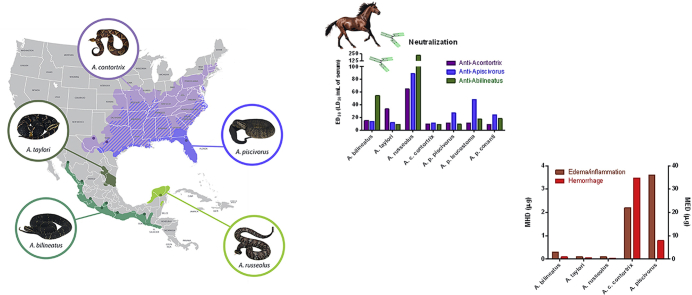
Abstract
In the present work, venoms from five species of the genus Agkistrodon were evaluated in terms of their enzymatic (Phospholipase A2 and caseinolytic) and biological (edema forming, hemorrhagic, procoagulant and lethal) effects. Horses were used to produce monovalent hyperimmune sera against each of three venoms (A. bilineatus, A. contortrix and A. piscivorus) and their neutralizing potency, expressed as Median Effective Dose (ED50), was determined against the venoms of all five species. In terms of PLA2 and caseinolytic activities, all venoms are extremely homogeneous. PLA2 activity is high, while caseinolytic activity is low when in contrast with that of the rattlesnake Crotalus simus. On the other hand, biological activities showed marked interspecific differences, particularly between the species from Mexico and those from the United States. Mexican species displayed higher edema-forming, hemorrhagic and lethal effects than US species, while none of the species studied presented procoagulant activity. All three monovalent hyperimmune sera showed good neutralizing potency against the analyzed venoms. Nonetheless, we observed relevant immunochemical differences among the venoms using ELISA and Western Blot assays. We conclude that the venoms of A. piscivorus (USA) and A. bilineatus would be ideal to use as immunogens for the production of a polyvalent antivenom with good neutralizing potency against the venoms of all the species of the genus.
Keywords: Agkistrodon venoms, Antivenom neutralization, Edema, Hemorrhage, Immunochemistry
Graphical abstract
Highlights
-
•
Venoms from Agkistrodon species from the US and Mexico are significantly different in terms of biological activities.
-
•
Edema, hemorrhage and procoagulant, as well as enzymatic activities are determined for five species of the genus.
-
•
Neutralization by three monospecific hyperimmune sera show there is significant cross reactivity between species.
-
•
Venoms from A. bilineatus and A. piscivorus are proposed as immunogens to generate a genus-wide neutralizing serum.
1. Introduction
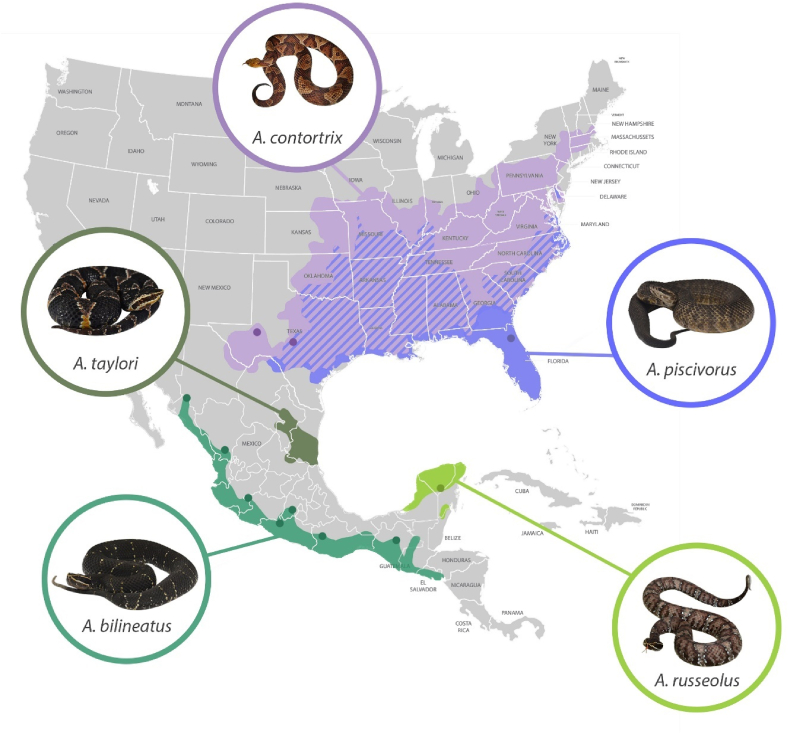
Subfamily Crotalinae is a group of snakes, within the family Viperidae, containing about 242 species grouped in 21 genera (Uetz et al., 2019). Snakes of this subfamily, also known as pit vipers, include some Asian genera as well as all the American Vipers; among the latter is the genus Agkistrodon. Campbell and Lamar, in 2004, reported 4 species in the genus: A. bilineatus (3 subspecies), A. contortrix (5 subspecies), A. piscivorus (3 subspecies) and A. taylori. Later, in 2013, Porras and collaborators elevated the subspecies of Agkistrodon bilineatus to species level: A. bilineatus, A. russeolus and A. howardgloydi, leaving the genus with six species distributed in North and Central America (Fig. 1) (Campbell and Lamar, 2004, Porras et al., 2013). The equine hyperimmunization protocols described in the present study were performed in 2014, and therefore this classification is used throughout the work. Nonetheless, it is important to note that in 2015, some of the subspecies within A. piscivorus and A. contortrix were elevated to species, leaving four species for the North American Agkistrodon: A. contortrix, A. laticinctus, A. piscivorus and A. conanti. For details on the new taxonomy see (Burbrink and Guiher, 2015).
Fig. 1.
Distribution of species of the genus Agkistrodon in North America. Colored areas represent the distribution of the species modified from (Campbell and Lamar, 2004, Porras et al., 2013). Diagonal lines represent areas where both A. contortrix and A. piscivorus are present. A. c. contortrix photo by Eric Centenero. (This map is not to scale; it is only meant for illustrative purposes).
In the United States, there are approximately 45,000 snakebites in humans every year. Among these, about 8,000 result in envenomations. Almost 2,000 are caused by snakes of the genus Agkistrodon (Dart and Gomez, 1996), making it one of the most medically relevant in the country. Accurate records regarding snakebite accidents in Mexico are very scarce, but it has been reported that about 4,000 envenomations occur per year, with the genus Agkistrodon also being one of the most medically significant (Chippaux, 2017).
The clinical syndrome caused in the USA by the copperhead (A. contortrix) is characterized by local symptoms, mainly pain, edema and ecchymosis. Permanent loss of function, necrosis and systemic symptoms are unusual (Scharman and Noffsinger, 2001). These envenomations are thus considered of low risk and antivenom is not always indicated (Mazer-Amirshahi et al., 2014, Walker and Morrison, 2011). Other species of the genus have been reported to cause much more severe envenomations, however. Venom of the Mexican cantil (A. bilineatus), for example, in addition to local edema and pain, can cause severe hemorrhages in experimental envenomations (Ownby et al., 1990).
In experimental envenomations, the main activities described for A. bilineatus are the generation of hemorrhage and edema and various hemorrhagic toxins have been isolated from Agkistrodon venoms (Imai et al., 1989, Ownby et al., 1990). The edema-forming activity of the venoms has been attributed to protein families including phospholipases A2 (PLA2s), snake venom metalloproteases (SVMPs) and snake venom serine proteases (SVSPs) (de Freitas Oliveira et al., 2009, Hati et al., 1999, Serrano, 2013).
Like most viper venoms, Agkistrodon venoms are composed mainly of proteins and peptides while non-proteic components are in lower proportion and include citrate, as well as various ions. Lomonte and collaborators (Lomonte et al., 2014), performed a proteomic analysis of the venoms from four species of the genus Agkistrodon and some of their subspecies: A. contortrix (five subspecies), A. piscivorus (three subspecies), A. bilineatus (two subspecies) and A. taylori. In that work, they reported that all the venoms have a high proportion of PLA2s (31.5–46.0%) and SVMPs (21.0–33.1%), followed by a lower but still important percentage of SVSPs (8.9–22.5%). Together, these three families account for over 60% of the venoms’ components, the rest is composed of other enzymes like L-amino acid oxidases (LAAOs) and non-enzymatic proteins like desintegrins, Cysteine-rich secretory proteins (CRISP) and C-type lectins (CTLs). The proportion of protein families present in the venoms was observed to be very similar, which suggests that they are very homogeneous in terms of composition. Still, the great diversity present within each protein family, complicates the prediction of biological activities or clinical syndromes, even when the proportion of protein families in a venom is known (Castro et al., 2013). Also, the abovementioned differences in severity of the clinical syndromes developed by species of this genus suggest differences in toxicity of individual proteins that are relevant during envenomation and possibly also for antivenom neutralization.
The aim of this work was to characterize the biochemical and biological activities of the venoms of five species of the genus Agkistrodon, as well as their immunochemical characteristics. This knowledge can be of importance in the development of antivenoms and in clinical management of envenomation.
2. Materials and methods
2.1. Ethics statement
All animal experiments were performed in compliance with the EU Directive 2010/63/EU for animal experiments (European Parliament, 2010), under the procedures and with the approval of the Institutional Bioethics Committee of the Biotechnology Institute of the National Autonomous University of Mexico (IBt-UNAM) project number 254: “Functional characterization of Agkistrodon venoms and equine immune response against them” (Caracterización funcional del veneno de Agkistrodon, así como la respuesta inmune en caballos contra los mismos).
ICR mice used in all experiments were obtained from the laboratory animal facility of IBt-UNAM. Animal facility staff as well as research staff was trained in the correct and humane handling of mice before the start of any procedure.
The use of human blood from a single healthy donor was also approved by the Institutional Bioethics Committee of IBt-UNAM, as a part of project 254. All residues that had been in contact with the blood were discarded in accordance to Institutional regulations.
Some individuals from the species A. bilineatus were collected under license number SGPA/DGVS/03459/15 and kept for successive venom extractions at the vivarium “Cantil: Herpetario del IBt” with registration number MOR-IN-166-07-04. All housing and handling procedures have been revised and approved by Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Mexico in strict accordance with the regulations of the National General Law of Wildlife (Congreso, 2018).
Horse housing and handling protocols were in strict accordance with Mexican and international animal welfare regulations and were approved by SAGARPA (Secretaría de Desarrollo Agrícola y Desarrollo Rural, Pesca y Alimentación; SENASICA –Dirección General de Salud Animal-) with permit number: DU00411. All handling of horses was performed by previously trained staff from ranch Ojo de Agua in Puebla, Mexico and submitted to monthly medical check by an in-house specialized veterinarian.
2.2. Venoms
Some venoms were from the venom bank of our laboratory at IBt-UNAM while others were obtained through collaborations with the following herpetariums, who kindly lent their snakes for venom extraction: Reptiles Fergo (license number: DGVS-PIMVS-EA-0084-MOR/08), UMA TSÁAB KAAN (license number UMA-IN-0183-YUC-10), Herpetario de la Facultad de Ciencias, UNAM and DeVal Animal (license number DGVS-CR-IN-0957-D.F./07). Pools from 2 to 5 individuals were used for each species, except in the case of A. taylori, where only one specimen was available for venom extraction. Table 1 and Fig. 1 detail the source of all the venoms analyzed in the present study. A pool from 18 adult specimens of Crotalus simus from Veracruz, Mexico, and a venom pool from the scorpion Centruroides limpidus (both from the venom bank at IBt-UNAM) were used for comparison or external controls when needed.
Table 1.
Individual venoms used for characterization and hyperimmunization pools.
| A. Venom characterization Pools | ||||
|---|---|---|---|---|
| Pool | Species | Herpetarium | State of origin | Venom dry weight (mg) |
| A. bilineatus | A. bilineatus | DeVal Animal | Colima, MX | 8 |
| A. bilineatus | DeVal Animal | Nayarit, MX | 8 | |
| A. bilineatus | IBt-UNAM | Nayarit, MX | 8 | |
| A. bilineatus | FC-UNAM | Chiapas, MX | 8 | |
| A. bilineatus | FC-UNAM | Chiapas, MX | 8 | |
| A. taylori | A. taylori | IBt-UNAM | Tamaulipas, MX | 6 |
| A. russeolus | A. russeolus | IBt-UNAM | Yucatán, MX | 3 |
| A. russeolus | IBt-UNAM | Yucatán, MX | 3 | |
| A. piscivorus | A. p. conantia | NNTRC | Texas, US | 1.5 |
| A. p. conanti | “TSÁAB KAAN” | Florida, US | 1.5 | |
| A. p. conanti | “TSÁAB KAAN” | Florida, US | 1.5 | |
| A. p. leucostomaa | NNTRC | Texas, US | 1.5 | |
| A. p. piscivorus | “Reptiles Fergo" | Unknown | 1.5 | |
| A. c. contortrix | A. c. contortrixa | NNTRC | Texas, US | 6 |
| B. Horse hyperimmunization Pools | ||||
|---|---|---|---|---|
| Pool | Species | Institution | Collection site | Venom dry weight (mg) |
| Imm-Abil | A. bilineatus | IBt-UNAM | Colima, MX | 8 |
| A. bilineatus | IBt-UNAM | Sinaloa, MX | 8 | |
| A. bilineatus | IBt-UNAM | Nayarit, MX | 8 | |
| A. bilineatus | FC-UNAM | Chiapas, MX | 8 | |
| A. bilineatus | FC-UNAM | Chiapas, MX | 8 | |
| Imm-Acont | A. c. contortrixa | NNTRC | Texas, US | 36 |
| Imm-Apisc | A. p. piscivorus | “Reptiles Fergo" | Unknown | 12 |
| A. p. conantia | NNTRC | Texas, US | 12 | |
| A. p. leucostomaa | NNTRC | Texas, US | 12 | |
Venom pool.
All venoms were obtained through manual extraction and were then washed from the extraction cup using low volumes of 20 mM ammonium acetate pH 4.7 (maximum proportion of ammonium acetate to venom was 1:10 v/v). They were subsequently centrifuged at 16,800 g for 3 min and the supernatant was stored at −70 °C for lyophilization. Finally, lyophilized venoms were stored at 4 °C until their use. Some of the venoms from A. p. conanti and all A. c. contortrix and A. p. leucostoma were pools purchased from the National Natural Toxin Research Center (NNTRC) in Texas, U.S.
2.3. Protein concentration
Protein concentration of the pooled and individual venoms was determined using a Pierce® Bicinchoninic Acid (BCA) Protein Assay (Thermo Scientific), with bovine serum albumin (BSA) as a standard, according to the manufacturer's protocols.
2.4. Biochemical characterization
2.4.1. SDS-PAGE
Twenty-five μg of each venom were loaded on 12.5% SDS-PAGE gels under reducing conditions. Samples were diluted using Sample buffer 5X (10% Glycerol, 2.5% SDS, Tris-HCl 50 mM pH 6.8, 5% 2-mercaptoethanol, 0.002% bromophenol blue) to a final volume of 20 μL and boiled for 5 min. Electrophoresis was performed with a constant voltage of 80 V for 15 min and then 100 V for approximately 60 min. Gels were stained with G-250 Coomassie Brilliant Blue. Apparent molecular weights were determined comparing migration distance with 5 μL of molecular weight markers (Precision Plus Protein Dual Xtra Standards, Bio-Rad) using ImageJ software version 1.50i.
2.4.2. RP-HPLC profiles
Venom samples (1 mg of dry weight) were dissolved in 1 mL of water containing 0.1% trifluoroacetic acid (TFA), centrifuged to remove debris and fractionated through RP-HPLC on a C18 column (4.6 × 250 mm, 5 μm particle size; Vydac®) using an Agilent 1100 chromatograph. Elution was performed at 1 mL/min by applying a gradient to solution B (acetonitrile, containing 0.1% TFA), as follows: 0% B for 5 min, 0–15% B over 15 min, 15–45% B over 60 min, 45–70% B over 12 min, and 70% B for 10 min.
2.4.3. PLA2 activity
PLA2 enzymatic activity of pooled venoms was determined using a titrimetric assay with a 10% egg yolk solution (0.1 M NaCl, 0.01 M CaCl2, 0.1% Triton-100 and 10% egg yolk) as substrate (Shiloah et al., 1973). The assay was performed on 500 μL of the previously described solution, stabilized in pH 8.05 with 50 mM NaOH. The solution was under constant stirring and mild N2 bubbling. 50 mM NaOH was also used for titration. Units of enzymatic activity (U) were defined as μmoles of NaOH consumed per minute and the results were reported in units per milligram of venom (U/mg).
2.4.4. Proteolytic activity
Proteolytic activity of the venoms was evaluated using a further modification of the method described by (Chen et al., 2004, Yang et al., 2015) and modified by (Gutiérrez et al., 2008). Briefly, azocasein was dissolved in a standard solution (50 mM Tris-HCl, 150 mM NaCl and 5 mM CaCl2) to a final concentration of 10 mg/mL. Afterwards, 20 μg of venom, dissolved in 20 μL of 150 mM NaCl, were added to 100 μL of the azocasein solution and incubated for 30 min at 37 °C. After incubation, the reaction was stopped by adding 200 μL of 5% trichloroacetic acid. Then, samples were centrifuged at 16,800 g for 5 min and 150 μL of the supernatant of each sample were added to 150 μL of 500 mM NaOH in a 96 well plate (NUNC). Finally, sample absorbance at 450 nm was determined. Units of enzymatic activity (U) are defined as the change of 0.2 in the absorbance of the sample per minute.
In order to verify the linearity of the observed reaction, proteolytic activity was determined through varying incubation times (30, 60 and 90 min). The selected incubation time was 30 min, because substrate was still in excess.
2.5. Biological characterization
2.5.1. Lethality
The median lethal dose (LD50) of each venom pool was determined intravenously (i.v.). Different venom doses in a total volume of 0.5 mL were inoculated through the tail vein to groups of five ICR mice between 18 and 20 g of body weight (4 groups per venom were used in average). The percentage of dead mice was measured 24 h after venom injection with monitoring intervals of approximately 3 h; mice that were evidently moribund were euthanized through cervical dislocation to minimize animal suffering. The obtained data were analyzed through a non-linear regression (variable slope, dose-response curve) using the software GraphPad Prism 6.01. LD50 was defined as the amount of venom that causes death to 50% of the mice population (Casasola et al., 2009).
2.5.2. Edema-forming activity
The edema-forming activity of the venom pools was analyzed through the determination of a Minimum Edema-forming Dose (MED), using the method described by (Gutiérrez et al., 1986). Throughout these experiments, groups of three ICR mice were subcutaneously (s.c.) injected in the left hind paw with different amounts of venom (4 groups per venom were used in average). Concentrations were calculated in order to always inoculate them with a volume of 50 μL of venom, resuspended in PBS. The right hind paw of every mouse was injected with 50 μL of PBS to use as individual control.
After venom inoculation, the diameter of both paws was measured every 10 min for the first hour and every 30 min for the next 2 h using a manual Vernier caliper. The increase in limb volume caused by the venom for each measured time was determined using the percentage of diameter increase of the envenomated paw compared to the control paw. In order to minimize animal suffering, all mice were euthanized immediately after conclusion of the experiment.
2.5.3. Procoagulant activity
The procoagulant activity of the venom pools was analyzed through the determination of a Minimum Procoagulant Dose in Plasma (MPD-P), using the method described by (Theakston and Reid, 1983). Different amounts of venom were added to glass tubes with 200 μL of citrated (sodium citrate, 3.8 g/dL) human plasma. Time was measured between venom addition and evident clot formation while gently moving the glass tube. Obtained data was processed with a linear regression, selecting only the initial section of the dose-response curve and verifying linearity (R2 > 0.9). The venom dose that generates a clot in 1 min was interpolated using the software GraphPad Prism 6.01. The MPD-P was defined as the amount of venom that induces the generation of an evident clot in 60 s.
In order to minimize variation, blood from the same human donor was used for all the experiments and the time measurements were always taken by the same observer.
2.5.4. Hemorrhagic activity
The hemorrhagic activity of the venom pools was analyzed through the determination of a Minimum Hemorrhagic Dose (MHD) using the method by (Gutiérrez et al., 1985) with some modifications. Briefly, groups of 5 CD1 mice were intradermally (i.d.) inoculated in the higher region of the back with 50 μL of venom resuspended in PBS with varying concentrations (4 groups per venom were used in average). Three hours after inoculation, mice were sacrificed through CO2 inhalation and their skins were removed. The hemorrhagic area (HA) around the injection point was measured using millimetric paper and the diameter (D) of the hemorrhagic halo was calculated using the following formula: (Varios, 2007). MHD was defined as the amount of venom that generates a hemorrhagic halo of 1 cm in diameter.
2.6. Immunochemical characterization
2.6.1. Production of horse hyperimmune sera
Three adult, male, crossbred horses kept in the farm “Ojo de Agua” in the community Venustiano Carranza (Puebla, Mexico), were inoculated with increasing amounts of venom, using the immunization scheme detailed in Supplementary Table 1. Each horse was identified with a number and inoculated with the venom from only one Agkistrodon species as follows: Horse 201 with A. c. contortrix, horse 202 with a pool of the three subspecies of A. piscivorus and horse 203 with A. bilineatus. The hyperimmunization pools used are detailed in Table 1.
2.6.2. Determination of titers of Agkistrodon hyperimmune horse sera
Maxisorp (Nunc Inc, USA) plates were coated with 100 μL/well of 5 μg/mL of the venoms, diluted in 100 mM carbonate/bicarbonate buffer, with pH 9.5 and incubated overnight at 4 °C. The plates were then washed 3 times with 250 μL/well of washing buffer (50 mM Tris/HCl, 150 mM NaCl, 0.05% Tween 20 and pH 8) in a microplate washer (BIO-RAD Immuno wash 1575). The remaining binding sites were blocked with 200 μL/well of blocking buffer (50 mM Tris/HCL, 5 mg/mL gelatin, 0.2% Tween 20 and pH 8) and incubated for 2 h at 37 °C. The plates were then washed 3 times as described before. Samples of anti-Agkistrodon horse serum were initially mixed with vehicle buffer (50 mM Tris–HCl, 0.5 M NaCl, 1 mg/mL gelatin and 0.05% Tween 20, pH 8.0), at a dilution of 1:300. This solution was serially diluted 1:3, with the same buffer on the ELISA plates. Plates were incubated for 1 h at 37 °C and, after washing them 3 times, plates were incubated for 1 h at 37 °C with 100 μL/well of peroxidase-conjugated goat anti horse IgG antibody (1:3000 dilution, Gene Tex). After washing them 5 times, 100 μL/well ABTS solution (Roche) were added and incubated for 10 min at 25 °C. When this timespan concluded, the reaction was stopped with 20 μL/well of 20% sodium dodecyl sulfate (SDS) and absorbances of wells were measured at 405 nm in a Microplate Reader. Sigmoidal dose-response curves were generated using non-linear regression with a variable slope with the software GraphPad Prism 4. Antibody titer is defined as the serum dilution at which 50% of the colorimetric response is obtained.
2.6.3. Competitive ELISA
We used two different plates, incubation and assay plates. Incubation plates (Maxisorp Nunc Inc, USA) were blocked with 200 μL/well of blocking buffer and incubated for 2 h at 37 °C. Samples of Agkistrodon venoms were prepared in vehicle buffer with a concentration of 300 μg/mL or 1000 μg/mL and serially diluted 1:3. Also, 100 μL/well of horse serum anti Agkistrodon were added to the plate using a dilution equivalent to the titer (EC50) of each serum for its homologous venom and incubated for 1 h at 37 °C.
Assay plates were coated with 100 μL/well of 5 μg/mL of venoms from A. bilineatus, A. c. contortrix or a mixture of A. piscivorus subspecies and incubated overnight at 4 °C. The remaining binding sites were blocked with 200 μL/well of blocking buffer, incubated for 2 h at 37 °C.
The mixture from the incubation plates was then added to the assay plates and these were incubated for 1 h at 37 °C. Finally, plates were incubated for 1 h at 37 °C with 100 μL/well of peroxidase-conjugated goat anti-horse IgG antibody (1.5 10−3 μg/mL, Gene Tex) and developed using 100 μL/well of ABTS solution (Roche) incubated for 10 min at 25 °C. After this timespan concluded, the reaction was stopped with 20 μL/well of 20% SDS. Each samples’ absorbance was determined at 405 nm in a Microplate Reader.
The serum titer for the homologous venom was used to define the serum dilution to add as primary antibody to each ELISA plate. The absorbance of the maximum venom dilution of the homologous venom for each plate was defined as 0% competition and that of the highest venom dilution, as the percent of maximum inhibition. Data analysis was performed using the software GraphPad Prism 4.0.
2.6.4. Neutralizing potency
Neutralizing potency of the horse hyperimmune sera was evaluated through the determination of a Median effective Dose (ED50) (Casasola et al., 2009). To this end, different serum volumes were incubated for 30 min at 37 °C with 3LD50s of Agkistrodon venom. Afterwards, groups of 5 mice between 18 and 20 g of body weight were inoculated i.v. with the samples (4 groups per venom were used in average). The survival rate was determined 24 h after venom injection with monitoring intervals of approximately 3 h; mice that were evidently moribund were euthanized through cervical dislocation to minimize animal suffering. Obtained data was processed through non-linear regression (variable slope, dose-response curve) using the software GraphPad Prism 6.01. The ED50 was defined as the serum volume capable of preventing death in 50% of envenomated mice.
2.7. Statistical analysis
In order to evaluate differences between treatments, a one way ANOVA and a post hoc Tukey test were performed for PLA2 and caseinolytic activities. Results were considered statistically different when P < 0.05.
3. Results
3.1. Biochemical and biological characterization
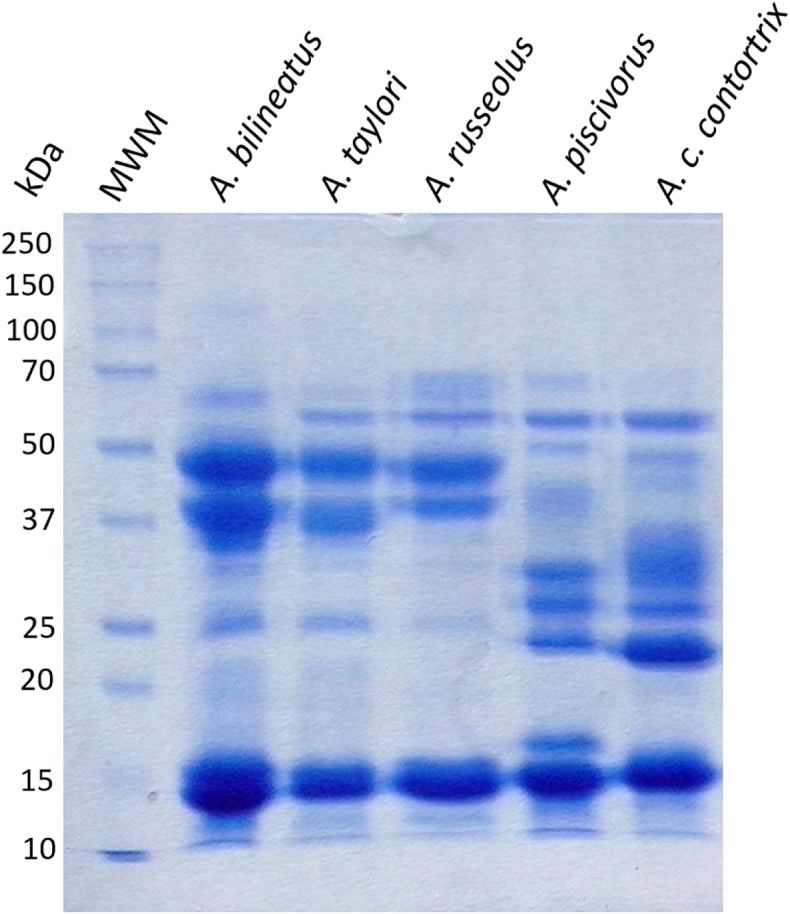
Electrophoretic profiles of Agkistrodon venoms are shown in Fig. 2. All analyzed venoms showed several conspicuous protein bands of 13.2 kDa–15.3 kDa. Venoms from the Mexican species (lanes 2 to 4) showed very similar patterns among them, with two more abundant protein bands of approximately 35.2 and 45.8 kDa and less abundant bands of around 27.1, 45.8 and 58.3 kDa. On the other hand, electrophoretic pattern of venoms from A. piscivorus and A. c. contortrix differed from the Mexican venom pattern and were also relatively different from each other. These venoms lacked the abundant high molecular weight proteins, or had them in very small proportion, and were instead rich in medium molecular weight protein bands, ranging from 24.9 to 35 kDa (three bands in A. piscivorus and four in A. c. contortrix).
Fig. 2.
SDS-PAGE profile of Agkistrodon venom pools. Reducing conditions (2 ME). MWM. Molecular weight markers.
RP-HPLC profiles also showed the Mexican species to be very similar to each other, with the most abundant components eluting between 60 and 70 min of retention time (RT) and a single, also very abundant peak at approximately 85 min. Profiles from A. c. contortrix and the subspecies of A. piscivorus were similar, but not identical, with some marked differences before 50 min RT; both species differed significantly from the profiles of the Mexican species. Some of the observed differences were a greater diversity of components around 85 min RT and the presence of one to three abundant components between 50 and 60 min RT (Fig. 3).
Fig. 3.
RP-HPLC of Agkistrodon venom pools on a C18analytic column.mAU. Milli Absorbance Units at 214 nm % B. Percentage of buffer B (CH3CN + 0.1% TFA).
In Table 2 we summarized the results obtained for the biochemical and biological activities of Agkistrodon venoms. The venom of C. simus was used for comparison where relevant.
Table 2.
Enzymatic and pharmacological activities of Agkistrodon venom pools.
| PLA2a |
Proteolysisb |
MEDc |
MHDd |
MPD-HPe |
LD50f |
||
|---|---|---|---|---|---|---|---|
| (U/mg ± SD)g | (U/mg ± SD)g | (μg ± SD) | (μg ± SD) | (μg) | (μg/mouse) | (μg/g) | |
| A. bilineatus | 383.2 ± 97.7 | 6.8 ± 1.3 | 0.2 ± 0.01 | 0.8 ± 0.04 | >100 | 36.5 (36–37.1) | 1.9 |
| A. taylori | 720.2 ± 81 | 6.4 ± 0.6 | 0.1 ± 0.01 | 0.7 ± 0.25 | >100 | 26.2 (22.7–30.6) | 1.4 |
| A. russeolus | 371.5 ± 146.3 | 5.2 ± 0.7 | 0.1 ± 0.03 | 0.3 ± 0.06 | >100 | 22.3 (22.2–22.3) | 1.2 |
| A. c. contortrix | 296.8 ± 64.9 | 7.0 ± 1.0 | 1.8 ± 1.2 | 33.8 ± 1.4 | >100 | 215.4 (197.0–235.5) | 11.3 |
| A. piscivorus | 231.9 ± 49.3 | 6.4 ± 0.6 | 3.6 ± 0.5 | 7.4 ± 0.74 | >100 | 96.6 (92.6–100.9) | 5.1 |
| C. simus | NDh | 11.2 ± 1.6 | 10.0 ± 1.2 | 25.1 ± 1.3i | NDh | 3.8 (3.4–3.9) | 0.2 |
Phospholipase A2 activity on 10% egg yolk.
Proteolytic activity on azocasein.
Minimum edema-forming dose.
Minimum hemorrhagic dose.
Minimum procoagulant dose on human plasma.
Median lethal dose, values in parenthesis represent 95% confidence intervals.
Units of enzymatic activity per milligram of venom ± standard deviation.
Not determined.
LD50s of the analyzed venoms ranged from 0.2 to 11.3 μg/g and are shown in Table 2. In terms of PLA2 activity, most Agkistrodon venoms showed no statistically significant difference between them (P ≥ 0.05), ranging between 231.9 and 383.2 U/mg; A. taylori was the only one with a significantly higher activity (720.2 U/mg). Proteolytic activity on azocasein substrate was also statistically the same for all the Agkistrodon venoms tested (P ≥ 0.05), ranging between 5.2 and 7 U/mg (Table 2).
In the current work, we observed that edema-forming activity was greatest in the venoms from A. bilineatus, A. taylori and A. russeolus, with MED ranging between 0.1 and 0.2 μg. In contrast, the venoms from A. c. contortrix and A. piscivorus showed MEDs of 1.8 and 3.6 μg respectively; between 7.3 and 36.0 times higher than those of the Mexican species (Table 2). Also, all venoms tested caused hemorrhage to some extent, yet once again, the venoms from A. bilineatus, A. taylori and A. russeolus had the highest activities with MHDs of 0.8, 0.7 and 0.3 μg respectively. The venoms from A. piscivorus and A. c. contortrix had comparatively less activity, with MHDs of 7.4 and 33.8 μg respectively (Table 2).
3.2. Immunochemical characterization
3.2.1. ELISA titers
To analyze the recognition of the produced hyperimmune sera, we determined ELISA titers using homologous and heterologous Agkistrodon venoms. Highest titers were obtained for serum 203 (anti-A. bilineatus) against the two other Mexican species (A. russeolus −121,043- and A. taylori −122,525-) while titers against A. c. contortrix and the subspecies of A. piscivorus ranged between 33,522 and 67,872. Interestingly, the titer against the homologous venom was relatively low (37,003) (Table 3).
Table 3.
Antibody titers and neutralization potency of produced sera against Agkistrodon venoms.
| LD50a |
Serum 201 [anti-A. c. contortrix] |
Serum 202 [anti-A. piscivorus] |
Serum 203 [anti-A. bilineatus] |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Titer | ED50b |
Titer | ED50b |
Titer |
ED50b |
|||||
| μg/g | mgV/mLSc | LD50/mLSd | mgV/mLSc | LD50/mLSd | mgV/mLSc | LD50/mLSd | ||||
| A. bilineatus | 1.9 | 18,467 | 0.5 (0.5–0.6) | 15.8 | 15,821 | 0.5 (0.4–0.6) | 14.2 | 37,003 | 2.0 (1.6–2.4) | 54.4 |
| A. taylori | 1.4 | 41,131 | 0.9 (0.9–0.9) | 33.5 | 44,780 | 0.3 (0.3–0.4) | 12.6 | 122,525 | 0.2 (0.2–0.3) | 9.4 |
| A. russeolus | 1.2 | 34,457 | 1.5 (1.4–1.5) | 65.1 | 49,880 | 2.0 (1.6–2.4) | 89.1 | 121,043 | 5.1 (4.9–5.4) | 229.5 |
| A. c. contortrix | 11.3 | 29,829 | 2.2 (2–2.3) | 10.2 | 30,528 | 2.5 (2.1–3.0) | 11.8 | 33,522 | 2.0 (1.7–2.4) | 9.4 |
| A. p. piscivorus | 5.1 | 23,278 | 1.1 (1.0–1.3) | 11.8 | 110,262 | 2.6 (2.4–2.9) | 27.3 | 43,676 | 0.9 (0.9–1.0) | 9.7 |
| A. p. leucostoma | 6.1 | 20,755 | 1.4 (1.3–1.5) | 11.8 | 83,123 | 5.7 (4.6–7.0) | 48.6 | 61,035 | 2.1 (1.6–2.8) | 17.7 |
| A. p. conanti | 6.3 | 29,829 | 1.1 (1.0–1.1) | 9.0 | 108,032 | 2.9 (2.8–3.1) | 24.5 | 67,872 | 2.2 (2.0–2.4) | 18.5 |
| Numbers in parenthesis represent 95% confidence intervals. Values in bold represent responses to the homologous venom. | ||||||||||
Median lethal dose.
Neutralization median effective dose, using 3LD50s of venom.
Milligrams of venom neutralized per milliliter of serum.
Median lethal doses of venom neutralized per milliliter of serum.
Serum 201 (anti–A. c. contortrix) had the lowest titers, ranging between 18,467 and 34,457 against the Mexican species and between 23,278 and 29,829 against species from the U.S., including the homologous venom. Finally, titers for serum 202 (anti-A. piscivorus) were high against the homologous subspecies (83,123 to 110,262) and relatively low against both A. c. contortrix and the Mexican species (15,821 to 49,880) (Table 3). Titers for the negative control venom, Centruroides limpidus, were 0 against all sera.
3.2.2. Competitive ELISA
In the plates with A. bilineatus venom and serum against A. bilineatus, we obtained highest percentages of competition with Mexican species of A. bilineatus, A. russeolus and A. taylori, while less was observed for species from USA, showing that Mexican species are immunochemically similar to each other. On the other hand, on the plate with A. piscivorus fixed venom, we obtained the maximum inhibition with homologous A. piscivorus venom and a moderate inhibition with A. c. contortrix venom (70.1%). The species of A. bilineatus, A. russeolus and A. taylori were bad competitors. Finally, venom from A. c. contortrix was the one that showed lowest competition values with all other species. Still, Mexican species share fewer epitopes with it than do the subspecies of A. piscivorus (Fig. 4).
Fig. 4.
Percentage of competition of each horse serum with Agkistrodon venoms, determined using a competitive ELISA. A higher inhibition percentage shows more shared epitopes between the homologous and tested venom.
There was no competition with the venom of the negative control, C. limpidus, in any of the analyzed sera, indicating that there is no non-specific recognition by equine immunoglobulins.
3.2.3. Neutralization
Monovalent sera, in general, presented a higher neutralization potency against the homologous venom than against the heterologous venoms. Serum 201 (anti-A. c. contortrix), showed an EC50 of 2.2 mgV/mLS (milligrams of venom per milliliter of serum) against the homologous venom, while this same value ranged between 0.6 and 1.5 mgV/mLS when tested against heterologous venoms. Serum 202 (anti-A. piscivorus) presented a neutralization potency between 2.6 and 5.7 mgV/mLS for the three subspecies of A. piscivorus and between 0.3 and 2.5 mgV/mLS for other species of the genus. On the other hand, serum 203 (anti-A. bilineatus) was an exception because it neutralized the venoms from A. russeolus, A. c. contortrix, A. p. leucostoma and A. p. conanti just as well or better than the homologous venom (5.1, 2.0, 2.1 and 2.0 mgV/mLS, respectively) (Table 3).
4. Discussion
General composition in viper venoms is broadly conserved in terms of protein families (Calvete et al., 2009, Lomonte et al., 2014), where the most abundant are usually SVMPs, PLA2s and SVSPs (Tasoulis and Isbister, 2017). Differences among the species of a genus such as Agkistrodon are generally given by variation in the proportions of the mentioned protein families and by the presence or absence of individual proteins which can have a strong effect in a particular venom activity (Borja et al., 2018, Castro et al., 2013, Glenn et al., 1994, Saldarriaga et al., 2003). Venom variation can be immunochemical, biochemical or in terms of its toxic activities and it can significantly affect neutralization by antivenoms (de Roodt et al., 2011, Gutiérrez et al., 2010, Neri-Castro et al., 2019). It has been shown that it can exist between species and within a single species, generated by ontogenetic changes or from one geographic location to another, among other factors (Borja et al., 2018, Borja et al., 2014, Borja et al., 2013, Castro et al., 2013, Durban et al., 2017, Glenn et al., 1994, Glenn et al., 1982, Mackessy et al., 2018, Rivas et al., 2017, Wilkinson et al., 2018, Zancolli et al., 2018, Zelanis et al., 2010).
Only interspecific variation was considered in the present study, while the possibility of the existence of intraspecific variation in Agkistrodon venoms was not evaluated, mainly due to sample availability. However, samples from a wide distribution range were available for the case of A. bilineatus (Fig. 1), so biochemical activities as well as SDS-PAGE were performed with 12 individual venoms. We observed that there is no evident intraspecific variation across the distribution range of this species (Supplementary Figure 1). Also, a pool of 9 neonate individuals from A. bilineatus (born in captivity) was analyzed using the same RP-HPLC method used for the pooled venoms and it proved to be almost identical to the adult pool (Fig. 3). Intraspecific variation has been also analyzed using the venom of A. contortrix by (Lagesse and Ford, 1996) and found the venom of this species to have only small variations across its distribution.
In the SDS-PAGEs performed in the present work, we observed that the Mexican species have similar electrophoretic profiles among them and are different from the species native to the U.S. (Fig. 2). This grouping can be also observed in the RP-HPLC profiles (Fig. 3). Except for a few small differences, the RP-HPLC profiles were extremely similar to the ones previously obtained by Lomonte and collaborators (Lomonte et al., 2014), during their proteomic characterization of these species (Fig. 3).
Proteins of the PLA2 families were very abundant in all the venoms studied in the present work. Within the PLA2 proteins that have been described in viper venoms there are two distinct groups: those with enzymatic activity, which have an aspartic acid in canonical position 49 (D49), and those with a substitution of this residue to lysine or serine (K49 or S49) and that are devoid of enzymatic activity (Fernández et al., 2013, Kini, 2003, Lomonte et al., 2003). Many among the second group are commonly referred to as true myotoxins because they act on cellular membranes of muscle cells causing lysis through direct damage (Lomonte and Rangel, 2012, Ward et al., 2002). D49 PLA2s have extremely variable pharmacological activities, often interfering in cellular signaling cascades or causing cell lysis through breakdown of membrane phospholipids (de Freitas Oliveira et al., 2009). In the particular case of Agkistrodon venoms, no relationship was observed between the presence of D49 or K49 PLA2s and the edema-forming activity of the complete venoms (Table 4).
Table 4.
Type of PLA2s present in Agkistrodon venoms.
| % D49a | % K49a | MEDb (μg) | PLA2c (U/mg) | |
|---|---|---|---|---|
| A. bilineatus | 16.9 | 17.9 | 0.2 | 383.2 |
| A. taylori | 26.5 | 7.8 | 0.1 | 720.2 |
| A. c. contortrix | 9.8 | 21.7 | 1.8 | 296.8 |
| A. p. piscivorus | 24.0 | 12.8 | 3.6d | |
| A. p. leucostoma | 28.6 | 14.6 | 231.9d | |
| A. p. conanti | 28.7 | 17.3 |
Percentage data were obtained from Lomonte et al. (2014).
Minimum Edema-forming Dose (micrograms).
PLA2 enzymatic activity (Units per milligram).
Activity determined using a pool of the three subspecies.
All venoms showed a similar proteolytic activity when using azocasein as substrate. This activity is due both by SVMPs and SVSPs (Serrano, 2013). SVMPs are found in similarly high proportions in all the species of the genus Agkistrodon as shown in the present work and in (Lomonte et al., 2014). Nonetheless, when performing a deeper analysis of the available proteomes, we observe than the Mexican species have a much higher amount of type P-II SVMPs while the ones present in A. c. contortrix and A. piscivorus are mainly of the type P–I (Table 5). These differences can also be observed in the electrophoretic profiles (Fig. 2).
Table 5.
Metalloprotease type in Agkistrodon venoms.
| Group | SVMPs % |
|
|---|---|---|
| A. c. contortrix | A. taylori | |
| P–I | 29.7 | 1.9 |
| P-II | 2.3 | 28.1 |
| P-III | 0.5 | 0.6 |
Adapted from Lomonte et al. (2014).
One of the main pharmacological activities that can be caused by SVMPs is hemorrhage (Fox and Serrano, 2005). In 1989, Imai et al. (Imai et al., 1989, Nikai et al., 2000) isolated and described a protein they called Bilitoxin-I (PII-SVMP, MW ≈ 48 kDa) from the venom of A. bilineatus. Although P-III SVMP have been described generally to be the most potent hemorrhagic SVMPs (Fox and Serrano, 2005), Bilitoxin-I has an extremely potent hemorrhagic activity, MHD of 0.008 μg, and it is likely responsible for the high hemorrhagic activity observed for the Mexican species (Table 2). For example, A. taylori, had a MHD of 0.7 μg and this protein and similar isoforms account for about 28% of total venom (Lomonte et al., 2014). A. russeolus has been minimally studied but its low MHD (0.3 μg), an abundant RP-HPLC peak with retention time of 83.6 min (consistent with Bilitoxin-I in A. bilineatus and A. taylori), and our observation of severe hemorrhages in envenomated mice, indicate Bilitoxin-I could also be present in high proportions in this venom. On the other hand, based on A. c. contortrix or A. piscivorus venom proteomes (Lomonte et al., 2014), these species contain less than 2% of Bilitoxin-I or similar proteins. These venoms are much less hemorrhagic, with MHDs of 33.8 and 7.4 μg respectively. Hemorrhages observed in these cases may therefore be caused by other, less potent, SVMPs.
Regarding the minimum procoagulant dose in human plasma (MPD-HP), no clot formation was observed for 10 min when adding 100 μg of any venom to 200 μL of citrated human plasma. Procoagulant activity is usually observed in viper venoms that have a high proportion of SVSPs with “thrombin-like” activity as well as other, less common, SVSPs and SVMP that act on different coagulation factors. Examples of these include prothrombin and factor X activators within the SVMP family (Ramos and Selistre-de-Araujo, 2006) and activators of factors V, VIII and also factor X and prothrombin within the SVSP family (Serrano, 2013). One thrombin-like SVSP, named Bilineobin, has been described in the venom of A. bilineatus (Komori et al., 1993), though it has low procoagulant activity when compared to that of other viper venoms. In our experiments, the presence of this protein does not appear to be relevant when the whole venom is tested (Table 2), but the formation of some fibrin fibers was observed. Previous reports have also shown a lack of procoagulant activity of various Agkistrodon venoms (Arce et al., 2003, Lomonte et al., 2014). Contrasting with this result, procoagulant activity has been previously reported for the venom of A. bilineatus (de Roodt et al., 2005), and a Protein C activator protein has been previously described in the venom of the same species (Nakagaki et al., 1990). Some procoagulant enzymes have also been described in the venoms of closely related genus of snakes (Li et al., 2018, Yukelson et al., 1991). On the other hand, a fibrinolytic SVMP without hemorrhagic activity has been described in the venom of A. contortrix laticinctus (Selistre De Araujo, 2013).
One of the most clinically conspicuous signs during Agkistrodon envenomation is edema and inflammation (Scharman and Noffsinger, 2001). Edema is the extravasation of fluid to the interstitial space, and even though it is often related to an inflammatory process, this is not always the case (Trigo and Valero, 2004). Inflammation is a complex cascade of biochemical and cellular processes initiated and regulated by the immune system. The classic manifestations of an inflammatory event are pain, heat, blushing, and local volume increase. Inflammation can be classified in acute and chronic, primarily based on duration and the presence of healing at the site of inflammation. The first is characterized by being short in duration (generally less than 4 h), usually generated by spontaneous damage, while the second is long in duration and characterized by the initiation of healing (Owen et al., 2014, Trigo and Valero, 2004).
The venom of several snakes has been reported to start a complex inflammatory process, associated with the liberation or synthesis of mediator molecules that recruit different cells, as well as pain and edema (Gutiérrez, 2002). The main protein families that have been described to cause inflammation are SVMPs and PLA2s (de Freitas Oliveira et al., 2009, Gutiérrez and Lomonte, 2013, Mackessy, 2009); both groups possess different mechanisms of activation of the immune response. Bothrops asper has one of the most studied venoms in this matter. Here, SVMPs favor edema by causing the rupture of blood vessels and therefore the extravasation of liquid to the interstitial space without the previous initiation of an inflammatory process (Rucavado et al., 2002, Rucavado et al., 1995); inflammation will start later in response to tissue damage. On the other hand, some PLA2s from this venom generate damage of muscle tissue (Zuliani et al., 2005), promoting an inflammatory response without an initial edema.
In the present work, the progression of edema/inflammation was different in both groups of venoms. Injection of venom from the Mexican species resulted in a slow increase in limb volume, with a maximum around 50 min, after which it started decreasing and returned to 0% about 3 h after subcutaneous venom injection (Fig. 5). This behavior could be caused by an initial rupture of the vascular endothelium by SVMPs generating tissue damage and starting an inflammatory response. Also, the venom of A. bilineatus has been described to have bradykinin potentiating factors (BPF) which directly increase vascular permeability (Munawar et al., 2016).
Fig. 5.
Progression of edema on mouse paw injected subcutaneously with different Agkistrodon venoms from Mexico (A) or the U.S. (B). Percentage represents increased diameter compared to the contralateral paw (See Materials and Methods).
On the other hand, both species from the U.S. showed a rapid increase in limb diameter, with a maximum at 10 min and a relatively fast decrease, returning to 0% around 2 h after inoculation (Fig. 5). This suggests the extravasation of liquid without the initiation of an intense inflammatory process.
More detailed studies, including histology of envenomated tissue and/or quantification of inflammation mediators, should be done in order to obtain a proper description of this process.
Another important factor to discuss is the individual variation in humoral immune response of horses used for hyperimmunization. It has been previously observed that horses submitted to the same immunization protocol and adjuvants can have different responses in terms of antibody titers as well as neutralization potencies against homologous venoms (Angulo et al., 1997, Calderón Corona, 2011, Estrada et al., 1989). In this work, only one horse per venom was used for the production of hyperimmune sera and therefore we have no way of verifying that the differences observed are due to the venom's properties as antigens and not to individual variation between horses. Further studies must be done in order to address this issue.
The venoms from Mexican species of this genus were more lethal to mice than those of both species from the U.S. (Table 2). Similar lethal potencies, of 4.6 and 9.1 μg/g, have been previously determined for A. piscivorus and A. c. contortrix, respectively (Arce et al., 2003). The high lethality of the Mexican species could also be explained by the presence of the aforementioned Bilitoxin-I, whose reported LD50 is 0.5 μg/g (Kuniko et al., 1989). In order to further analyze this, we fractionated the venom from A. bilineatus through gel filtration using a Sephadex G75 column (Supplementary Figure 2) and studied the neutralization of fraction A (mainly SVMPs) by sera 203 and 201. We observed that serum 203 (anti-A. bilineatus) was much better at neutralizing this fraction, which has an LD50 of 0.96 μg/g (ED50 = 15.6 mgV/mLS), than serum 201 (anti-A. c. contortrix) (ED50 < 0.9 mgV/mLS). Given that this fraction is mainly composed of Bilitoxin-I (data not shown), the neutralization difference provides strong evidence that the SVMPs in A. c. contortrix are immunochemically different from Bilitoxin-I, a finding that explains both the differences in neutralization potency of the tested sera against whole venoms and, at least in part, the differences in lethality.
It is important to note that a high ELISA titer does not necessarily reflect better neutralization. In fact, the sera with the highest titers were different from the ones with highest neutralization effective doses (Table 3).
The competitive ELISA experiments have also shown the presence of at least two groups with different immunochemical characteristics in Agkistrodon venoms, with the Mexican species in one and those from the U.S. in another. Additionally, the Mexican species appear to be more closely related to each other than A. c. contortrix and A. piscivorus, since these last two species show significantly lower competition percentage between them (Fig. 4).
Further observations can be made when neutralization potencies are studied in terms of the number of LD50s neutralized per mL of serum. When analyzed in that way, we can consider the variations in lethality of each venom. Interestingly, here, the venom of A. russeolus was better neutralized with all the analyzed sera. Previously, we mentioned that Bilitoxin-I is likely the main protein responsible for lethality of Mexican Agkistrodon venoms. Given that A. russeolus has approximately the same proportion of this toxin than A. bilineatus and A. taylori (16.8%, 15.3% and 17.5%, respectively), the higher lethal potency of the first could also be due to other toxins, which are less abundant in the latter. A good neutralization of these proteins by all the analyzed sera can be assumed, given their low effective dose against A. russeolus venom. However, a lot more studies should be performed to confirm this hypothesis.
The neutralizing potency of the equine monovalent sera generated in this work was equal to or higher than 1 mg of venom per milliliter of serum (1 mgV/mLS) for the heterologous venoms and higher than 2 mgV/mLS for the homologous venoms (Table 3). Judging from the experience of the authors, all sera have good neutralization potencies for antivenom production. Given that serum 203 (anti-A. bilineatus) was best at neutralizing the venoms from Mexican species and serum 202 (anti-A. piscivorus) was best at neutralizing all the analyzed venoms from the U.S., we consider that genus-wide neutralization can be achieved by the inclusion of venoms from A. bilineatus and A. piscivorus in equine immunization protocols.
5. Conclusion
Although proteomic analysis suggests that venoms are very similar in the composition of their protein families, our results demonstrate the presence of two groups in terms of immunochemistry and pharmacological activities. These differences have a significant influence on the cross neutralization of the produced sera. Therefore, the inclusion of one species from each group is important to generate a genus-wide neutralizing serum, efficient for treatment of envenomation by Agkistrodon species from both Mexico and the U.S.
Acknowledgements
The authors thank Fernando González, from “Reptiles Fergo”, Mónica Salmerón from the herpetarium at Facultad de Ciencias UNAM and Fernando del Valle and Gabriel del Valle from “DeVal Animal”, for allowing the venom extraction of snakes under their care. The authors also thank Timoteo Olamendi Portugal, Alejandro Olvera Rodríguez, Felipe Olvera Rodríguez, Ricardo Mondragón and Angélica Linares, for diverse technical assistance on experiments performed during this work.
We thank the team at ranch “Ojo de Agua” in Puebla, Mexico: Dr. Andrés Baltazar Alagón Cano, Jesús Pulido Hernández, Jesús Adalberto Pulido Carballo, Irma Yoana Pulido Carballo, Moisés Pelcastre Peñafiel, Julián Hernández Villegas, Emilio Márquez Diego and Yolanda González, for their work during hyperimmunization as well as the caring and handling of horses.
We also thank Dr. Leslie Boyer for her kind and thorough revision of the present manuscript.
Finally, we thank Paulina Bénard, Eduardo Berrón, Jaime Orea and Aline Bénard for their help on the preparation of figures and the distribution map on Fig. 1, as well as English language revision throughout the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2019.100013.
Funding
This work was supported by grants from Dirección General de Asuntos del Personal Académico (DGAPA-PAPIIT) [proyect numbers IN205214 and IN207218] and Consejo Nacional de Ciencia y Tecnología (CONACyT) [proyect numbers CB-2013-01 221343] granted to AA.
LRD, MBV, ENC and BGO are scholarship recipients from CONACyT. LRD was also awarded an undergraduate scholarship from Instituto de Biotecnología, UNAM (IBt, UNAM). HVL was supported by a postdoctoral scholarship from CONACyT. IA was supported by a scholarship from Veteria Labs S.A. de C.V.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
Conceived and designed the experiments: ENC MBV AA LRD. Performed the experiments: LRD ENC MBV BGO IA HVL. Analyzed the data: LRD MBV ENC AA. Contributed reagents/materials/analysis tools: AA JOM VP. Wrote and revised the paper: MBV LRD AA ENC.
Ethical statement
All animal experiments were performed under the procedures and with the approval of the Institutional Bioethics Committee of the Biotechnology Institute of the National Autonomous University of Mexico (IBt-UNAM) project number 254 (Caracterización funcional del veneno de Agkistrodon, así como la respuesta inmune en caballos contra los mismos).
ICR mice used in all experiments were obtained from the laboratory animal facility of IBt-UNAM. Animal facility staff as well as research staff is trained in the correct and humane handling of mice before the start of any procedure.
The use of human blood from a single healthy donor was also approved by the Institutional Bioethics Committee of IBt-UNAM, as a part of project 254. All residues that had been in contact with the blood were discarded in accordance to Institutional regulations.
Some individuals from the species A. bilineatus were collected under license number SGPA/DGVS/03459/15 and kept for successive venom extractions at the vivarium “Cantil: Herpetario del IBt” with registration number MOR-IN-166-07-04. All housing and handling procedures have been revised and approved by Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Mexico in strict accordance with the regulations of the National General Law of Wildlife (Congreso, 2018).
Horse housing and handling protocols were in strict accordance with Mexican and international animal welfare regulations and were approved by SAGARPA (Secretaría de Desarrollo Agrícola y Desarrollo Rural, Pesca y Alimentación; SENASICA –Dirección General de Salud Animal-) with permit number: DU00411. All handling of horses is performed by previously trained staff from ranch Ojo de Agua in Puebla, Mexico and submitted to monthly medical check by an in-house specialized veterinarian.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Angulo Y., Estrada R., Gutiérrez J.M. Clinical and laboratory alterations in horses during immunization with snake venoms for the production of polyvalent (Crotalinae) antivenom. Toxicon. 1997;35:81–90. doi: 10.1016/S0041-0101(96)00077-3. [DOI] [PubMed] [Google Scholar]
- Arce V., Rojas E., Ownby C.L., Rojas G., Gutiérrez J.M. Preclinical assessment of the ability of polyvalent (Crotalinae) and anticoral (Elapidae) antivenoms produced in Costa Rica to neutralize the venoms of North American snakes. Toxicon. 2003;41:851–860. doi: 10.1016/S0041-0101(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Borja M., Castañeda G., Espinosa J., Neri E., Carbajal A., Clement H., García O., Alagon A. Mojave rattlesnake (Crotalus scutulatus scutulatus) with type B venom from Mexico. Copeia. 2014;2014:7–13. doi: 10.1643/OT-12-041. [DOI] [Google Scholar]
- Borja M., Lazcano D., Martínez-Romero G., Morlett J., Sánchez E., Cepeda-Nieto A.C., Garza-García Y., Zugasti-Cruz A. Intra-specific variation in the protein composition and proteolytic activity of venom of Crotalus lepidus morulus from the Northeast of Mexico. Copeia. 2013;2013:702–716. doi: 10.1643/OT-13-005. [DOI] [Google Scholar]
- Borja M., Neri-Castro E., Castañeda-Gaytán G., Strickland J.L., Parkinson C.L., Castañeda-Gaytán J., Ponce-López R., Lomonte B., Olvera-Rodríguez A., Alagón A., Pérez-Morales R. Biological and proteolytic variation in the venom of crotalus scutulatus scutulatus from Mexico. Toxins. 2018;10:1–19. doi: 10.3390/toxins10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbrink F.T., Guiher T.J. Considering gene flow when using coalescent methods to delimit lineages of North American pitvipers of the genus Agkistrodon. Zool. J. Linn. Soc. 2015;173:505–526. doi: 10.1515/amsc-2016-0014. [DOI] [Google Scholar]
- Calderón Corona A. Universidad Nacional Autónoma de México; 2011. Caracterización de la respuesta inmune humoral en caballos contra el veneno de Micrurus tener. [Google Scholar]
- Calvete J.J., Sanz L., Angulo Y., Lomonte B., Gutiérrez J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009;583:1736–1743. doi: 10.1016/j.febslet.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Campbell J.A., Lamar W.W. Cornell University Press; Ithaca, New York, USA: 2004. The Venomous Reptiles of the Western Hemisphere. [Google Scholar]
- Casasola A., Ramos-Cerrillo B., de Roodt A.R., Carbajal Saucedo A., Chippaux J.P., Alagón A., Stock R.P. Paraspecific neutralization of the venom of African species of cobra by an equine antiserum against Naja melanoleuca: a comparative study. Toxicon. 2009;53:602–608. doi: 10.1016/j.toxicon.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Castro E.N., Lomonte B., del Carmen Gutiérrez M., Alagón A., Gutiérrez J.M. Intraspecies variation in the venom of the rattlesnake Crotalus simus from Mexico: different expression of crotoxin results in highly variable toxicity in the venoms of three subspecies. J. Proteomics. 2013;87:103–121. doi: 10.1016/j.jprot.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Chen Y.-H., Wang Y.-M., Hseu M.-J., Tsai I.-H. Molecular evolution and structure–function relationships of crotoxin-like and asparagine-6-containing phospholipases A 2 in pit viper venoms. Biochem. J. 2004;381:25–34. doi: 10.1042/BJ20040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.-P. Incidence and mortality due to snakebite in the Americas. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congreso . 2018. Ley General de Vida Silvestre. [Google Scholar]
- Dart R.C., Gomez H.F. Reptile bites and scorpion stings. In: Tintinalli M.S., Ruiz E., Krome R.L., editors. Emergency Medicine: A Comprehensive Study Guide. American College of Emergency Physicians. McGraw-Hill; New York: 1996. pp. 864–867. [Google Scholar]
- de Freitas Oliveira C., da Silva Lopes D., Mendes M.M., Homsi-Brandeburgo M.I., Hamaguchi A., de Alcântara T.M., Clissa P.B., de Melo Rodrigues V. Insights of local tissue damage and regeneration induced by BnSP-7, a myotoxin isolated from Bothrops (neuwiedi) pauloensis snake venom. Toxicon. 2009;53:560–569. doi: 10.1016/j.toxicon.2008.12.025. [DOI] [PubMed] [Google Scholar]
- de Roodt A.R., Estévez-Ramírez J., Paniagua-Solís J.F., Litwin S., Carvajal-Saucedo A., Dolab J.A., Robles-Ortiz L.E., Alagón A., Originales A., Cano A.A. 2005. Toxicidad de venenos de serpientes de importancia médica en México. [PubMed] [Google Scholar]
- de Roodt A.R., Lanari L.C., Costa De Oliveira V., Laskowicz R.D., Stock R.P. Neutralization of Bothrops alternatus regional venom pools and individual venoms by antivenom: a systematic comparison. Toxicon. 2011;57:1073–1080. doi: 10.1016/j.toxicon.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Durban J., Sanz L., Trevisan-Silva D., Neri-Castro E., Alagón A., Calvete J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult Mexican rattlesnakes Crotalus simus, C. Tzabcan, and C. Culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017;16:3370–3390. doi: 10.1021/acs.jproteome.7b00414. [DOI] [PubMed] [Google Scholar]
- Estrada R., Gutiérrez J.M., Alvarado J., Robles A., Avila C., Picado I.C., Microbiología F., Rica De, U.D.C., José S., Rica C., Picado C. Desarrollo de la respuesta de anticuerpos anti-fosfolipasa A , en caballos inoculados con veneno para la producción de suero antiofídico polivalente en Costa Rica El suero antiofídico polivalente se produce en Costa Rica desde 1967 para el lraU ! miem o d. Rev. Biol. Trop. 1989;37:187–191. [PubMed] [Google Scholar]
- European Parliament Directive 2010/63/EU - on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010;276:33–79. doi: 10.1111/32010L0063. [DOI] [Google Scholar]
- Fernández J., Caccin P., Koster G., Lomonte B., Gutiérrez J.M., Montecucco C., Postle A.D. Muscle phospholipid hydrolysis by Bothrops asper Asp49 and Lys49 phospholipase A 2 myotoxins - distinct mechanisms of action. FEBS J. 2013;280:3878–3886. doi: 10.1111/febs.12386. [DOI] [PubMed] [Google Scholar]
- Fox J.W., Serrano S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–985. doi: 10.1016/j.toxicon.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Glenn J.L., Straight R.C., Wolfe M.C., Hardy D.L. 1982. Geographical Variation in Crotalus Scutulatus Scutulatus (Mojave Rattlesnake) Venom Properties. [DOI] [PubMed] [Google Scholar]
- Glenn J.L., Straight R.C., Wolt T.B. Regional variation in the presence of canebrake toxin in Crotalus horridus venom. Comp. Biochem. Physiol. Part C Pharmacol. 1994;107:337–346. doi: 10.1016/1367-8280(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Comprendiendo los venenos de serpientes: 50 anos de investigaciones en America Latina. Rev. Biol. Trop. 2002;50:377–394. [PubMed] [Google Scholar]
- Gutiérrez J.M., Alberto Ponce-Soto L., Marangoni S., Lomonte B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: comparison between crotoxin, crotoxin B and a Lys49 PLA2homologue. Toxicon. 2008;51:80–92. doi: 10.1016/j.toxicon.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Gené A., Rodas G., Cerdas L., Picado I.C., Microbiologia F., Rica De, U.D.C., José S., Rica C., Rica I.N.C. 1985. Neutralization of Proteolytic and Hemorragic Activities of Costa Rican Snake Venoms by a Polyvalent Antivenom. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Lomonte B. Phospholipases A2: unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon. 2013;62:27–39. doi: 10.1016/J.TOXICON.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Rojas G., Lomonte B., Genb J.A., Cerdas L. Comparative study of the edema-forming activity of Costa Rican snake venom and its neutralization by a polyvalent antivenom. Biochem. Physiol. 1986 doi: 10.1016/0742-8413(86)90069-1. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Sanz L., Flores-Díaz M., Figueroa L., Madrigal M., Herrera M., Villalta M., León G., Estrada R., Borges A., Alape-Girón A., Calvete J.J. Impact of regional variation in Bothrops asper snake venom on the design of antivenoms: integrating antivenomics and neutralization approaches. J. Proteome Res. 2010;9:564–577. doi: 10.1021/pr9009518. [DOI] [PubMed] [Google Scholar]
- Hati R., Mitra P., Sarker S., Bhattacharyya K.K. Snake venom hemorrhagins. Crit. Rev. Toxicol. 1999;29:1–19. doi: 10.1080/10408449991349168. [DOI] [PubMed] [Google Scholar]
- Imai K., Nikai T., Sugihara H., Ownby C.L. Hemorrhagic toxin from the venom of Agkistrodon bilineatus (Common cantil) Int. J. Biochem. 1989;21:667–673. doi: 10.1016/0020-711X(89)90388-1. [DOI] [PubMed] [Google Scholar]
- Kini R.M. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Komori Y., Nikai T., Ohara A., Yagihashi S., Sugihara H. Effect of Bilineobin, a thrombin-like proteinase from the venom of common cantil (Agkistrodon bilineatus) Toxicon. 1993;31:257–270. doi: 10.1016/0041-0101(93)90144-8. [DOI] [PubMed] [Google Scholar]
- Kuniko I., Toshiaki N., Hisayoshi S., Ownby C.L. Hemorrhagic toxin from the venom of Agkistrodon bilineatus (Common cantil) Int. J. Biochem. 1989;21:667–673. doi: 10.1016/0020-711X(89)90388-1. [DOI] [PubMed] [Google Scholar]
- Lagesse L.A., Ford N.B. Ontogenetic variation in the diet of the southern copperhead, Agkistrodon contortrix, in northeastern Texas. Tex. J. Sci. 1996;48:48–54. doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- Li H., Huang Y., Wu X., Wu T., Cao Y., Wang Q., Qiu Y., Fu W., Zhang Q., Pang J. Effects of hemocoagulase agkistrodon on the coagulation factors and its procoagulant activities. Drug Des. Dev. Ther. 2018;12:1385–1398. doi: 10.2147/DDDT.S159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte B., Angulo Y., Calderón L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42:885–901. doi: 10.1016/j.toxicon.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Lomonte B., Rangel J. Snake venom Lys49 myotoxins: from phospholipases A 2 to non-enzymatic membrane disruptors. Toxicon. 2012;60:520–530. doi: 10.1016/j.toxicon.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Lomonte B., Tsai W.C., Ureña-Diaz J.M., Sanz L., Mora-Obando D., Sánchez E.E., Fry B.G., Gutiérrez J.M., Gibbs H.L., Sovic M.G., Calvete J.J. Venomics of new world pit vipers: genus-wide comparisons of venom proteomes across agkistrodon. J. Proteomics. 2014;96:103–116. doi: 10.1016/j.jprot.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackessy S., Leroy J., Mociño-Deloya E., Setser K., Bryson R., Saviola A. Venom ontogeny in the Mexican lance-headed rattlesnake (Crotalus polystictus) Toxins. 2018;10:271. doi: 10.3390/toxins10070271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackessy S.P. CRC Press. Taylor & Francis Group; Boca Raton, FL: 2009. Handbook of Venoms and Toxins of Reptiles. [Google Scholar]
- Mazer-Amirshahi M., Boutsikaris A., Clancy C. Elevated compartment pressures from copperhead envenomation successfully treated with antivenin. J. Emerg. Med. 2014 doi: 10.1016/j.jemermed.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Munawar A., Zahid A., Negm A., Akrem A., Spencer P., Betzel C. 2016. Isolation and Characterization of Bradykinin Potentiating Peptides from Agkistrodon Bilineatus Venom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagaki T., Latif Kazim A., Kisiel W. Isolation and characterization of a protein C activator from tropical moccasin venom. Thromb. Res. 1990 doi: 10.1016/0049-3848(90)90305-V. [DOI] [PubMed] [Google Scholar]
- Neri-Castro E., Hernández-Dávila A., Olvera-Rodríguez A., Cardoso-Torres H., Bénard-Valle M., Bastiaans E., López-Gutierrez O., Alagón A. 2019. Detection and Quantification of a β-neurotoxin (Crotoxin Homologs) in the Venom of the Rattlesnakes Crotalus Simus, C. Culminatus and C. Tzabcan from Mexico. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikai T., Taniguchi K., Komori Y., Masuda K., Fox J.W., Sugihara H. Primary structure and functional characterization of bilitoxin-1, a novel dimeric P-II snake venom metalloproteinase from Agkistrodon bilineatus venom. Arch. Biochem. Biophys. 2000 doi: 10.1006/abbi.2000.1795. [DOI] [PubMed] [Google Scholar]
- Owen J.A., Punt J., Stranford S., Jones P.P. McGraw-Hill Interamericana Editores; México, D.F: 2014. KUBY. Inmunología, 7e. [Google Scholar]
- Ownby C.L., Nika T., Imai K., Sugihara H. Pathogenesis of hemorrhage induced by bilitoxin, a hemorrhagic toxin isolated from the venom of the common cantil (Agkistrodon bilineatus bilineatus) Toxicon. 1990;28:837–846. doi: 10.1016/S0041-0101(09)80006-8. [DOI] [PubMed] [Google Scholar]
- Porras L.W., Wilson L.D., Schuett G.W., Reiserer R.S. A taxonomic reevaluation and conservation assessment of the common cantil, Agkistrodon bilineatus (Squamata: Viperidae): a race against time. Amphib. Reptile Conserv. 2013;7:48–73. [Google Scholar]
- Ramos O.H.P., Selistre-de-Araujo H.S. Snake venom metalloproteases--structure and function of catalytic and disintegrin domains. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;142:328–346. doi: 10.1016/j.cbpc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Rivas E., Neri-Castro E., Bénard-Valle M., Hernánez-Dávila A.I., Zamudio F., Alagón A. General characterization of the venoms from two species of rattlesnakes and an intergrade population (C. lepidus x aquilus) from Aguascalientes and Zacatecas, Mexico. Toxicon. 2017;138:191–195. doi: 10.1016/j.toxicon.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Rucavado A., Escalante T., Teixeira C.F.P., Fernándes C.M., Díaz C., Gutiérrez J.M. Increments in cytokines and matrix metalloproteinases in skeletal muscle after injection of tissue-damaging toxins from the venom of the snake Bothrops asper. Mediat. Inflamm. 2002;11:121–128. doi: 10.1080/09629350220131980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucavado A., Lomonte B., Ovadia M., Gutiérrez J.M. Local tissue damage induced by BaP1, a metalloproteinase isolated from Bothrops asper (Terciopelo) snake venom. Exp. Mol. Pathol. 1995;63:186–199. doi: 10.1006/exmp.1995.1042. [DOI] [PubMed] [Google Scholar]
- Saldarriaga M.M., Otero R., Núñez V., Toro M.F., Díaz A., Gutiérrez J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon. 2003;42:405–411. doi: 10.1016/S0041-0101(03)00171-5. [DOI] [PubMed] [Google Scholar]
- Scharman E.J., Noffsinger V.D. Copperhead snakebites: clinical severity of local effects. Ann. Emerg. Med. 2001;38:55–61. doi: 10.1067/mem.2001.116148. [DOI] [PubMed] [Google Scholar]
- Selistre De Araujo E. Handbook of Proteolytic Enzymes. 3° Ed. Elsevier Ltd; 2013. Venom metalloproteases of Agkistrodon contortrix laticinctus; pp. 977–980. [DOI] [Google Scholar]
- Serrano S.M.T. The long road of research on snake venom serine proteinases. Toxicon. 2013;62:19–26. doi: 10.1016/j.toxicon.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Shiloah J., Klibansky C., de Vries A., Berger A. Phospholipase B activity of a purified phospholipase A from Vipera palestinae venom. J. Lipid Res. 1973;14:267–278. [PubMed] [Google Scholar]
- Tasoulis T., Isbister G.K. A Review and Database of Snake Venom Proteomes. Toxins. 2017;vol. 9 doi: 10.3390/toxins9090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston R.D.G., Reid H.A. vol. 61. 1983. pp. 949–956. (Development of Simple Standard Assay Procedures for the Characterization of Snake Venoms *). [PMC free article] [PubMed] [Google Scholar]
- Trigo F., Valero G. fourth ed. Universidad Nacional Autónoma de México; México, D.F: 2004. Patología General Veteriaria. [Google Scholar]
- Varios . Universidad de Costa Rica, Facultad de Microbiología, Instituto Clodomiro Picado; 2007. Determinación de actividades tóxicas de venenos de serpientes y su neutralización por antivenenos. Manual de métodos de laboratorio. [Google Scholar]
- Walker J.P., Morrison R.L. Current management of copperhead snakebite. J. Am. Coll. Surg. 2011 doi: 10.1016/j.jamcollsurg.2010.12.049. [DOI] [PubMed] [Google Scholar]
- Ward R.J., Chioato L., De Oliveira A.H.C., Ruller R., Sa J.M. Active-site mutagenesis of a Lys 49-phospholipase A 2 : biological and membrane-disrupting activities in the absence of catalysis. Biochem. J. 2002 doi: 10.1042/0264-6021:3620089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J.A., Glenn J.L., Straight R.C., Sites J.W. 2018. Distribution and Genetic Variation in Venom A and B Populations of the Mojave Rattlesnake (Crotalus Scutulatus Scutulatus in Arizona Published By : Allen Press on Behalf of the Herpetologists ’ League Stable URL ; pp. 54–68.https://www.jstor.org/stable/3892815 REF 47 [Google Scholar]
- Yang Z.M., Guo Q., Ma Z.R., Chen Y., Wang Z.Z., Wang X.M., Wang Y.M., Tsai I.H. Structures and functions of crotoxin-like heterodimers and acidic phospholipases A2from Gloydius intermedius venom: insights into the origin of neurotoxic-type rattlesnakes. J. Proteomics. 2015;112:210–223. doi: 10.1016/j.jprot.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Yukelson L.Y., Tans G., Thomassen M.C.L.G.D., Hemker H.C., Rosing J. Procoagulant activities in venoms from central Asian snakes. Toxicon. 1991;29:491–502. doi: 10.1016/0041-0101(91)90023-K. [DOI] [PubMed] [Google Scholar]
- Zancolli A.G., Calvete J.J., Cardwell M.D., Greene H.W., Hayes K., Hegarty M.J., Herrmann H., Holycross A.T., Dominic I., Mulley J.F., Sanz L., Travis Z.D., Whorley J.R., Catharine E., Wüster W. When one phenotype is not enough-divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. bioRxiv. 2018:1–21. doi: 10.1101/413831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelanis A., Tashima A.K., Rocha M.M.T., Furtado M.F., Camargo A.C.M., Ho P.L., Serrano S.M.T. Analysis of the ontogenetic variation in the venom proteome/peptidome of Bothrops jararaca reveals different strategies to deal with prey. J. Proteome Res. 2010;9:2278–2291. doi: 10.1021/pr901027r. [DOI] [PubMed] [Google Scholar]
- Zuliani J.P., Fernandes C.M., Zamuner S.R., Gutiérrez J.M., Teixeira C.F.P. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A 2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45:335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Uetz, P., Freed, P., Hošek, J., (eds.), 2019. The Reptile Database, URL: http://www.reptile-database.org (accessed 3.31.19).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.