Abstract
In comparison with other animal venoms, fish venoms remain relatively understudied. This is especially true for that of the lesser Echiichthys vipera and greater weever fish Trachinus draco which, apart from the isolation of their unique venom cytolysins, trachinine and dracotoxin, respectively, remain relatively uncharacterised. Envenomation reports mainly include mild symptoms consisting of nociception and inflammation. However, like most fish venoms, if the venom becomes systemic it causes cardiorespiratory and blood pressure changes. Although T. draco venom has not been studied since the 1990's, recent studies on E. vipera venom have discovered novel cytotoxic components on human cancer cells, but due to the scarcity of research on the molecular make-up of the venom, the molecule(s) causing this cytotoxicity remains unknown. This review analyses past studies on E. vipera and T. draco venom, the methods used in the , the venom constituents characterised, the reported symptoms of envenomation and compares these findings with those from other venomous Scorpaeniformes.
Keywords: Echiichthys vipera, Trachinus draco, Trachinine, Dracotoxin, Weever fish venom, Cytolytic
Highlights
-
•
Research on the weever fish venoms Echiichthys vipera and Trachinus draco has
been scarce.
-
•
E. vipera and T. draco venoms elicit cardiorespiratory symptoms in victims.
-
•
E. vipera and T. draco contain unique cytolysins – Trachinine and Dracotoxin.
-
•
Dracotoxin is haemolytic and contains membrane depolarising activities.
-
•
E. vipera venom triggers apoptosis in human colon carcinoma cells.
1. Piscine venoms
Although over half of all living venomous vertebrates are fish (Smith and Wheeler, 2006), their venoms remain relatively understudied (Church and Hodgson, 2002, Ziegman and Alewood, 2015, Smith et al., 2016). Piscine venoms are hypothesised to have evolved on 18 separate occasions (Smith et al., 2016), with the evolution of the fish venom glands being hypothesised to be from the thickening and aggregation of epidermal cells near the spine apparatus that originally secreted anti-parasitic skin toxins (Harris and Jenner, 2019). Venomous fish are mainly distributed among the catfish (Siluriformes), six clades of spiny rayed fish (Acanthomorph), toadfish (Batrachoidiformes), scorpionfish (Scorpaeniformes and Scorpaenoidei), surgeonfish, scats, and rabbitfish (Perciformes: Acanthuroidei), saber-toothed blennies (Perciformes: Blennioidei), jacks (Perciformes: Percoidei), and stargazers and weevers (Perciformes: Trachinoidei) (Smith and Wheeler, 2006).
Most venomous fish (~95%) harbour spines which contain anterolateral grooves on their surface that encompass the venom glands (Sivan, 2009, Smith et al., 2016). Only a small proportion of venomous fish have evolved fangs (2%), cleithral spines (2%) and opercular/sub-opercular spines (1%) (Smith et al., 2016). Most fish venoms are used defensively (~95%) (Gomes et al., 2011, Smith et al., 2016); however, in some cases (~2%) they are used in predation to incapacitate prey (Smith et al., 2016). All types of fish venom apparatus lack musculature, therefore they cannot voluntarily control the secretion of their venom (Ziegman and Alewood, 2015). Thus, envenomation occurs when mechanical pressure is applied to the spine, breaking the integumentary sheath surrounding the spines, allowing venom to seep from the venomous cells into the victim (Sivan, 2009, Borges et al., 2018).
A characteristic fish venom symptom is that the immense pain inflicted is disproportionate to the size of the envenomation injuries, which are commonly small puncture wounds (Church and Hodgson, 2002). Fish venoms can cause cardiovascular, neuromuscular, oedematic and cytolytic symptoms with cardiovascular activity noted as the main effect after envenomation (Church and Hodgson, 2002, Sivan, 2009). The venoms are composed of a cocktail of toxins, enzymes, bioactive peptides and non-proteinaceous components such as serotonin, norepinephrine and acetylcholine (Ziegman and Alewood, 2015). Many fish venoms act in similar ways and have similar components, evidenced by the cross-reactivity of stonefish (Synanceia trachynis) anti-venom which can neutralise certain fish venoms (Church and Hodgson 2002, 2003; Sivan, 2009, Gomes et al., 2011). However, even though the venom components are similar between species, venoms often contain a lethal toxin unique to that species (Church and Hodgson, 2002, Ziegman and Alewood, 2015).
2. Trachinidae
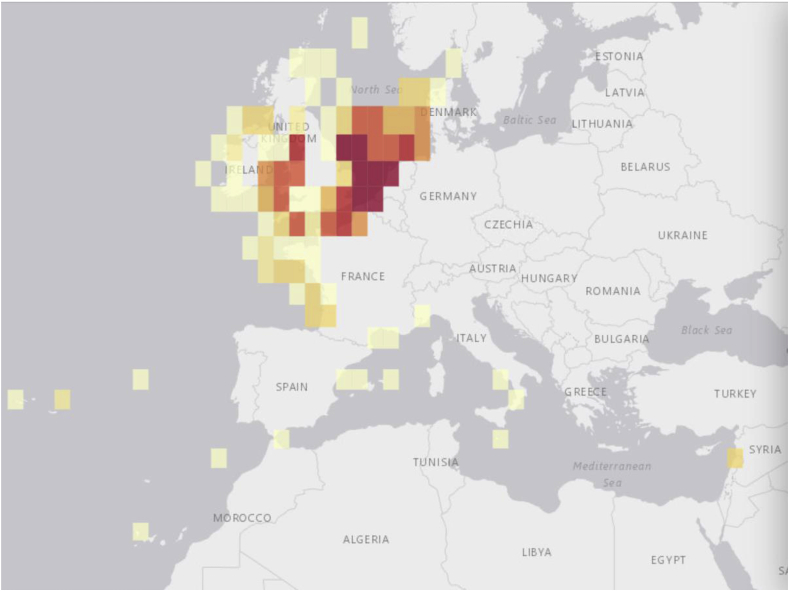
Weever fish (family Trachinidae) are venomous benthic fish which comprise nine extant species, split between two genera (Trachinus and Echiichthys). The two main venomous species of weever fish are the greater weever (T. draco) and the lesser weever (E. vipera - also referred to as Trachinus vipera). Unlike most venomous marine animals which are found in the Indo-pacific (Fenner, 2004), weevers are mainly found in temperate regions throughout the Mediterranean, North, Celtic and Irish Seas, expanding in some cases to the Atlantic coast of North Africa (Dehaan et al., 1991) (Fig. 1).
Fig. 1.
Native distribution of E. vipera. Map taken with permission from OBIS (2015). Darker shade represents a higher abundance. *Colour should be used in print*.
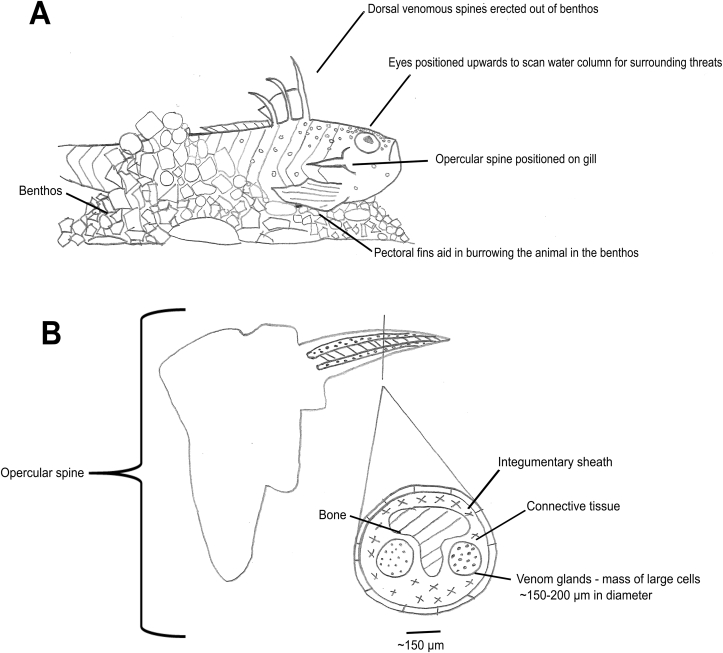
Weever fish typically are to be found on sandy substrata, against which they are well camouflaged. Frequently, they will partially bury themselves within the substrate, only revealing their anterior and dorsal portions of the body (Fig. 2A). These exposed anterior and dorsal body portions possess venomous dorsal and opercular spines, with one individual spine situated on each operculum and three spines on the dorsal fin (Ziegman and Alewood, 2015) (Fig. 2, Fig. 3).
Fig. 2.
Sketch A depicts the typical concealment and observation behaviour of a weever fish. Sketch B is based on histological photos and description of the venom gland architecture by Perriere and Michel (1986) in the opercular spine of E. vipera. Author's sketches.
Fig. 3.
Frozen specimen of an adult E. vipera collected from the Celtic Sea in November 2015. White arrows point to dorsal and opercular spines, respectively. *Colour should be used in print*. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Weevers, like other venomous fish e.g. stonefish, have convergently evolved dorsal spines housing multiple anterolateral grooves that contain venom glands, lined on the interior of the spine encompassing large glandular cells (Skeie, 1962b, Perriere and Michel, 1986, Smith et al., 2016) (Fig. 2B). It should be noted that many previous authors have studied the anatomy and histology of the venom apparatus and glands from T. draco and E. vipera (Allman, 1840, Byerley, 1849, Schmidt, 1874, Gressin, 1884, Parker, 1888, Borley, 1907). The venom gland cells are surrounded by supporting cells in the connective tissue (Fig. 2b) which are derived from epidermal cells (Perriere and Goudey-Perriere, 1989). The method of venom secretion from the weever venom glands has been reported as holocrine (Skeie, 1966). Unlike certain other venomous fish, weevers also possess opercular spines which do not differ in their histology from dorsal spines (Skeie, 1966). Most human envenomation cases result from being stung in the limbs, most often during handling by fishermen (mainly T. draco) or after having been stepped on in the shallow intertidal zone (Padin et al., 2018). Stepping injury is more common for E. vipera as it migrates to very shallow inshore waters in warmer months. In agreement with this, the probability of being stung by E. vipera increases with both increasing sea and air temperature (Padin et al., 2018). Approximately 10–12 cases of weever envenomation per year are reported in Denmark and Italy (Cain, 1983), 15 per 100,000 inhabitants along the south eastern Spanish coast (Strempel et al., 2009) with recent reports of 1005 cases in Northern Spain during one summer (equating to 0.3% of all bathers) (Padin et al., 2018). During the period 2003–2004, 17 cases of weever injury among fishermen were reported along the eastern Mediterranean coast (PMID: 19070287). Although the cases of envenomation are relatively frequent, there remains a paucity of contemporary information concerning the composition and activity of venom from the weever family. Prior to the most recent study by Fezai et al. (2017) the last research note on weever fish venom (T. draco) was published in 1992 (Chhatwal and Dreyer, 1992b).
3. Symptomology and treatment of envenomation
Commonly reported symptoms of E. vipera human envenomation include local pain, abdominal pain, inflammation, nausea, necrosis and vomiting (Skeie, 1966, Dehaan et al., 1991, Bonnet, 2000, Borondo et al., 2001, Chinellato and Savelli, 2015). T. draco envenomation also causes an inflammation reaction with a subsequent oedema occurring at the wound site (Dekker, 2001, Halpern et al., 2002, Łopaciński et al., 2009). The pain from a weever fish sting has been described as feeling more excruciating than that experienced from a stingray envenomation (Russell and Emery, 1960), although this comparison is challenging to conceptualise without personal experience. If the deposition of venom becomes systemic, weever fish envenomation can cause disturbances in cardiac rhythm (including increased heart rates and heart failure) and respiratory distress in mammals, from mice to humans (Evans, 1907, Russell and Emery, 1960, Carlisle, 1962, Skeie, 1962b, Russell, 1983, Borondo et al., 2001, Chinellato and Savelli, 2015).
Other reported symptoms include dermal fibrosis and hyperalgesia (Davies and Evans, 1996, Mulcah et al., 1996; Chinellato and Savelli, 2015), and Secondary Raynaud's Phenomena (reduced blood flow due to artery spasms, causing a white/blue colouration accompanied with a feeling of numbness or pain) (Evans, 1907, Carducci et al., 1996, Mayser et al., 2003), with the affected area being rendered useless from days to weeks after envenomation.
Only one human fatality has been attributed to weever envenomation (Borondo, et al., 2001). Post-mortem examination of the victim found a haematic coagulation within the epidermis near the puncture wound, a 5 cm haematoma within the left leg, several oedemas within the alveoli and fluid in the lungs. Before death the victim experienced respiratory distress, palpitations and vomiting, ultimately resulting in cardiorespiratory arrest.
Stonefish Synanceia trachynis anti-venom (SFAV) can be used to neutralise the symptoms for some piscine envenomation cases (Church and Hodgson, 2002, Sivan, 2009, Ziegman and Alewood, 2015) e.g. scorpionfish Scorpaena plumieri (Gomes et al., 2011), lionfish (Pterois volitans) and the soldierfish (Gymnapistes marmoratus) (Church and Hodgson, 2003), however it does not universally cross-react against all fish venoms. For instance, bullrout Notesthes robusta venom shows no antigenicity to SFAV (Hahn and O’Connor, 2000). Common treatment for victims of most piscine envenomations (including weevers) is hot water immersion (39°C–45 °C) for 20–90 min, as most piscine venoms are thermolabile, thus relieving the pain (Russell, 1983; Diaz, 2015; Ziegman and Alewood, 2015, Barnett et al., 2017). Another treatment involves administering painkillers or local anaesthetics to the victim in order to relieve the nociceptive symptoms (Ziegman and Alewood, 2015), for example some authors have reported lidocaine as an effective treatment for reducing the symptoms of weever envenomation (Zammit, 1974).
4. Comparison of Trachinus draco and Echiichthys vipera venoms
A seminal study on the bioactivity of T. draco and E. vipera, pooled the venom samples from both species in order to increase sample size (Russell and Emery, 1960) due to the similarity in the anatomies of the respective venom organs (Halstead and Modglin, 1958, Russell and Emery, 1960). Whether the pooling of venom in this study (Russell and Emery, 1960) has led to the inaccurate recording of bioactivity in E. vipera or T. draco venom is unclear. This study consisted of testing the chemistry of the venom and the mode of action of the venom in a range of non-human mammalians, recordings cardiovascular effects including disturbances in cardiac rhythm, ischemic injury, and respiratory distress. For the chemical studies analysis of the spines from six T. draco individuals were combined with the spines from 86 E. vipera individuals, whereas in the biological assays the authors did not specify whether they used venom from E. vipera opercula and dorsal spines or the venom from the spines of both E. vipera and T. draco (Russell and Emery, 1960). After venom injection into small mammals, a “staircase effect” on blood pressure (falling, then stabilising, then falling) was observed, with both changes to venous and arterial blood pressure. In addition, heart rate changes and shallowing of respiration were noted in the animals, with some fatalities. The mode of action was reported to be similar to that of stingray venom. The bioactive effects of the venom are mainly targeted to the cardiorespiratory system, similar to those other fish venoms (Church and Hodgson, 2002, Sivan, 2009, Ziegman and Alewood, 2015), however it is unclear which individual venom was responsible for the bioactivity recorded in Russell and Emery (1960) study.
4.1. Trachinus draco venom
Studies on the venom of T. draco date back to 1849, where Byerley published a paper on the anatomy of the venom glands in the opercular and dorsal spines in T. draco (Byerley, 1849). Phisalix (1899) demonstrated that T. draco venom elicited paralysis, local swelling, necrosis and, ultimately, death in guinea pigs. Evans (1907) found T. draco venom to be haemolytic on roach, perch, guinea-pig, sheep, ox, horse and human blood. Later, Evans attributed the haemolytic action to a complement-like substance and amboceptor within the venom (Evans, 1910). Similar to the biological activity reported in the study by Russell and Emery (1960), Evans (1907) observed respiratory, blood pressure and cardiac changes in both cats and rabbits after T. draco injection. De Marco (1936) hypothesised the venom to contain neurotoxic properties following observations of increased potassium permeability in a frog nervous system.
Skeie (1962a) was the first author to use quantitative titrations of T. draco venom and attribute them to the biological results produced in mice and guinea pigs. Mice were intravenously injected with crude venom (venom extracted from the glands of a venomous species without any subsequent purification) where the LD100 after 24 h was 0.4 μL. Skeie (1962a) also noted that the opercular glands contained the most venom as opposed to the dorsal spines in T. draco. Skeie (1962a) also used these titrations on chick fibroblasts where crude T. draco venom also had an LD100 of 0.4 μL. However, the occurrence of multiple vacuoles in the cytoplasm and profuse cell granulation was witnessed in much lower titrations (1:1024 dilution) of T. draco venom.
Skeie (1962b) also conducted in vivo bioassays on mice, guinea pigs and rabbits using T. draco venom. As witnessed in Russell and Emery (1960) Skeie (1962b) recorded similar blood pressure, respiration and cardiac changes after administering T. draco venom at lethal doses. Autopsies revealed all animals exhibited frothy discharge in the respiratory systems and contained heart anomalies with the left ventricle being devoid of blood whereas the right was full (Skeie, 1962b) indicating that the venom may have elicited heart failure, possibly due to high catecholamine levels (the so-called catecholamine storm), which has been reported for jellyfish, scorpion and spider envenomations (Abroug et al., 2015, Weisel-Eichler and Libersat, 2004). Skeie (1962b) attributed the oedema symptoms produced by the venom to be to the high content of hyaluronidase present (38,600 IU/mL). Antigenicity to the venom was also witnessed with the disappearance of necrotic symptoms after repeated sub-lethal injections in mice (Skeie, 1962b).
Skeie (1962c) was the first study to purify the lethal fraction within T. draco venom. Skeie (1962c) isolated the fraction by performing paper electrophoresis on the crude venom which revealed three fractions. Density and gradient zone electrophoresis was subsequently performed to further separate the fractions and these fractions were then used in toxicity bioassays (Skeie, 1962c). One of the six peaks found in the density and gradient zone electrophoresis contained seven fractions with five of these fractions containing 85% of the toxicity found in the crude venom (Skeie, 1962c). In the same study, the immunity of animals against T. draco venom was tested and after 60 days of venom injections, antigenicity against T. draco venom was achieved with lethal doses causing no reaction in mice (Skeie, 1962c).
Haavaldsen and Fonnum (1963) first confirmed venom components specific to T. draco, by using paper chromatography to reveal the presence of histamine and catecholamine, and then using a photofluorimetric method to further classify the catecholamines as adrenaline and noradrenaline. Venom homogenates were then tested for their cholinesterase activity and the venom was discovered to contain acetyl- and butyryl-cholinesterases (Haavaldsen and Fonnum, 1963). Even though venoms are a main source of non-synaptic acetylcholinesterase, the function of cholinesterase during envenomation is unclear (Ziegman and Alewood, 2015, Ahmed et al., 2016). In snake venoms it has been hypothesised that the presence of soluble acetylcholinesterase may be to hydrolyse acetylcholine in muscles disrupting the transmission at the neuromuscular junction of the prey, potentially leading to paralysis (Ahmed et al., 2016).
The 1990s saw renewed interest in weever venom. Chhatwal and Dreyer (1992a) showed that T. draco venom activated neurons, thus supporting the neurotoxin hypothesis proposed by De Marco (1938). The crude venom used in the study (Chhatwal and Dreyer, 1992a) had an LD₅₀ of 1.8 μg/g in mice and had an LD₅₀ 0.075 μg/mL on rabbit erythrocytes. Chhatwal and Dreyer (1992a) concluded that the crude venom was not proteolytic because it did not hydrolyse azocaesin.
Subsequently, Chhatwal and Dreyer (1992b) isolated a unique protein toxin (dracotoxin) from crude T. draco venom using 50% ammonium acetate precipitation of the crude venom followed by gel filtration using high performance liquid chromatography to separate the venom components. Chhatwal and Dreyer (1992b) calculated the molecular weight of dracotoxin by using SDS- polyacrylamide gel electrophoresis and by calculating the retention time of the gel filtration column in the high-performance liquid chromatography. Dracotoxin had a molecular weight of 105 kDa and possessed neuronal membrane depolarising activities, causing haemolysis of rabbit erythrocytes with an EC₅₀ of 0.003 μg/mL; 25 times more potent than the crude venom (Chhatwal and Dreyer, 1992b). Both crude T. draco venom (Chhatwal and Dreyer, 1992a) and dracotoxin (Chhatwal and Dreyer, 1992b) have potent cytolytic effects on rabbit and rat erythrocytes, but no impact on the erythrocytes of humans. Chhatwal and Dreyer (1992b) suggested that the haemolytic action was due to the interaction of dracotoxin with glycophorin in the rabbit erythrocyte membrane.
4.2. Echiichthys vipera venom
The first scientific interest in E. vipera venom dates back to 1840 when Allman (1840) described the histology of the opercular spines (Zammit, 1974). Following the study by Russell and Emery, 1960, Carlisle, 1962 conducted chemical and bioactivity assays on E. vipera venom. Carlisle separated the E. vipera venom into a dialysable and a non-dialysable fraction. Paper chromatography showed the dialysable fraction to contain both 5-hydroxytrypatmine (serotonin) and a histamine releaser. Bioactivity assays using both himself and a goby fish (Gobius ruthensparri) proved the dialysable fraction to be the non-lethal fraction and the main pain producing fraction of the venom. Carlisle concluded that the high levels of 5-hydroxytrypatmine (ranging from 0.1 to 2% of venom dry weight), which were some of the highest to be found in a living animal (Carlisle, 1962), to have a primary effect of inducing pain. In fish venoms 5-hydroxytrypatmine is hypothesised to be a major contributor to their nociceptive activity (Ziegman and Alewood, 2015). Contrastingly, no 5-hydroxytrypatmine was found in T. draco venom extracts (Haavaldsen and Fonnum, 1963), similar to S. verrucosa venom where the absence of 5-hydroxytrypatmine has also been reported (Garnier et al., 1996). Paper electrophoresis revealed that the non-dialysable fraction contained two separable albumins and a neutral amino mucopolysaccharide (Carlisle, 1962). However, Carlisle (1962) concluded that the lethal constituents found in the non-dialysed fraction (two albumins and an amino-polysaccharide) may represent a combined protein complex in the native venom and not necessarily separate constituents, as there was no evidence in the study supporting them being separate complexes. The non-dialysable fraction was lethal to G. ruthensparri and elicited respiratory distress and an increased heart rate in Carlisle upon self-injection. Due to the absence of haemolysis on citrated human blood, Carlisle (1962) concluded that the main bioactivity of the non-dialysed fraction was neurotoxicity.
Perriere (1985) analysed the enzyme presence within E. vipera venom and recorded the activity of: alkaline phosphatase; acid phosphatase; phosphoamidase; arylamidase; an ATPase; proteases; esterases; β glucuronidase; and, cytochrome oxidase - see Table 1 (Perriere and Goudey-Perriere, 1999). Enzymes are a common component of other fish venoms, allowing the deterioration of biological structures in the victim that may inhibit the spread of the venom itself (Ziegman and Alewood, 2015). Interestingly though, there was no presence of any hyaluronidase activity which is found in many Scorpaeniform venoms (Ziegman and Alewood, 2015). Hyaluronidase is a spreading factor and degrades hyaluronate, allowing venom to move through the victim's tissue quicker (Khoo, 2002). Hyaluronidase is present at higher quantities in stonefish venom than in snake venoms (Poh et al., 1992). Fish venom hyaluronidase has a low similarity to hyaluronidases found in other animals (<50%) however, there is a high homology in Scorpaeniformes with >72% identity between stonefish venom hyaluronidase (S. horrida and S. verrucosa) and that of lionfish (P. volitans and P. antennata) (Ziegman and Alewood, 2015).
Table 1.
The venom components and their respective bioactivity within E. vipera and T. draco venoms.
| Species | Venom constituents discovered | Bioactivity | Reference |
|---|---|---|---|
| E. vipera | Trachinine | Cytolysin | Perriere et al., 1988, Fezai et al., 2016 |
| 5-hydroxytrypatmine | Nociception | Carlisle, (1962) | |
| Histamine releaser | Inflammation | Carlisle, (1962) | |
| Enzymes (alkaline phosphatase; acid phosphatase; phosphoamidase; arylamidase; an ATPase; proteases; esterases; β glucuronidase; and, cytochrome oxidase) | Unknown | Perriere, 1985, Perriere and Goudey-Perriere, 1987 | |
| T. draco | Dracotoxin | Haemolysin, Cytolysin | Chhatwal and Dreyer, (1992b) |
| Cholinesterases (acetylcholinesterase and butyrylcholinesterase) | Unknown | Haavaldsen and Fonnum, (1963) | |
| Hyaluronidase | Spreading factor | Skeie, (1962b) | |
| Histamine | Inflammation | Haavaldsen and Fonnum, (1963) |
Proteases are abundant within fish venoms (Ziegman and Alewood, 2015), but as with many fish venom components, not many proteases from fish venoms have been isolated (Campos et al., 2016). Exceptions include Sp-GP from Scorpaena plumieri venom which exhibits gelatinolytic activity (Carrijo et al., 2005) and the Natterin protease family from Thalassophryne nattereri venom which exhibit kininogenase activity and degrade collagen, contributing to many symptoms witnessed after envenomation including necrosis, inflammation, nocieception and oedema (Magalhães et al., 2005).
Alkaline phosphatase and acid phosphatase were also found in the venoms of Scatophagus argus (Sivan et al., 2010), G. marmoratus and S. horrida (Hopkins and Hodgson, 1998). Although their full biological roles are not well known, in snake venoms acid and alkaline phosphatases are thought to play a role in liberating purines which produces a multi-toxin, possibly aiding in prey immobilisation upon envenomation (Dhananjaya and D'Souza, 2011). In addition, in bee venom, acid phosphatase is a potent allergen and histamine releaser (Grunwald et al., 2006). As with E. vipera venom (Perriere and Goudey-Perriere, 1987), arylamidase has been reported to be one of the components of the enzyme active fraction in honeybee venom, although its biological function upon envenomation is unknown (Zolfagharian et al., 2015).
Nucleosidases such as ATPase have been observed in snake venoms (Sales and Santoro, 2008, Dhananjaya and D'Souza, 2010) and their role is thought to help exacerbate the effects of the myotoxins also present in the venom (Caccin et al., 2013). Myotoxins produce ATP which nucleosidases then convert to ADP, ADMP and Adenosine (Caccin et al., 2013). High concentrations of adenosine cause hypotensive, anti-coagulant and paralysing effects in vivo, contributing to the pharmacological responses seen in snake envenoming (Caccin et al., 2013). Future research should aim to isolate and conduct bioassays on the enzymes present in E. vipera venom, in order to understand whether venom bioactivity can be attributed to certain venom constituents e.g. natterins in Thalassophryne nattereri venom (Magalhães et al., 2005).
Perriere et al. (1988) used gel electrophoresis to isolate two lethal fractions from E. vipera venom (a migratory and a non-migratory fraction); although this cannot necessarily be taken to suggest two separate active constituent fractions as they may combine to form a single protein. The crude venom used for separation in the gel electrophoresis was denatured, thus the proteins within the venom were not isolated in their native form. The migratory fraction was identified as trachinine, with an LD₁₀₀ of <100 μg/kg in mice. The molecular weight of trachinine was determined by comparing the mobility of trachinine in different percentage acrylamide gels (5, 6 and 7%) to the mobility of different bovine serum albumin polymers (monomer, dimer, trimer and tetramer) in these same gels, following the method by Hedrick and Smith (1968) (Perriere et al., 1988). This same method (Hedrick and Smith, 1968) was used to determine the molecular weight of Verrucotoxin from Synanceia verrucosa venom (Garnier et al., 1995). Trachinine was hypothesised to comprise four identical subunits, each of 81 kDa, giving a combined molecular mass of 324 kDa (Perriere, et al., 1988). However, it should be noted that this electrophoresis of trachinine was performed under denaturing conditions. The second non-migratory fraction was an aggregate of proteins ranging from 40 to 92 kDa (40, 48, 54, 61, 75, 84 and 92 kDa), however no further identification was conducted. No further analysis on the biological activity or structure was conducted on trachinine due to its high lability, losing toxicity within 1 h at room/body temperature (Perriere, et al., 1988).
After a pause in the research of E. vipera venom, Fezai et al., 2016, Fezai et al., 2017 measured the pharmacological potential of semi-purified E. vipera venom on human erythrocytes and colon carcinoma cells. E. vipera venom was semi-purified by first extracting the dorsal spines, and then sonicating the spines to release the cell constituents (Fezai et al., 2016, Fezai et al., 2017). A dialysis cellulose membrane with an 8 kDa cut-off was then used to dialyse the venom and the dialysate was then filtered through a 0.22 μm membrane, lyophilised, and stored at - 20 °C until further use (Fezai et al., 2016, Fezai et al., 2017). The semi-purified E. vipera venom (500 μg/mL) triggered eryptosis by cell shrinkage, calcium entry and phosphatidylserine translocation to the cell surface (Fezai et al., 2016). Eryptosis is the death of erythrocytes by an apoptotic-like pathway (Repsold and Joubert, 2018). Furthermore, the E. vipera venom caused cell death of the colon carcinoma cells (HCT116 and RKO) by triggering the mitochondrial intrinsic pathway of apoptosis (Fezai et al., 2016). These findings may explain necrotic symptoms following envenomation by E. vipera (Dehaan et al., 1991). Additionally, colon carcinoma cells were arrested in the G1 phase of the cell cycle when exposed to semi-purified E. vipera venom, inhibiting their proliferation (Fezai et al., 2016). This venom also affected cell motility and reduced the migration of treated colon cancer cells through inducing marked changes in the cell shape and size with a huge reorganization of actin and tubulin (cytoskeletal proteins) (Fezai et al., 2017). Other venoms which show components with apoptotic and inhibitory cell proliferation mechanisms such as bee venom (Son et al., 2007) and snake venom (Vyas et al., 2013) have been hypothesised to be novel targeted cancer therapies. In addition to cancer, venoms with apoptotic bioactivity have been shown to target other autoimmune diseases such as rheumatoid arthritis. For example, bee venom was shown to inhibit the proliferation of human rheumatoid synovial fibroblasts after 24 h through the caspase 3 activated apoptosis (Hong et al., 2005). Since the induction of apoptosis is one of the most important functions of anti-cancer therapies (Vyas et al., 2013), these studies (Fezai et al., 2016, Fezai et al., 2017) highlight the possible pharmaceutical potential of E. vipera venom and its constituents.
4.3. Trachinine and dracotoxin
In piscine venoms most of the large proteinaceous toxins display cytolytic/haemolytic activity and function by forming pores within cell membranes, leading to apoptosis and other effects (Ziegman and Alewood, 2015) (Table 2). Weever fish (Family: Trachinidae) form part of the order Scorpaeniformes, which also contains some of the world's most dangerous fish including stonefish and scorpionfish (Smith et al., 2016, Malacarne et al., 2018). The cytolysins from certain species of Scorpaeniformes have been shown to have genetic and protein domain similarity (Chuang and Shiao, 2014) and are hypothesised to be evolutionarily related (Malacarne, et al., 2018). Thus, unsurprisingly, it has been hypothesised (Ziegman and Alewood, 2015) that dracotoxin and trachinine may be related to the cytolysin toxins found in other Scorpaeniformes due to their similar structure and biological activities.
Table 2.
Toxins isolated from piscine venoms. Adapted from Ziegman and Alewood (2015) and Church and Hodgson (2002).
| Species | Common name | Toxin | Molecular Weight | Bioactivity of toxin | Citation |
|---|---|---|---|---|---|
| Scorpaena guttata | California scorpionfish | 50–800 kDa toxin | N/A | Schaeffer et al. (1971) | |
| Echiichthys vipera | Lesser weever | Trachinine | 324 kDa | N/A | Perriere et al. (1988) |
| Synanceia horrida | Estuarine stonefish | Stonustoxin | 148 kDa | Haemolysis, oedematic | Poh et al. (1991) |
| Trachinus draco | Greater weever | Dracotoxin | 105 kDa | Haemolysis, neuronal activation | Chhatwal and Dreyer (1992b) |
| Plotosus canius | Grey eel catfish | Toxin-PC | 15 kDa | Neuromuscular blocking activity in cardiac tissues | Auddy et al. (1995) |
| Synanceia verrucosa | Reef stonefish | Verrucotoxin | 322 kDa | Haemolysis, cardioactive | Garnier et al. (1995) |
| Neoverrucotoxin | 166 kDa | Haemolysis | Ueda et al. (2006) | ||
| Cardioleputin | 46 kDa | Cardioactive | Abe et al. (1996) | ||
| Synanceia horrida | Estuarine stonefish | Trachynilysin | 158 kDa | Cardioactive | Colasante et al. (1996) |
| Notesthes robusta | Bullrout | Nocitoxin | 169.8–174.5 kDa | Nociceptive, haemolysis | Hahn and O’Connor (2000) |
| Scatophagus argus | Spotted scat | SA-HT | 18 kDa | Haemorrhagic, oedema, muscle contraction/relaxation, capillary permeability | Karmakar et al. (2004) |
| Thalassophryne maculosa | Cano toadfish | TmC4-47.2 | Unknown | Myotoxic | Sosa-Rosales et al. (2005) |
| Hypodytes rubripinnis | Redfin velvetfish | Karatoxin | 110 kDa | Cytolytic, mitogenic, chemotactic | Nagasaka et al. (2009) |
| a | 160 kDa | N/A | Kiriake et al. (2013) | ||
| Scorpaena plumieri | Spotted scorpionfish | Plumieribetin | 14 kDa | Weakens cell-collagen contacts, reduces cell spreading, alters actin cytoskeleton | de Santana Evangelista et al. (2009) |
| Sp-CTx | 121 kDa | Neurotoxic, cardiotoxic, inflammatory, vaso-relaxant, haemolytic | Andrich et al. (2010) | ||
| SP-CL 1-5 | 16.8–17 kDa | Haemolytic, biphasic vasoactivity | Andrich et al. (2015) | ||
| Pterois volitans | Red lionfish | a | 2 subunits, both ~75 kDa | N/A | Kiriake and Shiomi (2011) |
| Pterois antennata | Spotfin lionfish | a | 2 subunits, both ~75 kDa | N/A | Kiriake and Shiomi (2011) |
| Thalassophryne nattereri | Toadfish | Nattectin | 15 kDa | Hemagglutination | Lopes-Ferreira et al. (2011) |
| Cathorops spixii | Madamango sea catfish | Wap65 | 54 kDa | Pro-inflammatory action | Ramos et al. (2012) |
| Pterois lunulata | Luna lionfish | a | 160 kDa | N/A | Kiriake et al. (2013) |
| Inimicus japonicus | Devil stinger | a | 160 kDa | N/A | Kiriake et al. (2013) |
Unnamed toxin similar to stonefish (based on SNTX and VTX).
Most cytolysisns within piscine venoms are around 150 kDa and possess one alpha and one beta subunit (Andrich et al., 2010, Ziegman and Alewood, 2015). However, like verrucotoxin (VTX), trachinine's structure and hypothesised molecular weight is much larger than other conventional fish venom haemolysins, with both toxins being tetramers and 322 kDa and 324 kDa in size, respectively (Garnier, et al., 1995). It is possible that this stark difference in the molecular weight of trachinine compared with other haemolysins (including dracotoxin) is due to the method used to estimate the molecular weight. Both VTX and trachinine had their molecular weight predicted using the same method by Hedrick and Smith (1968) and coincidentally are the haemolysins that are hypothesised to have the largest size (Perriere et al., 1988, Garnier et al., 1995). Contrastingly, the molecular weight of dracotoxin was confirmed with high performance liquid chromatography (Chhatwal and Dreyer, 1992b). Thus, it is recommended that future studies use methods such as column chromatography to isolate trachinine and confirm its molecular weight.
Dracotoxin possesses severe haemolytic activity on rabbit erthyrocytes (Chhatwal and Dreyer, 1992b). This biological activity is similar to that of Verrucotoxin (Garnier, et al., 1995), neoverrucotoxin (Ueda, et al., 2006), Stonustoxin (Poh et al., 1991), Sp-CTX (Andrich, et al., 2010) and Nocitoxin (Hahn and O’Connor, 2000) which all also possess haemolytic activities.
Dracotoxin was reported have membrane depolarising activities which did not involve sodium channels or potassium channels (Chhatwal and Dreyer, 1992b). The mode of action of how dracotoxin elicits membrane depolarisation and/or haemolysis was not investigated, however it may be due to pore formation in cell membranes as reported for several cytolysins from fish venoms (Desong et al., 1997, Ouanounou et al., 2002, Church et al., 2003, Andrich et al., 2010).
As with dracotoxin, the mode of action of trachinine was not studied, therefore it is recommended that future work should conduct bioassays of dracotoxin and trachinine on cells with and without osmoprotectants to check for pore-formation activity of the toxins (see Desong et al., 1997, Ramírez-Carreto et al., 2019). In conjunction with bioassays, future work should also focus on investigating the genetic similarities between both trachinine and dracotoxin, and other venomous fish toxins from the order Scorpaeniformes to help us understand their functions.
5. Piscine venom stabilisation
Throughout the last century high lability has been agreed by authors as a common problem with fish venoms (Evans, 1910, Malacarne et al., 2018) and therefore makes their pharmacological activities hard to investigate. Furthermore, their mucus-rich composition makes it difficult to separate proteins within the venom (Baumann et al., 2014). Thus, the high lability of fish venoms presents a major bottleneck for research, slowing the throughput of venom prospecting. This is especially true in the case of E. vipera venom, which has been speculated to be one of the most fragile fish venoms (Perriere and Goudey-Perriere, 1999).
Despite the known loss of crude bioactivity, freezing is a commonly practised method of stabilising crude fish venoms (Skeie, 1962a, Chhatwal and Dreyer, 1992a, Ueda et al., 2006, Prithiviraj, 2012). However, other methods such as ammonium sulphate precipitation have been shown to maintain the toxicity of T. draco crude venom for several weeks if kept at 4 °C, preserving the bioactivity as optimally as freezing (Chhatwal and Dreyer, 1992a). Furthermore, ammonium sulphate and acetone precipitation has been used to remove mucus contamination from the venom of blue-spotted stingray (Baumann, et al., 2014).
Isolating and preserving the individual toxins present within piscine venoms is just as challenging as preserving the crude venom bioactivity, due to their difficulty in handling and extreme lability out of their native form (Malacarne, et al., 2018). Nevertheless, recently Malacarne et al. (2018) investigated the optimal preservation of fish venom toxin Sp-CTx from Scorpaena plumieri venom, and concluded that if it was kept at – 80 °C or −196 °C in the presence of 10% glycerol at pH 7.4 its biological activity can remain for up to 60 days. Similar to Malacarne et al., 2018, Perriere et al., 1988 reported an increase in trachinine's shelf life if preserved in glycerol (50%) from 1 h to 24 h. Future research should aim to use methods from Perriere et al., 1988, Chhatwal and Dreyer (1992a) and Malacarne et al., 2018 to further extend the shelf life of both dracotoxin and trachinine.
6. Conclusions
Previous studies on E. vipera venom have been infrequent, with a 20+ year gap between the some of the most recent publications (Perriere and Goudey-Perriere, 1989, Fezai et al., 2016, Fezai et al., 2017), whilst T. draco venom has not been studied since the 1990's (Chhatwal and Dreyer, 1992b). During this gap the field of venomics has developed significantly, allowing more rigorous and detailed dissection of piscine venoms using complementary bioactive and proteomic studies, resulting in an increase in the discovery of many bioactive peptides. In the scorpion fish Scorpaena plumieri, venomics has allowed toxins in both the venom and skin mucus to be directly related to their biological activity (Borges et al., 2018), whereas in stonefish (Synanceia horrida) it has allowed an expansion in discovered venom constituents from only hyaluronidase and Stonustoxin (SNTX), to a full transcriptome of putative venom constituents where only 0.4% had previously been associated with piscine venoms (Ziegman et al., 2019), further emphasising the untapped source of toxins and proteins. As molecules within venoms are bioactive and often novel, many venoms are attracting interest for their pharmaceutical properties, with certain venom molecules being synthesised into drugs. As recent findings from Fezai et al., 2016, Fezai et al., 2017 demonstrate the cytotoxic bioactivity of E. vipera venom on human cancer cells, and as it is still unclear what components are present within E. vipera or its relative T. draco venom, complementary proteomic analysis and venom gland transcriptomics (see Xie et al., 2019) should be a focus for future research.
Ethical statement
The research that prompted the forming of this review was approved by the Newcastle University Ethics in Research Committee and was conducted in compliance with the UK Animals (Scientific Procedures) Act, 1986.
Declaration of competing interest
The authors have no conflicts of interest.
References
- Abe T., Sumatora M., Hashimoto Y., Yoshihara J., Shimamura Y., Fukami J. Purification and properties of a cardioactive toxin, cardioleputin, from stonefish, Synanceja verrucosa. J. Venom. Anim. Toxins. 1996;2:135–149. [Google Scholar]
- Abroug F., Souheil E., Ouanes I., Dachraoui F., Fekih-Hassen M., Ouanes Besbes L. Scorpion-related cardiomyopathy: clinical characteristics, pathophysiology, and treatment. Clin. Toxicol. 2015;53:511–518. doi: 10.3109/15563650.2015.1030676. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Rocha J.B.T., Morsch V.M., Schetinger M.R. vol. 207. 2016. (9 Snake Venom Acetylcholinesterase. Handbook of Venoms and Toxins of Reptiles). [Google Scholar]
- Allman G.J. XX.—on the stinging properly of the lesser weever-fish (Trachinus vipera.) J. Nat. Hist. 1840;6(36):161–165. [Google Scholar]
- Andrich F., Carnielli J., Cassoli J., Lautner R., Santos R., Pimenta A., de Lima M., Figueiredo S. A potent vasoactive cytolysin isolated from Scorpaena plumieri scorpionfish venom. Toxicon. 2010;56:487–496. doi: 10.1016/j.toxicon.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Andrich F., Richardson M., Naumann G., Cordeiro M., Santos A., Santos D., Oliveira J., de Lima M., Figueiredo S. Identification of C-type isolectins in the venom of the scorpionfish Scorpaena plumieri. Toxicon. 2015;95:67–71. doi: 10.1016/j.toxicon.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Auddy B., Muhuri D.C., Alam M.I., Gomes A. A lethal protein toxin (toxin-PC) from the Indian catfish (Plotosus canius, Hamilton) venom. Nat. Toxins. 1995;3:363–368. doi: 10.1002/nt.2620030507. [DOI] [PubMed] [Google Scholar]
- Barnett S., Saggiomo S., Smout M., Seymour J. Heat deactivation of the stonefish Synanceia horrida venom-implications for first-aid management. Diving and hyperbaric medicine. 2017;47(3):155. doi: 10.28920/dhm47.3.155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K., Casewell N.R., Ali S.A., Jackson T.N., Vetter I., Dobson J.S., Cutmore S.C., Nouwens A., Lavergne V., Fry B.G. A ray of venom: combined proteomic and transcriptomic investigation of fish venom composition using barb tissue from the blue-spotted stingray (Neotrygon kuhlii) J. Proteomics. 2014;109:188–198. doi: 10.1016/j.jprot.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Bonnet M.S. The toxicology of Trachinus vipera: the lesser weeverfish. British Homoeopathic J. 2000;89:84–88. doi: 10.1054/homp.1999.0359. [DOI] [PubMed] [Google Scholar]
- Borges M.H., Andrich F., Lemos P.H., Soares T.G., Menezes T.N., Campos F.V., Neves L.X., Castro-Borges W., Figueiredo S.G. Combined proteomic and functional analysis reveals rich sources of protein diversity in skin mucus and venom from the Scorpaena plumieri fish. J. Proteomics. 2018;187:200–211. doi: 10.1016/j.jprot.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Borley J.O. The poison apparatus of the weever. Trans. Norfolk & Norwich Naturalists' Soc. 1907;8:369–373. [Google Scholar]
- Borondo J.C., Sanz P., Nogue S., Poncela J.L., Garrido P., Valverde J.L. Fatal weeverfish sting. Hum. Exp. Toxicol. 2001;20:118–119. doi: 10.1191/096032701668435659. [DOI] [PubMed] [Google Scholar]
- Byerley I. On the Trachinus draco, or otterpike, sting fish, or weever. Proc. Literary and Philosoph. Soc. of Liverpool. 1849;5:156–168. [Google Scholar]
- Caccin P., Pellegatti P., Fernandez J., Vono M., Cintra-Francischinelli M., Lomonte B., Gutiérrez J.M., Di Virgilio F., Montecucco C. Why myotoxin-containing snake venoms possess powerful nucleotidases? Biochem. Biophys. Res. Commun. 2013;430(4):1289–1293. doi: 10.1016/j.bbrc.2012.11.129. [DOI] [PubMed] [Google Scholar]
- Cain D. Weever fish sting: an unusual problem. Br. Med. J. 1983;287:406. doi: 10.1136/bmj.287.6389.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F.V., Menezes T.N., Malacarne P.F., Costa F.L., Naumann G.B., Gomes H.L., Figueiredo S.G. A review on the Scorpaena plumieri fish venom and its bioactive compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016;22(1):35. doi: 10.1186/s40409-016-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci M., Mussi A., Leone G., Catricala C. Raynaud's phenomenon secondary to weever fish stings. Arch. Dermatol. 1996;132:838. [PubMed] [Google Scholar]
- Carlisle D.B. On the venom of the lesser weeverfish, Trachinus vipera. J. Mar. Biol. Assoc. U. K. 1962;42:155–162. [Google Scholar]
- Carrijo L.C., Andrich F., De Lima M.E., Cordeiro M.N., Richardson M., Figueiredo S.G. Biological properties of the venom from the scorpionfish (Scorpaena plumieri) and purification of a gelatinolytic protease. Toxicon. 2005;45(7):843–850. doi: 10.1016/j.toxicon.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Chhatwal I., Dreyer F. Biological properties of a crude venom extract from the greater weever fish Trachinus draco. Toxicon. 1992;30:77–85. doi: 10.1016/0041-0101(92)90503-w. [DOI] [PubMed] [Google Scholar]
- Chhatwal I., Dreyer F. Isolation and characterization of dracotoxin from the venom of the greater weever fish Trachinus draco. Toxicon. 1992;30:87–93. doi: 10.1016/0041-0101(92)90504-x. [DOI] [PubMed] [Google Scholar]
- Chinellato M., Savelli M.M. Une piqûre de vive de localisation inhabituelle. Annales Françaises de Médecine d'Urgence. 2015;5:59–60. [Google Scholar]
- Chuang P.S., Shiao J.C. Toxin gene determination and evolution in scorpaenoid fish. Toxicon. 2014;88:21–33. doi: 10.1016/j.toxicon.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Church J.E., Hodgson W.C. The pharmacological activity of fish venoms. Toxicon. 2002;40:1083–1093. doi: 10.1016/s0041-0101(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Church J.E., Hodgson W.C. Stonefish (Synanceia trachynis) antivenom: in vitro efficacy and clinical use. J. Toxicol. - Toxin Rev. 2003;22(1):69–76. [Google Scholar]
- Church J.E., Moldrich R.X., Beart P.M., Hodgson W.C. Modulation of intracellular Ca2+ levels by Scorpaenidae venoms. Toxicon. 2003;41(6):679–689. doi: 10.1016/s0041-0101(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Colasante C., Meunier F.A., Kreger A.S., Molgó J. Selective depletion of clear synaptic vesicles and enhanced quantal transmitter release at frog motor nerve endings produced by trachynilysin, a protein toxin isolated from stonefish (Synanceia trachynis) venom. Eur. J. Neurosci. 1996;8:2149–2156. doi: 10.1111/j.1460-9568.1996.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Davies R.S., Evans R.J. Weever fish stings: a report of two cases presenting to an accident and emergency department. J. Accid. Emerg. Med. 1996;13:139. doi: 10.1136/emj.13.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco R. Ricerche sulla permeabilita dell’asse cerebrospinale di Bufo viridis sotto l’azione del veleno di Trachinus draco e di Scorpaena scropha. Boll. Soc. Ital. Biol. Sper. 1936;11:767–768. [Google Scholar]
- De Marco R. Effetti del veleno di Trachinus sulla capacita di lavoro del gastrocnemio di rana. Riv. Biol. 1938;25:225–234. [Google Scholar]
- De Santana Evangelista K., Andrich F., de Rezende, F F, Niland S., Cordeiro M.N., Horlacher T., Castelli R., Schmidt-Hederich A., Seeberger P.H., Sanchez E.F. Plumieribetin, a fish lectin homologous to mannose-binding B-type lectins, inhibits the collagen-binding α1β1 integrin. J. Biol. Chem. 2009;284:34747–34759. doi: 10.1074/jbc.M109.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaan A., Ben-Meir P., Sagi A. A "scorpion fish"(Trachinus vipera) sting: fishermen's hazard. Br. J. Ind. Med. 1991;48:718–720. doi: 10.1136/oem.48.10.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker C.J. Chronic pain and impairment of function after a sting by the great weever fish (Trachinus draco) Ned. Tijdschr. Geneeskd. 2001;145:881. [PubMed] [Google Scholar]
- Desong C.H.E.N., Kini R.M., Raymond Y.U.E.N., Khoo H.E. Haemolytic activity of stonustoxin from stonefish (Synanceja horrida) venom: pore formation and the role of cationic amino acid residues. Biochem. J. 1997;325(3):685–691. doi: 10.1042/bj3250685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhananjaya B.L., D'Souza C.J. The pharmacological role of nucleotidases in snake venoms. Cell Biochem. Funct.: Cellular Biochemistry and its Modulation by Active Agents or Disease. 2010;28(3):171–177. doi: 10.1002/cbf.1637. [DOI] [PubMed] [Google Scholar]
- Dhananjaya B.L., D'Souza C.J. The pharmacological role of phosphatases (acid and alkaline phosphomonoesterases) in snake venoms related to release of purines‐a multitoxin. Basic Clin. Pharmacol. Toxicol. 2011;108(2):79–83. doi: 10.1111/j.1742-7843.2010.00630.x. [DOI] [PubMed] [Google Scholar]
- Diaz J.H. Marine scorpaenidae envenomation in travelers: epidemiology, management, and prevention. J. Trav. Med. 2015;22(4):251–258. doi: 10.1111/jtm.12206. [DOI] [PubMed] [Google Scholar]
- Evans H.M. Observations on the poisoned spines of the weever fish (Trachinus draco) Br. Med. J. 1907;1:73. doi: 10.1136/bmj.1.2402.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H.M. Further studies in haemolysis by weever venom. Br. Med. J. 1910;1(2573):982. doi: 10.1136/bmj.1.2573.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner P.J. Venomous marine animals. J. South Pacific Underwater Med. Soc. 2004;34:196–202. [Google Scholar]
- Fezai M., Slaymi C., Ben-Attia M., Lang F., Jemaà M. Purified lesser weever fish venom (Trachinus vipera) induces eryptosis, apoptosis and cell cycle arrest. Sci. Rep. 2016;6:39288. doi: 10.1038/srep39288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezai M., Slaymi C., Ben-Attia M., Kroemer G., Lang F., Jemaà M. Inhibition of colon carcinoma cell migration following treatment with purified venom from lesser weever fish (Trachinus vipera) Cell. Physiol. Biochem. 2017;41:2363–2373. doi: 10.1159/000475646. [DOI] [PubMed] [Google Scholar]
- Garnier P., Goudey-Perriere F., Breton P., Dewulf C., Petek F., Perriere C. Enzymatic properties of the stonefish (Synanceia verrucosa Bloch and Schneider, 1801) venom and purification of a lethal, hypotensive and cytolytic factor. Toxicon. 1995;33:143–155. doi: 10.1016/0041-0101(94)00151-w. [DOI] [PubMed] [Google Scholar]
- Garnier P., Grosclaude J.M., Goudey-Perrière F., Gervat V., Gayral P., Jacquot C., Perrière C. Presence of norepinephrine and other biogenic amines in stonefish venom. J. Chromatogr. B Biomed. Sci. Appl. 1996;685(2):364–369. doi: 10.1016/s0378-4347(96)00203-4. [DOI] [PubMed] [Google Scholar]
- Gomes H.L., Menezes T.N., Carnielli J.B., Andrich F., Evangelista K.S., Chávez-Olórtegui C., Vassallo D.V., Figueiredo S.G. Stonefish antivenom neutralises the inflammatory and cardiovascular effects induced by scorpionfish Scorpaena plumieri venom. Toxicon. 2011;57(7–8):992–999. doi: 10.1016/j.toxicon.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Gressin L. Contribution a l'étude de l'appareil a venin chez les Poissons du genre" vive"(Trachynus drago, Trachynus vipera, Trachynus radiatus, Trachynus araneus) Thèse Fac. Méd. 1884;51 [Google Scholar]
- Grunwald T., Bockisch B., Spillner E., Ring J., Bredehorst R., Ollert M.W. Molecular cloning and expression in insect cells of honeybee venom allergen acid phosphatase (Api m 3) J. Allergy Clin. Immunol. 2006;117(4):848–854. doi: 10.1016/j.jaci.2005.12.1331. [DOI] [PubMed] [Google Scholar]
- Haavaldsen R., Fonnum F. Weever venom. Nature. 1963;199:286–287. doi: 10.1038/199286a0. [DOI] [PubMed] [Google Scholar]
- Hahn S., O’Connor J. An investigation of the biological activity of bullrout (Notesthes robusta) venom. Toxicon. 2000;38:79–89. doi: 10.1016/s0041-0101(99)00135-x. [DOI] [PubMed] [Google Scholar]
- Halpern P., Sorkine P., Raskin Y. Envenomation by Trachinus draco in the eastern mediterranean. Eur. J. Emerg. Med. 2002;9:274–277. doi: 10.1097/00063110-200209000-00014. [DOI] [PubMed] [Google Scholar]
- Halstead B.W., Modglin R.F. Weever fish stings and the venom apparatus of weevers (Trachinus) Zeitschrift fur Tropenmedizin und Parasitologie. 1958;9:129–146. [PubMed] [Google Scholar]
- Harris R.J., Jenner R.A. Evolutionary ecology of fish venom: adaptations and consequences of evolving a venom system. Toxins. 2019;11(2):60. doi: 10.3390/toxins11020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J.L., Smith A.J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch. Biochem. Biophys. 1968;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Rim G.S., Yang H.I., Yin C.S., Koh H.G., Jang M.H., Kim C.J., Choe B.K., Chung J.H. Bee venom induces apoptosis through caspase-3 activation in synovial fibroblasts of patients with rheumatoid arthritis. Toxicon. 2005;46(1):39–45. doi: 10.1016/j.toxicon.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Hopkins B.J., Hodgson W.C. Enzyme and biochemical studies of stonefish (Synanceja trachynis) and soldierfish (Gymnapistes marmoratus) venoms. Toxicon. 1998;36:791–793. doi: 10.1016/s0041-0101(97)00167-0. [DOI] [PubMed] [Google Scholar]
- Karmakar S., Muhuri D., Dasgupta S., Nagchaudhuri A., Gomes A. Isolation of a haemorrhagic protein toxin (SA-HT) from the Indian venomous butterfish (Scatophagus argus, Linn) sting extract. Indian J. Exp. Biol. 2004;42:452–460. [PubMed] [Google Scholar]
- Khoo H.E. Bioactive proteins from stonefish venom. Clin. Exp. Pharmacol. Physiol. 2002;29(9):802–806. doi: 10.1046/j.1440-1681.2002.03727.x. [DOI] [PubMed] [Google Scholar]
- Kiriake A., Shiomi K. Some properties and cDNA cloning of proteinaceous toxins from two species of lionfish (Pterois antennata and Pterois volitans) Toxicon. 2011;58:494–501. doi: 10.1016/j.toxicon.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Kiriake A., Suzuki Y., Nagashima Y., Shiomi K. Proteinaceous toxins from three species of scorpaeniform fish (lionfish Pterois lunulata, devil stinger Inimicus japonicus and waspfish Hypodytes rubripinnis): close similarity in properties and primary structures to stonefish toxins. Toxicon. 2013;70:184–193. doi: 10.1016/j.toxicon.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Lopes-Ferreira M., Magalhaes G.S., Fernandez J.H., Junqueira-de-Azevedo I.D.L.M., le Ho P., Lima C., Valente R.H., Moura-da-Silva A.M. Structural and biological characterization of Nattectin, a new C-type lectin from the venomous fish Thalassophryne nattereri. Biochimie. 2011;93:971–980. doi: 10.1016/j.biochi.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Magalhães G.S., Lopes-Ferreira M., Junqueira-de-Azevedo I.L., Spencer P.J., Araújo M.S., Portaro F.C.V., Ma L., Valente R.H., Juliano L., Fox J.W., Ho P.L. Natterins, a new class of proteins with kininogenase activity characterized from Thalassophryne nattereri fish venom. Biochimie. 2005;87(8):687–699. doi: 10.1016/j.biochi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Malacarne P.F., Menezes T.N., Martins C.W., Naumann G.B., Gomes H.L., Pires R.G., Figueiredo S.G., Campos F.V. Advances in the characterization of the Scorpaena plumieri cytolytic toxin (Sp-CTx) Toxicon. 2018;150:220–227. doi: 10.1016/j.toxicon.2018.06.065. [DOI] [PubMed] [Google Scholar]
- Mayser P., Dreyer F., Repp H. Persistent skin reaction and Raynaud phenomenon after a sting by Echiichthys draco (great weever fish) Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2003;54:633–637. doi: 10.1007/s00105-002-0463-4. [DOI] [PubMed] [Google Scholar]
- Mulcah D.M., Devitt A., Shannon F., Reidy D., Callagy G., Walsh M. Case report: weever fish sting—an unusual cause of foot pain. Ir. J. Med. Sci. 1996;165:153–154. doi: 10.1007/BF02940238. [DOI] [PubMed] [Google Scholar]
- Nagasaka K., Nakagawa H., Satoh F., Hosotani T., Yokoigawa K., Sakai H., Sakuraba H., Ohshima T., Shinohara M., Ohura K. A novel cytotoxic protein, Karatoxin, from the dorsal spines of the redfin velvetfish, Hypodytes rubripinnis. Toxin Rev. 2009;28:260–265. [Google Scholar]
- OBIS . Intergovernmental Oceanographic Commission of UNESCO; 2015. Global Map Showing Geogrpahic Distirbution of Echiichthys vipera.https://obis.org/taxon/150630 Accessed: 30.07.19. (Available : Ocean Biogeographic Information System. [Google Scholar]
- Ouanounou G., Malo M., Stinnakre J., Kreger A.S., Molgó J. Trachynilysin, a neurosecretory protein isolated from stonefish (Synanceia trachynis) venom, forms nonselective pores in the membrane of NG108-15 cells. J. Biol. Chem. 2002;277(42):39119–39127. doi: 10.1074/jbc.M203433200. [DOI] [PubMed] [Google Scholar]
- Padin X.A., Alonso-Fernández A., Lijó A., Otero V., Otero J. Environmental drivers of lesser weever stings on the northeast Atlantic coast (A Lanzada beach, Spain) Ecol. Indicat. 2018;95:242–249. [Google Scholar]
- Parker W.N. On the poison‐organs of Trachinus. Proc. Zool. Soc. Lond. 1888;56(1):359–367. [Google Scholar]
- Perriere C. [S.l.]; 1985. Les glandes venimeuses et le venin de la petite vive (Trachinus vipera C.V.) : etude cytologique et données biochimiques, these de doctorat de l’universite Paris-VI, specialite cytologi. 176. [Google Scholar]
- Perriere C., Goudey-Perriere F. 53A. Physiologie; 1987. (Activités enzymatiques dans le venin de la petite vive Trachinus vipera (Téléostéen, Perciforme)). [Google Scholar]
- Perriere C., Goudey-Perriere F. Origin and function of supporting cells in the venom glands of the lesser weeverfish (Trachinus vipera) Toxicon. 1989;27(3):287–295. doi: 10.1016/0041-0101(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Perrière C., Goudey-Perrière F. Particularités des venins de Poissons. In Annales de l'Institut Pasteur/Actualites. 1999;10(2):253–272. [Google Scholar]
- Perriere C., Michel C. Les glandes operculaires de la petite vive, Trachinus vipera C. V. (Teléosteens, Trachinoidea, Trachinidae). I – Étude cytologique. Cah. Biol. Mar. 1986;27:469–490. [Google Scholar]
- Perriere C., Goudey-Perriere F., Petek F. Purification of a lethal fraction from the venom of the weever fish. Trachinus vipera C.V. Toxicon. 1988;26:1222–1227. doi: 10.1016/0041-0101(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Phisalix C. Experiences sur le venin des vives (Trachinus vipera et Trachinus draco) Bull. Museum Nat. Hist. Nat. (Paris) 1899;5:256–258. [Google Scholar]
- Poh C., Yuen R., Khoo H., Chung M., Gwee M., Gopalakrishnakone P. Purification and partial characterization of stonustoxin (lethal factor) from Synanceja horrida venom. Comp. Biochem. Physiol. B Comp. Biochem. 1991;99:793–798. doi: 10.1016/0305-0491(91)90143-2. [DOI] [PubMed] [Google Scholar]
- Poh C.H., Yuen R., Chung M.C.M., Khoo H.E. Purification and partial characterization of hyaluronidase from stonefish (Synanceja horrida) venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1992;101:159–163. doi: 10.1016/0305-0491(92)90172-n. [DOI] [PubMed] [Google Scholar]
- Prithiviraj N. Bioactive properties of the stone fish Synanceia horrida thomas 1984 spine venom. International Journal of Pharmaceutical and Biological Archive. 2012;3(5):1217–1221. [Google Scholar]
- Ramírez-Carreto S., Vera-Estrella R., Portillo-Bobadilla T., Licea-Navarro A., Bernaldez-Sarabia J., Rudiño-Piñera E., Verleyen J.J., Rodríguez E., Rodríguez-Almazán C. Transcriptomic and proteomic analysis of the tentacles and mucus of Anthopleura dowii verrill, 1869. Mar. Drugs. 2019;17(8):436. doi: 10.3390/md17080436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A.D., Conceição K., Silva P.I., Jr., Richardson M., Lima C., Lopes-Ferreira M. Specialization of the sting venom and skin mucus of Cathorops spixii reveals functional diversification of the toxins. Toxicon. 2012;59:651–665. doi: 10.1016/j.toxicon.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Repsold L., Joubert A.M. Eryptosis: an erythrocyte’s suicidal type of cell death. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/9405617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F.E. Weever fish sting: the last word. Br. Med. J. 1983;287:981. doi: 10.1136/bmj.287.6397.981-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F.E., Emery J.A. Venom of the weevers Trachinus draco and Trachinus vipera. Ann. N. Y. Acad. Sci. 1960;90:805–819. doi: 10.1111/j.1749-6632.1960.tb26424.x. [DOI] [PubMed] [Google Scholar]
- Sales P.B.V., Santoro M.L. Nucleotidase and DNase activities in Brazilian snake venoms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008;147(1):85–95. doi: 10.1016/j.cbpc.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Schaeffer R.C., Jr., Carlson R.W., Russell F.E. Some chemical properties of the venom of the scorpionfish Scorpaena guttata. Toxicon. 1971;9:69–78. doi: 10.1016/0041-0101(71)90045-6. [DOI] [PubMed] [Google Scholar]
- Schmidt F.T. Om fjarsingens stik og giitredskaber. Nord. Med. Arkiv. 1874;6 [Google Scholar]
- Sivan G. Fish venom: pharmacological features and biological significance. Fish Fish. 2009;10(2):159–172. [Google Scholar]
- Sivan G., Venketasvaran K., Radhakrishnan C. Characterization of biological activity of Scatophagus argus venom. Toxicon. 2010;56:914–925. doi: 10.1016/j.toxicon.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Skeie E. Weeverfish toxin: extraction methods, toxicity determinations and stability examinations 1. Acta Pathol. Microbiol. Scand. 1962;55(2):166–174. [PubMed] [Google Scholar]
- Skeie E. Toxin of the weeverfish (Trachinus draco) Experimental studies on animals. Acta Pharmacol. Toxicol. 1962;19(2):107–120. doi: 10.1111/j.1600-0773.1962.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Skeie E. Weeverfish toxin: some physico‐chemical and immunological observations. Acta Pathol. Microbiol. Scand. 1962;56(2):229–238. [PubMed] [Google Scholar]
- Skeie E. Weeverfish stings. Frequency, occurrence, clinical course, treatment and studies on the venom apparatus of the weeverfish, the nature of the toxin and immunological aspects. Dan. Med. Bull. 1966;13(4):119–121. [PubMed] [Google Scholar]
- Smith W.L., Wheeler W.C. Venom evolution widespread in fishes: a phylogenetic road map for the bioprospecting of piscine venoms. J. Hered. 2006;97(3):206–217. doi: 10.1093/jhered/esj034. [DOI] [PubMed] [Google Scholar]
- Smith W.L., Stern J.H., Girard M.G., Davis M.P. Evolution of venomous cartilaginous and ray-finned fishes. Integr. Comp. Biol. 2016;56:950–961. doi: 10.1093/icb/icw070. [DOI] [PubMed] [Google Scholar]
- Son D.J., Lee J.W., Lee Y.H., Song H.S., Lee C.K., Hong J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007;115(2):246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Sosa-Rosales J.I., Piran-Soares A.A., Farsky S.H., Takehara H.A., Lima C., Lopes-Ferreira M. Important biological activities induced by Thalassophryne maculosa fish venom. Toxicon. 2005;45:155–161. doi: 10.1016/j.toxicon.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Strempel A.P., Ceballos E.H., Strempel J.P. Incidencia de picaduras de peces venenosos en la costa de Málaga, España, durante la época estival. Emerge: Revista de la Sociedad Española de Medicina de Urgencias y Emergencias. 2009;21:32–35. [Google Scholar]
- Ueda A., Suzuki M., Honma T., Nagai H., Nagashima Y., Shiomi K. Purification, properties and cDNA cloning of neoverrucotoxin (neoVTX), a hemolytic lethal factor from the stonefish Synanceia verrucosa venom. Biochim. Biophys. Acta Gen. Subj. 2006;1760(11):1713–1722. doi: 10.1016/j.bbagen.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Vyas V.K., Brahmbhatt K., Bhatt H., Parmar U. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pacific journal of Tropical Biomedicine. 2013;3(2):156–162. doi: 10.1016/S2221-1691(13)60042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel-Eichler A., Libersat F. Venom effects on monoaminergic systems. J. Comp. Physiol. 2004;190:683–690. doi: 10.1007/s00359-004-0526-3. [DOI] [PubMed] [Google Scholar]
- Xie B., Yu H., Kerkkamp H., Wang M., Richardson M., Shi Q. Comparative transcriptome analyses of venom glands from three scorpionfishes. Genomics. 2019;111(3):231–241. doi: 10.1016/j.ygeno.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Zammit L. More about weever fish and their toxic stings. The St. Luke's Hospital Gazette. 1974;9(2):108–113. [Google Scholar]
- Ziegman R., Alewood P. Bioactive components in fish venoms. Toxins. 2015;7:1497–1531. doi: 10.3390/toxins7051497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegman R., Undheim E.A., Baillie G., Jones A., Alewood P.F. Investigation of the estuarine stonefish (Synanceia horrida) venom composition. J. Proteomics. 2019;201:12–26. doi: 10.1016/j.jprot.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Zolfagharian H., Mohajeri M., Babaie M. Honeybee venom (Apis mellifera) contains anticoagulation factors and increases the blood-clotting time. J. Pharmacopuncture. 2015;18(4):7. doi: 10.3831/KPI.2015.18.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łopaciński B., Bak M., Fiszer M., Czerniak P., Krakowiak A. Poisoning with weever fish venom: a case report. Przegl. Lek. 2009;66:464. [PubMed] [Google Scholar]