and significant medial temporal atrophy may indicate concomitant AD-related pathological change (Burton et al., 2009). Studies reporting measures of cortical thickness (CTh) in DLB have shown changes in parietal, occipital, temporal and frontal cortices but to a lesser extent than in AD (Watson et al., 2015). Cortical thinning has also been shown to be associated with cognitive impairment and dementia in PD (Hwang et al., 2013; Rektorova et al., 2014; Zarei et al., 2013), with cortical changes becoming more widespread as the disease progresses.
Our aim was to determine patterns of cortical thinning in a large and well-characterised cohort of older people with AD, DLB and PDD. Atrophic changes in DLB may lie somewhere between AD and PDD, possibly reflecting increased AD co-pathology, but no study has made direct comparisons between all the three dementia groups. We, therefore, utilised FreeSurfer to estimate CTh from structural MRI data to contrast patterns across these groups. We also investigated the clinical correlates of CTh in order to probe the clinical significance of the neurodegenerative process in these conditions.
Methods
Participants
The investigation consisted of 245 individuals gathered from four independent study samples: 76 probable AD (McKhann et al., 1984), 65 probable DLB (McKeith et al., 2005), 29 PDD (Emre et al., 2007) and 76 older controls. Sample characteristics were the following: study 1 (13 controls, 30 AD, 21 DLB and 16 PDD), study 2 (31 controls, 32 AD and 31 DLB), study 3 (17 controls, 13 AD and 13 DLB) and study 4 (15 controls and 13 PDD). People with probable AD, DLB and PDD were recruited from a community-dwelling population of patients referred to local old age psychiatry, geriatric or neurology services. Older cognitively normal control participants were recruited from patient spouses, friends and volunteers. Approval was sought from the Newcastle, North Tyneside and Northumberland research ethics committees. All participants and/or nearest relatives gave written informed consent. All participants had clinical, cognitive, neurological and neuropsychiatric assessments. Cognitive function was evaluated with the mini-mental state examination (MMSE) (Folstein et al., 1975) and Cambridge Cognitive Examination (CAMCOG) (Roth et al., 1986) tests, while motor parkinsonism was assessed using the unified Parkinson’s disease rating scale (UPDRS-III) (Fahn et al., 1987). For AD, DLB and PDD participants, neuropsychiatric features and cognitive fluctuations were measured with the neuropsychiatric inventory (NPI) (Cummings et al., 1994) and the clinician assessment of fluctuations (CAF) scale (Walker et al., 2000).
Study exclusion criteria across samples included contraindications for MRI, history of alcohol or substance misuse, severe neurological or psychiatric illness, focal brain lesions or presence of other severe or medical illness.
MRI
Within 2 months of their respective study assessments, all subjects underwent T1-weighted MRI on a 3-T MRI system (8-channel head coil, Intera Achieva scanner; Philips Medical Systems, Eindhoven, The Netherlands), although there were slight variations in the three-dimensional (3D) MPRAGE sequence across samples. Study 1: sagittal acquisition, matrix 216 (anterior–posterior) × 208 (superior–inferior) × 180 (right–left), repetition time (TR) = 8.3 ms, echo time (TE) = 4.6 ms, flip angle = 8°, SENSE factor = 2, voxel output 1.0 × 1.0 × 1.0 mm3. Study 2: sagittal acquisition, matrix 240 × 240 × 180, TR = 9.6 ms, TE = 4.6 ms; flip angle = 8°; SENSE factor = 2, voxel output 1.0 × 1.0 × 1.0 mm3. Study 3: sagittal acquisition, matrix 240 × 240 × 150, TR = 9.6 ms, TE = 4.6 ms, flip angle = 8°, SENSE factor = 2, voxel output 0.94 × 0.94 × 1.2 mm3. Study 4: sagittal acquisition, matrix 240 × 240 × 180, TR = 8.3 ms, TE = 4.6 ms, flip angle = 8°, SENSE factor = 2, voxel output 1.0 × 1.0 × 1.0 mm3. Image volumes were then orientated such that the axial plane was standardised to align with the anterior commissure-posterior commissure (AC-PC) line.
Cortical thickness
CTh estimates were derived for each participant from cortical surface reconstructions of the T1 images using FreeSurfer (version 5.1), the methodological details of which have been described (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 1999). In short, the analysis pipeline included non-uniform intensity correction, Talairach transformation, removal of non-brain tissue, segmentation of the white matter (WM) and deep grey matter (GM) structures, triangular tessellation of the WM–GM boundary and then surface warping by tracking intensity gradients to optimally identify the WM–GM and GM–CSF (cerebrospinal fluid)/pial borders (Dale et al., 1999; Fischl and Dale, 2000). Once cortical models were produced, surface inflation, spherical atlas registration and parcellation of the cerebral cortex into gyral and sulcal structures were performed, (Makris et al., 2006) thereby generating a series of surface data. Representations of CTh were then calculated as the closest distance from the WM–GM to pial boundaries at each vertex on the tessellated surface (Fischl et al., 2004). Examination at each step of image processing was performed to confirm the accuracies of segmentation, WM/pial surfaces and tissue classifications. Images with errors that could not be corrected were excluded. Images were then aligned to a common surface space (MNI 305) and smoothed with a 20-mm full width at half maximum (FWHM) surface–based Gaussian kernel.
Statistical analyses
Demographic and behavioural variables were tested for normality and variance homogeneity using Shapiro–Wilk and Levene’s tests, respectively. Where applicable, the data were examined using parametric (analysis of variance [ANOVA] F, Welch’s ANOVA W) and non-parametric (Kruskal–Wallis H, χ2) tests and interpreted as significant if p ⩽ 0.05. Analysis was conducted using IBM SPSS v.23.0.0.3.
Group effects and behavioural correlates on CTh were assessed vertex wise using the general linear model (GLM). CTh was modelled as a function of each group controlling for the effects of age, gender and MRI sequence (analysis of covariance [ANCOVA]), CTh = β1.Con + β2.AD + β3.DLB + β4.PDD + β5.age + β6.gender + β7.MRI_sequence + μ + ε (where μ is a constant and ε is error). An F contrast was derived to test whether any differences in mean CTh at each vertex existed between the four groups. The resultant surface map, converted to a binary brain mask, defined the surface subspace from which subsequent post hoc analyses were performed to establish which groups differed from each other. In particular, CTh was then modelled within the surface subspace as a function of two groups along with the nuisance covariates, where T contrasts generated specific pairwise comparisons (two-tailed t tests): Con vs AD, Con vs DLB, Con vs PDD, AD vs DLB, AD vs PDD and DLB vs PDD. Effects of measures (MMSE, CAMCOGtotal, CAMCOGsubscales, NPItotal, NPIhall and CAFtotal) on CTh were also investigated separately in AD, DLB and PDD. CTh was modelled as a function of covariate of interest, and where appropriate, controlling for effects of age, gender and MRI sequence. These contrasts tested whether the clinical variables significantly correlated with CTh in these conditions. For all analyses, a false discovery rate (FDR) (Genovese et al., 2002) probability threshold was applied (PFDR ⩽ 0.05), controlling the expected proportion of type I errors among the suprathreshold vertices due to multiple testing across the surfaces.
Results
Demographics and behavioural characteristics
Table 1 depicts the study characteristics. Age was broadly similar across groups; however, PDD patients were slightly younger than DLB patients. The male-to-female ratio was much greater in PDD than in AD, DLB and control participants. Global cognition (MMSE, CAMCOGtotal) and executive function were similar across AD, DLB and PDD, whereas memory (CAMCOGmem), although comparable between DLB and PDD, was, as expected, more impaired in AD. Neuropsychiatric symptoms, hallucinations and cognitive fluctuations were more severe in DLB and PDD than in AD, while UPDRS-III scores, again as expected, were significantly higher in DLB and PDD relative to AD and controls.
Table 1.
Demographic and group characteristics.
| Controls | AD | DLB | PDD | Statistic, p value | |
|---|---|---|---|---|---|
| n | 76 | 75 | 65 | 29 | |
| Age (years) | 76.1 ± 5.8 | 77.9 ± 7.5 | 78.2 ± 6.4 | 74.5 ± 5.6 | F3, 241 = 3.1, 0.03a |
| Gender (m:f) | 45:31 | 47:28 | 46:19 | 26:3 | χ2 = 9.9, 0.02 |
| MMSE | 29.0 ± 1.3 | 20.4 ± 4.3 | 20.3 ± 5.2 | 21.2 ± 4.3 | H3 = 144.3, <0.001b |
| CAMCOGtotal | 96.2 ± 4.8 | 67.4 ± 13.0 | 67.8 ± 15.2 | 71.8 ± 14.3 | H3 = 147.1, <0.001b |
| CAMCOGmem | 23.4 ± 1.7 | 10.5 ± 4.6 | 15.5 ± 4.9 | 17.3 ± 4.8 | H3 = 159.6, <0.001c |
| CAMCOGexec | 22.3 ± 3.1 | 13.7 ± 4.6 | 11.7 ± 5.2 | 12.4 ± 3.0 | W3, 105.6 = 132.2, <0.001 d |
| NPItotal | Na | 11.7 ± 10.9 | 18.2 ± 15.2 | 20.8 ± 14.4 | H2 = 13.2, 0.001e |
| NPIhall | Na | 0.3 ± 0.8 | 2.4 ± 2.5 | 2.3 ± 2.3 | H2 = 62.9, <0.001e |
| CAFtotal | Na | 1.7 ± 3.2 | 6.3 ± 4.4 | 6.8 ± 3.9 | H2 = 55.5, <0.001e |
| UPDRS-III | 1.6 ± 2.0 | 3.6 ± 3.7 | 25.6 ± 12.8 | 36.9 ± 17.6 | H3 = 175.4, <0.001f |
| Duration of PD (years) | Na | Na | Na | 8.9 ± 5.1 | |
| gCThleft | 2.44 ± 0.06 | 2.28 ± 0.06 | 2.31 ± 0.05 | 2.40 ± 0.07 | F3, 241 = 112.4, <0.001g |
| gCThright | 2.45 ± 0.05 | 2.30 ± 0.06 | 2.31 ± 0.05 | 2.40 ± 0.06 | F3, 241 = 123.2, <0.001h |
| gCThleft (%)i | Na | −6.6% | −5.3% | −1.6% | |
| gCThright (%)i | Na | −6.1% | −5.7% | −2.0% |
Values are expressed as mean ± 1 SD.
AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; PDD: Parkinson disease dementia; PD: Parkinson’s disease; SD: standard deviation; DLB: dementia with Lewy bodies; PDD: Parkinson disease dementia; MMSE: mini-mental state examination; CAMCOGtotal: Cambridge Cognitive Examination total score; CAMCOGmem: Cambridge Cognitive Examination memory subdomain; CAMCOGexec: Cambridge Cognitive Examination executive function subdomain; NPItotal: neuropsychiatric inventory total score; NPIhall: neuropsychiatric inventory hallucinations; CAFtotal: clinical assessment of fluctuation total score; UPDRS-III: unified Parkinson’s disease rating scale (section III); gCTh: global cortical thickness (age, gender and sequence adjusted); Na: not applicable; Ns: not significant (p > 0.05).
Bold text denotes statistical significance.
Post hoc tests:
DLB > PDD (p = 0.05); otherwise Ns (Gabriel’s).
Con > AD, DLB, PDD (p < 0.001); otherwise Ns (Mann–Whitney U).
Con > AD, DLB, PDD (p < 0.001); DLB, PDD > AD (p < 0.001); otherwise Ns (Mann–Whitney U).
Con > AD, DLB, PDD (p < 0.001); otherwise Ns (Games-Howell).
DLB, PDD > AD (p < 0.03); otherwise Ns (Mann–Whitney U).
DLB, PDD > Con, AD (p < 0.001); otherwise Ns (Mann–Whitney U).
Con > AD, DLB, PDD (p < 0.006); PDD > AD, DLB (p < 0.001), otherwise Ns (Gabriel’s).
Con > AD, DLB, PDD (p < 0.001); PDD > AD, DLB (p < 0.001), otherwise Ns (Gabriel’s).
% reduction relative to controls.
Cortical thickness
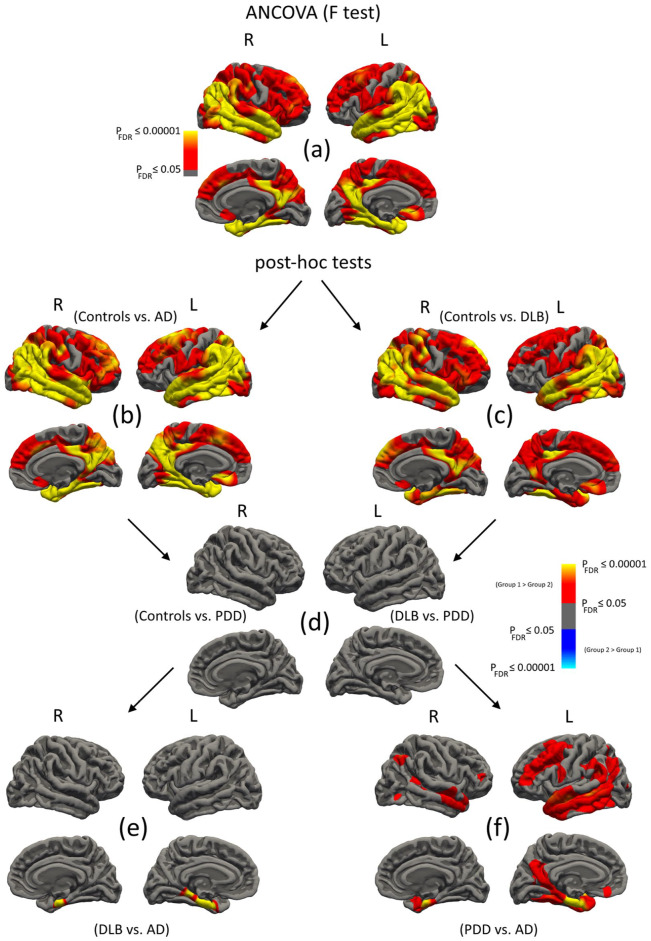
Figure 1(a) illustrates the ANCOVA analysis (n = 245), and Table 2 summarises the cluster data. Across groups, most of the cerebral cortex showed significant changes in CTh apart from areas of the inferior frontal, pre-post central gyri, paracentral, anterior cingulate, orbitofrontal and medial occipital lobes, where regional CTh was similar. The greatest differences were in the entorhinal cortices. Figure 1(b)–(f) present the pairwise post hoc results. Controls vs AD: Cortical thinning was bilaterally diffuse and involved the temporal, parietal and frontal lobes (Figure 1(b)). As expected, the most affected areas were the entorhinal cortices, parahippocampal gyri, posterior cingulate, lateral temporal and inferior parietal lobules. Table 2 details the significant clusters. Controls vs DLB: Cortical thinning was similarly widespread, i.e. in temporal, parietal and frontal lobes (Figure 1(c)). As in AD, areas of greatest change were in the posterior cingulate, lateral temporal and inferior parietal lobules. Entorhinal and parahippocampal changes were also apparent but to a lesser extent than AD. Table 2 summarises the cluster data. Controls vs PDD, DLB vs PDD: In PDD, CTh did not differ from healthy controls or participants with DLB (Figure 1(d)). DLB vs AD: Areas of significant thinning were confined only to the bilateral entorhinal cortex and left parahippocampal gyrus in AD compared to DLB. PDD vs AD: CTh was reduced in AD relative to PDD in temporal, frontal and parietal regions (Figure 1(f)). The most affected structures were the entorhinal cortices (Table 2).
Figure 1.
Regional vertex-wise analyses of cortical thickness (CTh) in controls, AD, DLB and PDD. (a) ANCOVA analysis across groups. (b–f) Subsequent pairwise post hoc tests. Maps are displayed at PFDR ⩽ 0.05 (L: left hemisphere; R: right hemisphere).
Table 2.
Cortical thickness results (PFDR ⩽ 0.05).
| Area | NVtxs | −log10 P | MNI | Annotation | |
|---|---|---|---|---|---|
| (a) F test | 56,262 | 110,469 | 24.7 | −23, −17, −27 | L entorhinal |
| 1146 | 2330 | 5.1 | −7, 24, −12 | L medial orbitofrontal | |
| 54,877 | 108,443 | 15.9 | 22, −12, −28 | R entorhinal | |
| 1729 | 4065 | 3.8 | 29, −21, 66 | R medial orbitofrontal | |
| 292 | 630 | 2.7 | 6, 16, −8 | R rostral anterior cingulate | |
| (b) Con vs AD | 56,067 | 110,172 | 22.9 | −23, −17, −27 | L entorhinal |
| 1039 | 2089 | 5.8 | −8, 26, −13 | L medial orbitofrontal | |
| 54,698 | 108,056 | 16.4 | 25, −12, −29 | R entorhinal | |
| 285 | 616 | 3.0 | 6, 12, −9 | R medial orbitofrontal | |
| 560 | 1350 | 2.4 | 13, −18, 68 | R precentral | |
| (c) Con vs DLB | 48,173 | 95,704 | 9.3 | −58, −60, 5 | L middle temporal |
| 1146 | 2330 | 4.6 | −8, 25, −13 | L medial orbitofrontal | |
| 51,366 | 101,023 | 8.8 | 54, −52, 32 | R inferior parietal | |
| 1729 | 4065 | 4.5 | 20, −20, 70 | R precentral | |
| 200 | 423 | 2.4 | 7, 24, −16 | R medial orbitofrontal | |
| (d) Con vs PDD DLB vs PDD |
No suprathreshold clusters | ||||
| (e) DLB vs AD | 1462 | 3454 | 10.5 | −23, −16, −27 | L entorhinal |
| 328 | 776 | 16.4 | 24, −13, −28 | R entorhinal | |
| (f) PDD vs AD | 19,844 | 39,429 | 11.2 | −23, −18, −25 | L entorhinal |
| 7364 | 13,008 | 3.6 | −39, 49, 4 | L rostral middle frontal | |
| 113 | 353 | 2.3 | −29, 26, 9 | L pars triangularis | |
| 170 | 266 | 2.1 | −7, 35, −18 | L medial orbitofrontal | |
| 86 | 266 | 2.1 | −33, −22, 18 | L insula | |
| 3164 | 5988 | 5.3 | 24, −12, −29 | R entorhinal | |
| 867 | 1928 | 4.4 | 46, −57, 45 | R inferior parietal | |
| 279 | 446 | 3.3 | 41, 40, 2 | R rostral middle frontal | |
| 137 | 165 | 2.8 | 43, −75, −6 | R lateral occipital | |
AD: Alzheimer’s disease; DLB: Dementia with Lewy bodies; PDD: Parkinson disease dementia.
Table depicts cluster data, i.e. maximum vertex significance (−log10 P units), surface area (Area, mm2), number of vertices (NVtxs), MNI coordinates (x, y, z; mm) of peak vertex and its annotation. −log10 P = 1.3, 2.0, 3.0 → P = 0.05, 0.01, 0.001, respectively.
To quantify the CTh results, we calculated for each subject, global cortical thickness (gCTh) by averaging across the surface subspaces (left, right) defined by the significant F-contrast correcting for age, gender and MR sequence. Table 1 depicts the gCTh for each hemisphere and group, where the global profile was controls > PDD > DLB > AD. For gCTh, dementia groups were lower than controls (p ⩽ 0.006); PDD was greater than both DLB and AD (p < 0.001) but similar between DLB and AD. Percentage wise, the average (left and right hemispheres combined) reduction in gCTh relative to controls was −6.4%, −5.5% and −1.8% for AD, DLB and PDD, respectively.
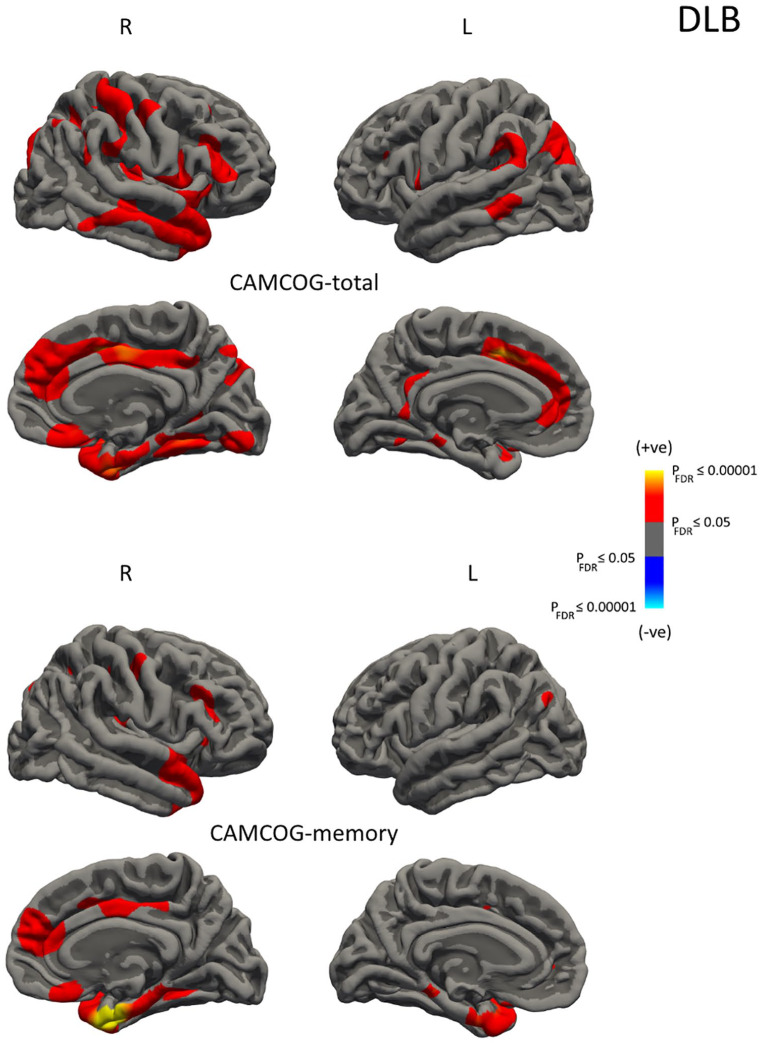
Figure 2 and Table 3 summarise the correlation results in AD, DLB and PDD. Significant effects on CTh were found for CAMCOGtotal and CAMCOGmem in DLB. Lower CAMCOGtotal scores were associated with a pattern of decreased thickness in the bilateral medial frontal, middle temporal, inferior frontal and inferior parietal as well as right insula, right posterior cingulate, right fusiform and right medial orbitofrontal structures. Lower CAMCOGmem scores were associated with a pattern of decreased thickness in the bilateral medial temporal/temporal poles, left inferior parietal, right cingulate, right superior temporal and right superior/orbito frontal regions. Interestingly, there were no significant relationships between CTh and any of the behavioural measures in AD or PDD.
Figure 2.
Vertex-wise analyses of cortical thickness (CTh) as a function of CAMCOGtotal and CAMCOGmem in DLB. Maps are displayed for PFDR ⩽ 0.05 (L: left hemisphere; R: right hemisphere).
Table 3.
Correlations in AD, DLB and PDD (PFDR ⩽ 0.05).
| Area | NVtxs | −log10 P | MNI | Annotation | |
|---|---|---|---|---|---|
| AD | No suprathreshold correlations | ||||
| DLB-CAMCOGtotal | 2020 | 3858 | 4.3 | −11, 11, 43 | L superior frontal |
| 263 | 583 | 3.8 | −17, 15, −19 | L rostral middle frontal | |
| 1103 | 1810 | 3.4 | −33, −80, 30 | L inferior parietal | |
| 512 | 1102 | 3.4 | −59, −48, 28 | L supramarginal | |
| 479 | 971 | 3.2 | −18, −35, −11 | L parahippocampal | |
| 232 | 574 | 3.1 | −39, −18, 33 | L precentral | |
| 104 | 241 | 3.0 | −37, 1, −25 | L superior temporal | |
| 277 | 628 | 3.0 | −8, −44, 31 | L isthmuscingulate | |
| 409 | 754 | 3.0 | −59, −41, −7 | L middle temporal | |
| 117 | 254 | 2.7 | −51, 6, 7 | L pars opercularis | |
| 5983 | 12,575 | 4.4 | 41, −2, −20 | R insula | |
| 3312 | 7185 | 4.2 | 5, −4, 38 | R posterior cingulate | |
| 2243 | 3487 | 4.1 | 32, −53, −12 | R fusiform | |
| 603 | 1282 | 3.6 | 7, 22, −16 | R medial orbitofrontal | |
| 2848 | 5600 | 3.6 | 35, −53, 38 | R inferior parietal | |
| 2156 | 5337 | 3.2 | 46, −24, 44 | R postcentral | |
| 239 | 577 | 3.1 | 26, −55, 5 | R lingual | |
| 969 | 1736 | 2.8 | 45, 31, 11 | R pars triangularis | |
| 345 | 799 | 2.5 | 45, −9, 42 | R precentral | |
| 166 | 340 | 2.1 | 53, −42, 32 | R supramarginal | |
| 105 | 202 | 2.1 | 32, 29, 40 | R rostral middle frontal | |
| DLB-CAMCOGmem | 649 | 1430 | 4.3 | −38, 4, −28 | L superior temporal |
| 131 | 305 | 4.3 | −18, −34, −12 | L parahippocampal | |
| 106 | 230 | 3.9 | −17, 15, −19 | L lateral orbitofrontal | |
| 102 | 179 | 3.4 | −41, −75, 31 | L inferior parietal | |
| 38 | 115 | 3.3 | −38, −18, 33 | L precentral | |
| 20 | 54 | 3.3 | −11, 4, 42 | L superior frontal | |
| 11 | 24 | 3.2 | −14, 47, 1 | L medial orbitofrontal | |
| 3984 | 8490 | 6.0 | 28, −5, −34 | R entorhinal | |
| 751 | 1838 | 3.7 | 8, 1, 40 | R posterior cingulate | |
| 1195 | 2065 | 3.5 | 11, 36, 15 | R rostral anterior cingulate | |
| 549 | 998 | 3.0 | 40, 29, 19 | R rostral middle frontal | |
| 314 | 723 | 3.0 | 43, −10, 43 | R precentral | |
| 205 | 409 | 2.9 | 6, 25, −19 | R medial orbitofrontal | |
| 89 | 207 | 2.6 | 31, 28, −4 | R lateral orbitofrontal | |
| 26 | 65 | 2.5 | 28, −53, 50 | R superior parietal | |
| 13 | 28 | 3.6 | 45, −25, 46 | R postcentral | |
| PDD | No suprathreshold correlations | ||||
AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; PDD: Parkinson disease dementia; CAMCOGtotal: Cambridge Cognitive Examination total score; CAMCOGmem: Cambridge Cognitive Examination memory subdomain; NVtxs: number of vertices.
Discussion
Using an ANCOVA approach followed by pairwise post hoc testing, we investigated the pattern of cortical thinning associated with AD, DLB and PDD. A largely similar pattern of regional cortical thinning was apparent between AD and DLB relative to controls apart from a more severe loss within the entorhinal and parahippocampal structures in AD. In PDD, regional CTh was indistinguishable from controls and DLB, implying either a heterogeneous disease group or one with a degree of ‘intermediate’ cortical change. By averaging the regional values across the cortical surfaces, we derived measures of gCTh in these groups, where DLB, PDD and AD showed significantly lower gCTh than controls; however, PDD was also greater than DLB and AD, suggesting an ‘intermediary’ level of global cortical change in PDD. In percentage terms, the average reduction in gCTh compared to controls was −1.8%, −5.5% and −6.4% for PDD, DLB and AD, respectively. Cortical thinning was also associated with cognitive decline in DLB but not in PDD or AD.
In AD, there was a widespread pattern of change in CTh with the lateral temporoparietal and medial temporal areas particularly affected. Cortical thinning in AD has been extensively studied, and our results are consistent with those of previous reports (Du et al., 2007; Lehmann et al., 2011). Relative to controls, a similar picture was associated with DLB, with evidence of some medial temporal thinning but to a lesser extent than AD. When AD and DLB are contrasted, the results suggest a largely shared pattern with the exception of the entorhinal and parahippocampal structures which are more affected in AD. It, therefore, seems that for established AD and DLB with similar levels of global cognition, DLB resembles an AD-like pattern but slightly upstream in terms of cortical thinning. An investigation of prodromal patients found CTh changes relative to controls in AD but not DLB (Blanc et al., 2015), while longitudinal studies in DLB have shown significantly lower rates of global atrophy compared to AD (Mak et al., 2015b), with higher rates of atrophy associated with increased concurrent levels of AD co-pathology (β-amyloid) (Sarro et al., 2016), which appears to support this view.
Cortical degeneration in DLB is heterogeneous and not entirely understood, although a contributory factor may be the presence of coexisting AD-related pathology (β-amyloid, tau). Positive emission tomography (PET) imaging has shown that around 50–60% of people with DLB have significant amyloid deposition (Donaghy et al., 2015), with a high burden predicting a greater rate of cortical atrophy (Sarro et al., 2016). More recently, tau deposition has been reported in parietal structures in DLB utilising the tau PET tracer 18F-AV-1451 (Gomperts et al., 2016; Smith et al., 2018). Amyloid-positive DLB cases had marked increases in cortical tau (18F-AV-1451 uptake) (Lee et al., 2018), and similarly, others found increased temporoparietal and occipital tau binding correlated with global Pittsburgh compound B (PiB) in DLB (Kantarci et al., 2017). It seems that in DLB, people with a higher amyloid burden have additional cortical tau pathology which may then accelerate or contribute to the neurodegenerative process in DLB. Longitudinal studies utilising molecular imaging techniques (tau and amyloid) may offer insight into the evolutionary interaction of these pathologies and their effects on the clinical features, cortical atrophy and outcomes.
The pattern of regional cortical thinning associated with PDD was indistinguishable from healthy controls and patients with DLB, indicating either a heterogeneous group or one with ‘intermediate’ levels of cortical degeneration. In vivo Alzheimer proteinopathies are less common in PDD than in DLB, where studies have shown higher cortical amyloid deposition (Bohnen et al., 2017) and more transitional tau retention in the former (DLB > PDD > controls) (Smith et al., 2018). Pathologically, studies have also reported higher cortical β-amyloid and tau loads in DLB compared to PDD, which contribute to cortical neurodegeneration (Jellinger, 2018). Therefore, less cortical thinning in PDD than in DLB is consistent with this notion. However, significant cortical thinning has been previously reported in people with mild cognitive impairment and dementia in PD relative to controls, in contrast to our results (Danti et al., 2015; Gasca-Salas et al., 2017; Hwang et al., 2013; Kim et al., 2017; Mak et al., 2015a; Pagonabarraga et al., 2013; Pereira et al., 2014; Segura et al., 2014; Zarei et al., 2013). Results of structural MRI change have been variable across studies utilising voxelwise, region of interest and CTh measures in PDD. Some report very little change, while others have reported widespread cortical deficits. This may be partly due to the differences in analysis methods. Some reported either uncorrected or Monte Carlo cluster-wise statistics. Cluster-wise approaches provide lower spatial sensitivity and infer a signal somewhere within a cluster. For liberal cluster thresholds, large clusters are often generated where it is then difficult to locate regions representing the true effects, and consequently, the degree of reported change may be overstated. One study that reported vertex-wise FDR-corrected results showed cortical thinning in the frontal, temporal and posterior parietal cortices in PDD (n = 15) (Zarei et al., 2013). Notably, average gCTh in PDD was similar in both studies, CTh (present study) 2.40 ± 0.07 and CTh (Zarei et al.) 2.34 ± 0.05, and was greater than that in DLB, CTh (present study) 2.31 ± 0.05. Variation in results may be partly due to global cognition (MMSEpresent = 21.2, MMSEZarei = 18.3), differences in cohort size and comparison group but also highlights disease heterogeneity in PDD. Longitudinal studies with larger sample sizes are needed to accurately assess heterogeneity in PDD.
Cortical thinning was associated with cognitive decline in DLB. Memory (CAMCOGmem) related to a CTh pattern involving predominantly the temporal lobes and right frontal regions, whereas for global cognition (CAMCOGtotal), there was also a correlation with parietal and medial frontal areas. We did not detect significant effects between CTh and any of the behavioural measures in either AD or PDD. For AD, the lack of association may be due to floor effects as cortical degeneration occurs much earlier in the disease course. Cholinergic dysfunction is strongly associated with cognitive deficits, fluctuating cognition and visual hallucinations in PDD (Aarsland et al., 2003) and may suggest that strategic loss of key corticopetal afferents from the basal forebrain rather than broad-scale cortical neuronal loss underpins cognitive impairment in this group. Despite similar levels of global cognition, the variation in the pattern and extent of cortical thinning suggests differing mechanisms underlying cognitive decline among AD, DLB and PDD.
Strengths of the study include the following: a large group of probable AD, DLB and PDD patients who were matched for global cognition (MMSE and CAMCOG) and the reporting of FDR-corrected results. While combining datasets has the important advantage of statistical power, one of the disadvantages is in differing assessment procedures. This included the lack of common measures of everyday function and assessment of duration of illness. Duration of illness can be difficult to accurately assess in dementia and, especially, in DLB and PDD where diagnostic delays are common (Galvin et al., 2010). Other study limitations include the following: diagnoses were clinical rather than neuropathological and there were no measure of tau or amyloid. The effect of using slightly different MRI sequences across the study cohorts did not appear to significantly affect the vertex-wise analyses and, therefore, results but was included as a nuisance covariate for completeness. Further, CTh data from study 2 have been published (Watson et al., 2015), while the data from studies 1, 3 and 4 have not. Future work could investigate the patterns of cortical thinning in DLB and PDD, stratified according to high/low amyloid and tau, in order to study the AD pathological burden and their effects on cortical degeneration in Lewy body disease.
Conclusion
Our findings suggest a specific profile of cortical thinning across healthy controls, PDD, DLB and AD. The pattern of structural change in DLB was similar to that in AD, to a slightly lesser extent, while PDD appeared to demonstrate an ‘intermediate’ degree of regional and global cortical thinning. In these samples, effects of CTh on cognition were confined to DLB that may support the notion of differing processes underlying cognition across Lewy body diseases.
Footnotes
Author Contributions: S.J.C. co-designed the study, conducted image and data analyses and co-wrote the manuscript. R.W. supported the recruitment and coordination of study participants, interpreted results and co-wrote the manuscript. Professor A.M.B. reviewed the manuscript and assisted with project funding. Professor J.T.O.B. reviewed the manuscript and assisted with project funding. Professor J.-P.T. supported the recruitment and coordination of study participants, interpreted results, co-wrote the manuscript and assisted with project funding.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: S.J.C., R.W. and Professor A.M.B. report no disclosures. Professor J.T.O.B. has been a consultant for GE Healthcare, Lilly, TauRx, Axon and Eisai and has received honoraria for talks from GE Healthcare, Lilly and Novartis. Professor J.-P.T. has received honoraria for talks from GE Healthcare and Flynn pharmaceuticals.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Financial support was from the National Institute for Health Research (NIHR) Research for Public Benefit, Wellcome Trust (WT088441MA Fellowship funding J-P.T). NIHR Biomedical Research Centre at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge. The NIHR Newcastle Biomedical Research Centre in Ageing and Chronic Disease and Biomedical Research Unit in Lewy Body Dementia based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The study was also funded by the Sir Jules Thorn Charitable Trust (Grant ref: 05/JTA).
ORCID iD: Rosie Watson  https://orcid.org/0000-0002-4235-3803
https://orcid.org/0000-0002-4235-3803
References
- Aarsland D, Litvan I, Salmon D, et al. (2003) Performance on the Dementia Rating Scale in Parkinson’s disease with dementia and dementia with Lewy bodies: Comparison with progressive supranuclear palsy and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry 74: 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer MK, Larsen JP, Aarsland D. (2007) Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology 69: 747–754. [DOI] [PubMed] [Google Scholar]
- Blanc F, Colloby SJ, Philippi N, et al. (2015) Cortical thickness in dementia with Lewy bodies and Alzheimer’s disease: A comparison of prodromal and dementia stages. PLoS ONE 10: e0127396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller M, Frey KA. (2017) Molecular imaging and updated diagnostic criteria in Lewy body dementias. Current Neurology and Neuroscience Reports 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. (2009) Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain 132: 195–203. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, et al. (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Danti S, Toschi N, Diciotti S, et al. (2015) Cortical thickness in de novo patients with Parkinson disease and mild cognitive impairment with consideration of clinical phenotype and motor laterality. European Journal of Neurology 22: 1564–1572. [DOI] [PubMed] [Google Scholar]
- Donaghy P, Thomas AJ, O’Brien JT. (2015) Amyloid PET imaging in Lewy body disorders. American Journal of Geriatric Psychiatry 23: 23–37. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, et al. (2007) Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 130: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, et al. (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders 22:1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. and Members of the UPDRS Development Committee (1987) Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden C, Calne D, et al. (eds) Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health Care Information, pp. 153–164. [Google Scholar]
- Fischl B, Dale AM. (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. (1999) Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, et al. (2004) Automatically parcellating the human cerebral cortex. Cerebral Cortex 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. (1975) ‘Mini-mental state’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Duda JE, Kaufer DI, et al. (2010) Lewy body dementia: The caregiver experience of clinical care. Parkinsonism & Related Disorders 16: 388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasca-Salas C, Garcia-Lorenzo D, Garcia-Garcia D, et al. (2017) Parkinson’s disease with mild cognitive impairment: Severe cortical thinning antedates dementia. Brain Imaging and Behavior 13: 180–188. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Goldman JG, Williams-Gray C, Barker RA, et al. (2014) The spectrum of cognitive impairment in Lewy body diseases. Movement Disorders 29: 608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Locascio JJ, Makaretz SJ, et al. (2016) Tau Positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol 73: 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang KS, Beyer MK, Green AE, et al. (2013) Mapping cortical atrophy in Parkinson’s disease patients with dementia. Journal of Parkinson’s Disease 3: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. (2018) Dementia with Lewy bodies and Parkinson’s disease-dementia: Current concepts and controversies. Journal of Neural Transmission (Vienna) 125: 615–650. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Lowe VJ, Boeve BF, et al. (2017) AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Annals of Neurology 81: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lee D, Cho KH, et al. (2017) Cognitive and neuroanatomical correlates in early versus late onset Parkinson’s disease dementia. Journal of Alzheimer’s Disease 55: 485–495. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cho H, Choi JY, et al. (2018) Distinct patterns of amyloid-dependent tau accumulation in Lewy body diseases. Movement Disorders 33: 262–272. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Crutch SJ, Ridgway GR, et al. (2011) Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer’s disease. Neurobiology of Aging 32: 1466–1476. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, et al. (2005) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology 65: 1863–1872. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, et al. (2015. a) Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain 138: 2974–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, et al. (2015. b) Longitudinal assessment of global and regional atrophy rates in Alzheimer’s disease and dementia with Lewy bodies. NeuroImage 7: 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kaiser J, Haselgrove C, et al. (2006) Human cerebral cortex: A system for the integration of volume- and surface-based representations. NeuroImage 33: 139–153. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, et al. (2013) Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson’s disease. PLoS ONE 8: e54980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Svenningsson P, Weintraub D, et al. (2014) Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 82: 2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rektorova I, Biundo R, Marecek R, et al. (2014) Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS ONE 9: e85595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Tym E, Mountjoy CQ, et al. (1986) CAMDEX: A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. British Journal of Psychiatry 149: 698–709. [DOI] [PubMed] [Google Scholar]
- Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, et al. (2009) Correlations between gray matter reductions and cognitive deficits in dementia with Lewy Bodies and Parkinson’s disease with dementia. Movement Disorders 24: 1740–1746. [DOI] [PubMed] [Google Scholar]
- Sarro L, Senjem ML, Lundt ES, et al. (2016) Amyloid-β deposition and regional grey matter atrophy rates in dementia with Lewy bodies. Brain 139: 2740–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura B, Baggio HC, Marti MJ, et al. (2014) Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Movement Disorders 29: 1495–1503. [DOI] [PubMed] [Google Scholar]
- Smith R, Schöll M, Londos E, et al. (2018) 18F-AV-1451 in Parkinson’s disease with and without dementia and in dementia with Lewy bodies. Scientific Reports 8: 4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CW, Burton EJ, McKeith IG, et al. (2005) Temporal lobe atrophy on MRI in Parkinson disease with dementia: A comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 64: 861–865. [DOI] [PubMed] [Google Scholar]
- Walker MP, Ayre GA, Cummings JL, et al. (2000) The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. British Journal of Psychiatry 177: 252–256. [DOI] [PubMed] [Google Scholar]
- Watson R, Colloby SJ, Blamire AM, et al. (2015) Assessment of regional gray matter loss in dementia with Lewy bodies: A surface-based MRI analysis. American Journal of Geriatric Psychiatry 23: 38–46. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Graff-Radford J, Tosakulwong N, et al. (2018) Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer’s disease. Alzheimer’s & Dementia 14: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. (2013) Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry 84: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]