Abstract
Objectives
Obesity is a well-recognised risk factor for osteoarthritis (OA). Our aim is to characterise body mass index (BMI)-associated pathological changes in the osteochondral unit and determine if obesity is the major causal antecedent of early joint replacement in patients with OA.
Methods
We analysed the correlation between BMI and the age at which patients undergo total knee replacement (TKR) in 41 023 patients from the Australian Orthopaedic Association National Joint Replacement Registry. We then investigated the effect of BMI on pathological changes of the tibia plateau of knee joint in a representative subset of the registry.
Results
57.58% of patients in Australia who had TKR were obese. Patients with overweight, obese class I & II or obese class III received a TKR 1.89, 4.48 and 8.08 years earlier than patients with normal weight, respectively. Microscopic examination revealed that horizontal fissuring at the osteochondral interface was the major pathological feature of obesity-related OA. The frequency of horizontal fissure was strongly associated with increased BMI in the predominant compartment. An increase in one unit of BMI (1 kg/m2) increased the odds of horizontal fissures by 14.7%. 84.4% of the horizontal fissures were attributable to obesity. Reduced cartilage degradation and alteration of subchondral bone microstructure were also associated with increased BMI.
Conclusions
The key pathological feature in OA patients with obesity is horizontal fissuring at the osteochondral unit interface. Obesity is strongly associated with a younger age of first TKR, which may be a result of horizontal fissures.
Keywords: knee osteoarthritis, obesity, total knee replacement, horizontal fissuring, osteochondral unit
Key messages.
What is already known about this subject?
Obesity is a risk factor for osteoarthritis (OA) but the mechanism of obesity-related OA is complex and controversial. It is still not clear if obesity heralds an early total knee replacement (TKR) in patients with OA nor are the underlying pathological features of obesity-related OA well documented.
What does this study add?
Based on assessment of the registry data of over 40 000 cases, more than 57% of patients in Australia who had a TKR were obese.
Patients classified as overweight, obese class I & II and obese class III received a TKR 2, 4 and 8 years earlier than patients with normal weight.
Horizontal fissuring at the osteochondral interface caused by mechanical overload is a novel pathological feature in OA patients with obesity.
How might this impact on clinical practice or future development?
Obesity heralds an early TKR in patients with OA. Horizontal fissuring on the osteochondral interface is the major pathological feature of OA patients with obesity. Appreciation of such a pathogenetic mechanism may help in developing new interventions in the management of obesity-related OA.
Introduction
Obesity affects more than a quarter of the global population. Despite numerous public health initiatives aimed at tackling this issue, the incidence of obesity continues to rise.1 Obesity is a well-recognised risk factor for osteoarthritis (OA) and contributes to 45% of the OA burden in Australia.2–5 Obese patients with total knee replacement (TKR) have a high risk of perioperative complications, which may lead to surgical revision, and pose a high direct medical cost.6–9
The underlying mechanism of obesity-related OA is complex and controversial.10 Abnormal mechanical loading and meta-inflammation are factors considered to contribute to the pathogenesis of OA.11 Studies on the aetiology of obesity-related OA among obese animal models generally suggested metainflammation as the main cause of obesity-related OA.12–14 On the other hand, epidemiological studies on human OA suggested that excessive abnormal mechanical loading on articular joints is the main risk factor on OA progression.2 15 A recent population-based cohort study in the Netherlands also supported the concept that obesity-related mechanical stress is the most important risk factor for knee OA.15
While current understanding on the impact of abnormal mechanical loading in patients with obesity-related OA is mostly limited to epidemiological data, we proposed that the pathological changes in the osteochondral units in the joints of these patients could provide insights into the mechanism underlying OA in obesity. A functional osteochondral unit plays a central role on biomechanical function as it distributes the loading on the joint surface.16 The interface also prevents movement of large molecules between these structural components.17 During OA development, the integrity of the osteochondral unit is breached by neurovascular invasion, increasing the crosstalk between cartilage and subchondral bone.18 19 At present, however, the pathological changes at the osteochondral interface in OA patients with obesity remain unclear.
To determine the impact of obesity on OA, we analysed the correlation between body mass index (BMI) and the age at which patients undergo TKR in 41 023 cases of patients from the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). We then investigated the effect of BMI on histopathological changes of the knee joint in a representative subset of the registry, to search for a link between BMI, histopathological changes of the osteochondral unit and its interface, and the subsequent age at which first TKR was performed.
Methods
Study population
We obtained deidentified data on all primary TKR procedures performed for primary OA from 1 September 1999 to 31 December 2015 from the AOANJRR. Summary data, including age, BMI and gender, for 41 023 cases were collected. Data were categorised by BMI using the following WHO definition: ‘Underweight and Normal weight’ (<24.9 kg/m2), ‘Overweight’ (25–29.9 kg/m2), ‘Obese class I & II’ (30–39.9 kg/m2) and ‘Obese class III’ (≥40 kg/m2).
We also collected specimens from 88 patients with primary OA who had a TKR as a representative subset from this registry. We selected patients from each of the four categories of BMI without the knowledge of the registry cohort data. Samples size in each group was determined by availability and patient consent. Comparison between the cases and the registry cohort showed that the average age at the time of TKR in each category was similar, but the percentages of cases in each BMI category between the groups were different. Patient clinical data including BMI, age, gender and radiographic information were recorded. The diagnosis of OA was made according to the classification of the American College of Rheumatology.20 All radiographs were assessed according to the Kellgren and Lawrence criteria by an experienced assessor, who was blinded to patients’ BMI.21 The most severe OA compartment, either medial or lateral, was identified as the predominant compartment.22 Informed written consent was obtained from all patients.
Micro-CT
A cylindrical specimen of osteochondral tissue was extracted from both the centre one-third of the medial and lateral tibial plateaus by a precision bone trephine under continuous water irrigation.23 After fixation, all tissue blocks were examined using a micro-CT scanner (Skyscan 1174, Bruker, Kontich, Belgium) according to previous publication.24 25
The following parameters were calculated: bone volume fraction (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), trabecular number (Tb.N, 1/mm), structure model index (SMI), degree of anisotropy (DA) and connectively density (Conn.Dn, 1/mm3). Bone mineralised density (BMD, mg/cm3) of subchondral bone (SCB) was calibrated by using the attenuation coefficient of two defined BMD phantoms of 0.25 and 0.75 g/cm3.
Histological specimen processing
All tissue blocks were processed for histological evaluation. Each was infiltrated and embedded in methyl-methacrylate; 5 μm thickness sections were obtained and stained with Goldner’s Trichrome for the morphological and histomorphometric examination of SCB and the osteochondral interface and Safranin-O/Fast Green for evaluation of cartilage degradation.24 25
Histomorphometric measurements of SCB
Histomorphometry was performed using Bioquant Osteo Histomorphometry software (Bioquant Osteo, Nashville, Tennessee, USA). The following parameters were measured: thickness of osteoid (O.Th, μm), per cent osteoid volume (OV/BV, %), per cent osteoid surface (OS/BS, %), specific osteoid surface (OS/BV, mm2/mm3), per cent eroded surface (ES/BS, %), specific eroded surface (ES/BV, mm2/mm3), eroded surface in bone tissue volume (ES/TV, mm2/mm3) and the ratio of osteoid surface to eroded surface (OS/ES).24
Histological evaluation of articular cartilage and osteochondral interface
The assessment was performed by histopathologists (MZ and JP), blinded to the BMI of each patient, using the Osteoarthritis Research Society International (OARSI) grading system.26 A fissure at the osteochondral interface was defined as a gap in the cartilage–bone interface containing at least two of the following characteristics: (1) cartilage erosion; (2) free cartilage/bone debris; (3) fibrogranulation tissue infiltration; (4) rupture of microcapillaries and the presence of red blood cells and leucocytes within the fissures. The definition and location of the fissures were mutually agreed by qualified investigators (MZ, LC and JP). The length is characterised by the surface of the calcified cartilage immediately beneath the separation. The area of fissures is characterised by an area of the splitting at the osteochondral interface. These two parameters were measured by Bioquant Osteo Histomorphometry software.
Statistical analysis
Registry cohort data were expressed as mean±SD. Data were tested for normality using the Shapiro-Wilks test. One-way analysis of variance (ANOVA) followed by post-hoc comparison was applied to analyse differences in age at TKR between the four weight categories.
Among the 88 cases collected for microstructural and pathological analyses, an independent sample t-test was applied to compare the mean age at TKR between each category and its represented category. Fisher’s exact test and one-way ANOVA were used to, respectively, compare the categorical and continuous variables of the demographic characteristics among four categories based on BMI in the case series subset.
Univariable and multivariable general linear regression models were used to investigate the association between BMI and the parameters of bone microstructure, remodelling and cartilage OARSI grading (all as continuous variables). Univariable and multivariable logistic regression models were applied to explore the effect of BMI on the frequency of the fissures in osteochondral interface. In logistic regression models, we defined dummy variables using 0 to indicate cases without horizontal fissures and 1 to indicate cases with horizontal fissures. Multivariable linear regression and logistic regression were adjusted for sex and age. OR and 95% CIs are reported. The relationship between horizontal fissures (including area and length) and BMI was assessed by Spearman’s correlation.
To estimate the proportion of horizontal fissures attributable to obesity, we calculated the attributable fraction among the exposure (AFex) by using the formula AFex= (OR−1)/OR.27–29 The OR value was obtained by multivariable logistic regression to estimate the ORs for the association between the horizontal fissures and normal weight, overweight and obesity.
Variables were expressed as mean±SD, median (25th, 75th percentiles) and frequencies (percentage), respectively. All statistical methods were applied by Prism 7 software and IBM SPSS Statistics (V.25). All hypotheses were two-tailed tested, and p<0.05 was considered statistically significant.
Results
OA Patients with obesity received TKR at younger age than those of normal weight
We analysed the characteristics of 41 023 cases of patients with primary OA receiving TKR and grouped them into 4 categories according to their BMI. As shown in table 1, 57.58% of patients who had a TKR were obese. The age at which OA patients had a TKR differed significantly within their BMI groups. A negative correlation between age at TKR and BMI category was observed (p<0.0001) with post hoc testing showing the age at TKR significantly decreased with each increase in BMI category. Patients in the overweight, obese class I&II and class III categories received a TKR 1.89, 4.48 and 8.08 years earlier than patients in the normal weight category, respectively. The association was independent of sex. Comparison of age at TKR between the case series and AOANJRR cohort showed no significant age differences in each category (online supplementary figure 1). Table 2 summarises the demographic characteristics of the case series subset.
Table 1.
Relationship between body mass index and mean age of patients undergoing total knee replacement
| Normal weight | Overweight | Obese class I & II | Obese class III | One-way ANOVA P (age) |
|||||
| N (%) | Age | N (%) | Age | N (%) | Age | N (%) | Age | ||
| Overall | 4 533 (11.05) | 71.70±9.43 | 12 867 (31.37) | 69.81±9.07 | 19 432 (47.37) | 67.22±8.34 | 4 191 (10.21) | 63.62±7.77 | <0.0001 |
| Male | 1 797 (9.9) | 71.77±9.75 | 6 808 (37.4) | 69.24±9.08 | 8 441 (46.4) | 66.63±8.27 | 1 149 (6.3) | 63.60±7.80 | <0.0001 |
| Female | 2 736 (12.0) | 71.66±9.42 | 6 059 (26.5) | 70.45±8.56 | 10 991 (48.1) | 67.67±8.39 | 3 042 (13.3) | 63.63±8.27 | <0.0001 |
Data are represented as mean±SD or number (percentage). Mean ages among four categories in each registry were significantly different at p<0.001 by post-hoc comparison.
ANOVA, analysis of variance.
Table 2.
Demographic and clinical characteristics of the subset of patients undergoing total knee replacement
| Normal weight | Overweight | Obese class I & II | Obese class III | P value | |
| (n=26) | (n=19) | (n=31) | (n=12) | ||
| Women (N/%) | 8 (30.8) | 12 (63.2) | 15 (48.4) | 8 (66.7) | 0.413 |
| Age/year* | 72.46±8.31 | 69.00±8.03 | 66.84±9.28 | 61.42±6.49 | 0.003 |
| X-ray/ K-L† | 2.5 (2 to 3) | 2 (1 to 3) | 2 (2 to 4) | 2 (2 to 3.75) | 0.725 |
| Predominant compartment | 0.093 | ||||
| Medial (N/%) | 17 (65.4) | 18 (94.7) | 24 (77.4) | 8 (66.7) | |
| Lateral (N/%) | 9 (34.6) | 1 (5.3) | 7 (22.6) | 4 (33.3) |
The comparisons of continuous parameters were performed using one-way analysis of variance. Comparison of categorical data was performed using Fisher’s exact test.
*Values presented as mean±SD.
†Value presented as median (25th, 75th percentiles).
K-L, Kellgren and Lawrence.
annrheumdis-2020-216942supp001.pdf (399.5KB, pdf)
Increased BMI is associated with less articular cartilage degradation
We then investigated the histopathology of a representative subset of 88 cases from the AOANJRR. Patients with higher BMI displayed less disruption and matrix loss in the articular cartilage surface in the predominant compartment, as well as overall in both medial and lateral compartments, when compared with patients with lower BMI. Patients in the normal weight category displayed cartilage erosion and degenerative changes, starting from the superficial layer and extending to the deeper layers, while patients in the obese categories had less cartilage erosion on the articular surface (table 3). In the predominant compartment, lower OARSI scores were associated with increased BMI in unadjusted (p=0.003) and multivariable linear regression (p adjusted=0.004). Similar results were obtained in both medial and lateral compartments (medial p=0.011, p adjusted =0.016 and lateral p=0.030, p adjusted=0.044). These results suggested that OA patients with obesity have less cartilage degradation compared with OA patients of normal weight.
Table 3.
Body mass index-associated changes of cartilage degradation and subchondral bone (SCB) remodelling
| Unadjusted | Age and sex adjusted | |||||
| β (95% CI) | P value | β (95% CI) | P value | |||
| Predominant | Cartilage evaluation | OARSI | −0.046 (−0.076 to −0.016) | 0.003 | −0.048 (−0.081 to −0.016) | 0.004 |
| Bone formation | OV/BV (%) | 0.001 (0.000 to 0.001) | 0.019 | 0.001 (0.000 to 0.001) | 0.014 | |
| OS/BS (%) | 0.004 (0.001 to 0.007) | 0.022 | 0.004 (0.001 to 0.008) | 0.024 | ||
| O.Th (µm) | 0.327 (0.161 to 0.492) | 0.000 | 0.293 (0.112 to 0.454) | 0.002 | ||
| Bone resorption | ES/BS (%) | 0.000 (−0.001 to 0.000) | 0.349 | 0.000 (−0.001 to 0.000) | 0.254 | |
| ES/BV (mm2/mm3) | −0.003 (−0.009 to 0.002) | 0.228 | −0.004 (−0.010 to 0.002) | 0.178 | ||
| ES/TV (mm2/mm3) | −0.001 (−0.003 to 0.001) | 0.190 | −0.001 (−0.003 to 0.001) | 0.173 | ||
| OS/BV (mm2/mm3) | −0.148 (−0.015 to 0.069) | 0.199 | 0.032 (−0.015 to 0.079) | 0.181 | ||
| OS/ES | 0.776 (−0.080 to 1.632) | 0.075 | 0.788 (−0.171 to 1.747) | 0.106 | ||
| Medial | Cartilage evaluation | OARSI | −0.035 (−0.062 to −0.008) | 0.011 | −0.037 (−0.066 to −0.007) | 0.016 |
| Bone formation | OV/BV (%) | 0.001 (0.000 to 0.001) | 0.014 | −0.001 (0.000 to 0.001) | 0.011 | |
| OS/BS (%) | 0.004 (0.001 to 0.007) | 0.014 | 0.004 (0.001 to 0.008) | 0.015 | ||
| O.Th (µm) | 0.345 (0.181 to 0.510) | 0.000 | 0.311 (0.133 to 0.490) | 0.001 | ||
| Bone resorption | ES/BS (%) | 0.000 (0.000 to 0.000) | 0.579 | 0.000 (0.000 to 0.000) | 0.596 | |
| ES/BV (mm2/mm3) | −0.002 (−0.007 to 0.002) | 0.237 | −0.002 (−0.007 to 0.002) | 0.310 | ||
| ES/TV (mm2/mm3) | −0.001 (−0.002 to 0.001) | 0.396 | −0.001 (−0.002 to 0.001) | 0.423 | ||
| OS/BV (mm2/mm3) | 0.030 (−0.012 to 0.071) | 0.163 | 0.036 (−0.011 to 0.082) | 0.399 | ||
| OS/ES | 0.772 (−0.084 to 1.629) | 0.077 | 0.764 (−0.197 to 1.724) | 0.118 | ||
| Lateral | Cartilage evaluation | OARSI | −0.033 (−0.062 to −0.003) | 0.030 | −0.033 (−0.064 to −0.001) | 0.044 |
| Bone formation | OV/BV (%) | 0.001 (9.915E−5 to 0.001) | 0.020 | 0.001 (0.000 to 0.001) | 0.009 | |
| OS/BS (%) | 0.004 (0.001 to 0.007) | 0.024 | 0.004 (0.001 to 0.008) | 0.017 | ||
| O.Th (µm) | 0.320 (0.161 to 0.479) | 0.000 | 0.319 (0.143 to 0.495) | 0.001 | ||
| Bone resorption | ES/BS (%) | 0.000 (−0.001 to 0.000) | 0.226 | 0.000 (−0.001 to 0.000) | 0.235 | |
| ES/BV (mm2/mm3) | −0.004 (−0.010 to 0.001) | 0.137 | −0.004 (−0.011 to 0.002) | 0.167 | ||
| ES/TV (mm2/mm3) | −0.001 (−0.003 to 0.001) | 0.153 | −0.001 (−0.003 to 0.001) | 0.199 | ||
| OS/BV (mm2/mm3) | 0.027 (−0.016 to 0.069) | 0.218 | 0.034 (−0.013 to 0.081) | 0.149 | ||
| OS/ES | 0.768 (−0.087 to 1.632) | 0.078 | 0.823 (−0.119 to 1.766) | 0.086 | ||
Dependent variable: histomorphomectric parameters (continuous variable). Independent variable: BMI (kg/m2, continuous variable). Method: linear regression.
ES/BS, per cent eroded surface; ES/BV, specific eroded surface; ES/TV, eroded surface in bone tissue volume; OARSI, Osteoarthritis Research Society International Grading; OS/BS, per cent osteoid surface; OS/BV, specific osteoid surface; OS/ES, the ratio of osteoid surface to eroded surface; O.Th, thickness of osteoid; OV/BV, per cent osteoid volume.
Impact of increased BMI on SCB microstructure
We next examined if increased BMI had an impact on SCB microstructure. In the predominant compartment, linear regression adjusted for sex and age showed that BV/TV and BMD were negatively associated with BMI, while SMI was positively associated with BMI. Interestingly, without justification of the predominant compartment, there was nonlinear correlation between BMI and the microarchitectural parameters by both univariable and multivariable linear regression in both medial and lateral compartments (online supplementary table 1).
Histomorphometric assessment revealed that BMI was significantly and positively associated with parameters representing the formation of osteoid, including OV/BV, OS/BS and O.Th in both univariable and multivariable linear regression models in the predominant, lateral and medial compartments (table 3). BMI was not associated with bone surface erosion, as demonstrated by bone resorption parameters, including ES/BS, ES/BV, ES/TV and OS/ES, in neither predominant, medial nor lateral compartments. These results showed that while obesity increased osteoid formation and SMI in SCB, total bone volume and BMD were decreased.
Obesity causes horizontal fissuring and cartilage erosion at the osteochondral interface
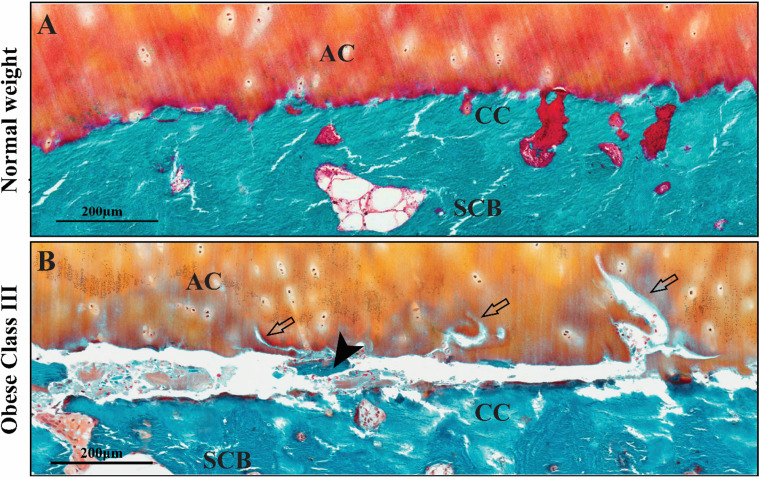
We next examined the integrity of the osteochondral interface between cartilage and SCB.30 We observed that patients with obesity displayed horizontal fissures with cartilage erosion at the osteochondral interface (figure 1), characterised by irregular cartilage erosion, fibrogranulation tissue infiltration, the presence of cartilage/bone debris and rupture of microcapillaries within the fissures at the osteochondral interface (figure 2). The presence of red blood cells and leucocytes within the fissures situated between the non-mineralised and calcified cartilage regions indicated that the fissures were not artefacts or damage during sample preparation. While not all patients with obesity had these pathological changes, patients with obesity showed higher frequency of horizontal fissuring, compared with those with normal weight. Horizontal fissures were a unique feature of OA pathology in patients with obesity. In the predominant compartment, 75% of patients in the obese class III category displayed horizontal fissures with cartilage erosion at the osteochondral interface of the predominant compartment, compared with only 7.7% of patients in the normal weight group. Over 58% of patients in the obese class III category displayed fissuring in both medial and lateral compartments of the tibial plateau, compared with none in the medial compartment and 19.2% in the lateral compartment of patients with normal weight (table 4). Measurement of the length and area of fissures showed that there were significant correlations between fissures and BMI in the predominant compartment or medial and lateral compartments without justification of the predominant compartment (table 5). The association between BMI and the presence of horizontal fissuring was significant after adjusting for sex and age (table 5). An increase in one unit of BMI (1 kg/m2) increased the odds of horizontal fissures by 14.7% (OR (95% CI) 1.147 (1.055 to 1.246)) in the predominant compartment. Subanalysis of attributable fraction showed that obesity increases the attributable fraction of horizontal fissures. The AFex of obesity was 84.4%, estimating that over 80% of the horizontal fissures in the predominant compartment were attributable to obesity. Together, these results suggested that abnormal overload in patients with obesity strongly contribute to horizontal fissures at the osteochondral interface.
Figure 1.
Representative images of horizontal fissures in the osteochondral units from OA patients with normal weight and morbid obesity. In patients with normal weight (A), cartilage is firmly attached on calcified cartilage (CC). In patients with obese class III (B), a horizontal fissure is observed at the osteochondral interface between the articular cartilage (AC) and subchondral bone (SCB). Free bone debris (black arrowhead) and cartilage erosion (empty arrow) are presented within the fissure.
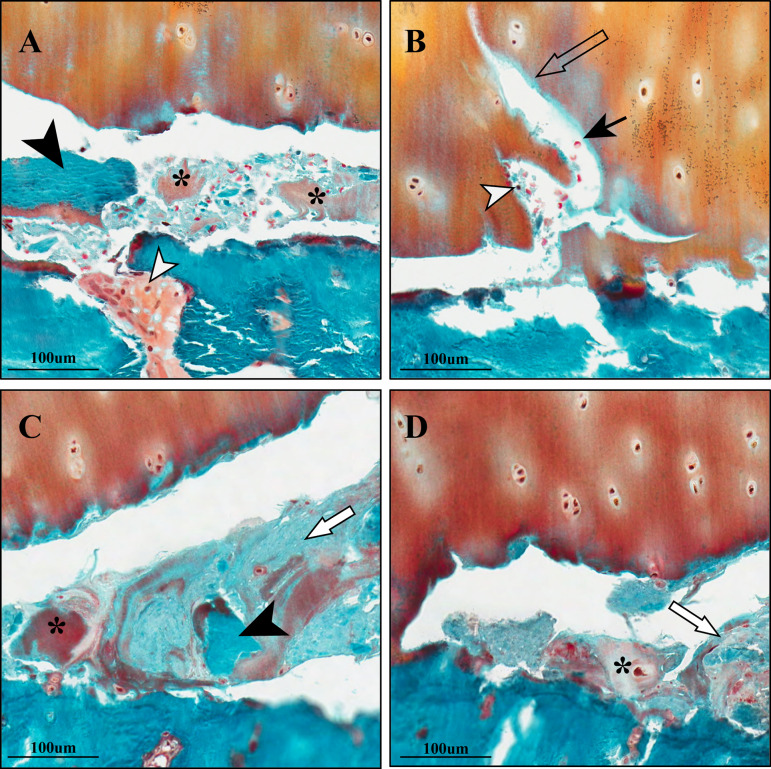
Figure 2.
Representative cases of horizontal fissures at the osteochondral interface. (A) Free cartilage (*) and bone debris (black arrowhead) are observed in the horizontal fissures. Presence of plasma cells (white arrowhead) is seen. (B) Cluster of red blood cells (black arrow) and leucocyte (white arrowhead) within the fissure. Cartilage erosion towards the articular surface (empty arrow) was observed in the horizontal interface between hyaline cartilage and the mineralised tissue. (C) Fibrous infiltration (white arrow), cartilage (*) and bone debris (black arrowhead) were observed within the fissures. (D) Fibrogranulation tissue infiltration (white arrow) was present in the horizontal fissures. Free cartilage debris (*) was also seen. Staining method: Goldner’s Trichrome.
Table 4.
Pathological changes of horizontal fissuring in osteochondral interface among body mass index categories
| Cartilage erosion N (%)* |
Fibrogranulation tissue infiltration N (%)* |
RBC/leucocyte infiltration N (%)* |
Bone/cartilage debris N (%)* |
Total† N (%) |
Length of fissures (μm) |
Area of fissures (mm2) |
|||
| Mean | Median‡ | Mean | Median ‡ | ||||||
| Predominant | |||||||||
| Normal weight | 2 (7.7%) | 2 (7.7%) | 0 (0) | 1 (3.8%) | 2 (7.7%) | 27.11±97.02 | 0 (0 to 0) | 0.002±0.006 | 0 (0 to 0) |
| Overweight | 3 (15.8%) | 3 (15.8%) | 0 (0) | 3 (15.8%) | 3 (15.8%) | 58.70±171.08 | 0 (0 to 0) | 0.01±0.03 | 0 (0 to 0) |
| Obese class I & II | 6 (19.4%) | 7 (22.6%) | 1 (3.2%) | 7 (22.6%) | 7 (22.6%) | 72.75±143.29 | 0 (0 to 30.25) | 0.01±0.03 | 0 (0 to 0.002) |
| Obese class III | 9 (75.0%) | 6 (50.0%) | 1 (8.3%) | 8 (66.7%) | 9 (75.0%) | 483.38±493.48 | 423.30 (0 to 693.60) | 0.02±0.03 | 0.02 (0 to 0.04) |
| Medial | |||||||||
| Normal weight | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 (0 to 0) | 0 | 0 (0 to 0) |
| Overweight | 2 (10.5%) | 2 (10.5%) | 0 (0) | 2 (10.5%) | 2 (10.5%) | 55.62±166.81 | 0 (0 to 0) | 0.006±0.020 | 0 (0 to 0) |
| Obese class I & II | 8 (25.8%) | 8 (25.8%) | 1 (3.2%) | 8 (25.8%) | 9 (29.0%) | 87.28±163.30 | 0 (0 to 133.31) | 0.01±0.03 | 0 (0 to 0.004) |
| Obese class III | 7 (58.33%) | 6 (50%) | 1 (8.3%) | 5 (41.7%) | 7 (58.33%) | 434.65±470.20 | 411.31 (0 to 693.60) | 0.03±0.03 | 0.014 (0.096) |
| Lateral | |||||||||
| Normal weight | 4 (15.4%) | 5 (19.2%) | 0 (0) | 3 (11.5%) | 5 (19.2%) | 66.85±146.38 | 0 (0 to 0) | 0.003±0.01 | 0 (0 to 0) |
| Overweight | 4 (21.1%) | 5 (26.3%) | 0 (0) | 4 (21.1%) | 5 (26.3%) | 118.98±243.15 | 0 (0 to 0) | 0.01±0.03 | 0 (0 to 0.012) |
| Obese class I & II | 9 (29.0%) | 10 (32.3%) | 0 (0) | 7 (22.6%) | 10 (32.3%) | 147.52±348.71 | 0 (0 to 253.65) | 0.02±0.04 | 0 (0 to 0.016) |
| Obese class III | 7 (58.33%) | 4 (33.3%) | 0 (0) | 7 (58.33%) | 7 (58.33%) | 364.67±431.92 | 221.51 (0 to 789.39) | 0.03±0.03 | 0.025 (0 to 0.047) |
*Data were presented as number (N) and percentage (%) of cases with the described pathology.
†Total number of cases with horizontal fissures.
‡Data were presented as median and IQR.
RBC, red blood cells.
Table 5.
Body mass index (BMI)-associated horizontal fissuring in osteochondral interface
| Unadjusted | P value | Age and sex adjusted | P value | |
| Predominant | ||||
| Incidence of fissures (OR (95% CI))* | 1.144 (1.061 to 1.233) | <0.001 | 1.147 (1.055 to 1.246) | 0.001 |
| Length of fissures (µm) (R)† | 0.403 | <0.001 | 0.417 | <0.001 |
| Area of fissures (mm2) (R)‡ | 0.400 | <0.001 | 0.391 | <0.001 |
| Medial | ||||
| Incidence of fissures (OR (95% CI))* | 1.173 (1.081 to 1.273) | <0.001 | 1.186 (1.081 to 1.302) | <0.001 |
| Length of fissures (µm) (R)† | 0.463 | <0.001 | 0.507 | <0.001 |
| Area of fissures (mm2) (R)‡ | 0.467 | <0.001 | 0.518 | <0.001 |
| Lateral | ||||
| Incidence of fissures (OR (95% CI))* | 1.086 (1.017 to 1.159) | 0.013 | 1.078 (1.004 to 1.157) | 0.038 |
| Length of fissures (µm) (R)† | 0.264 | 0.013 | 0.240 | 0.027 |
| Area of fissures (mm2) (R)‡ | 0.286 | 0.007 | 0.224 | 0.040 |
*Logistic regression between BMI (kg/m2, continuous variable) and incidence of fissures.
†Spearman’s rank correlation analysis between BMI (kg/m2) and the length of fissures (µm).
‡Spearman’s rank correlation analysis between BMI (kg/m2) and the area of fissures (mm2).
Discussion
We showed that 57.58% of patients in Australia who had a TKR were obese. Patients with overweight, obese class I & II or obese class III received a TKR 1.89, 4.48 and 8.08 years earlier than patients with normal weight, respectively. While the altered microstructure in SCB and the reduced cartilage degradation were observed in patients with higher BMI, horizontal fissuring at the osteochondral interface was the key pathological feature of obesity-induced OA. The frequency of horizontal fissures at the osteochondral interface was positively associated with increased BMI. Subanalysis of attributable fraction showed that 84.4% of the horizontal fissures in the predominant compartment were attributable to obesity.
Previous studies have shown that more than two-thirds of obese individuals have OA and eventually undergo TKR.2 31 32 Changulani et al showed that the mean age at which morbidly obese (BMI ≥40 kg/m2) patients underwent TKR was 13 years younger than patients with normal BMI, based on data from 344 patients.33 The data are similar with our large cohort study that age at TKR in patients with class III obesity was nearly 8 years younger than in those of normal weight. Due to the life span of knee prostheses, our study suggested that a weight-loss strategy prior to TKR in patients with obesity is warranted.
Abnormal mechanical loading is an important mechanism in accelerating the progression of obesity-related OA.34–36 The present study has shown that patients with obesity have more mechanically related pathological changes at the osteochondral interface. The osteochondral unit is a functional complex with cartilage having a higher Poisson’s ratio than the underlying mineralised bone.37 38 Under high compression loading, cartilage displays a larger lateral deformation; however, the deformation is restrained by its underlying calcified bed and the SCB.39 This results in the generation of secondary shear force to the cartilage–bone interface, creating fissures and exacerbating OA progression.39 Interestingly, the pathologesis of horizontal splitting of osteochondral interface in human OA was initially reported in 1915 and later confirmed by Meachim and Bentley in 1978. Animal studies in the 1980s of the overload-induced OA also showed induction of horizontal splitting of osteochondral interface.40 41 However, little attention has been paid to this observation over the last two decades. It is also noteworthy that horizontal fissures differ from cartilage delamination. The latter is caused by acute trauma in younger adults due to the sudden introduction of shear forces to the subchondral interface.42 43 Delamination is an isolated chondral lesion, normally causing complete separation of cartilage from the subchondral bone and results in the formation of a cartilage flap in the joint.44 45 On the other hand, horizontal fissures are a condition of chronic inflammation, causing cartilage erosion at the subchondral interface due to secondary shear force generated by compressive stress. No cartilage flaps or complete separation is seen. Our study showed that microscopic horizontal fissures are characterised by horizontal cartilage erosion, inflammation and rupture of microcapillaries at the osteochondral interface.
The effect of obesity on the microstructure of SCB seems somewhat contradictory.46 47 Previously, it has been shown that lower subchondral thickness in the SCB of femoral heads from patients with overweight/obesity compared with normal-weight patients.48 On the other hand, Reina et al showed that increased bone volume and more plate-like trabeculae was associated with increased BMI.49 In our study, we observed that patients with obesity have relatively higher osteoid formation and lower BV/TV in predominant compartment as compared with those in non-obese patients, suggesting that obesity alters the SCB microstructure in OA patients.
There are, however, limitations in this study. First, the sample size collected for histopathological evaluation was relatively small as compared with the registry cohort and selection of cases was intentional, according to the category of BMI. Nevertheless, the mean age of the case series in all four categories was identical to those from the registry data, suggesting insignificant impact bias on sample size and collection. Second, there is lack of control subjects. The main source of control subjects in this setting can only be cadaveric specimens or those obtained as results of amputation, which are often unsatisfactory specimen, and cannot be reliably controlled. Third, this is a cross-sectional observational study.
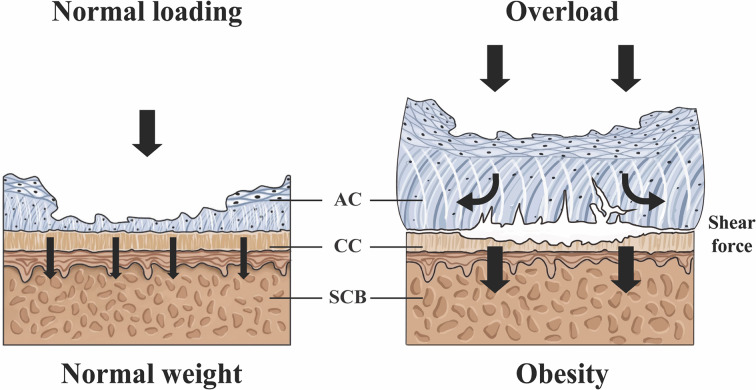
We hypothesise that obesity induces shear forces on the osteochondral interface causing horizontal fissures in patients with OA (figure 3). As hyaline cartilage, calcified cartilage and SCB have different mechanical properties; high compression loading generates excessive shear forces and thus induces fissuring at the interface. The structural deterioration enhances the metainflammatory reactions, attracts the ingrowth of vessels and nerves into the cartilage and further aggravates the symptoms of patients with obesity during joint motion.
Figure 3.
Hypothetic model of shear force-induced horizontal fissures in osteoarthritis (OA) patients with obesity. Osteochondral unit is a multiphasic materials structure. The unit may receive direct loading from cartilage to subchondral bone (SCB) in OA patients with normal weight. However, in patients with obesity, articular cartilage (AC) experiences a large lateral deformation due to the high compressive stress, the deformation being restrained by the underlying calcified cartilage (CC) and SCB, which generates secondary shear stress to induce horizontal fissures and cartilage erosion at the osteochondral interface.
Our study has shown that the key pathological feature in OA patients with obesity is horizontal fissuring at the osteochondral interface. Obesity is strongly associated with a younger age of first TKR, and may be due to horizontal fissures. While the causal relationship between this pathological change and earlier TKR seen in patients with obesity remains unclear, our observation provides a possible explanation between BMI and age of OA patients undergoing TKR.
Acknowledgments
The authors would like to acknowledge Diana Patalwala, Alysia Hubbard and Ivan Lozic for the facilities and the scientific and technical assistance at the National Image Facility at the Centre for Microscopy, Characterization & Analysis, The University of Western Australia, Oliver Beaumont from Oxford University hospitals. This manuscript has been corrected by professional medical writer Dr Alison Carleton in Australia.
Footnotes
Handling editor: Josef S Smolen
Contributors: Conception and design of the study: MZ, CJ, LC and FY. Acquisition of data: LC, FY, PC, EL-B, PV, ML, SG and RCS. Analysis and interpretation of data: LC, MB, GL, TW, MF, and JG. Drafting of article or revising it critically for important intellectual content: LC, JJYZ, GL, YH, JP, CZ, DW, CJ and MZ. Final approval of the version of the article to be published: all authors.
Funding: The work is supported by the general funding of the University of Western Australia and International Cooperation and Exchange of the National Natural Science Foundation of China (Grant Number: 81820108020 and 81802214). Lianzhi Chen has received Scholarship for International Research Fees from the University of Western Australia.
Competing interests: The conduct of work is supported by the general funding of the University of Western Australia. LC has received Scholarship for International Research Fees from the University of Western Australia. All authors declare no conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The Human Research Ethics Committee of the University of Western Australia approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All original datasets (including deidentified participant data) collected for this study will be available upon request to the corresponding author, Minghao Zheng. Registry data can be reused under the approval from the Australian Orthopaedic Association.
References
- 1. Hales CM, Fryar CD, Carroll MD, et al. . Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–5. 10.1001/jama.2018.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reyes C, Leyland KM, Peat G, et al. . Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol 2016;68:1869–75. 10.1002/art.39707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Yin J, Gao J, et al. . Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther 2013;15:223. 10.1186/ar4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization Obesity and overweight, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Accessed 11 Nov 2019].
- 5. Australia Government Australia Institute of Health and Welfare Impact of overweight and obesity as a risk factor for chronic conditions, 2017. Available: https://www.aihw.gov.au/reports/burden-of-disease/impact-of-overweight-and-obesity-as-a-risk-factor-for-chronic-conditions/contents/table-of-contents
- 6. Kremers HM, Visscher SL, Kremers WK, et al. . The effect of obesity on direct medical costs in total knee arthroplasty. J Bone Joint Surg Am 2014;96:718–24. 10.2106/JBJS.M.00819 [DOI] [PubMed] [Google Scholar]
- 7. Carr AJ, Robertsson O, Graves S, et al. . Knee replacement. The Lancet 2012;379:1331–40. 10.1016/S0140-6736(11)60752-6 [DOI] [PubMed] [Google Scholar]
- 8. Wallace G, Judge A, Prieto-Alhambra D, et al. . The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage 2014;22:918–27. 10.1016/j.joca.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 9. Li Q, Blume SW, Huang JC, et al. . Prevalence and healthcare costs of obesity-related comorbidities: evidence from an electronic medical records system in the United States. J Med Econ 2015;18:1020–8. 10.3111/13696998.2015.1067623 [DOI] [PubMed] [Google Scholar]
- 10. Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol 2013;9:225–35. 10.1038/nrrheum.2012.224 [DOI] [PubMed] [Google Scholar]
- 11. Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol 2011;7:65–8. 10.1038/nrrheum.2010.123 [DOI] [PubMed] [Google Scholar]
- 12. Griffin TM, Huebner JL, Kraus VB, et al. . Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum 2009;60:2935–44. 10.1002/art.24854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C-L, McNeill J, Goon K, et al. . Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage Fas-induced Apoptosis-Transgenic mice. Arthritis Rheumatol 2017;69:1772–83. 10.1002/art.40161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larrañaga-Vera A, Lamuedra A, Pérez-Baos S, et al. . Increased synovial lipodystrophy induced by high fat diet aggravates synovitis in experimental osteoarthritis. Arthritis Res Ther 2017;19:264 10.1186/s13075-017-1473-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Visser AW, de Mutsert R, le Cessie S, et al. . The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the Neo study. Ann Rheum Dis 2015;74:1842–7. 10.1136/annrheumdis-2013-205012 [DOI] [PubMed] [Google Scholar]
- 16. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol 2016;12:632–44. 10.1038/nrrheum.2016.148 [DOI] [PubMed] [Google Scholar]
- 17. Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone 2012;51:204–11. 10.1016/j.bone.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 18. Suri S, Gill SE, Massena de Camin S, et al. . Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis 2007;66:1423–8. 10.1136/ard.2006.063354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol 2012;8:390–8. 10.1038/nrrheum.2012.80 [DOI] [PubMed] [Google Scholar]
- 20. Altman R, Asch E, Bloch D, et al. . Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis & Rheumatism 1986;29:1039–49. 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- 21. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunter DJ, Gerstenfeld L, Bishop G, et al. . Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther 2009;11:R11. 10.1186/ar2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Huang Y-C, Yan CH, et al. . Abnormal subchondral bone remodeling and its association with articular cartilage degradation in knees of type 2 diabetes patients. Bone Res 2017;5:17034 10.1038/boneres.2017.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li G, Ma Y, Cheng TS, et al. . Identical subchondral bone microarchitecture pattern with increased bone resorption in rheumatoid arthritis as compared to osteoarthritis. Osteoarthritis Cartilage 2014;22:2083–92. 10.1016/j.joca.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 25. Li G, Zheng Q, Landao-Bassonga E, et al. . Influence of age and gender on microarchitecture and bone remodeling in subchondral bone of the osteoarthritic femoral head. Bone 2015;77:91–7. 10.1016/j.bone.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 26. Pritzker KPH, Gay S, Jimenez SA, et al. . Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 2006;14:13–29. 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 27. Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health 2001;55:508–14. 10.1136/jech.55.7.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steenland K, Armstrong B. An overview of methods for calculating the burden of disease due to specific risk factors. Epidemiology 2006;17:512–9. 10.1097/01.ede.0000229155.05644.43 [DOI] [PubMed] [Google Scholar]
- 29. Park-Wyllie LY, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011;305:783–9. 10.1001/jama.2011.190 [DOI] [PubMed] [Google Scholar]
- 30. Campbell SE, Ferguson VL, Hurley DC. Nanomechanical mapping of the osteochondral interface with contact resonance force microscopy and nanoindentation. Acta Biomater 2012;8:4389–96. 10.1016/j.actbio.2012.07.042 [DOI] [PubMed] [Google Scholar]
- 31. Leyland KM, Judge A, Javaid MK, et al. . Obesity and the relative risk of knee replacement surgery in patients with knee osteoarthritis: a prospective cohort study. Arthritis Rheumatol 2016;68:817–25. 10.1002/art.39486 [DOI] [PubMed] [Google Scholar]
- 32. Murphy L, Schwartz TA, Helmick CG, et al. . Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 2008;59:1207–13. 10.1002/art.24021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Changulani M, Kalairajah Y, Peel T, et al. . The relationship between obesity and the age at which hip and knee replacement is undertaken. J Bone Joint Surg Br 2008;90:360–3. 10.1302/0301-620X.90B3.19782 [DOI] [PubMed] [Google Scholar]
- 34. Messier SP, Mihalko SL, Legault C, et al. . Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the idea randomized clinical trial. JAMA 2013;310:1263–73. 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen R, Bartels EM, Astrup A, et al. . Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2007;66:433–9. 10.1136/ard.2006.065904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radin E, Paul I, Rose R. Role of mechanical factors in pathogenesis of primary osteoarthritis. The Lancet 1972;299:519–22. 10.1016/S0140-6736(72)90179-1 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Fan Y, Zhang M. Comparison of stress on knee cartilage during kneeling and standing using finite element models. Med Eng Phys 2014;36:439–47. 10.1016/j.medengphy.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 38. Lai Y-S, Chen W-C, Huang C-H, et al. . The effect of graft strength on knee laxity and graft in-situ forces after posterior cruciate ligament reconstruction. PLoS One 2015;10:e0127293. 10.1371/journal.pone.0127293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Radin EL, Burr DB, Caterson B, et al. . Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum 1991;21:12–21. 10.1016/0049-0172(91)90036-Y [DOI] [PubMed] [Google Scholar]
- 40. Radin EL, Martin RB, Burr DB, et al. . Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res 1984;2:221–34. 10.1002/jor.1100020303 [DOI] [PubMed] [Google Scholar]
- 41. Meachim G, Bentley G. Horizontal splitting in Patellar articular cartilage. Arthritis Rheum 1978;21:669–74. 10.1002/art.1780210610 [DOI] [PubMed] [Google Scholar]
- 42. Levy AS, Lohnes J, Sculley S, et al. . Chondral delamination of the knee in soccer players. Am J Sports Med 1996;24:634–9. 10.1177/036354659602400512 [DOI] [PubMed] [Google Scholar]
- 43. Kendell SD, Helms CA, Rampton JW, et al. . Mri appearance of chondral delamination injuries of the knee. AJR Am J Roentgenol 2005;184:1486–9. 10.2214/ajr.184.5.01841486 [DOI] [PubMed] [Google Scholar]
- 44. Anderson LA, Peters CL, Park BB, et al. . Acetabular cartilage delamination in femoroacetabular impingement. risk factors and magnetic resonance imaging diagnosis. J Bone Joint Surg Am 2009;91:305–13. 10.2106/JBJS.G.01198 [DOI] [PubMed] [Google Scholar]
- 45. Jannelli E, Parafioriti A, Acerbi A, et al. . Acetabular delamination: epidemiology, histological features, and treatment. Cartilage 2019;10:314–20. 10.1177/1947603518768096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lim YZ, Wang Y, Wluka AE, et al. . Association of obesity and systemic factors with bone marrow lesions at the knee: a systematic review. Semin Arthritis Rheum 2014;43:600–12. 10.1016/j.semarthrit.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 47. Felson DT. Weight and osteoarthritis. Am J Clin Nutr 1996;63:430S–2. 10.1093/ajcn/63.3.430 [DOI] [PubMed] [Google Scholar]
- 48. Philp AM, Collier RL, Grover LM, et al. . Resistin promotes the abnormal type I collagen phenotype of subchondral bone in obese patients with end stage hip osteoarthritis. Sci Rep 2017;7:4042 10.1038/s41598-017-04119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reina N, Cavaignac E, Pailhé R, et al. . BMI-related microstructural changes in the tibial subchondral trabecular bone of patients with knee osteoarthritis. J Orthop Res 2017;35:1653–60. 10.1002/jor.23459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-216942supp001.pdf (399.5KB, pdf)