Abstract
Recent developments in the generation of neuronal population-specific, genetically-modified mouse lines have allowed precise identification and selective stimulation of cholinergic neurons in vivo. Although considerably less laborious than studies conducted with post-hoc identification of cholinergic neurons by immunostaining, it is not known whether the genetically based labeling procedures that permit in vivo identification are electrophysiologically benign. In this study we use mice carrying a BAC transgene that drives expression of a tau-GFP fusion protein specifically in cholinergic neurons. This allowed us to visualize basal forebrain cholinergic neurons in acute slice preparations. Using whole cell, patch clamp electrophysiological recording in acute brain slices, here we present original data about the basic electrical properties of these genetically tagged cholinergic neurons including firing rate, resting membrane potential, rheobase and various characteristics of their action potentials and after hyperpolarization potentials. The basic electrical properties are compared (1) with non-cholinergic neurons in the same brain regions; (2) in cholinergic neurons between immature animals and young adults; and (3) with cholinergic neurons that are expressing light sensitive channels. Our conclusions based on these data are (a) cholinergic neurons are less excitable then their non-cholinergic neighbors, (b) the basic properties of cholinergic neurons do not significantly change between adolescence and young adulthood and (c) these properties are not significantly affected by chronic expression of the excitatory opsin, oChIEF.
Introduction
Cholinergic neurons of the basal forebrain are thought to play a pivotal role in the modulation of attention and memory related behaviors and are renowned for their well-documented demise with the cognitive impairments characteristic of Alzheimer’s and other neurodegenerative disorders (Picciotto et al. 2012, Ballinger et al. 2016). Previous data on the electrophysiological profile of cholinergic vs. non cholinergic neurons in the basal forebrain have primarily utilized post-hoc identification with choline acetyl-transferase (ChAT) or vesicular acetylcholine transporter (VAChT) immunohistochemistry (e.g. (Markram and Segal (1990), Serafin et al. 1996, Bengtson & Osborne 2000, Hedrick & Waters 2010). More recent studies have employed genetically encoded markers to identify and stimulate cholinergic neurons with optogenetic probes such as ChR2, oChIEF and/or with subsequent monitoring of activity profiles based on spike characteristics (Jiang et al. 2014, Luchicchi et al. 2014, Hangya et al. 2015, Jiang et al. 2016, Ballinger et al. 2016, Hedrick et al. 2016). Although the latter approaches have the advantage of online identification and stimulation, there is little documentation as to how the expression and activation of these markers might alter the properties of cholinergic basal forebrain neurons. This study assesses this issue directly and in comparison with the reported literature.
Experimental procedures
Study design
These experiments used one and two month old male BAC-transgenic mice expressing a tau-GFP fusion protein under control of the ChAT promoter (ChAT tau-GFP; gift from S.Vijayaraghavan, Univ. Colorado, (Grybko et al. 2011)). For a subset of experiments these mice were crossed with a ChAT Cre recombinase knock–in line (B6;129S6-Chattm2(cre)Lowl/J, Jax stock number: 006410,(Rossi et al. 2011). Animals were housed in a temperature and humidity controlled facility with free access to food and water. Lights were turned on at 7 AM and off at 7 PM. Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the SUNY Research Foundation at Stony Brook University.
For the studies carried out for Figures 1 and 2, we compared basic electrical properties of both cholinergic and non-cholinergic neurons in mice of identical genetic background. Whenever possible, all male litter mates were used in these experiments and data from cholinergic and non-cholinergic neurons were collected from the same animal. For the studies shown in Figure 3, we crossed the ChAT-tau-GFP mice with ChAT-Cre mice. Genotypes of offspring were determined by PCR of DNA isolated from tail biopsies. All animals were injected with AAV9-CAG-DIO-oChIEF-tdTomato. Infected cholinergic neurons were identified by tdTomato / GFP double labeling visualized in acute slice. Non-infected neurons were GFP positive and tdTomato negative. Primary measures included resting membrane potential, rheobase and maximum firing rate. Other measures reported are properties of the action potentials generated following either current injection or optical stimulation, as noted.
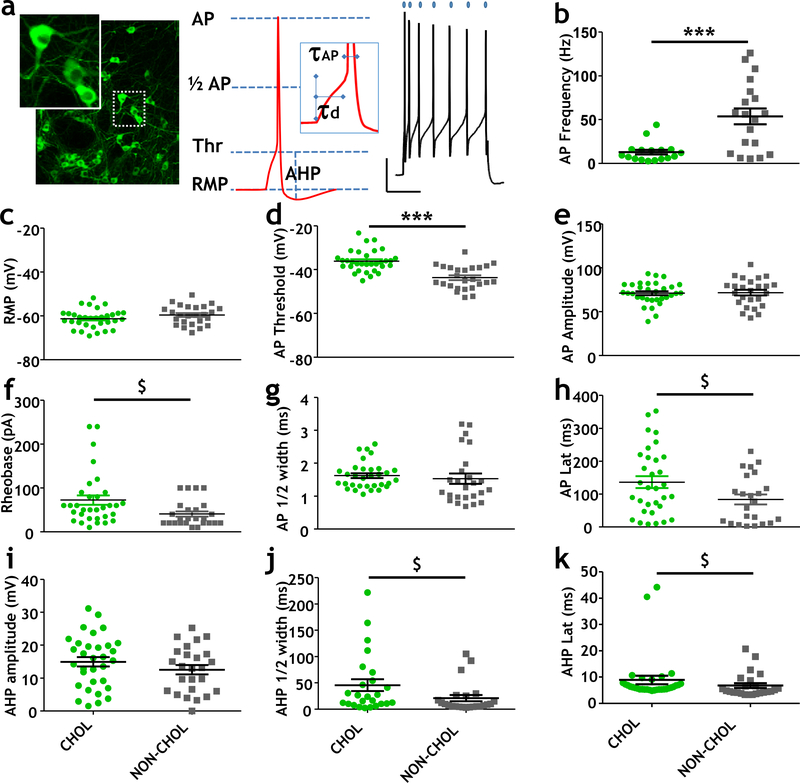
Figure 1. Comparison of electrical properties of cholinergic and non-cholinergic neurons in the NBM region of the basal forebrain.
a (left) Fluorescence photomicrograph of cholinergic (ChAT tau-GFP positive) neurons in the NBM region of the basal forebrain. Inset shows enlarged view of ChAT tau-GFP positive neurons. (middle) Illustration of parameters of electrical excitability that were measured in identified cholinergic and non-cholinergic neurons in the NBM. Parameters indicated here include AP spike threshold (Thr), resting membrane potential (RMP), AP amplitude (AP), AP latency (td), AP half-width (1/2 AP), and AHP amplitude (AHP) (right) Sample recordings showing the rapid accommodation of AP firing in cholinergic neurons despite continued depolarization for 500 ms. Calibration bar is 15mV X 200ms. See Methods for details.
b Scatter plots showing distribution of AP firing rates during a 500 ms duration depolarizing step in cholinergic neurons vs non-cholinergic neurons (Mann-Whitney, p=0.001).
c-e Scatter plots showing resting membrane potential (c); AP threshold values (d), and AP amplitude values of cholinergic and non-cholinergic neurons in the NBM. There was no significant difference between cholinergic and non-cholinergic neurons in RMP or AP amplitude. AP thresholds were significantly different between cholinergic and non-cholinergic NBM neurons (unpaired T-test, p< 0.001, power = 1).
f-h Scatter plots of all rheobase (f), AP ½-width (g) and AP latency (h) values for cholinergic and non-cholinergic neurons in the NBM. There were no significant differences in these properties, although there was a trend for increased rheobase ($: Mann Whitney test p=0.01, power = 0.51) and AP latency in the cholinergic neurons ($: Mann Whitney test, p=0.047, power=0.035) compared to the non-cholinergic neurons.
i-k Scatter plots showing features of the after-hyperpolarization potential (AHP) spike between cholinergic and non-cholinergic neurons in the NBM. There was no significant difference in the AHP spike amplitude (i), but there were trends towards increased AHP half-width (j; Mann Whitney test, p = 0.03, power = 0.51) and latency (k; Mann Whitney test p = 0.015, power = 0.18) in the cholinergic compared to the non-cholinergic neurons in the NBM.
The action potential properties were evaluated at rheobase current injection. Data from cholinergic neurons (ChAT tau-GFP positive) are represented by green-filled circles (n = 32 neurons, 11 mice) and non-cholinergic neurons (ChAT tau-GFP negative) are indicated by gray-filled squares (n = 24 neurons, 10 mice). A subset of the data presented here had been included in the supplementary figure 1 of (Jiang et al. 2016).
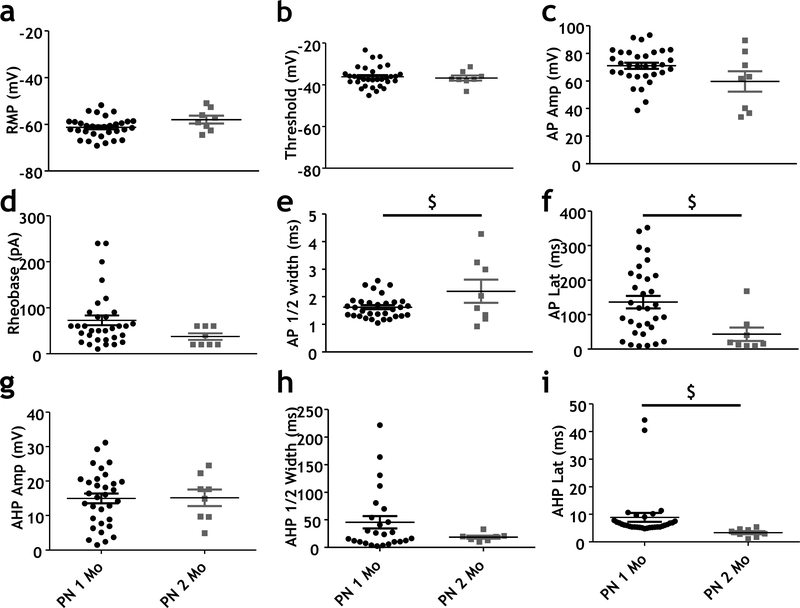
Fig 2. Comparison of electrical properties of cholinergic neurons in 1 month and 2 month-old animals in the NBM region of the basal forebrain.
a-c Scatter plots showing all RMP (a), AP threshold (b) and AP amplitude (c) values for ChAT tau-GFP positive neurons in 1 month and 2 month-old mice. There were no significant differences in these properties between 1 month and 2 month-old mice.
d-f Scatter plots showing rheobase (d), AP ½-width (e) and AP latency (f) values of ChAT tau-GFP positive neurons at 1 month and 2 months-old in the NBM. There were trends towards differences in AP ½-width (e; Unpaired t-test, 0.025; power =0.61) and AP latency (f; Mann Whitney test p = 0.01; power = 0.6) between 1 month and 2 month-old mice.
g-i Scatter plots showing features of the AHP spike between cholinergic and non-cholinergic neurons in the NBM between ChAT tau-GFP positive neurons in the NBM of 1 month and 2 month-old mice. There was a trend towards a significant difference in AHP latency between 1 month and 2 month-old mice (i; Mann Whitney test p <0.001; power = 0.37). There was no significant difference in AHP amplitude (g) or AHP half-width (h).
Action potential properties were evaluated at rheobase current injection. Data from 1 month-old ChAT tau-GFP positive neurons are represented by black-filled circles (n = 32 neurons, 11 mice) and 2 month-old ChAT tau-GFP positive neurons are indicated by gray filled squares (n = 8 neurons, 2 mice). Note that the data on 1 month old mice is from Figure 1.
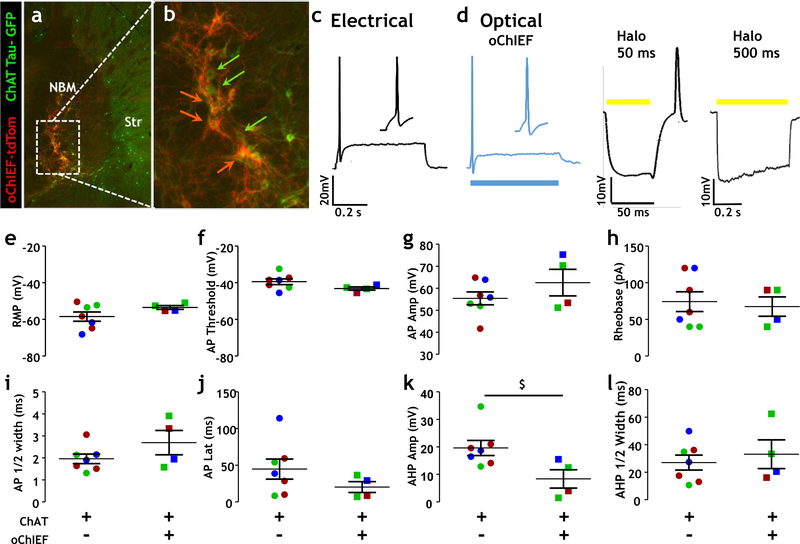
Fig 3. Comparison of electrical properties of opsin negative and opsin labeled cholinergic neurons in the NBM.
a Fluorescence photomicrograph of cholinergic (ChAT tau-GFP positive) neurons in the NBM region of the basal forebrain overlapping with tdTomato tagged cholinergic neurons expressing a viral vector encoding a ChR2 variant, oChIEF.
b Enlarged view of fluorescence photomicrograph from panel a. Green arrows label cholinergic neurons (ChAT tau-GFP labeled) and orange arrows represent ChAT tau-GFP and oChIEF labeled (overlap between ChAT tau-GFP neurons labeled with opsin, orange cell bodies/processes).
c,d Sample recording depicting AP firing in cholinergic neurons (and inset showing AP waveform) using both (c) electrical stimulation and (d) optical stimulation, following depolarization for 500 ms. Activation of halorhodopsin on NBM cholinergic neurons by 50 ms of yellow light stimulation (d middle panel) results in hyperpolarization during light stimulation and produces an off-response AP following termination of light. Activation by 500 ms of yellow light stimulation (d right panel) results in persistent hyperpolarization with decay, with no off-response AP observed.
e-h Scatter plots showing resting membrane potential (e), AP threshold (f), AP amplitude (g) and rheobase (h) of ChAT tau-GFP/oChIEF negative neurons and ChAT tau-GFP/oChIEF positive neurons. There was no significant difference in these measures between groups.
i-l Scatter plots showing all AP ½-width (i), AP latency (j), AHP amplitude (k) and AHP ½-width (l) values for ChAT tau-GFP/oChIEF negative neurons and ChAT tau-GFP/oChIEF positive neurons. There was no significant difference in AP ½-width or latency or AHP ½-width between groups. There was a trend towards a reduced AHP amplitude (k) in the ChAT tau-GFP/oChIEF positive neurons compared to the ChAT tau-GFP/oChIEF negative neurons ($; Mann Whitney test, p = 0.024, power = 0.44)
Action potential properties were evaluated at rheobase current injection. Data from ChAT tau-GFP/oChIEF negative neurons (n = 7 neurons, 3 mice) are displayed on the left side of graphs, and ChAT tau-GFP/oChIEF labeled (n = 4 neurons, 3 mice) are displayed on the right side of graphs. Colors indicate data points obtained from the same mouse.
Viral delivery
Four-week-old ChAT Cre x ChAT tau-GFP mice were anesthetized and stereotaxically injected bilaterally into the nucleus basalis of Meynert (NBM) with 0.5 μl of AAV9-CAG-DIO-oChIEF-tdTomato or AAV9-Ef1α-DIO-eNpHR3.0-eYFP. High titer recombinant AAV stocks (1013 – 1014 genome copies/ml) were prepared by the University of North Carolina vector core.
Brain slice preparation
Approximately 3 weeks after virus injection animals were anesthetized and transcardially perfused with cutting solution (in mM: sucrose 248, KCl 2, MgSO4 3, KH2PO4 1.25, NaHCO3 26, glucose 10, sodium ascorbate 0.4 and sodium pyruvate 1, bubbled with 95% O2 and 5% CO2), and maintained at 0~4°C. The brain was removed and sliced coronally (300 μm). Slices were equilibrated with an oxygenated incubation solution containing (in mM): sucrose 110, NaCl 60, KCl 2.5, MgCl2 7, NaH2PO4 1.25, NaHCO3 25, CaCl2 0.5, MgCl2 2, glucose 25, sodium ascorbate 1.3 and sodium pyruvate 0.6 at room temperature (24–26°C) for at least 1 hour.
Electrophysiological recording
During recording, slices were continuously superfused with oxygenated artificial cerebrospinal fluid (Jiang et al. 2016). NBM cholinergic neurons were identified by GFP and/or tdTomato expression. Signals were recorded using patch electrodes (resistance: 4–6 MΩ), a Multi Clamp 700B amplifier and pClamp10 software (Molecular Devices, Inc.). The pipette solution contained (in mM): 125 K-gluconate, 3 KCl, 1 MgCl2, 10 HEPES, 0.2 CaCl2, 0.1 EGTA, 2 Mg-ATP and 0.2 Na-GTP (pH=7.3).
Optogenetic stimulation
oChIEF or eNpHR3.0 (halorhodopsin) were activated with either a 50 or 500 ms pulse of 480 nm or 630nm light, respectively, of 1–3 mW/mm2 intensity.
Data Analysis
Data were filtered at 2 kHz and analyzed using Clampfit10 (Molecular Device, Inc.). Inclusion criteria used were: assessment of membrane potential (less than or equal to −50mV), input resistance (IR) (between 100 MΩ and 300 MΩ), series resistance (<10MΩ) that was unaltered throughout the recording and firing at least a 45mV AP at rheobase.
Depolarizing current steps of 10–500 ms were delivered to trigger action potentials. Action potential (AP) frequency was calculated from the depolarization step which yielded the maximum spike number divided by the duration of the depolarizing step. Resting membrane potential was measured after 10 minutes without current injection. AP threshold was the membrane potential at the point where the rising slope is larger than 10 mV/ms at rheobase. All the other AP properties were measured at rheobase. AP amplitude was measured from the threshold to the peak. AP half-width was calculated as the duration at half maximal AP amplitude. AP latency was the time between the onset of rheobase current and AP threshold. IR was calculated at rheobase by dividing the change in membrane potential by the current injected. The after hyperpolarization potential (AHP) amplitude was the difference between AP threshold and the most hyperpolarized potential reached. AHP latency was the time between the AP threshold (from AP repolarizing phase) and the most hyperpolarized potential. The AHP half-width was the duration at half maximal AHP amplitude.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc.), Sigmaplot 12.5 (Systat Software, Inc.) and OriginPro 9.1 (Origin Lab Corporation). A one way ANOVA or Student’s t-test was used when the data passed the normality test. If the data failed the normality test, a nonparametric Ranks Sum Test or Mann-Whitney test was used (see details in the text and Figure legends for each experiment.). Power analysis was performed with Sigmaplot, <0.9 was considered as underpowered. Statistical significance was designated at p < 0.05 and all data are presented as mean ± standard error of the mean unless non-parametric analyses were used.
Results
Our first goal was to assess the physiological properties of cholinergic neurons (identified in acute slice by their ChAT-promoter driven expression of a tau-GFP fusion protein), with neighboring neurons from the same animals that were not labeled, but were identified with differential interference contrast (DIC) optics. The latter group were classified as “non-cholinergic” (Fig 1). The active and passive membrane properties assessed are presented schematically in Figure 1a, and as delineated in the Methods. All individual data points are shown for resting membrane potential, spike threshold, spike timing at rheobase (latency, half width), spike frequency with prolonged depolarization and characteristics of the after spike-hyperpolarization.
Cholinergic neurons and non-cholinergic neurons differ dramatically in a variety of electrical properties (Fig 1). The most striking differences were in spike frequency (Fig 1b; cholinergic: 13.3 ± 2.7 Hz, non-cholinergic: 53.4 ± 9.5 Hz) and spike threshold (Fig 1d: cholinergic: −36.2 ± 0.9 mV, non-cholinergic: −43.7 ± 1.1 mV), with a trend towards greater rheobase (Fig 1f: cholinergic: 72.5 ± 10.6; non-cholinergic: 40.8 ± 5.9) and AP latency (Fig 1h: cholinergic: 136 ± 17.9 ms, non-cholinergic: 83.7 ± 15.3 ms), with no significant difference in resting membrane potential, (Fig 1c), AP ½ width (Fig 1g) or aspects of the AHP (Fig 1i –k). Increasing current injection elicited a dramatic increase in the spike frequency of non-cholinergic neurons that this was not seen in cholinergic neurons (Sup. Fig 1). Overall these data reveal that cholinergic basal forebrain neurons in the NBM are further from AP threshold, fire more slowly and with considerably more spike adaptation than their non-cholinergic neighbors, an overall pattern that is consistent with prior reports using post-hoc immunohistochemical or single cell RT-PCR identification (Markram & Segal 1990, Serafin et al. 1996, Bengtson & Osborne 2000, Sotty et al. 2003, Hedrick & Waters 2010).
We also examined whether differences in excitability of basal forebrain cholinergic neurons might be a feature of young adult neurons, as the bulk of studies completed to date have focused on early postnatal animals (PND 6 – 35). Comparison of passive and active membrane properties at 1 vs. 2 months postnatal are presented in Fig 2. All neurons included were identified as cholinergic, again, based on their expression of the ChAT promotor driven tau-GFP (Grybko et al. 2011). Comparison of these data from a total of 40 neurons revealed no significant differences in active membrane properties between cholinergic neurons assayed in 1 vs. 2 month old animals. There were trends for developmental changes in AP half-width, AP latency and in the timing of the after spike hyperpolarization (p=0.025, power=0.61; p<0.1, power = 0.06; and p<0.01, power =0.37, respectively).
We also compared the properties of responses elicited by electrical stimulation vs. those elicited by activation of either excitatory (oChiEF) or inhibitory (eNpHR3.0) light sensitive channels (Fig 3a–d). Action potential profiles for electrical vs. optogenetic stimulation of the same neurons revealed little difference in spike amplitude, shape or duration. Assay of a variety of protocols for optogenetically induced hyperpolarization revealed that short flashes of appropriate wavelength for activation of halorhodopsin transiently hyperpolarizes, but then depolarizes ChAT+ neurons, i.e. there is a reliable off-response action potential evoked by activation of halorhodopsin for <100 msec (50 msec shown in Fig 3d). In contrast, flashes of 500 msec duration or longer elicit sustained hyperpolarization without consequent off-responses (Fig 3d). Longer flash durations revealed that the extent of hyperpolarization during the flash declines with a time constant of ~1.5 sec consistent with the idea that hyperpolarizing flash durations of more than 3 seconds do not significantly enhance the extent of hyperpolarization.
Finally, we evaluated whether the expression of oChIEF significantly altered membrane properties of cholinergic neurons and compared our findings with prior studies using other methods to identify the cholinergic neurons (Fig 3 and Table 1). In direct (within mouse; Figure 3) comparisons of ChAT neurons with and without oChIEF co-expression, the only difference detected was a trend towards decreased amplitude of the after spike hyperpolarization (p=0.024, power = 0.44; Fig 3k).
Table 1.
summarizes AP properties of cholinergic neurons across multiple studies. Data reported includes median/mean, 95% confidence interval/range, and cell number (n) where possible.
| REF | AREA | ChAT+ Identification Species & Age | RMP (mV) | Firing Rate (Hz) | AP Amplitude (mV) | AP Threshold (mV) | AP Half-Width (ms) | AHP Amplitude (mV) | Input Resistance (MΩ) |
|
Median [95% CI] (n) |
Median [95% CI] (n) |
Median [95% CI] (n) |
Median [95% CI] (n) |
Median [95% CI] (n) |
Median [95% CI] (n) |
Median [95% CI] (n) |
|||
| This paper & Jiang et al. 2016 | NBM | ChAT tau-GFP tg, mouse PND 60+ | −58.1 [−61.2,−54.71 (8) |
4.7 [0, 43.8] (7) |
62.4 [45.2,74.2] (8) |
−37.3 [−39.2,−34.4] (8) |
1.8 [1.4,3.0] (8) |
16.3 [10.5,19.8] (8) |
288 [281,309] (8) |
| NBM | ChAT tau-GFP/ChAT-Cre tg + DIO-oChIEF, mouse PND 60+ | −59 [−63.2,−57.8] (19) |
2 [2.9, 7.3] (17) |
61.3 [49.8,62.4] (21) |
−41.6 [−43.0,−36.7] (19) |
2.5 [2.4,4.1] (21) |
8.8 [7.0,14.0] (20) |
162 [151,366] (18) |
|
| NBM | ChAT-Cre tg + DIO-oChIEF, mouse PND 60+ | −59.2 [−62.5,−57.2] (16) |
4.2 [3.9, 11.65] (16) |
65.3 [53.6,71.7] (16) |
−45.6 [−49.1,−41.3] (16) |
1.9 [1.7,3.4] (16) |
12.2 [7.9,14.0] (16) |
198 [153,268] (15) |
|
| Mean ± SEM (n) |
Mean ± SEM [Range] (n) |
Mean ± SEM [Range] (n) |
Mean ± SEM (n) |
Mean ± SEM [Range] (n) |
Mean ± SEM [Range] (n) |
Mean ± SEM [Range] (n) |
|||
| Hedrick et al. 2010 | NBM | C57/BL6 + ChAT Immunohistochemistry, mouse PND 42–54 | −55.4 ± 4.8 (7) | 20 | 64.0 ± 3.4 (9) | −31.5 ± 2.8 (9) | 0.52 ± 0.04 (9) | sAHP: 13.3 ± 2.2 (9) fAHP: 16.6 ± 3.5 (9) |
251 ± 17.7 (9) |
| Bengtson et al. 2000 | NBM | Wistar Rat + ChAT Immunohistochemistry, PND 6–18 | −65.3 ± 2.7 (13) | [6–13] | 60 ± 9.0 (13) | −41 ± 1.0 (8) | 1.3 ± 0.1 (13) | 23 ± 1.7 (13) | ND |
| Markram et al. 1989 | MS | Rat + ChAT Immunohistochemistry, PND 6–18 | −65 ± 7 (7) | [4–10] | 76.1 ± 4.2 (7) | ND | ND | [15–20] | 135 ± 53 (7) |
| Serafin et al. 1996 | MS | Rat/Guinea Pig, PND 14–20 (rat)/100–150g (guinea pig) | −60.2 ± 0.34 [−58 – −62] |
8.5 ± 0.2 [7.3–10] (13) |
69.6 ± 1.8 [60–80] (13) |
ND | 1.18 ± 0.1 [9–1.5] (13) |
18.6 ± 1.0 [16–22] (13) |
143.2 ± 9.1 [96–186] (12) |
| Sotty et al. 2003 | MS | SD Rat + single cell RT-PCR PND 13–19 | ND | 16.3 ± 1.6 (14) | ND | ND | ND | 7.4 ± 0.7 (14) | ND |
| Hedrick et al. 2016 | NBM | ChAT-Cre/Ai32 (ChR2-YFP), mouse PND 21–35 | −44.8 ± 2.1 (10) | [15–20] | 61.9 ± 5.2 (10) | −27.9 ± 1.0 (10) | 0.58 ± 0.03 | sAHP: 13.5 ± 2.7 (10) fAHP: 16.8 ± 2.5 (10) |
163.4 ± 26.7 (10) |
| Hedrick et al. 2016 | NBM | ChAT-Cre/Ai35 (Arch-GFP), mouse PND 21–35 | −49.2 ± 4.5 (7) | -- | 62.1 ± 4.4 (9) | −28.2 ± 1.5 (9) | 0.61 ± 0.03 | sAHP: 19.6 ± 2.9 (8) fAHP: 10.4 ± 16 (8) |
233.6 ± 45.4 (9) |
Discussion
Table 1 summarizes our findings and compares the passive and active membrane properties and action potential parameters assayed in this study with those obtained by others in their studies in both mouse and rat using genetic and non-genetic techniques for cholinergic neuron identification. Overall, this assessment reveals that while there might be subtle changes in membrane properties as a function of developmental stage, cholinergic neurons in older animals appear to have similarly low excitability profiles. In addition, the expression of and identification by optogenetic labeling had minimal effect on passive and active membrane properties of the cholinergic neurons (Table 1). This is in marked contrast to the reports of increased cholinergic tone associated with a 4 – 6 fold elevation in VAChT expression from BAC-ChAT derived transgenes (Nagy & Aubert 2012, Kolisnyk et al. 2013). We have not quantified levels of VAChT protein or mRNA in this study and cannot rule out that elevated VAChT contributes to the subtle changes in the passive and active membrane properties between our results and those of others (Table 1). However, we do not think that elevated VAChT expression affects the properties measured in this study because results we have obtained from recordings of cholinergic neurons identified post-hoc by ChAT mRNA expression, using single cell RT-PCR, are equivalent to the values reported here (RMP: −61.8 ± 3.3 mV; Thr: −43.0 ± 0.9 mV; IR: 259 ± 52 MΩ; ½ AP: 1.6 ± 0.2 ms; AP Amplitude: 53.9 ± 4.1 mV; AHP Amplitude: 11.8 ± 1.0 mV).
Although cholinergic properties as evaluated with in vivo or post-hoc identification methods appear to be reasonably similar, this and prior studies have indicated substantial differences in the properties of ChAT-positive neurons compared with noncholinergic neurons (i.e. ChAT negative). Perhaps the most striking differences are in the spike properties, with cholinergic neurons typically exhibiting much lower firing rates than non-cholinergic neurons within the same regions of basal forebrain (Markram & Segal 1990, Serafin et al. 1996, Bengtson & Osborne 2000, Hedrick & Waters 2010). Zaborszky and colleagues have further delineated that cholinergic neurons comprise two subpopulations with respect to spike rate and spike accommodation profiles (Unal et al. 2012). These differences, although much less dramatic than the differences between cholinergic and non-cholinergic neurons, are worth examining in more detail given the suggestion that these different activity profiles could underlie the differences in faster, more transient ACh release vs. longer lasting, slower transmitter release (Unal et al. 2012, Unal et al. 2015).
It should be noted that although the qualitative nature of the differences between cholinergic and non-cholinergic neurons is consistent with prior studies, the absolute values of a subset of the membrane properties of ChAT+ neurons obtained with live markers were different from those reported from studies using post-hoc identification (Bengtson & Osborne 2000, Sotty et al. 2003, Hedrick & Waters 2010). Some aspects of these differences may have to do with expected differences in rat vs. mouse, may be a function of the mouse lines and background strains used and/or may be due to differences in the precise location, and hence neuronal subtypes, of the respective recordings. These considerations need to be kept in mind when comparing results between different lines of genetically modified mice and markers for in vivo and in vitro studies of cholinergic neurons.
In contrast to results with ChR2, optogenetic labeling of ChAT+ neurons with oChIEF provides a reliable and consistent method for repeat photo-stimulation that elicits action potentials that are very similar to those evoked by direct electrical stimulation (Lin et al. 2009, Hedrick et al. 2016). The prolonged action potential duration seen with ChR2 probes might reflect its longer opening and closing kinetics as well as its higher permeability to calcium.
Supplementary Material
Figure legend for Supplemental Fig 1
Comparison of AP frequency with increased depolarization current injection between cholinergic (ChAT tau-GFP positive) neurons and non-cholinergic (ChAT tau-GFP negative) neurons.
Acknowledgments and conflict of interest disclosure
This research was supported by R01 NS 22061 (LWR); DP1 OD007014 (LWR), K12GM102778 (GYL-H), F30 MH105087 (EB).
Abbreviations
- ChAT
choline acetyl-transferase
- VAChT
vesicular acetylcholine transporter
- ChR2
channelrhodopsin-2
- oChIEF
mammalian codon optimized chimera EF with I170V mutation
- eNpHR3.0
Natronomonas pharaonis-derived halorhodopsin version3.0
- AAV9
Adeno-associated virus serotype 9
- CAG
Cytomegalovirus enhancer, chicken beta-Actin promoter and rabbit beta-Globin splice acceptor site
- Ef1a
elongation factor-1alpha
- DIO
double-floxed inverse open reading frame
- BAC
bacterial artificial chromosomes
- GFP
green fluorescent protein
- eYFP
enhanced yellow fluorescent protein
- tdTomato
tandem dimer tomato
- Cre
Cre recombinase; causes recombination
- NBM
nucleus basalis of Meynert
- DIC
differential interference contrast
- AP
Action Potential
- AHP
after hyperpolarization potential
- Thr
AP threshold
- RMP
resting membrane potential
- td
AP latency
- ½ AP
AP half-width
- IR
input resistance
Footnotes
The authors declare no competing financial interests.
All experiments were conducted in compliance with the ARRIVE guidelines.
References
- Ballinger EC, Ananth M, Talmage DA and Role LW (2016) Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron, 91, 1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP and Osborne PB (2000) Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices. Journal of neurophysiology, 83, 2649–2660. [DOI] [PubMed] [Google Scholar]
- Grybko MJ, Hahm ET, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE and Vijayaraghavan S (2011) A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. The European journal of neuroscience, 33, 1786–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M and Kepecs A (2015) Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell, 162, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick T, Danskin B, Larsen RS, Ollerenshaw D, Groblewski P, Valley M, Olsen S and Waters J (2016) Characterization of Channelrhodopsin and Archaerhodopsin in Cholinergic Neurons of Cre-Lox Transgenic Mice. PloS one, 11, e0156596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick T and Waters J (2010) Physiological properties of cholinergic and non-cholinergic magnocellular neurons in acute slices from adult mouse nucleus basalis. PloS one, 5, e11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, Lopez-Hernandez GY, Ballinger EC, Wang S, Talmage DA and Role LW (2016) Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits. Neuron, 90, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Lopez-Hernandez GY, Lederman J, Talmage DA and Role LW (2014) Optogenetic studies of nicotinic contributions to cholinergic signaling in the central nervous system. Reviews in the neurosciences, 25, 755–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolisnyk B, Guzman MS, Raulic S, Fan J, Magalhaes AC, Feng G, Gros R, Prado VF and Prado MA (2013) ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33, 10427–10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P and Tsien RY (2009) Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophysical journal, 96, 1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A, Bloem B, Viana JN, Mansvelder HD and Role LW (2014) Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Frontiers in synaptic neuroscience, 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H and Segal M (1990) Electrophysiological characteristics of cholinergic and non-cholinergic neurons in the rat medial septum-diagonal band complex. Brain research, 513, 171–174. [DOI] [PubMed] [Google Scholar]
- Nagy PM and Aubert I (2012) Overexpression of the vesicular acetylcholine transporter increased acetylcholine release in the hippocampus. Neuroscience, 218, 1–11. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ and Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron, 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D et al. (2011) Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell metabolism, 13, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin M, Williams S, Khateb A, Fort P and Muhlethaler M (1996) Rhythmic firing of medial septum non-cholinergic neurons. Neuroscience, 75, 671–675. [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R and Williams S (2003) Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. The Journal of physiology, 551, 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Golowasch JP and Zaborszky L (2012) Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Frontiers in behavioral neuroscience, 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Pare D and Zaborszky L (2015) Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience, 35, 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure legend for Supplemental Fig 1
Comparison of AP frequency with increased depolarization current injection between cholinergic (ChAT tau-GFP positive) neurons and non-cholinergic (ChAT tau-GFP negative) neurons.