Abstract
Circulating tumour cells (CTCs) have potential utility in various clinical applications for cancer management. The present study focused on evaluating the diagnostic role of CTCs in colorectal cancer (CRC). A total of 89 blood samples from 59 patients diagnosed with CRC and 30 healthy individuals were collected for CTC detection. The Cyttel method is an improved CTC detection strategy, which combines negative enrichment with immunofluorescence and fluorescence in situ hybridization. This method effectively detected a significant increase in total CTCs in patients with CRC (49/59) compared with those in healthy controls (3/30). A cut-off value of 2 CTCs/3.2 ml blood yielded a sensitivity of 83.05% and a specificity of 100%. Additionally, three traditional serum tumour markers, namely carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9) and CA72-4, were examined by immunoassays. The diagnostic sensitivity of CTCs was much higher than that of CEA, CA19-9 and CA72-4 alone or in combination, particularly in patients with early stage CRC. The combined sensitivity of CTCs and CEA reached 91.53%, which was only slightly lower than the sensitivity of all four markers combined (CTCs + CEA + CA19-9 + CA72-4). CTCs with aneuploidy of chromosome 7 or 8 were carefully distinguished, and the associations among different types of CTCs, clinicopathological characteristics and overall survival were statistically analysed. Total CTCs were revealed to be significantly associated with tumour differentiation and nerve invasion. CTCs were more likely to be detected in poorly differentiated CRC tumours than in well- and moderately-differentiated tumours (P=0.026). Furthermore, to the best of our knowledge, the present study was the first to report that CTCs with multiploidy of chromosome 7 were significantly associated with TNM stage. These CTCs exhibited a high chance of being identified in the peripheral blood of patients with late-stage CRC (stage III–IV; P=0.031). The present study suggests that the combination of CTCs and CEA may serve as an effective potential diagnostic and prognostic indicator in patients with CRC. Detection of CTCs with aneuploidy may have increased specificity in predicting highly malignant and invasive tumours in CRC management.
Keywords: circulating tumour cells, colorectal cancer, Cyttel method, aneuploidy, tumour markers
Introduction
Colorectal cancer (CRC) is the third most common type of cancer in both men and women, and it is the fourth most common cause of cancer-associated mortality in the United States (1). In 2018, Siegel (2) reported that clinical outcomes of American patients with CRC vary depending on the stage of cancer at diagnosis, with a 5-year survival rate of ~90% for localized disease, 71% for regional disease and only 14% for distantly metastatic CRC (mCRC). Therefore, detecting CRC at an early stage is crucial to reduce the cancer-specific mortality rate. Currently, diagnostic imaging examinations, including CT and MRI, have the ability to depict the size and location of CRC, but have limited potential in detecting small primary tumours and metastatic lesions (3). Colonoscopy and biopsy are considered the gold standard for confirming the diagnosis of CRC. However, biopsy specimens may fail to provide a definitive diagnosis if the viable tumour tissue is difficult to obtain or if the tissue samples are extensively ulcerated or necrotic (4). Additionally, several serum tumour markers, including carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9) and cancer antigen 72-4 (CA72-4), are widely used for the screening, diagnosis and postoperative surveillance of gastrointestinal cancer, but have insufficient sensitivity and specificity (5). In addition, patients with CRC diagnosed in the early stages often receive surgery to remove the original tumour and the nearby lymph nodes, yet 20–30% of these patients suffer from recurrence or metastasis within 5 years of radical resection, indicating the presence of minimal residual disease (6) Consequently, developing a less invasive and reliable method for the early diagnosis and dynamic management of CRC is urgently required.
Circulating tumour cells (CTCs) are tumour cells that are shed from the primary tumour and metastatic foci, and enter the peripheral bloodstream (7); they can be classified into signal CTCs and CTC clusters (8). Unlike the conventional theory that the metastatic dissemination of cancer cells represents the final stage of a deteriorating process, CTCs often disseminate at the beginning during the process of tumorigenesis, with some of them invading distant organs and eventually developing into overt metastatic lesions (9). Therefore, the detection of CTCs in the circulation may represent a feasible way to improve the early diagnosis and treatment of patients with CRC prior to metastasis. Clinical studies have suggested that CTCs are significantly associated with poor progression-free survival (PFS) and overall survival (OS) time in patients with CRC. For example, Bork et al (10) revealed that >1 CTC per 7.5 ml blood in the blood was significantly associated with worse OS time (38.4 months vs. 49.8 months; P<0.001) in non-metastatic patients with CRC (UICC I–III), as well as in the complete cohort (33.6 months vs. 48.4 months; P<0.001), compared with non-detected group. Furthermore, Cohen et al (11) detected CTCs from 7.5 ml blood of 430 patients with metastatic CRC. It suggested that patients with >3 CTCs had shorter PFS time (4.4 months vs. 7.8 months, P=0.004) and OS time (9.4 months vs. 20.6 months, P<0.0001) compared with those whose CTCs was <3.
Since there may only be 1 CTC in 1×107 leukocytes per ml of blood, it is challenging to isolate CTCs from peripheral blood. The principles of CTC isolation involve CTC enrichment followed by detection. The former is achieved by means of physical properties of the cells, such as size, density or specific biological features, whereas the latter is commonly achieved by immunostaining and microscopy, or by PCR-based methods (12). The most frequently used CTC detection technology reported in these studies is the CellSearch® system (Janssen Diagnostics). This system enriches CTCs using ferromagnetic beads coated with antibodies that target epithelial cell adhesion molecule (EpCAM), and defines CTCs according to their morphological characteristics, positive expression of cytokeratin and absence of CD45 (also known as leukocyte common antigen). However, certain CTCs may lose epithelial cell markers during the process of epithelial-to-mesenchymal transition, resulting in a reduced positive rate and accuracy of the CellSearch® system (13). The advantage of the Cyttel method (14) is that the enrichment of CTCs does not rely on the expression of EpCAM, and the enriched cells can be used for subsequent experiments, including cell culture and other tests. The Cyttel method involves a leukocyte depletion mechanism. After collecting a peripheral blood sample, erythrocytes can be removed by hypotonic haemolysis. Since all leucocytes express CD45, these can be removed using anti-CD45 antibody-conjugated magnetic beads.
Abnormal chromosome numbers (aneuploidy) are invariably found in the pleomorphic cells of malignant tumours and have been recognized as a common feature of cancer. This type of somatic copy number alteration has been proposed to drive tumourigenesis (15). Aneuploidy of chromosomes 7 and 8 is commonly observed in patients with CRC, with a high frequency of numerical abnormalities of the entire chromosome 7, as well as loss, gain or amplification of specific regions of chromosome 8 in primary CRCs with associated metastases (16). Detecting aneuploidy in peripheral blood cells may represent a novel approach for CTC detection, and assessing aneuploidy of chromosomes 7 and 8 at diagnosis may be of great clinical significance in patients with CRC. Therefore, the Cyttel method uses immunofluorescence and fluorescence in situ hybridization (imFISH) on the remaining cells, using DNA probes for chromosome 7 (CEP7), chromosome 8 (CEP8) and human CD45. Only cells that are CD45-negative and that emit signals (>2) of CEP7 or CEP8 are recognized as CTCs. The Cyttel method may allow the preservation of the rare karyocytes in the peripheral blood. By combining the detection of multiple molecular markers and abnormal chromosome alterations in cancer cells, the detection rate and accuracy of diagnostic tests may be improved.
In the present study, a total of 89 blood samples from 59 patients diagnosed with CRC and 30 healthy individuals were collected for single CTC identification using the Cyttel method. Subsequently, the diagnostic sensitivities of CTCs and serum tumour markers (CEA, CA19-9 and CA72-4) were compared to assess the efficacy of CTCs in detecting CRC. Furthermore, the associations between total and aneuploid CTCs and the clinicopathological characteristics of patients with CRC were explored.
Materials and methods
Subjects and blood sample collection
A total of 59 patients with newly diagnosed CRC were enrolled in the present study at Beijing Shijitan Hospital (Beijing, China) between July 2016 and August 2017. These patients had no history of any other malignancies during the previous 5 years. Blood samples were collected at the diagnosis of CRC. Patients received standard surgical resection of tumours and other adjuvant therapy according to the National Comprehensive Cancer Network (NCCN) guidelines for CRC (17). Patients with stage IV CRC also received surgery due to bowel obstruction or intestinal haemorrhage. The 59 patients with CRC were followed up until December 2018 (primary endpoint). The medical records of these patients were carefully reviewed to obtain clinicopathological data and follow-up information. A total of 30 healthy donors, including 17 males and 13 females with an average age of 62.7 years (range, 44–84 years), were included as controls. None of the controls had tumours, a family history of cancer or other systemic diseases. Written informed consent was obtained from all subjects. The present study was approved by the Ethics Review Committee of Beijing Shijitan Hospital.
Peripheral blood (3.2 ml) was collected into acid citrate dextrose (ACD) anticoagulant tubes (Becton, Dickinson and Company) and stored at 4°C. The blood sample had to be processed for CTC detection within 24 h. To avoid bias, CTC detection and clinicopathological data collection were blindly and independently performed by different researchers.
CTC detection using the Cyttel method
The collected blood (4 ml, including 0.8 ml of ACD) was added with PBS to 45 ml in a centrifuge tube and then centrifuged at 600 × g for 5 min at 4°C to separate cells from plasma. The supernatant was discarded, and the remaining cells were resuspended in 45 ml erythrocyte lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.). The centrifuge tube was placed in a vertical mixer to ensure the red blood cells would be fully lysed by hypotonic haemolysis, at room temperature. The aforementioned centrifugation process was repeated, and the supernatant was discarded. Residual cell particles were resuspended in 300 µl PBS and incubated with 20 µl anti-CD45 antibody-conjugated magnetic beads (Catalog no. 11153D, Thermo Fisher Scientific, Inc.) for 30 min at room temperature, with gentle tilting and rotation. The suspension was subsequently added to 3 ml isolation buffer (Thermo Fisher Scientific, Inc.), prior to centrifugation at 300 × g for 5 min at 4°C, and the supernatant was subsequently discarded. The remaining suspension was added with 100 µl PBS and placed in a magnetic stand (Promega Corporation) for 2 min to separate the magnetic beads. Following centrifugation at 2,070 × g for 3 min at 4°C, the supernatant was discarded. The 100 µl suspension was mixed with 100 µl fixative solution (Thermo Fisher Scientific, Inc.). The solution containing CTCs was transferred onto superfrost plus slides (Thermo Fisher Scientific, Inc.) and dried overnight at 33°C, in the oven. The prepared slides were immersed in saline-sodium citrate (SSC) buffer (Beijing Solarbio Science & Technology Co., Ltd.). at 37°C for 15 min, and then dehydrated by increasing concentrations of ethanol (75, 85 and 100%) for 3 min each. A total of 10 µl hybridization solution containing centromere DNA probes of chromosome 7 (green) and 8 (orange) (Abbott Pharmaceutical Co. Ltd.) was added to the slides, which were mounted and incubated in a StatSpin ThermoBrite hybridization oven (Abbott Pharmaceutical Co. Ltd.) at 37°C for 90 min. Following hybridization, the slides were put in the working fluid of formamide at 43°C for 15 min to wash the probes, and also washed with 2× SSC twice. The Alexa Fluor 594-conjugated anti-human CD45 antibody (1:100; cat. no. FAB1430T; R&D Systems, Inc.) was added to the slides, incubated at 33°C for 1 h in the oven and subsequently removed. The nucleus was stained with 10 µl nuclear dye DAPI (Vector Laboratories, Inc.; Maravai LifeSciences) at room temperature for 5 min. The sealing slides were automatically scanned and analysed using the Cyttel PathfinderTM system (Cyttel Biosciences Inc.) in the high-power field (10×40).
Measurement of serum tumour markers
Peripheral blood (3 ml) was collected into tubes without anticoagulant and centrifuged at 1,500 × g and 4°C for 10 min. The expression levels of CEA, CA19-9 and CA72-4 in serum samples were determined using an automatic immunoassay analyser (Cobas e601; Roche Diagnostics). The normal reference values for the three serum tumour markers were 0–5 ng/ml for CEA, 0–37.0 U/ml for CA19-9 and 0–6.9 U/ml for CA72-4. Exceeding the upper limit of the normal threshold was considered positive. For the combined detection of two or more tumour markers, an elevated result of any of the tumour markers was considered positive. The diagnostic sensitivity was calculated by dividing the number of positive cases by the total number of CRC cases.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software (v.19.0; IBM Corp.). Data are presented as the mean ± standard deviation. A receiver operating characteristic (ROC) curve was plotted to determine the sensitivity and specificity of CTC detection for diagnosis. Differences between groups were determined using two-tailed unpaired Student's t-test. The χ2 test or Fisher's exact test was performed to explore the associations between total and aneuploid CTCs and patient clinicopathological characteristics. Kaplan-Meier analysis and the log-rank test were employed to evaluate the prognostic significance of CTCs in patient survival. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of CTCs in patients with CRC and in healthy individuals
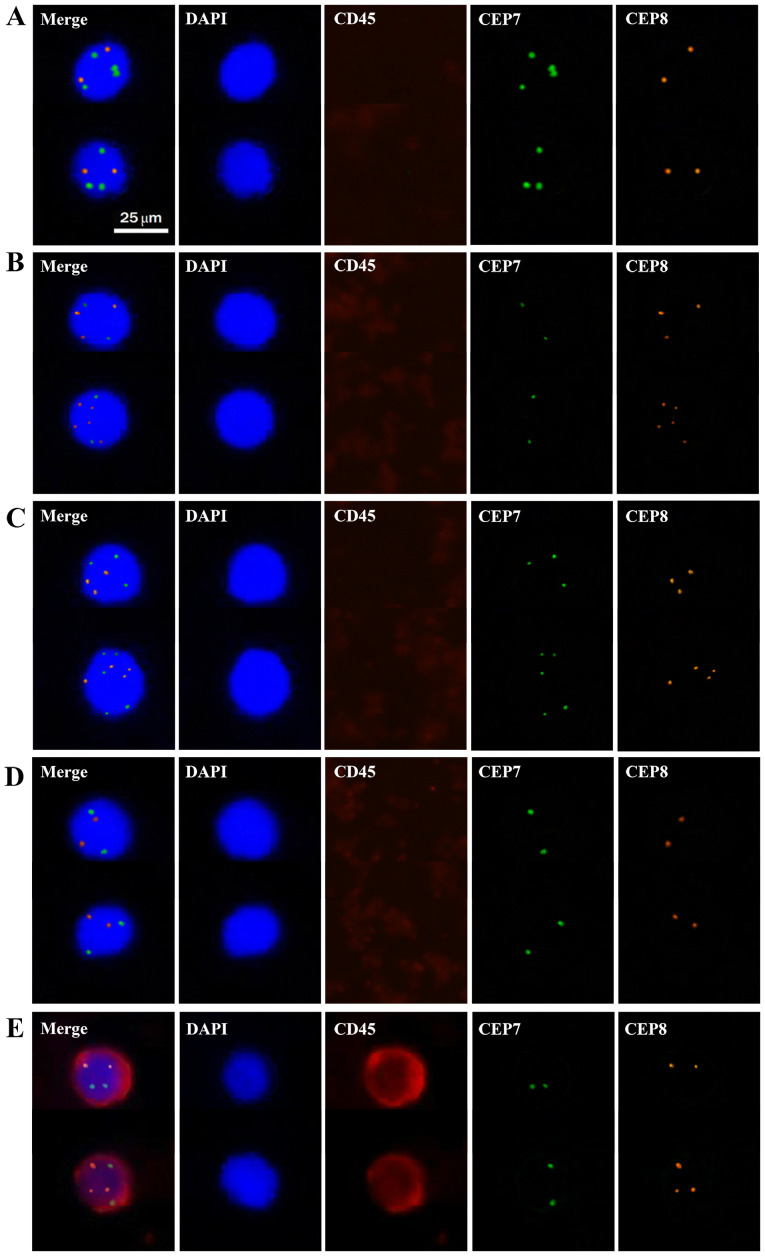
Cells enriched from the peripheral blood of patients with CRC and from healthy individuals were examined for the presence of cell nuclei (DAPI-stained) and chromosome ploidy (CEP7- and/or CEP8-stained). In addition, immunofluorescence staining of CD45 was applied to exclude leukocytes. Based on the staining results of DAPI, CD45, CEP7 and CEP8, cells were divided into five groups: Group A, CD45−, DAPI+ and CEP7>2/CEP8=2 (the number of hybridization signals for CEP7>2 or CEP8=2); Group B, CD45−, DAPI+ and CEP7=2/CEP8>2; Group C, CD45−, DAPI+ and CEP7>2/CEP8>2; Group D, CD45−, DAPI+ and CEP7=2/CEP8=2; and Group E, CD45+, DAPI+ and CEP7≥2/CEP8≥2 (Fig. 1). Cells in groups A-C characterized as nucleated cells with aneuploidy of chromosomes 7 and/or 8 but without CD45 expression were defined as CTCs, whereas cells in groups D and E were defined as indeterminate cells (normal leukocytes).
Figure 1.
Representative images of cells detected in the peripheral blood of patients with colorectal cancer. Cell nuclei stained with DAPI appear blue. Cytokines stained with CD45 antibody appear red. Chromosomes stained with CEP7 appear green. Chromosomes stained with CEP8 appear orange. (A) CD45-, DAPI+ and CEP7>2/CEP8=2; (B) CD45-, DAPI+ and CEP7=2/CEP8>2; (C) CD45-, DAPI+ and CEP7>2/CEP8>2; (D) CD45-, DAPI+ and CEP7=2/CEP8=2; (E) CD45+, DAPI+ and CEP7≥2/CEP8≥2. Cells in groups A-C were defined as CTCs, whereas cells in groups D and E were defined as normal leukocytes. Magnification, ×400. CTC, circulating tumour cell; CEP7, chromosome 7; CEP8, chromosome 8.
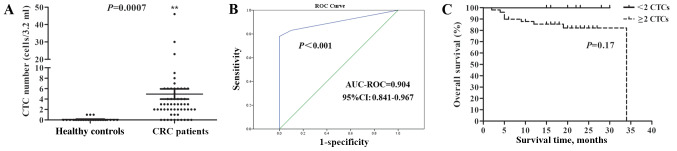
Based on the aforementioned CTC evaluation criteria, CTCs were identified in the peripheral blood of 3/30 (10.00%) healthy individuals, and their CTC count was 1. However, CTCs were identified in 49/59 (83.05%) patients with CRC, with a range of 2–46 cells/3.2 ml, which was significantly higher than in the control group (Fig. 2A). The ROC curve was then plotted to characterize the discriminating power of CTCs for the diagnosis of CRC. According to Youden's index, a cut-off value of 2 cells/3.2 ml yielded a sensitivity of 83.05% and a specificity of 100% (area under the curve, 0.904; 95% CI, 0.841–0.967; Fig. 2B). Although there was no significant association observed between CTC increase and overall survival according to the Kaplan-Meier analysis (Fig. 2C), there was a trend showing that patients with CRC with <2 CTCs may have an improved prognosis compared with those with ≥2 CTCs, since all 10 patients with <2 CTCs were alive at the primary endpoint. The association between CTC numbers and TNM staging, and between CTCs with multiploidy of chromosomes 7 or 8 and prognosis in patients with CRC were also investigated, but there were no significant differences identified (data not shown).
Figure 2.
Detection of CTCs in patients with CRC and healthy individuals. (A) Comparison of CTC numbers between healthy individuals and patients with CRC. (B) ROC curves for the ability of CTCs to discriminate between patients with CRC and healthy individuals. (C) Kaplan-Meier survival curves showing overall survival of patients with CRC based on the number of CTCs. CTC, circulating tumour cell; CRC, colorectal cancer; ROC, receiver operating characteristic; AUC, area under the curve.
Diagnostic sensitivity of CTCs and serum biomarkers in the diagnosis of CRC
Several serum tumour markers, such as CEA, CA19-9 and CA72-4, have been suggested as candidate biomarkers for diagnosis, prognosis, monitoring recurrence and guiding treatment in patients with CRC (4). Therefore, the present study aimed to compare the sensitivities of CTCs and tumour markers to diagnose CRC. The diagnostic sensitivity was used to represent the rate of correctly classified positive cases. Among the three individual serum tumour markers, CEA had the highest diagnostic sensitivity (50.85%) in the 59 patients with CRC, followed by CA72-4 (28.81%) and CA19-9 (23.73%; Table I). Combinations of any two or all three of the serum tumour markers produced sensitivities that were markedly improved (range, 40.68–64.41%) compared with those of the single tumour markers. In addition, the diagnostic sensitivity of CTCs was 83.05%, which was notably higher than that of each traditional serum tumour marker and of the combinations. The sensitivity reached 93.22% when combining CTCs and all three biomarkers. Interestingly, the sensitivity of CTCs + CEA (91.53%) was only slightly lower than the combined sensitivity of all four markers, indicating that CTCs + CEA may be an effective combination of serum tumour markers for the diagnosis and prognosis of CRC.
Table I.
Diagnostic sensitivity of CTCs and serum tumour markers in patients with colorectal cancer.
| Serum tumour markers | Diagnostic sensitivity (positive/total), % |
|---|---|
| CEA | 50.85 (30/59) |
| CA19-9 | 23.73 (14/59) |
| CA72-4 | 28.81 (17/59) |
| CEA + CA19-9 | 59.32 (35/59) |
| CEA + CA72-4 | 61.02 (36/59) |
| CA19-9 + CA72-4 | 40.68 (24/59) |
| CEA + CA19-9 + CA72-4 | 64.41 (38/59) |
| CTCs | 83.05 (49/59) |
| CTCs + CEA | 91.53 (54/59) |
| CTCs + CA19-9 | 84.75 (50/59) |
| CTCs + CA72-4 | 88.14 (52/59) |
| CTCs + CEA + CA19-9 + CA72-4 | 93.22 (55/59) |
CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; CA72-4, cancer antigen 72-4; CTC, circulating tumour cell.
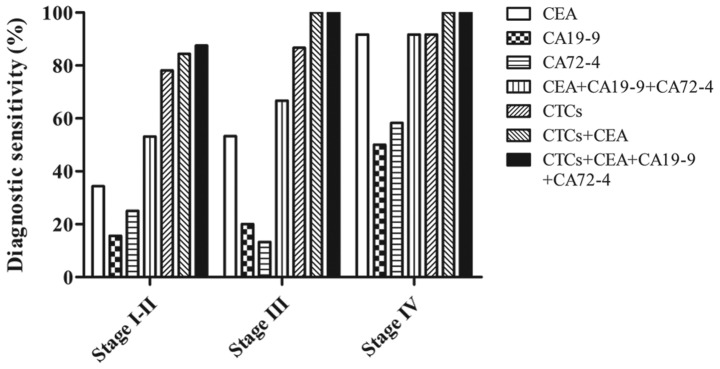
Furthermore, an association between tumour staging and the detection rate of CTCs and tumour markers was observed. As shown in Fig. 3, positive CEA expression (≥5 ng/ml) were detected in 34.38% of stage I–II patients with CRC, 53.33% of stage III patients and 91.67% of stage IV patients, and these rates were higher than those for CA19-9 and CA72-4 at all pathological stages. The combined diagnostic sensitivity of CEA + CA19-9 + CA72-4 was further increased in patients with stage I–II (53.13%), stage III (66.67%) and stage IV (91.67%, same as CEA alone) CRC. Additionally, increased CTC levels (≥2 cells/3.2 ml) were observed in patients with CRC compared with normal individuals, with increased CTC number in 78.13% of patients with stage I–II disease, 86.67% of patients with stage III disease and 91.67% of patients with stage IV disease. This corresponded to the elevated diagnostic sensitivity of different stages of cancer. Furthermore, when combining CTCs and CEA, the detection rate in stage I–II patients reached 84.38%. The increase in the detection sensitivity of CTCs suggested that CTCs may be used as an efficient biomarker in the detection of all stages of CRC, particularly early stage tumours.
Figure 3.
Diagnostic sensitivity of CEA, CA19-9, CA72-4 and CTCs (alone or in combination) in 59 patients with different stages of colorectal cancer. CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; CA72-4, cancer antigen 72-4; CTC, circulating tumour cell.
Association of total or aneuploid CTCs with clinicopathological characteristics of patients with CRC
As shown in Table II, there was a significant difference in CTC status between the well-, moderately and poorly differentiated CRC groups. CTCs were more likely to be detected in poorly differentiated CRC tumours (12/12) than in well- or moderately differentiated tumours. Furthermore, the results suggested that there was a significant association between CTCs and nerve invasion (pathological diagnosis). The detection rate of CTCs could be remarkably increased with cancer progression, such as invasion and metastasis.
Table II.
Association of total or aneuploid CTCs with the clinicopathological characteristics of patients (n=59) with colorectal cancer.
| Total CTCs, n | CTCs with multiploidy of chromosome 7, n | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Number of patients | Negative | Positive | P-value | Negative | Positive | P-value |
| Age, years | 0.396 | 0.157 | |||||
| <60 | 23 | 3 | 20 | 7 | 16 | ||
| ≥60 | 36 | 7 | 29 | 17 | 19 | ||
| Sex | 0.307 | 0.075 | |||||
| Male | 34 | 7 | 27 | 17 | 17 | ||
| Female | 25 | 3 | 22 | 7 | 18 | ||
| Tumour size, cm | 0.604 | 0.533 | |||||
| <5 | 36 | 6 | 30 | 15 | 21 | ||
| ≥5 | 23 | 4 | 19 | 9 | 14 | ||
| Locationa | 0.262 | 0.115 | |||||
| Left-sided | 43 | 6 | 37 | 20 | 23 | ||
| Right-sided | 16 | 4 | 12 | 4 | 12 | ||
| Histological differentiation | 0.026b | 0.163 | |||||
| Well | 9 | 4 | 5 | 4 | 5 | ||
| Moderate | 38 | 6 | 32 | 18 | 20 | ||
| Poor | 12 | 0 | 12 | 2 | 10 | ||
| Primary tumour | 0.894 | 0.841 | |||||
| T1-T2 | 8 | 1 | 7 | 4 | 4 | ||
| T3 | 26 | 5 | 21 | 10 | 16 | ||
| T4 | 25 | 4 | 21 | 10 | 15 | ||
| Lymph node | 0.350 | 0.111 | |||||
| N0 | 35 | 7 | 28 | 17 | 18 | ||
| N1-N2 | 24 | 3 | 21 | 7 | 17 | ||
| Distant metastasis | 0.343 | 0.406 | |||||
| M0 | 47 | 9 | 38 | 20 | 27 | ||
| M1 | 12 | 1 | 11 | 4 | 8 | ||
| TNM staging | 0.229 | 0.031b | |||||
| I–II | 32 | 7 | 25 | 17 | 15 | ||
| III–IV | 27 | 3 | 24 | 7 | 20 | ||
| Venous invasion | 0.293 | 0.311 | |||||
| Absent | 46 | 9 | 37 | 20 | 26 | ||
| Present | 13 | 1 | 12 | 4 | 9 | ||
| Nerve invasion | 0.008b | 0.306 | |||||
| Absent | 45 | 4 | 41 | 17 | 28 | ||
| Present | 14 | 6 | 8 | 7 | 7 | ||
| CEA, ng/ml | 0.612 | 0.438 | |||||
| <5 | 29 | 5 | 24 | 11 | 18 | ||
| ≥5 | 30 | 5 | 25 | 13 | 17 | ||
| CA19-9, U/ml | 0.248 | 0.020b | |||||
| <37 | 45 | 9 | 36 | 22 | 23 | ||
| ≥37 | 14 | 1 | 13 | 2 | 12 | ||
| CA72-4, U/ml | 0.600 | 0.177 | |||||
| <6.9 | 42 | 7 | 35 | 15 | 27 | ||
| ≥6.9 | 17 | 3 | 14 | 9 | 8 | ||
| Ki-67 index, % | 0.602 | 0.257 | |||||
| <70 | 11 | 2 | 9 | 3 | 8 | ||
| ≥70 | 48 | 8 | 40 | 21 | 27 | ||
| PD-L1 expression | 0.507 | 0.493 | |||||
| Negative | 21 | 4 | 17 | 8 | 13 | ||
| Positive | 38 | 6 | 32 | 16 | 22 | ||
Right-sided: Cecum, ascending colon and transverse colon; left-sided: Descending colon and sigmoid colon. bP<0.05. CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; CA72-4, cancer antigen 72-4; CTC, circulating tumour cell; PD-L1, programmed death-ligand 1.
As shown in Fig. 1, CTCs detected in the peripheral blood of patients with CRC had triploidy, tetraploidy or multiploidy (≥5 copies of chromosomes 7 or 8), indicating the existence of heterogeneous polysomic chromosomes in CTCs. The association between CTCs with multiploidy of chromosomes 7 or 8 and clinicopathological characteristics was evaluated. CTCs with aneuploidy of chromosome 8 were not significantly associated with any of the clinicopathological characteristics presented in Table II (data not shown). However, CTCs with multiploidy of chromosome 7 were significantly associated with TNM stage and serum CA19-9 levels (Table II). This type of CTC was more commonly observed in the peripheral blood of patients with late-stage CRC.
Discussion
CTC detection is a non-invasive approach that has potential utility for clinical diagnosis, prognosis and evaluation of therapeutic responses for different solid tumours. Therefore, it is important to establish a reliable CTC detection strategy. Various CTC detection methods, including the CellSearch® system, the AdnaTest, isolation by size of epithelial tumour cells (ISET®) and nested reverse transcription-quantitative (RT-q)PCR-based techniques, have been reported in CRC studies (10,12). The CellSearch® system is currently the only method for CTC detection approved by the FDA. However, the CTC-positive rates obtained using the CellSearch® system are low, ranging between 18.8 and 33% in patients with mCRC according to different studies (10,18). Furthermore, CTCs are barely detectable using this system in patients with non-mCRC (10). Gorges et al (19) identified CTCs using the CellSearch® technology and/or the AdnaTest in parallel, and demonstrated that a combined analysis with both methods leads to an elevated rate of CTC detection in patients with mCRC (positive rate, 33% for CellSearch®; 30% for the AdnaTest; 50% for both combined). The methods for CTC detection have been recently improved. Chen et al (20) introduced a novel isolation method, known as the ISET® system, involving automatic isolation and staining procedures to capture CTCs, and revealed that CTCs were detected in 38/72 (52.8%) patients with CRC. In another study based on nested RT-qPCR, epithelial cell transforming 2 (ECT2) was screened as a candidate marker gene for quantifying CTCs, with a detection rate of ECT2 of ~60% in patients with different stages of CRC (21).
Combining negative enrichment and imFISH, a strategy that is independent of epithelial marker expression and tumour size, has shown great potential for CTC detection, yielding diagnostic sensitivities of 84, 76.2 and 69.4% for detecting CTCs in lung, ovarian and ampullary cancer, respectively (22–24). In the present study, the Cyttel method (combining probes for CEP7 and CEP8 with an anti-CD45 antibody) was designed to detect CTCs in patients with CRC and in healthy controls. This strategy achieved a diagnostic sensitivity of 83.05% and a specificity of 100%, when using a cut-off value of 2 CTCs/3.2 ml of peripheral blood. The detection rate of CTCs with this method was higher than that of the CellSearch® system, which has been reported to be 15.33% (10), higher than that of the ISET® method, which has been reported to be 35% (20), and higher than that of the approach using RT-qPCR and ECT2, which has been reported to be 59% (21). Furthermore, the Cyttel method requires less peripheral blood (3.2 ml) than the CellSearch® system (7 ml). The present study suggested that the Cyttel method may be a sensitive and convenient strategy that could be helpful in improving the CTC detection rate in CRC.
In the present study, CTCs were identified in 3/30 (10.00%) healthy donors. In theory, CTCs should not be present in healthy individuals. However, Miller et al (25) demonstrated that CTCs detected by the CellSearch® technique are extremely rare in healthy volunteers (<3.5% for a threshold of ≥1 CTC per 7.5 ml of blood) and patients with benign disease (<7.5% for a threshold of ≥1 CTC per 7.5 ml of blood), and Ilie et al (26) also successively detected CTCs in patients with chronic obstructive pulmonary disease 1–4 years before they were diagnosed with lung cancer. To explain the above question, it is hypothesised that the epithelial cells isolated using different CTC detection methods may not all be the real CTCs, but instead include few cells with metabolic abnormality. These cells may come from donors with unhealthy lifestyles and eating habits, such as long-term smoking or high fat diet. Thus, it is possible to identify CTC-like cells in healthy people. However, the number of these abnormal cells is very low. Only one CTC was identified in the three healthy donors in the present study, and a cut-off value (2 CTCs/3.2 ml blood) was determined for the accurate distinction of tumour patients from healthy individuals. In conclusion, CTCs may be a useful tool for the early diagnosis and risk assessment of patients with cancer and healthy individuals.
At present, traditional serum tumour markers, such as CEA, CA19-9 and CA72-4, are widely used in the clinical management of CRC. According to the American Society of Clinical Oncology, CEA detection is recommended to monitor recurrence following curative surgery in patients with stage II and III CRC (27). The preoperative serum CA19-9 levels have been reported to be a prognostic indicator in patients with CRC (28). Additionally, a recent study demonstrated that CA72-4 can supplement CEA in evaluating CRC recurrence and metastasis (29). Early detection of tumour markers before distal metastasis occurs may have a significant impact on medical decision making and clinical outcomes of patients, particularly in the early stages. Furthermore, preoperative or postoperative serial CTC measurements may provide a method to predict or monitor early relapse in patients with non-mCRC (10). Based on the aforementioned results, the detection sensitivities of CTCs and three serum tumour markers were analysed simultaneously in patients with different stages of CRC to develop a non-invasive, effective and reliable biomarker detection strategy for the early diagnosis of CRC.
In the present study, the diagnostic sensitivities of single and combined serum tumour markers ranged between 40.68 and 64.41%, respectively, which was consistent with the findings of Gao et al (30). However, the sensitivity of the CTC detection method reached 83.05%, which was higher than that of the traditional tumour markers, even when combining CEA, CA19-9 and CA72-4. A similar result was obtained in a previous study using the same CTC detection method in patients with lung cancer (22). For patients with stage I–II CRC, CEA had the highest sensitivity (34.38%) among the three individual serum tumour markers, while the detection rate of the CTC method was 78.13%. It was clear that the CTC method was more sensitive for detecting early stage CRC compared with the use of other serum biomarkers. Furthermore, combining the CTC method with CEA achieved a sensitivity of 91.53%, which was only slightly lower than that for the combination of the four biomarkers (93.22%). This finding suggests that the combination of CTCs and CEA may be an effective and convenient routine detection strategy for diagnosing CRC, and for predicting and monitoring early recurrence and metastasis in patients with CRC.
The current study revealed novel insights into the potential diagnostic application of a CTC detection method based on aneuploidy detection. Davoli et al (31) revealed that an abnormally high number of chromosomes was commonly associated with high-grade tumours and poor prognosis. Papazachariou et al (32) have identified that the number of CTCs with chromosome 7 aneuploidy is significantly involved with late stage tumours and poor prognosis. In the present study, the association between total and aneuploid CTCs and patient clinicopathological characteristics was carefully analysed. Total CTCs were found to be significantly associated with tumour differentiation and nerve invasion. This finding indicated that the detection rate of CTCs increased along with tumour progression, which was consistent with the aforementioned results regarding the detection rate of CTCs in different stages of CRC. Additionally, there was a significant difference in CTCs with multiploidy of chromosome 7 between different TNM stages. These CTCs were more likely to be detected in patients with stage III–IV CRC than in patients with stage I–II CRC. The present study suggested that detection of CTCs based on aneuploidy detection could be more specific for predicting highly malignant and invasive tumours in CRC management than detection using current serum markers. A careful classification of aneuploid CTCs may provide novel insights for improved prediction of CRC.
In conclusion, the current data fully support the potential clinical value of total and aneuploid CTCs as identified with the Cyttel method in CRC diagnosis. The combination of CTCs and CEA has the potential to become an effective and convenient routine screening strategy for predicting CRC at different stages. However, the present study was performed at a single medical centre with a small sample size, which may limit the power of the statistical analysis. The dynamic change in the number of CTCs at different stages of treatment and the association between CTCs and patient survival should be studied in future research.
Acknowledgements
Not applicable.
Funding
This research was supported by grants from the National Natural Science Foundation of China (grant nos. 81372585, 81772557 and 81902360), the Beijing Health System High Level Training Plan of Health Technical Personnel (grant no. 2014-3-048), the Beijing Municipal Administration of Hospital's Clinical Medicine Development of Special Funding Support (grant no. XMLX201708), the Scientific Research and Development Program of Beijing Railway Corporation of China (grant no. J2017Z605), the Science Nurturing Foundation of Capital Medical University (grant no. PYZ2017151) and the Youth Fund of Beijing Shijitan Hospital (grant nos. 2017-q02 and q13).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HG and LD designed the study. HY and LM performed the experiments and wrote the initial draft of the manuscript. WL and YZ contributed to the analysis and interpretation of data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Review Committee of Beijing Shijitan Hospital [Beijing, China; approval no. 2016KY(55)]. Research has been conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Kekelidze M, D'Errico L, Pansini M, Tyndall A, Hohmann J. Colorectal cancer: Current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J Gastroenterol. 2013;19:8502–8514. doi: 10.3748/wjg.v19.i46.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponz de Leon M, Di Gregorio C. Pathology of colorectal cance. Dig Liver Dis. 2001;33:372–388. doi: 10.1016/S1590-8658(01)80095-5. [DOI] [PubMed] [Google Scholar]
- 5.Shah R, Jones E, Vidart V, Kuppen PJ, Conti JA, Francis NK. Biomarkers for early detection of colorectal cancer and polyps: Systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:1712–1728. doi: 10.1158/1055-9965.EPI-14-0412. [DOI] [PubMed] [Google Scholar]
- 6.Ryuk JP, Choi GS, Park JS, Kim HJ, Park SY, Yoon GS, Jun SH, Kwon YC. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res. 2014;86:143–151. doi: 10.4174/astr.2014.86.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda T, Hayashi N, Iguchi T, Ito S, Eguchi H, Mimori K. Clinical and biological significance of circulating tumor cells in cancer. Mol Oncol. 2016;10:408–417. doi: 10.1016/j.molonc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C, Stirnimann CU, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176:98–112.e14. doi: 10.1016/j.cell.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bork U, Rahbari NN, Schölch S, Reissfelder C, Kahlert C, Büchler MW, Weitz J, Koch M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: A prospective study. Br J Cancer. 2015;112:1306–1313. doi: 10.1038/bjc.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 12.Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Zhao M, He S. Study on the clinical significance of detection of Cyttel circulating tumor cells in peripheral blood of patients with gastric cancer. J Clin Exp Med. 2018;7:772–774. [Google Scholar]
- 15.Rajagopalan H. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 16.Sayagués JM, Abad MM, Melchor HB, Gutiérrez ML, González-González M, Jensen E, Bengoechea O, Fonseca E, Orfao A, Muñoz-Bellvis L. Intratumoural cytogenetic heterogeneity of sporadic colorectal carcinomas suggests several pathways to liver metastasis. J Pathol. 2010;221:308–319. doi: 10.1002/path.2712. [DOI] [PubMed] [Google Scholar]
- 17.Provenzale D, Gupta S, Ahnen DJ, Markowitz AJ, Chung DC, Mayer RJ, Regenbogen SE, Blanco AM, Bray T, Cooper G, et al. NCCN guidelines insights: Colorectal cancer screening, Version 1.2018. J Natl Compr Canc Netw. 2018;16:939–949. doi: 10.6004/jnccn.2018.0067. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Li J, Jin L, Wang D, Zhang J, Wang J, Zhao X, Wu G, Yao H, Zhang Z. Independent correlation between Ki67 index and circulating tumor cells in the diagnosis of colorectal cancer. Anticancer Res. 2017;37:4693–4700. doi: 10.21873/anticanres.11874. [DOI] [PubMed] [Google Scholar]
- 19.Gorges TM, Stein A, Quidde J, Hauch S, Röck K, Riethdorf S, Joosse SA, Pantel K. Improved detection of circulating tumor cells in metastatic colorectal cancer by the combination of the CellSearch® system and the AdnaTest®. PLoS One. 2016;11:e155126. doi: 10.1371/journal.pone.0155126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen F, Wang S, Fang Y, Zheng L, Zhi X, Cheng B, Chen Y, Zhang C, Shi D, Song H, et al. Feasibility of a novel one-stop ISET device to capture CTCs and its clinical application. Oncotarget. 2017;8:3029–3041. doi: 10.18632/oncotarget.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CJ, Sung WW, Chen HC, Chern JY, Hsu TH, Min Lin Y, Lin SH, Peck K, Yeh TK. Early assessment of colorectal cancer by quantifying circulating tumor cells in peripheral blood: ECT2 in diagnosis of colorectal cancer. Int J Mol Sci. 2017;18:743. doi: 10.3390/ijms18040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YY, Xu GB. Effect of circulating tumor cells combined with negative enrichment and CD45-FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. Med Oncol. 2014;31:240. doi: 10.1007/s12032-014-0240-0. [DOI] [PubMed] [Google Scholar]
- 23.Ning N, Zhan T, Zhang Y, Chen Q, Feng F, Yang Z, Liu Z, Xu D, Wang F, Guo Y, et al. Improvement of specific detection of circulating tumor cells using combined CD45 staining and fluorescence in situ hybridization. Clin Chim Acta. 2014;433:69–75. doi: 10.1016/j.cca.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Sun B, Liu H, Wang S, Xiang J, Liu X. Prognostic impact of circulating tumor cells in patients with ampullary cancer. J Cell Physiol. 2018;233:5014–5022. doi: 10.1002/jcp.26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast, colorectal and prostate cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilie M, Hofman V, Long-Mira E, et al. Clinical and pathological characteristics of CTC-positive COPD patients) PLoS One. 2014;9:e111597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–4470. doi: 10.1200/JCO.2013.50.7442. [DOI] [PubMed] [Google Scholar]
- 28.Abe S, Kawai K, Ishihara S, Nozawa H, Hata K, Kiyomatsu T, Tanaka T, Watanabe T. Prognostic impact of carcinoembryonic antigen and carbohydrate antigen 19-9 in stage IV colorectal cancer patients after R0 resection. J Surg Res. 2016;205:384–392. doi: 10.1016/j.jss.2016.06.078. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Dai W, Li Y, Xu Y, Li X, Cai S. Nomograms for predicting the prognostic value of serological tumor biomarkers in colorectal cancer patients after radical resection. Sci Rep. 2017;7:46345. doi: 10.1038/srep46345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. 2018;8:2732. doi: 10.1038/s41598-018-21048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355(pii):eaaf8399. doi: 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papazachariou I, Tsiambas E, Koliopoulou A, Tsounis D, Manaios M, Fotiades PP, Karameris A, Patsouris E. Chromosomes 7, 16 numerical aberrations are poor prognostic factors in colorectal adenocarcinoma: A tissue microarray analysis. Basic App Pathol. 2008;1:125–130. doi: 10.1111/j.1755-9294.2008.00027.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HG and LD designed the study. HY and LM performed the experiments and wrote the initial draft of the manuscript. WL and YZ contributed to the analysis and interpretation of data. All authors read and approved the final manuscript.