Abstract
Objectives
Holistic needs assessment (HNA) and care planning are proposed to address unmet needs of people treated for cancer. We tested whether HNA and care planning by an allied health professional improved cancer-specific quality of life for women following curative treatment for stage I–III gynaecological cancer.
Methods
Consecutive women were invited to participate in a randomised controlled study (HNA and care planning vs usual care) at a UK cancer centre. Data were collected by questionnaire at baseline, 3 and 6 months. The outcomes were 6-month change in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 (version 3), global score (primary) and, in EORTC subscales, generic quality of life and self-efficacy (secondary). The study was blinded for data management and analysis. Differences in outcomes were compared between groups. Health service utilisation and quality-adjusted life years (QALY) (from Short Form-6) were gathered for a cost-effectiveness analysis. Thematic analysis was used to interpret data from an exit interview.
Results
150 women consented (75 per group); 10 undertook interviews. For 124 participants (61 intervention, 63 controls) with complete data, no statistically significant differences were seen between groups in the primary endpoint. The majority of those interviewed reported important personal gains they attributed to the intervention, which reflected trends to improvement seen in EORTC functional and symptom scales. Economic analysis suggests a 62% probability of cost-effectiveness at a £30 000/QALY threshold.
Conclusion
Care plan development with an allied health professional is cost-effective, acceptable and useful for some women treated for stage I–III gynaecological cancer. We recommend its introduction early in the pathway to support person-centred care.
Keywords: gynaecological cancer, care plans, holistic needs assessment, quality of life, cost effectiveness
Introduction
Gynaecological cancer incidence and survival are increasing in the UK.1 Many women now live with gynaecological cancer as a long-term condition, with 5-year expected survival ranging from 40% for ovarian cancer (all stages) to 77% for endometrial cancer (all stages).1 Longitudinal studies report 25%–69% of women having physical, social or psychological unmet needs due to the consequences of cancer and its treatment.2 3 Reductions in health status, difficulty returning to previous levels of social functioning and work have been highlighted as some of the issues.4–6 The inability to return to work may affect people’s economic status, which in turn can negatively affect their level of physical and psychosocial functioning.6 7 Some women have reported their perception that follow-up oncology appointments are more concerned with survival than the experience of living with and beyond cancer and that their own primary care team lacks the expertise and time to address their range of unmet needs and concerns, leaving them unsure as to how to resolve them.8
Cancer rehabilitation aims to reduce the extent to which cancer interferes with an individual’s physical, psychosocial and economic functioning.9 By targeted assessment, treatment and advice the therapeutic interventions seek to help people live as fully as possible with the effects of the disease and its treatment. The aim is to improve quality of life (QoL), maximising ability to function by promoting independence and adaptation.9 This has been shown to have socioeconomic benefits with reduced use of the healthcare services10 and to support people to work toward societal reintegration.11
A preliminary qualitative interview study by our research group found that women treated for gynaecological cancers reported unmet rehabilitation care needs, both physical and psychosocial.12 Participants reported that they were unaware of how to resolve these needs or how to access help at the end of treatment and identified that individualised, tailored support at the end of treatment may meet these needs.12
Following the publication of ‘Improving Supportive and Palliative Care for Adults with Cancer’ by the Cancer Action Team,9 the holistic needs assessment (HNA) of supportive and palliative care needs for adults with cancer has been recommended as usual UK practice.13 The National Cancer Survivorship Initiative has promoted the development of various models of care to improve outcomes and experience and aims to promote supported self-management.14 It is intended that the opportunity to complete structured HNA should be offered to all adults with cancer at key points in their pathway.15 These include at diagnosis, end of treatment, any other time the patient requests or as healthcare professionals judge necessary. However, neither the clinical nor cost effectiveness of this has been evaluated for women following treatment for gynaecological cancers.
This mixed-methods study explored whether the development of a care plan with an experienced rehabilitation allied health professional (AHP), which incorporated an HNA, would improve cancer-specific health-related QoL over 6 months when compared with usual care for women who have completed primary treatment for stage I, II or III gynaecological cancer. It sought to measure objective changes in QoL. In addition, it aimed to explore and understand the perspective of those receiving the intervention and describe the cost implications for health and social care.
The specific objectives were as follows:
To compare changes in global cancer-specific health-related QoL over 6 months for women receiving an HNA to develop a care plan (intervention group) with changes for women receiving usual rehabilitation care (control group) (primary outcome).
To compare changes between groups in functioning, symptoms, general self-efficacy and generic health-related QoL (secondary outcomes).
To explore the impact of HNA and care planning on health and social care provision and conduct a cost-effectiveness analysis.
To understand the impact of HNA and care planning for people.
Methods
Design
A mixed-methods design was used with content and process evaluation. A single-centre, randomised, controlled, two-arm study was conducted at a UK tertiary cancer centre. Women’s experiences of the intervention were explored through nested semi-structured interviews.
Participants
The sample size (n=150) was chosen pragmatically, based on an anticipated recruitment rate, to estimate differences in scores between arms and to provide data to power potential future studies. Inclusion criteria were that participants must be 18 years or over, have recently completed first-line treatment with radical intent for stage I, II or III gynaecological cancer and reported physical or psychosocial need resulting from the disease or its treatment. Patients eligible for end-of-life care and those lacking capacity to give informed consent were excluded.
The original protocol included only women with stages I–II. This was amended to include patients with stage III disease to reflect the high chance of survival to 5 years expected at the treatment centre.
Procedure
Consecutive women who had successfully completed first-line treatment, reported physical or psychosocial needs and met the eligibility criteria were approached by clinical staff at outpatient clinic appointments and, if willing, were given information regarding the study. A research team member contacted them at least 24 hours later to discuss queries about the study, consent forms were returned by post and reasons for not consenting were recorded if given. Following receipt of informed written consent, participants were randomised by the Institute of Cancer Research Trials Line by minimisation (1:1 allocation) into control or intervention groups. Balancing factors were as follows: tumour site (ovarian, endometrial, cervical, vaginal or vulval), age (<40 years, 40–60 years, >60 years) and primary treatment (surgery, radiotherapy or chemotherapy).16 The women were then contacted to inform them of their group allocation, and appointments were made with those in the intervention group.
Interventions
Usual care in the trust consisted of referrals to appropriate members of the rehabilitation team, psychological care or external agencies if and when needs were identified. Rehabilitation needs were identified by a healthcare professional, patient or family member during a routine outpatient appointment. For example, people might be referred to a physiotherapist for individualised exercise advice, to a cancer support centre for peer support and activity groups or to a dietitian for specific nutritional queries.
In addition to usual care, women in the intervention group were offered face-to-face or telephone consultations with an AHP experienced in multiprofessional rehabilitation and familiar with behavioural change principles. Participants in the intervention group were sent a London HNA17 in advance of the first appointment, which provided the basis for a discussion and development of a collaborative care plan. Face-to-face discussions took place in a quiet consultation room in the rehabilitation department at the centre. The HNA and collaborative care plan were subsequently reviewed with the woman after an agreed interval of time, usually 3 months. Further details about the conduct of the intervention are available from the corresponding author.
Data collection
Women in both groups were asked to complete an outcome measures pack at baseline following randomisation, at 3 and 6 months. Packs were distributed by mail. A stamped addressed envelope was provided for return of the completed questionnaires to the research team. Non-responders were contacted by telephone after 2 weeks.
The pack contained measures of cancer-specific QoL (European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 (QLQ-C30) version 3),18 generic health-related QoL (Short Form-36 RANDversion 2, SF-36),19 self-efficacy (General Self Efficacy Scale, GSES)20 and use of health and social services (for the health economic evaluation). The self-reported data for the health economic analysis collected over the trial period included general practitioner (GP) visits, nurse visits, telephone calls to the nurse, other clinical consultations, complementary therapies, hospital outpatient visits, day cases, inpatient stays and accident and emergency (A&E) visits.
Demographic, cancer diagnosis, International Federation of Gynaecology (FIGO) tumour staging and treatment details were recorded from patient notes. Contacts as face-to-face, telephone or non-contact time were also extracted.
Group concealment
It was not possible to blind the therapists or participants to the group allocation. To minimise bias, the data were input by a data manager before being analysed by a senior statistician and health economist.
Ethical considerations
National Research Ethics Committee London–West London gave a favourable ethical opinion (11/LO/1065); the trial was conducted as per the Declaration of Helsinki.21
Analysis
Primary outcome
Standard scoring methods were used to calculate the mean change, with 95% CI, from baseline to 6 months in EORTC global QoL score (range 0 (worst) to 100 (best), ie, a high score represents a high QoL) for each arm for those with completed scores at both time points. Analysis of covariance (ANCOVA) was used to test for difference in global score at 6 months between groups, corrected for baseline score. A threshold of 0.05 was used to define significance. A sensitivity analysis was then performed in which women with missing scores at baseline or 6 months were assigned a change in score of ‘0’ and the difference in mean change between arms with 95% CI was recalculated.
Secondary outcomes
Descriptive statistics, means and 95% CIs, were used to summarise the outcomes. The functional scale is scored ‘0’ worst to ‘100’ best. Symptom and single item EORTC scores range ‘0’ best to ‘100’ worst, that is, a higher score represents a higher reporting of that symptom. GSES comprises 10 questions, each scored ‘1’ worst to ‘4’ best, that is, a higher score demonstrates better self-efficacy. SF-36 is scored over eight dimensions each on a scale of ‘0’ (worst) to ‘100’ (best). Missing data were not replaced. The number and percentage of women with an improvement in global EORTC score of >10 points at any time post-randomisation was recorded.
An exploratory linear regression analysis was performed to investigate the effect of arm, age, diagnosis, primary treatment type and FIGO stage on EORTC global QoL score at 6 months, as per the balancing factors for randomisation.
Health economics
Self-reported resource use including the type and number of sessions for each item (eg, GP visits, counselling sessions) was recorded per time-point per participant as frequency data to calculate the total economic costs from a National Health Service (NHS) payer perspective. Of the data captured, A&E visits, day cases and hospital inpatient stays were not included in the calculation of costs, as these healthcare items would not reasonably be induced or prevented by the intervention itself.
Unit costs for each healthcare resource were sourced from the personal and social services research unit (PSSRU)22 and the NHS reference costs.23 In the case of some alternative complementary medical treatments, not routinely offered through the NHS, other public sources were used to identify unit costs.24 Total costs of service use (excluding intervention) were calculated for each participant. The cost of the intervention was calculated separately at an individual participant level. This was based on the duration in minutes of the initial appointment at baseline, non-patient facing follow-up time and an additional appointment at 3 months for those women who requested it. Clinical contact was costed assuming, in practice, a Band 6 AHP or specialist nurse would accurately reflect the clinician delivering the intervention.
The main health outcome for the economic analysis is the quality-adjusted life year (QALY). This was estimated from responses to the SF-36 RAND questionnaire. Responses were converted to the Short Form-6D (SF-6D) from which a utility index value was estimated25 for each follow-up point. The QALY was calculated by integrating the index scores for each of the follow-up points over the 6 months of the trial using the area under the curve (AUC) method.
Missing data were assessed for pattern and level. Data appeared to be missing at random; however, due to high levels of missing data (approximately 20% for economic outcomes), imputation was not applied. A simple approach such as mean imputation would have biased variability relative to sample size downward, misrepresenting uncertainty in the results. More sophisticated multiple imputation methods were rejected as there were insufficient predictor variables with complete data with which to construct a suitable model. For these reasons, a pragmatic decision to focus on a complete case analysis was followed.26
To control for differences in baseline health between the intervention and control groups, cost and QALYs were estimated using multivariate linear regression (ordinary least squares (OLS)), with baseline health utility index included as a covariate. White adjusted SEs were used to control for unobserved heterogeneity. The mean incremental differences in costs and QALYs resultant from this regression analysis were used to calculate the point estimate of the incremental cost-effectiveness ratio (ICER). The ICER, assuming an intervention is more expensive but leads to better health outcomes than the alternative treatment, is the ratio which shows the cost, on average, per each additional year of life gained in perfect health. Given the typical non-normality of the distribution of cost and QALY data, uncertainty around these estimates was handled non-parametrically. A non-parametric bootstrap approach was used to resample with replacement from the trial data to estimate an empirical distribution of ICER estimates.27 Results were presented on the cost-effectiveness plane (CEP) showing the scatter of the mean difference in cost and effect for each of the bootstrap samples. These results were further combined to form the cost-effectiveness acceptability curve to determine the probability of the intervention being deemed cost-effective at a given willingness to pay per QALY.
Semi-structured interviews
Ten participants representative of those recruited (spanning ages and diagnosis), who had completed the intervention arm of the study, were selected by purposive sampling. They were invited to explore their accounts of the intervention, its impact for them and the aspects important to them, using a semi-structured qualitative interview conducted by an independent clinical researcher not known to them (box 1). The data were transcribed verbatim and analysed by two independent researchers, forming preliminary codes and notes. A prior meeting agreed the process of analysis and interpretations following Boyatzis’s latent thematic analysis.28 Similar codes were considered for overlap and descriptive text drawn from the transcripts to establish and clarify meanings and similarities. Quotes are presented to illustrate these themes.
Box 1. Topic schedule for the semi-structured interviews.
What were the priorities for you at the end of treatment?
A lot of people have challenges at the end of treatment. What were the biggest challenges for you?
How did you find the contact you had with the rehabilitation therapist?
How much do you feel the contact helped you identify and move toward resolving your concerns?
Looking back since the treatment ended, how have things changed?
Which help have you had as a result of your meeting with the research therapist?
If you had the choice where would you prefer to have been seen: locally or at the cancer treatment centre (considering quality of service, why and so on?)
How would you say the services you have been referred to have helped with the concerns you had?
What could have been improved?
Has anything changed for you as a result of being involved in the study?
Is there something else you would like to tell me about the research?
Results
Characteristics of participants
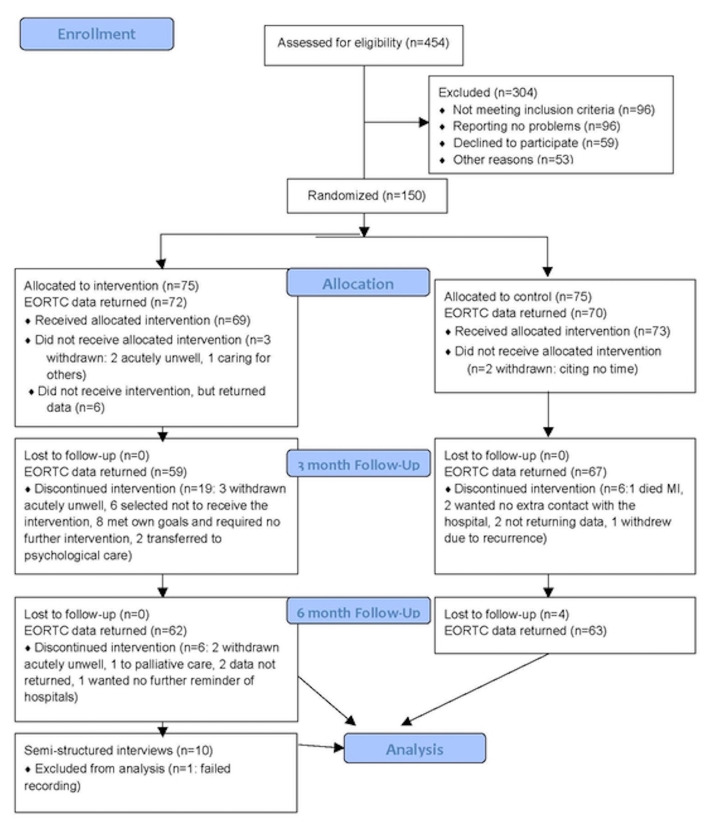
A total of 150 women gave informed written consent for the trial between August 2011 and September 2014, 75 to each group. Participant flow for those completing the primary endpoint EORTC global QoL score is reported in figure 1. Of the 150 patients recruited, 124 had complete data (63 for the control and 61 for the intervention group) for the primary endpoint; two had missing baseline scores and 24 had missing scores at 6 months. Participant demographics were balanced between groups (see table 1). No adverse events were reported as a result of the intervention.
Figure 1.
Study flow diagram.
Table 1.
Demographic data in the full study and interview populations
| Control group (n=75) | Intervention (n=75) |
Total (n=150) |
Interview (n=10) |
||
| Ethnicity | White | 62 (83%) | 66 (88%) | 128 (85%) | 9 (90%) |
| Asian/Asian British | 2 (3%) | 1 (1%) | 3 (2%) | ||
| Black/Black British | 4 (5%) | 3 (4%) | 7 (5%) | 1 (10%) | |
| Mixed | 1 (1%) | 2 (3%) | 3 (2%) | ||
| Other | 6 (8%) | 3 (4%) | 9 (6%) | ||
| Age | <40 | 14 (19%) | 14 (19%) | 28 (19%) | 1 (10%) |
| 40–60 | 35 (47%) | 34 (45%) | 69 (46%) | 6 (60%) | |
| >60 | 26 (35%) | 27 (36%) | 53 (35%) | 3 (30%) | |
| Diagnosis | Cervical | 14 (19%) | 16 (21%) | 30 (20%) | 2 (20%) |
| Endometrial | 24 (32%) | 27 (36%) | 51 (34%) | 4 (40%) | |
| Ovarian | 29 (39%) | 27 (36%) | 56 (37%) | 3 (30%) | |
| Vulval | 8 (11%) | 5 (7%) | 13 (9%) | 1 (10%) | |
| Primary treatment | Chemotherapy | 9 (12%) | 9 (12%) | 18 (12%) | 1 (10%) |
| Radiotherapy | 9 (12%) | 10 (13%) | 19 (13%) | 2 (20%) | |
| Surgery | 57 (76%) | 56 (75%) | 113 (75%) | 7 (70%) | |
| FIGO stage | I | 39 (52%) | 36 (48%) | 75 (50%) | 1 (10%) |
| II | 19 (25%) | 23 (31%) | 42 (28%) | 6 (60%) | |
| III | 17 (23%) | 16 (21%) | 33 (22%) | 3 (30%) | |
| Weeks from last treatment | Mean | 20.52 | 20.21 | 20.21 | 10.4 |
| Minimum | 0 | 0 | 0 | 2 | |
| Maximum | 178 | 44 | 178 | 23 |
Interview: those participants who completed the study in the intervention arm and were purposively sampled and invited to attend a semi-structured interview exploring their experiences and impact of the intervention.
FIGO: International Federation of Gynaecology tumour stages I–III.
Primary endpoint
Data revealed no difference between the groups. Mean baseline EORTC global QoL scores were 64.2 (SD 21.8) for the intervention group and 66.7 (SD 19.7) for the control group. The difference between groups in mean change in score was +1.5 points in favour of the control group, with a 95% CI from −5.7 (favouring intervention) to +8.7 (favouring control). Similar results were found after replacing missing data (not shown). An exploratory ANCOVA was performed to model global health score at 6 months as a function of arm and baseline score. This showed a significant relationship between score at baseline and at 6 months (p<0.0001), but no difference in score between arms (p=0.6223). A total of 22/75 (30%) of patients in the control arm and 23/75 (31%) in the intervention arm recorded an improvement in EORTC global QoL score of >10 points at 3 or 6 months. Baseline score was the only factor (not age, tumour site or stage) predictive of EORTC global QoL score at 6 months.
Secondary endpoints
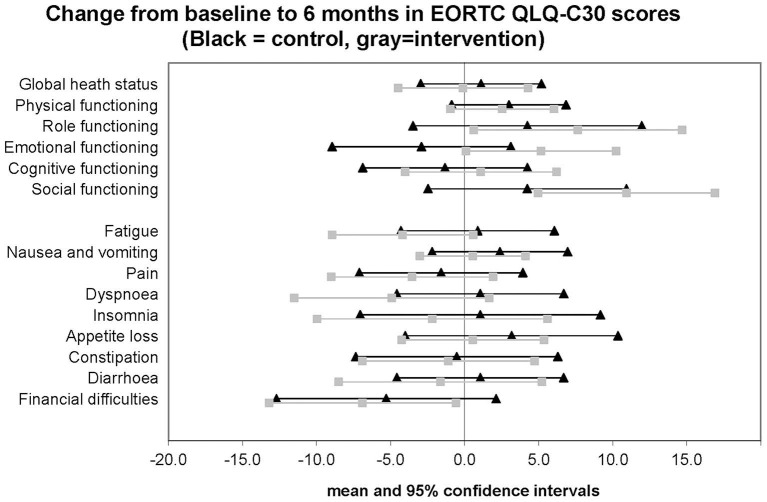
No statistically significant differences were found in the secondary endpoints (see table 2), although trends to improvement were seen in intervention group on the EORTC scales for emotional, role and social functioning (non-significant difference in change at 6 months of +3.5 points or more in favour of intervention) and some improvements were seen in the symptom scales for fatigue, dyspnoea and insomnia (non-significant difference in change at 6 months of at least −3 points in favour of intervention) (see figure 2).
Table 2.
Values at baseline, 3 and 6 months, for global, functional, symptom and single-item EORTC QLQ-C30 scores (version 3), General Self Efficacy Scale, Short Form-36 (version 2) scores and Short Form-36 utility index score
| Control | Intervention | |||||||||||||||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |||||||||||||
| n | Mean | SD | n | mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (version 3) | ||||||||||||||||||
| Global heath status/QoL (revised) | 70 | 66.7 | 19.7 | 67 | 67.3 | 22.0 | 64 | 69.4 | 21.1 | 72 | 64.2 | 21.8 | 59 | 66.2 | 19.1 | 62 | 67.5 | 22.6 |
| Physical functioning (revised) | 70 | 75.5 | 22.3 | 67 | 79.0 | 20.0 | 64 | 79.2 | 19.9 | 72 | 77.2 | 20.2 | 59 | 80.1 | 21.4 | 62 | 81.7 | 18.1 |
| Role functioning (revised) | 70 | 70.7 | 28.2 | 67 | 70.1 | 30.5 | 64 | 75 | 28.8 | 72 | 69.9 | 28.5 | 59 | 75.4 | 26.1 | 62 | 78.2 | 23.9 |
| Emotional functioning | 70 | 67.9 | 23.4 | 67 | 66.4 | 26.2 | 64 | 66.7 | 28.6 | 72 | 67.1 | 25.3 | 59 | 70.0 | 23.9 | 62 | 75.5 | 20.2 |
| Cognitive functioning | 70 | 74.3 | 24.5 | 67 | 74.4 | 25.7 | 64 | 73.4 | 26 | 72 | 77.3 | 22.1 | 59 | 79.7 | 20.8 | 62 | 80.9 | 20.9 |
| Social functioning | 70 | 70.0 | 29.0 | 67 | 74.9 | 29.8 | 64 | 74.2 | 28.9 | 72 | 74.1 | 26.7 | 59 | 85.0 | 21.4 | 62 | 86.0 | 18.9 |
| Fatigue | 70 | 37.1 | 23.2 | 67 | 38.1 | 27.5 | 64 | 37.0 | 23.9 | 72 | 36.7 | 24.1 | 59 | 32.4 | 21.7 | 62 | 30.5 | 20.1 |
| Nausea and vomiting | 70 | 8.6 | 13.8 | 67 | 8.2 | 14.6 | 64 | 10.4 | 20.5 | 72 | 5.8 | 13.7 | 59 | 4.2 | 11.0 | 62 | 4.6 | 11.0 |
| Pain | 70 | 26.7 | 28.3 | 67 | 28.1 | 31.9 | 64 | 23.4 | 25.8 | 72 | 25.7 | 28.4 | 59 | 22.6 | 28.3 | 62 | 21.8 | 25.0 |
| Dyspnoea | 70 | 15.7 | 25.2 | 67 | 18.9 | 28.0 | 64 | 15.6 | 25.2 | 72 | 19.0 | 26.1 | 59 | 14.7 | 22.5 | 62 | 13.4 | 17.6 |
| Insomnia | 70 | 41.0 | 32.7 | 67 | 47.3 | 32.9 | 64 | 41.7 | 32.0 | 72 | 37.0 | 33.4 | 59 | 33.3 | 29.0 | 62 | 32.8 | 28.0 |
| Appetite loss | 70 | 12.4 | 24.2 | 67 | 11.4 | 22.1 | 64 | 14.1 | 25.1 | 72 | 10.2 | 20.7 | 59 | 5.6 | 14.1 | 62 | 9.7 | 19.5 |
| Constipation | 70 | 16.2 | 25.2 | 67 | 15.9 | 26.2 | 64 | 15.6 | 25.2 | 72 | 16.7 | 26.2 | 59 | 11.3 | 22.0 | 62 | 12.9 | 22.9 |
| Diarrhoea | 70 | 15.7 | 25.8 | 67 | 11.9 | 23.0 | 64 | 15.1 | 27.2 | 72 | 14.4 | 22.9 | 59 | 11.9 | 19.3 | 62 | 13.4 | 26.6 |
| Financial difficulties | 70 | 26.7 | 32.9 | 67 | 21.4 | 30.0 | 64 | 19.8 | 26.4 | 71 | 21.1 | 33.9 | 58 | 11.5 | 22.1 | 60 | 11.1 | 21.8 |
| General Self Efficacy Scale scores | ||||||||||||||||||
| General Self-Efficacy Scale | 69 | 3.1 | 0.6 | 67 | 3.1 | 0.6 | 66 | 3.1 | 0.5 | 72 | 3.1 | 0.5 | 59 | 3.1 | 0.5 | 61 | 3.1 | 0.5 |
| Short Form-36 RAND (version 2) | ||||||||||||||||||
| Physical functioning | 70 | 65.1 | 28.8 | 67 | 69.7 | 26.5 | 65 | 69.6 | 28.0 | 72 | 62.8 | 29.4 | 59 | 72.1 | 26.4 | 62 | 68.7 | 29.5 |
| Role limitations due to physical health | 69 | 40.5 | 43.0 | 67 | 48.9 | 44.5 | 65 | 48.8 | 44.9 | 72 | 41.2 | 43.4 | 59 | 58.9 | 42.0 | 62 | 53.6 | 41.7 |
| Role limitation due to emotional problems | 69 | 56.5 | 45.1 | 67 | 60.7 | 41.8 | 66 | 61.6 | 44.6 | 72 | 58.8 | 43.2 | 59 | 70.1 | 40.4 | 61 | 64.5 | 41.7 |
| Energy/fatigue | 70 | 50.6 | 23 | 67 | 51.1 | 25.4 | 66 | 54.5 | 24.7 | 72.0 | 47.1 | 23.6 | 59 | 51.7 | 21.0 | 62 | 52.2 | 20.6 |
| Emotional well being | 70 | 67.7 | 20.4 | 67 | 69.8 | 19.7 | 66 | 69.0 | 21.2 | 72.0 | 65.6 | 21.1 | 59 | 68.0 | 19.5 | 62 | 72.0 | 17.8 |
| Social functioning | 70 | 68.0 | 26.3 | 67 | 69.8 | 30.3 | 66 | 71.4 | 26.8 | 72.0 | 63.7 | 26.7 | 59 | 73.7 | 24.5 | 62 | 75.4 | 23.4 |
| Pain | 70 | 67.5 | 26.9 | 67 | 67.7 | 28.6 | 66 | 74.5 | 25.4 | 72.0 | 65.7 | 26.0 | 59 | 73.6 | 25.6 | 62 | 73.4 | 24.2 |
| General Health | 70 | 59.6 | 22.4 | 67 | 57.8 | 21.5 | 66 | 58.9 | 22 | 72.0 | 53.0 | 23.9 | 59 | 58.1 | 19.7 | 62 | 59.4 | 22.2 |
| Short Form-36 RAND (version 2) utility index scores | ||||||||||||||||||
| Utility Index Score | 64 | 0.66 | 0.11 | 64 | 0.68 | 0.11 | 62 | 0.68 | 0.12 | 64 | 0.64 | 0.11 | 57 | 0.68 | 0.11 | 57 | 0.69 | 0.11 |
| QALYs Accrued by group | Control group: n=55, mean 0.3397, SD 0.051 | Intervention group: n=47, mean 0.342, SD 0.045 | ||||||||||||||||
QALY, quality-adjusted life years; QoL, quality of life.
Figure 2.
EORTC QLQ-C30 change from baseline scores at six months
Health economics results
Not all participants had sufficiently completed SF-36 results with which to estimate a health utility index. At baseline 128 (control 64, intervention 64), at 3 months 121 (control 64, intervention 57) and at 6 months 119 (control 62, intervention 57) had complete data. Furthermore, to estimate the QALYs accrued for each participant over the 6-month trial period, a utility index score at all time-points is required. Those with complete SF-36 data at all time-points reduced the sample to 102 (control 55, intervention 47). This sample size was further reduced when limiting the sample to those observations that also had complete resource use data, giving a complete case sample n=82 (control 41, intervention 41). Comparison between those participants with full data included in the analysis and those excluded due to missing cost or utility data revealed no significant differences with respect to baseline utility or age.
Based on the complete case data, the average duration of contact time for the first interview was 59 min, with 26 min of non-patient facing work. For patients who had a second interview at 3 months, the average contact time was 36 min with 19 min of non-patient facing work, equating to a modest average intervention cost per patient (table 3). There was wide variation in health service utilisation and costs across both groups. The control arm, on average, had higher healthcare use, but overall, there was no statistically significant difference between groups. The raw mean incremental difference in total cost favoured the control group; however, this difference was marginal at £57.
Table 3.
Total costs relating to the intervention and indirect healthcare resource use
| Control group | Intervention group | |||
| n | Mean (SD) | n | Mean (SD) | |
| Cost of intervention | n/a | n/a | 41 | £140 (69) |
| Cost of resource use | 41 | £387 (383) | 41 | £304 (288) |
| Total | 41 | £387 (383) | 41 | £444 (320) |
Costs are calculated from a National Health Service perspective, based on complete case data n=82 (control group 41 cases, intervention group 41 cases).
Utility index scores measure general health status and health-related QoL and are used as a component of QALY calculation. Participants in the control group reported higher utility index scores than the intervention group at baseline. At 3 and 6 months following the intervention, the intervention group reported higher utility scores than the control group. However, none of these differences were statistically significant (table 4).
Table 4.
Health outcomes for health-related quality of life with data estimates based on the Short Form-36 RAND responses
| Health-related quality of life Data estimates based on SF-36 RAND responses | |||||||
| Control group | Intervention group | Difference | p Value | Test | |||
| Health utility index | n | Mean (SD) | n | Mean (SD) | Mean (SE) | ||
| Utility at baseline | 64 | 0.656 (0.109) | 64 | 0.643 (0.108) | −0.012 (0.019) | 0.521 | t-test |
| Utility at 3 months | 64 | 0.680 (0.114) | 57 | 0.683 (0.108) | 0.003 (0.020) | 0.869 | t-test |
| Utility at 6 months | 62 | 0.678 (0.120) | 57 | 0.687 (0.106) | 0.008 (0.020) | 0.684 | t-test |
| QALY (AUC) | 55 | 0.340 (0.051) | 47 | 0.342 (0.045) | 0.003 (0.009) | 0.776 | t-test |
| QALY difference (controlling for baseline utility) | 55 | 47 | 0.006 (0.006) | 0.286 | OLS regression (white adjusted SE) | ||
Utility index scores calculated at a patient level, from SF-36, reduced to SF-6D using published utility weights.25 Quality of life (area under curve) estimated as the time integral of the utility scores over the 6-month trial period; quality of life difference estimated parametrically using ordinary least squares, controlling for baseline utility score.
AUC, area under the curve; SF-36, Short Form-36; QALY, quality-adjusted life years.
With the incremental difference in costs of £57 and 0.006 incremental QALY gain from treatment, the mean ICER was estimated as £57/0.006=£9500. This average cost per QALY of £9500 falls well below the National Institute for Health and Care Excellence (NICE) threshold of £20 000. The QALY is estimated as the AUC translated by the utility index scores and was higher over the 6-month treatment period for the intervention group. On average, over the 6-month trial period, the raw mean difference in reported QALYs was 0.003 in favour of the intervention group. The conditional mean difference in QALY outcomes as estimated using OLS regression, controlling for differences in baseline QoL,29 increased to 0.006. This was not statistically significantly different from 0. Uncertainty around the average cost per QUALY must be considered given the non-significance of the incremental differences in costs and health outcomes between treatment and control groups.
Uncertainty in the health economic findings was handled non-parametrically. A total of 20 000 samples of equal size were taken from the dataset. For each of these 20 000 samples, a mean difference in cost and QALYs was estimated. The results of these 20 000 samples are plotted on the CEP (figure 3).
Figure 3.
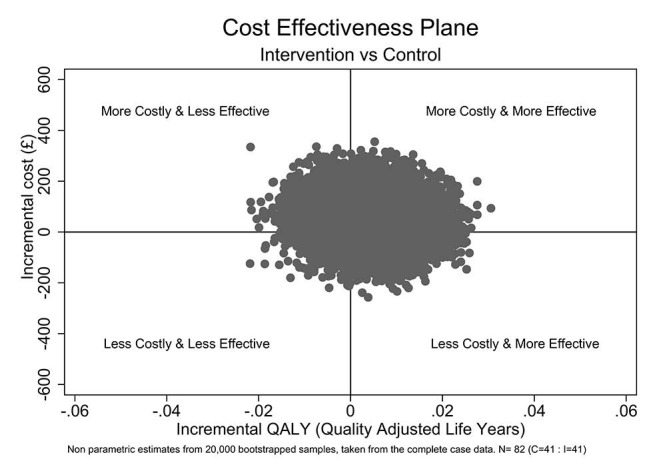
Incremental quality-adjusted life year (QALY) (£). Non-probability estimates based on 20 000 bootstrap samples, taken from the complete case data; n=82 (control 41, intervention 41).
Points in the lower right quadrant represent realisations where the intervention was both cheaper, on average, than the control arm and led to better health outcomes. Points in the top left quadrant represent realisations where the intervention was both more expensive and less effective than the control arm. Points in either the top right or bottom left quadrants are less clear. Here, the treatment was either more expensive and more effective or less effective and cheaper than current practice (control). To assess whether points in these two quadrants are favourable, we must decide how much we are willing to pay for the health gains or how much health we are willing to forgo for the potential savings. This threshold is implicitly set by NICE as £20 000 – £30 000 per QALY gained.30
As can be seen, with most realisations resulting in small incremental gains in reported QoL and only a marginal incremental cost, overall, the intervention on balance appears to be favoured (figure 4).
Figure 4.
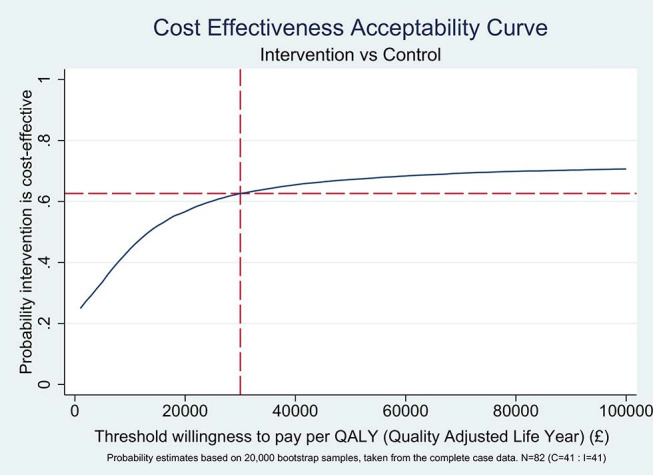
Threshold willingness to pay per quality-adjusted life year (QALY) (£). Probability estimates based on 20 000 bootstrap samples, taken from the complete case data; n=82 (control 41, intervention 41).
When a threshold willingness to pay of £30 000 per QALY is applied to the bootstrapped data, approximately 62% of all realisations are deemed cost-effective in favour of the intervention (figure 4). That is to say, if society is willing to pay £30 000 for each QALY gained, there is a 62% probability that this intervention, in practice, would be cost-effective.
Interview analysis data
Ten interviews were conducted between October 2012 and November 2013. All participants invited to take part in the qualitative interviews agreed and gave informed consent. The interviews lasted from 14 to 37 min and were digitally recorded. Field data were also used in the analysis. All (100%) reported to be happy to have the intervention delivered from the treating hospital, 20% preferred telephone contact and 10% suggested email communication for future delivery of this intervention.
Overarching theme: dislocation, isolation, uncertainty and vulnerability at the end of treatment
The data gave a sense of the uncertainty and isolation felt by participants. This was expressed as a vacuous space that was surprising and unexpected, following the highly structured experience of being ‘taken’ or ‘guided’ through treatment.
Participants expressed their experience of treatment for cancer in a range of ways. At the end of treatment, this included feelings of vulnerability, being “left reeling…and there is nothing else” (participant 47), experiencing acute isolation having “no-one to turn to” (participant 01). Powerful expressions of uncertainty about their future “you just don’t know what tomorrow will bring” (participant 87), “you are just sent off for 3 months, with no instructions…nothing” (participant 46) and a sense of abandonment, being “cast adrift” (participant 64) at the end of treatment “So, effectively you are on your own” (participant 09). This came at a time where many participants were considering the meaning of their lives and attempting to make adjustments following their cancer experience, attempting to establish a ‘new normal’. “It was like I was a new, completely new person” (participant 24). This was expressed in the broadest sense: physically, emotionally and how people perceived their identity through work and other roles: “Lifestyle has definitely changed. It’s not as it was before” (participant 24) and “(after leaving work) I needed to think about filling my life with other things.” (participant 24)
Some (40%) expressed that a lack of continuity in contact with health professionals meant physical, emotional and psychological issues were often not addressed.
“I felt…once the treatment had finished it would have been nice to have had somebody from here to speak to if I needed to. But there wasn’t anybody…that I felt I could come in to talk to.” (participant 09)
“Well, at the end of treatment, it’s like, go home, you’ve got people with you at home for a while…and then as far as the medical thing goes, you are quite abandoned, there isn’t, apart from your 20 min or whatever you get when you go to see the consultant that’s really it, and actually, my family doctor wasn’t terrific because they don’t really understand, they think, oh yes she’s had a hysterectomy, well I’ve a bit more than a hysterectomy but you know they don’t really understand, you know how you’re feeling.” (participant 03)
Theme: space to be heard and understood by a ‘trustworthy’, independent professional
Many participants (80%) described the importance of having the opportunity to explore issues and have them validated by someone ‘outside of the medical team’ (participant 87). They described the added value of talking with someone knowledgeable (about their cancer), professional and empathetic. Participant 87 described how the intervention had linked up her care in a way not offered in the clinic setting:
“The nurse was very nice and she listened and supported me, but she didn’t tell me about any of the other services I could have…without the study I would not have got any of this and I wouldn’t be where I am now.” (participant 87)
Comments tended to stress the value of having someone available to talk to who had an understanding of what they had experienced and an ability to listen. The nature and communication skills of the person doing the post-treatment assessment appeared crucial to service users, specifically the ability to listen and understand before signposting on. Participants reported that the person delivering the care needed to be ‘knowledgeable, professional and empathetic’ (participant 64) and that these qualities engendered a perception of that professional having a ‘trustworthiness’ to identify and resolve the issues that were important to them at a pace that suited them. This encouraged and facilitated participants to re-establish their belief in their own ability to self-manage with tailored information, support and treatment. Affiliation with the team, but distance from it, allowed participants to seek advice about concerning medical issues which they felt unable to ask their medical teams. Patients reported that they wanted to be able to dissociate and concentrate fully on this intervention at another time (participants 47 and 46).
“Because she understood most of the stuff I was saying. So I did find it very, very helpful…That’s what’s changed, that, you know, you do feel like there’s someone who’s going to answer a question.” (participant 03)
“I definitely appreciated it as soon as my treatment ended. So for me that was probably the most important time I had someone like (the researcher) to talk to. And it was nice to know that there was someone who actually understood.what I had just…done…what I had just been through…” (participant 05)
The interventions were valued for different reasons with participants expressing that it offered an opportunity to explore and express the psychological impacts, to have those expressions validated and to identify other issues in a safe and supported way:
“I’ve not talked about it enough with uhm people in general. I found…I was able to express myself, about concerns that I had, without sort of, any judgemental attitude. So she was of great benefit…I just think the talking. Uhm, was beneficial.” (participant 24)
“…Getting it out of my system and move on. I don’t have anybody like that…And I’m gradually discovering, that I do need to get it out of my system.” (participant 01)
The majority (60%) described the importance of exploring things outside the family, and this related to feelings of being a burden to family members.
“It was a turning point really because it did let me, let it all out if you like. Uhm and I really needed it. Having someone to talk to about it is great…someone independent who, will just listen, is fantastic. It really does help…I think…being able to offload to someone not in your own family helps.” (participant 46)
Others benefited from the support and reassurance to access the available support services (relaxation, complementary therapy services and psychological care) feeling that they were not eligible or should leave the services for those in a worse situation/more deserving:
“And although you get lots and lots of booklets and leaflets and so on. Being referred by (the researcher) was much easier than having to seek it out myself. Really. And it made me feel like…uhm…it was appropriate for me to have that referral because she suggested it, whereas, you know if it had been down to me, I would have thought ‘Oh, there’s people worse off than me, so I shouldn’t be bothering them and taking up their time. There’s people in a much worse situation.’” (participant 46)
“I sounded quite sort of…you know…depressed…and she said ‘There’s a…do you mind if uhm…if I bring somebody else in?’ Uhm, I can’t remember exactly what the date was. So that was immediately, I sort of thought…‘Thank goodness’…her professionalism and her knowledge and her empathy.” (participant 64)
Theme: moving toward to self-supported management
Some of the interviewees reported that the value of the intervention was clearer at the end of participation in the study, when they were able to reflect on the difference the support had made for them. Many described previous gaps in their knowledge and the absence of tailored information, making it hard for them to self-manage. Some found it invaluable for signposting and facilitating access to support services including relaxation, psychological support, physiotherapy and, in one instance, back to acute care services. This therapeutic rapport appeared to be a precursor to accessing other services. Participant 17 reported that it had given her the confidence to focus on certain issues at a difficult time, ‘to identify a lot and to break things down to manageable chunks.’ Similarly, participant 64 said:
“I think it was very good at identifying from the point of identifying issues…it’s very good for, I mean uhm, identifying actually what you don’t want. That was great because you know, it just set you know, the kind of small targets and things to do.”
Continuity and availability were important features of the contact; the importance of support with focusing, target or goal setting, and follow-up was highlighted by 90% of participants.
This worked well when the goals and barriers had been well identified and there was timely follow-up:
“It sort of made me…do some target setting…and then she rang me to see if I had done them.” (participant 87)
“She put me in touch with all sorts of different things, actually, and er, gave me some leaflets and all sorts, so er, yes it did help. Certain things helped, some things didn’t.” (participant 47)
The information received and services recommended and trialled were not always perceived to have been of benefit to that individual at that time. Thirty per cent of participants reported instances where the plans and recommendations followed had not led to progress. Participant 09 found that the services signposted to did not help. She felt that this reflected an absence of valuable support with the return-to-work issues sought.
“It is very; it has been very frustrating trying to get things sorted out in different areas. Because there just isn’t anywhere to turn to. She suggested I try a few things like going through Macmillan and all that, but, there was nothing there. So effectively you are on your own…there is nothing!” (participant 09)
At the end of the interview, most of the participants became emotional when expressing what the intervention had meant for them at the final question ‘Is there something else?’, one summed up their experience as:
“This should be available to everybody… if it can get people feeling more positive and getting on with trying to look after themselves better…and…sort of tackling…I don’t know that we can really do anything to stop ourselves getting recurrences…but I think that it can’t hurt to be physically better. And if I am physically stronger and I’m eating well, and exercising and so on, then if I do get a recurrence I’ll be in a better physical position to deal with it I presume. Uhm, so I think if other people could have just, I mean even one or two appointments of the sitting down making their own targets for what they are going to do, to deal with the situation from here on, then I think that it shows that someone else is interested, and puts you in control which is great.” (participant 46)
Discussion
Where an HNA was used to develop a care plan with women at the end of treatment for gynaecological cancer, there was no evidence in the primary endpoint (EORTC global QoL status scores) to favour the intervention. However, a trend to improvement over 6 months in the EORTC subscales of emotional, role, social functioning and symptom scales for fatigue, insomnia and breathlessness was seen only in the intervention group. This suggestion of improvement in function and symptoms was corroborated by the nested interviews. The interviews also gave insights about the significant impact of cancer and treatment for some women. The experience of receiving the intervention and specifically how it had the treatment impact for them indicates that the intervention was well received by the majority of the women interviewed. The intervention was inexpensive, and a reduced use of health and social care resources was shown in the intervention group. The health economic analysis was based on a reduced sample size; however, it suggests a high probability that the intervention was cost-effective according to NICE guidelines.
Biomedical clinical environments are inherently cancer treatment focused, and the technical aspects of management can ostracise patients from a truly active role in their care because of the power imbalances this creates.31–33 Despite screening tools and communication training, clinicians can struggle to recognise and engage effectively with peoples’ supportive care needs through treatment.34 35 Clinicians report barriers to effective implementation of supportive care screening tools to be time constraints and perceiving that it takes them beyond their scope of practice while also recognising the benefits to their practice through improved communication and rapport with people.34
Where clinicians are able to use a person-centred approach, people are more able to engage in positive health behaviours and have better health outcomes, better adjustment to illness, less uncertainty, less symptom distress with better reports of mental and physical QoL over time.36–38 Our findings of improvements in these important aspects of the EORTC subscales agree with these previous findings in our HNA-naive population. Ideally, early discussion of psychosocial issues at each consultation may support people’s understanding that they can have the conversation and complete the assessment as required with their clinical teams.
Since the study started, a number of key interventions, such as ‘the Recovery Package’, have been developed to support a shift toward shared decision making and supporting self-management. This has been developed with strong user involvement39 and will form a mandatory part of a person’s package of care by 2020.13 It supports the use of the HNA in response to an individual’s need and aims to empower people to know that these conversations should happen regularly and that they can initiate them. However, despite this, there remains a persistent gap between the commitment to person-centred care and its practice.40 We support the inclusion of and open invitation for the completion of an HNA to be included in an admission pack to empower patients to initiate conversations.
In this study, the initial HNA assessment and care plan development took an average of 59 min to complete with 26 min of non-patient facing time. Subsequent assessments took 36 min with 19 min of non-patient facing time. We note that this is far greater than the 30 min suggested in the guidance, and while we agree with the intention not to overburden the person, we also recognise that to assess concerns and to support people to make and be able to achieve care plans takes time.15 We would suggest that staff workloads be organised to allocate realistic amounts of time into other prescheduled appointments to deliver these interventions effectively.
The AHP delivering the intervention was experienced in multiprofessional rehabilitation and familiar with behavioural change principles; additional training and information were needed for the purposes of the study to develop skills and resources to competently cover areas which were beyond the scope of their professional training. We welcome the publication of accessible resources such as the ‘Prompt Tools’ recently published by the London Cancer Alliance.41 These support clinicians to be consistent and current in their information giving and onward referral in response to people’s needs.
The study recruited pragmatically, and one-third of those approached agreed to participate, with less than a fifth (96 women) reporting no problems when discussing the purpose of the study (see figure 1). This proportion reflects the reports of unmet physical and psychosocial needs and those requesting help from other similar groups.3 42 43 Our study agreed with the findings of Greimel et al 44 in a similar population that the EORTC global QoL score is not predictable using demographic and clinical variables.
Women treated for gynaecological cancer have often experienced complex and lengthy treatment pathways, and many have pre-existing comorbidities. This study demonstrates that experienced AHPs in the cancer care setting are able to identify patient needs that may respond to rehabilitation interventions and can appropriately risk stratify patients so that they receive care in the safest and most cost-efficient setting. The approach used to deliver the intervention in the study differed from the usual biomedical model. It enabled people to identify their needs and focus on accessing appropriate support and tapping into their own supports in a guided way.42 45 The HNA has so far lacked evidence to suggest how it is best delivered and whether this intervention is valued by people receiving it. The study provides some insight into the positive impact of a brief intervention with an experienced AHP.
The study was pragmatic and reflected the clinical use of the HNA, which was offered equitably to all at the end of treatment irrespective of their global QoL scores. It was not possible to detect change in those scoring high on the scale at the start of the study. Due to the large numbers required to power a definitive study, this was designed as a descriptive study. It was not powered to detect a difference between arms, and as such, there was no expectation that the selected validated measures would statistically significantly support the changes reported by interview participants.
Conclusion
This mixed-methods study has shown that women treated for gynaecological cancers can be supported at the end of treatment to adjust to their new normal and are seen to trend toward improvement in EORTC subscales when trained health professionals make use of person-centred approaches alongside usual care.
This intervention has been shown to be a cost-effective intervention to support self-management that is effective for some on the basis of the EORTC subscale and interview data. We suggest that, on balance, this should be made available to this group of women and a similar approach tested earlier in the pathway.
“I can’t say I feel happy…but I do now feel content.” (participant 87)
Acknowledgments
The authors thank the patients and the gynaecological unit at the Royal Marsden NHS Foundation Trust for supporting this study, with particular thanks to Nancy Hollondener and the other patient representatives, the Royal Marsden Therapies Department and the gynae-oncology clinical nurse specialists for support during the development and conduct of this study and also to Kath Malhotra for conducting a number of the interviews and to Professor Theresa Wiseman for ongoing support and advice.
Catherine Sandsund, Ruth Tigue and Amyn Lalji receive funding from the Royal Marsden Cancer Charity, and the authors acknowledge support from the National Institute for Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Centre. The Royal Marsden Charity grant was used to fund the health economists’ input.
Thanks to RAND for the use of SF-36 RAND (version 2). The SF-36 health survey was developed at RAND as part of the medical outcome survey. Thanks to the EORTC for permissions to use the EORTC-QLQ-C30 (version 3).
Footnotes
Contributors: This work was undertaken as a collaborative clinical research project. The contributions of the authors are outlined below. Study design: CAS, CS, RT, ND, AF and HG (additional contributions from patient representatives, therapies department, psychological support services and the gynaeocology clinical nurse specialists). Patient information sheet, consent form, study poster design: CAS, CS and patient representatives. Recruitment: CAS and RCT. Interventions: CAS and RCT. Semi-structured interviews: Kath Malhotra, RCT. Data management: AL. Statistical analysis: KT. Health economics analysis: HG and JJ. Interview analysis: RT and CAS. Manuscript preparations: CAS, CS, JJ, KT, RT, HG, RCT, ND, AF and patient representative (NH).
Funding: This study was funded by the Royal Marsden Charity.The study is reported according to CONSORT guidelines extension for non-pharmacological treatments.
Competing interests: None declared.
Patient consent: No individuals' identifiable information is included in this paper.
Ethics approval: National Research Ethics Committee London – West London.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Cancer Research UK. Cancer Survival Statistics. Secondary cancer survival statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival#heading-Zero
- 2. Wenzel L, DeAlba I, Habbal R, et al. . Quality of life in long-term cervical cancer survivors. Gynecol Oncol 2005;97:310–7. 10.1016/j.ygyno.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 3. Beesley VL, Price MA, Webb PM, et al. . Changes in supportive care needs after first-line treatment for ovarian cancer: identifying care priorities and risk factors for future unmet needs. Psychooncology 2013;22:1565–71. 10.1002/pon.3169 [DOI] [PubMed] [Google Scholar]
- 4. de Boer AG, Frings-Dresen MH. Employment and the common cancers: return to work of cancer survivors. Occup Med 2009;59:378–80. 10.1093/occmed/kqp087 [DOI] [PubMed] [Google Scholar]
- 5. Grunfeld EA, Cooper AF. A longitudinal qualitative study of the experience of working following treatment for gynaecological cancer. Psychooncology 2012;21:82–9. 10.1002/pon.1874 [DOI] [PubMed] [Google Scholar]
- 6. Greenwald HP, McCorkle R, Baumgartner K, et al. . Quality of life and disparities among long-term cervical cancer survivors. J Cancer Surviv 2014;8:419–26. 10.1007/s11764-014-0352-8 [DOI] [PubMed] [Google Scholar]
- 7. Kennedy F, Haslam C, Munir F, et al. . Returning to work following cancer: a qualitative exploratory study into the experience of returning to work following cancer. Eur J Cancer Care 2007;16:17–25. 10.1111/j.1365-2354.2007.00729.x [DOI] [PubMed] [Google Scholar]
- 8. Snyder CF, Dy SM, Hendricks DE, et al. . Asking the right questions: investigating needs assessments and health-related quality-of-life questionnaires for use in oncology clinical practice. Support Care Cancer 2007;15:1075–85. 10.1007/s00520-007-0223-1 [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Clinical Excellence. Improving supportive and palliative care for adults with cancer: National Institute for Clinical Excellence. 2004.
- 10. Yabroff KR, Lawrence WF, Clauser S, et al. . Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst 2004;96:1322–30. 10.1093/jnci/djh255 [DOI] [PubMed] [Google Scholar]
- 11. Yadav R. Rehabilitation of surgical cancer patients at University of Texas M. D. Anderson Cancer Center. J Surg Oncol 2007;95:361–9. 10.1002/jso.20775 [DOI] [PubMed] [Google Scholar]
- 12. Sandsund C, Pattison N, Doyle N, et al. . Finding a new normal: a grounded theory study of rehabilitation after treatment for upper gastrointestinal or gynaecological cancers - the patient’s perspective. Eur J Cancer Care 2013;22:232–44. 10.1111/ecc.12016 [DOI] [PubMed] [Google Scholar]
- 13. Taskforce IC. Achieving world class cancer outcomes: a strategy for England 2015-2020. 2015.
- 14. NCSI Research Workstream. NCSI Research Work Stream Survivorship Journey mapping project. Summary and reports for bowel cancer. Breast Cancer, Lung Cancer and Prostate Cancer 2009. [Google Scholar]
- 15. Cancer Action Team. Holistic Common Assessment of supportive and Palliative Care needs for adults with Cancer. London, 2007:21. [Google Scholar]
- 16. MINIM [program], 2000.
- 17. London Cancer Alliance. Holistic Needs Assessment. Secondary holistic needs assessment. 2013. http://www.londoncanceralliance.nhs.uk/media/60440/London%20Holistic%20Needs%20Assessment_print%20version_2013.pdf
- 18. Osoba D, Zee B, Pater J, et al. . Psychometric properties and responsiveness of the EORTC Quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res 1994;3:353–64. 10.1007/BF00451727 [DOI] [PubMed] [Google Scholar]
- 19. McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- 20. Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale : Johnston M, Wright SC, Weinman S J, Measures in health psychology: a user’s portfolio. Causal and control beliefs. Windsor: NFER-NELSON, 1995:35–-37. [Google Scholar]
- 21. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects secondary World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects 22.10. 2008. http://www.wma.net/en/30publications/10policies/b3/index.html
- 22. Curtis L. Unit Costs of Health and Social Care: DH Reference costs In: Health Do. 2010. [Google Scholar]
- 23. Department of Health. NHS Reference costs 2013-14. Secondary NHS Reference costs 2013-14. 2015. https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014
- 24. Cancer Research UK. Individual therapies. secondary individual therapies. 2015. http://www.cancerresearchuk.org/about-cancer/cancers-in-general/treatment/complementary-alternative/therapies/
- 25. Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851–9. 10.1097/01.mlr.0000135827.18610.0d [DOI] [PubMed] [Google Scholar]
- 26. Sterne JA, White IR, Carlin JB, et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327–40. [DOI] [PubMed] [Google Scholar]
- 28. Boyatzis RE. Transforming qualitative information: thematic analysis and code development. Thousand Oaks, Calif; London: Sage Publications, 1998. [Google Scholar]
- 29. Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487–96. 10.1002/hec.944 [DOI] [PubMed] [Google Scholar]
- 30. Towse A, Devlin N. Cost effectiveness thresholds: economic and ethical issues : Office for Health Economics, ed. What is NICE’sthreshold? An external view. London: The Kings Fund, 2002. [Google Scholar]
- 31. Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature Social Science & Medicine. 2000;51:1087–110. [DOI] [PubMed] [Google Scholar]
- 32. Lepledge A, Gzi lF, iM C, et al. . Person-centredness: a conceptual framework and review of the empirical literature. Social Science & Medicine 2007;51. [DOI] [PubMed] [Google Scholar]
- 33. Pearson A, Vaughn B, FitzGerald M. Nursing Models for practice. 3rd edn: Elsevier Health Sciences, 2004. [Google Scholar]
- 34. Ristevski E, Breen S, Regan M. Incorporating supportive care into routine cancer care: the benefits and challenges to clinicians' practice. Oncol Nurs Forum 2011;38:E204–E211. 10.1188/11.ONF.E204-E211 [DOI] [PubMed] [Google Scholar]
- 35. Doyle N, Henry R. Holistic needs assessment: rationale and practical implementation. Cancer Nursing Practice 2014;13:16–21. 10.7748/cnp.13.5.16.e1099 [DOI] [Google Scholar]
- 36. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff 2013;32:207–14. 10.1377/hlthaff.2012.1061 [DOI] [PubMed] [Google Scholar]
- 37. McCorkle R, Dowd M, Ercolano E, et al. . Effects of a nursing intervention on quality of life outcomes in post-surgical women with gynecological cancers. Psychooncology 2009;18:62–70. 10.1002/pon.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bakker DA, Fitch MI, Gray R, et al. . Patient-health care provider communication during chemotherapy treatment: the perspectives of women with breast cancer. Patient Educ Couns 2001;43:61–71. 10.1016/S0738-3991(00)00147-6 [DOI] [PubMed] [Google Scholar]
- 39. Department of Health. Macmillan Cancer support, NHS Improvement. National Cancer survivorship Initiative Vision. 2010:1–82.
- 40. The Health Foundation. Person-centred care: from ideas to action. 2014. [Google Scholar]
- 41. Alliance LC. Holistic Needs Assessment Prompt Tools. 2016. [Google Scholar]
- 42. Urbaniec OA, Collins K, Denson LA, et al. . Gynecological cancer survivors: assessment of psychological distress and unmet supportive care needs. J Psychosoc Oncol 2011;29:534–51. 10.1080/07347332.2011.599829 [DOI] [PubMed] [Google Scholar]
- 43. Armes J, Crowe M, Colbourne L, et al. . Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol 2009;27:6172–9. 10.1200/JCO.2009.22.5151 [DOI] [PubMed] [Google Scholar]
- 44. Greimel E, Lahousen M, Dorfer M, et al. . Patients' view of routine follow-up after gynecological cancer treatment. Eur J Obstet Gynecol Reprod Biol 2011;159:180–3. 10.1016/j.ejogrb.2011.06.027 [DOI] [PubMed] [Google Scholar]
- 45. Ahmed N, Ahmedzai SH, Collins K, et al. . Holistic assessment of supportive and palliative care needs: the evidence for routine systematic questioning. BMJ Support Palliat Care 2014;4:238–46. 10.1136/bmjspcare-2012-000324 [DOI] [PubMed] [Google Scholar]