Sex differences in the manifestations of clinical cardiovascular disease become more evident with advancing age and are likely related to sexual dimorphism in risk factors such as high blood pressure (BP).1 Accordingly, we and others have observed marked differences between females and males in trajectories of BP elevation, a surrogate of vascular aging, beginning early in life.2–4 In effect, previously reported evidence has shown that females tend to exhibit a more accelerated rise in BP in early-to-mid life such that their BP levels catch up to those of men by later life.2–4 Such overt sex differences in patterns of BP elevation are likely related to a variety of underlying mechanisms including variable associations with cardiometabolic risk factors, which are also known to differ between women and men. Therefore, we sought to examine sex differences in the extent to which cardiometabolic risk traits are related to BP elevation over the life course.
We used serially examined BP measurements collected longitudinally from participants of four community cohorts with study designs described previously:5 Framingham Heart Study (FHS) offspring cohort (Exams 1–9), Atherosclerosis Risk in Communities (ARIC) Study (Exams 1–4), Coronary Artery Risk Development in Young Adults (CARDIA) Study (Exams 1–8), and Multi-Ethnic Study of Atherosclerosis (MESA, Exams 1–4). Each participant provided informed consent, and institutional review boards approved the protocol at each study site. We excluded observations if concurrent data were missing for BP measures, antihypertensive medication, or key covariates: body mass index (BMI), smoking status, diabetes mellitus (DM) and total cholesterol (TC). Thus, our final study sample included N=32,833 unique participants who contributed 136,869 observations over a 43-year period (1971–2014) with ages spanning 5 to 98 years.
As part of harmonizing data across cohort studies, we corrected systolic BP measures to readings from mercury column sphygmomanometer after adjusting for previously described between-method differences.6–8 To account for the effects of antihypertensive therapy,9 we imputed BP values to be 10 mm Hg higher in estimated systolic BP and 5 mm Hg higher in estimated diastolic BP for individuals on antihypertensive therapy.10,11 In secondary analyses, we applied alternate approaches to imputing effects of medication therapy and we also conducted analyses without imputation but with adjustment for medication use in multivariable modeling. Higher versus lower risk burden was defined as presence of ≥2 versus <2 of the following cardiometabolic risk factors assessed at each visit: smoking status (current smoker), diabetes mellitus, obesity (BMI ≥30 kg/m2), or hypercholesterolemia (total cholesterol ≥200 mg/dL). We categorized participant-observations (N=136,869) into 4 categories: (1) women with higher risk burden, (2) women with lower risk burden, (3) men with higher risk burden, (4) men with lower risk burden. For each category, we fit multilevel linear regression models to display longitudinal BP trajectories, with age used as the timescale for all analyses. Each model included age as a fixed effect, participant IDs as random intercepts, and BP measure as the outcome.
Given that the associations of the predictor variable (age) with the outcome variable (systolic BP) were non-linear, we used restricted cubic splines with 4 knots to allow for nonlinearity of relationships. Under the premise of sex-specific physiology,12,13 we then calculated BP change from the baseline BP level (i.e. mean BP level at 18 years of age) for each category. Differences between higher and lower risk burden in the relationships between BP measures and age were tested via likelihood ratio test between models with and without parameters representing the interaction between risk burden and the cubic spline variable representing age. Differences in systolic BP trajectories between higher and lower risk burden over time were shown as the area between the BP curves with higher and lower risk burden. Graphically quantitative difference were then plotted as area chart above x-axis. In all fitted splines, data were truncated over an age range of 18 (0.5th percentile) to 85 (99.5th percentile) years. We then categorized participant-observations according to age tertiles (i.e. 18~49; 50~59; 60~85). To quantify the difference of BP trajectories between higher and lower risk burden, in each age tertile, we compared rates of BP increase with age (mmHg per decade) in the presence of higher versus lower risk burden using multilevel linear regression models adjusted for diabetes mellitus, smoking status, obesity, hypercholesterolemia, race, and cohort. We then compared differences by sex in the risk-related differences in BP rate of increase (difference in difference). All analyses were performed using R v3.5.1 (R Foundation for Statistical Computing, Vienna) and STATA v15 (StataCorp, College Station, TX).
In our multi-cohort sample, the mean age at baseline was 36.8±8.8 years in women and 36.6±8.8 years in men; 36% of women and 32% of men were of non-white race/ethnicity. During the follow-up period, 29.0% of women and 39.2% of men died. To discern the risk profiles among the survived females and males, we compared and observed that older women versus older men (i.e. participant-observations in the 60–80 year age range) had greater body mass index, systolic BP (SBP), and total cholesterol; however, they also had lower frequency of current smoking and diabetes (P<0.001 for all).
In analyses of systolic SBP trajectories over time, we observed that SBP levels increased with age to a greater extent in the higher than in the lower risk burden groups (Figure 1A), both in women and in men (P<0.001 for both). When trajectories of SBP elevation for both sexes and both risk groups are displayed in relation to adult baseline levels (i.e. from 18 years of age, allowing for SBP comparisons in older individuals to their younger selves over time), differences in risk-related SBP elevation are further clarified (Figure 1B). In particular, risk-stratified analyses demonstrate that the presence of a greater risk factor burden was associated with more accentuated SBP elevation and greater overall SBP load in women than in men throughout the adult life course (Figures 1C and 1D). Accordingly, as shown in Figure 1E, presence of a greater risk factor burden was associated higher multivariable-adjusted rates of SBP increase (i.e. calculated as slopes of BP rise), especially in early adulthood. Notably, risk-related SBP elevation was more profound in women than in men especially in younger adulthood (P=0.014). In analyses using different approaches to accounting for the effects of antihypertensive medication use, results were similar (data not shown). In analyses of diastolic BP, mean arterial pressure, results were similar (data not shown).
Figure 1. Sex Differences in the Cardiometabolic Risk-Related Systolic Blood Pressure Elevations Over the Life Course.
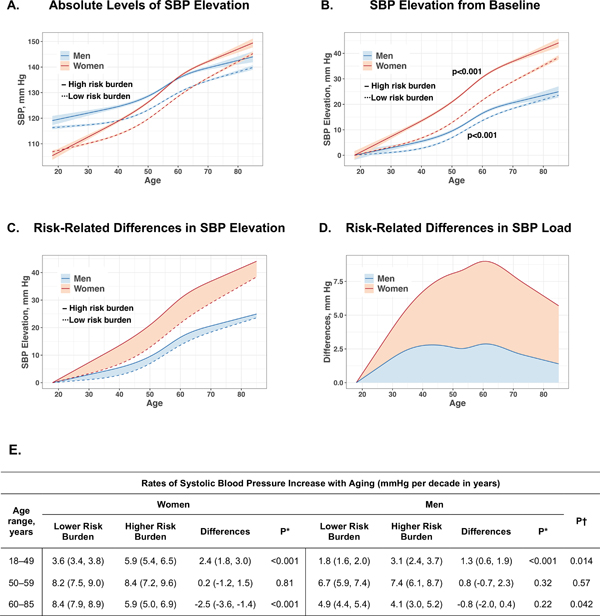
Sex-specific systolic blood pressure (SBP) trajectories are displayed for all participants stratified by presence of higher versus lower risk factor burden (≥2 versus <2 of the following risk factors: obesity, diabetes mellitus, hypercholesterolemia, current smoker). In Panel A, trajectories of SBP in the presence of higher risk factor burden are displayed as bolder curves (with darker shading for error limits), overlaid with trajectories for lower risk factor burden displayed as less bold curves (with lighter shading for error limits). In Panel B, data was displayed with sex-specific values set to represent change from baseline SBP level (i.e. “elevation from baseline”), allowing comparison of older individuals to their younger selves over time. The presence of a greater risk factor burden was associated with steeper increase of SBP in women and men (P<0.001 for both). In Panel C, sex-specific differences in SBP load between having higher versus lower risk factor burden shown as the area between the curves. In Panel D, the relative difference in SBP load between having higher versus lower risk factor burden is shown as area between the curve and the x-axis. In Panel E, risk-related SBP elevation (i.e. calculated as difference in slopes of SBP rise between higher and lower risk burden) was greater in women than in men at young age. Overall findings demonstrate greater differences in both SBP trajectories and SBP load over time (i.e. with aging) in women versus men, particularly in the presence of higher risk at young age. The P values in Panel B denoted with an asterisk are for sex differences in SBP elevation rate shown for lower risk and higher risk participant observations, respectively. *P values are for differences in rate (i.e. slope) of BP increase in the setting of <2 versus ≥2 of the following risk factors: obesity, hypercholesterolemia, diabetes mellitus, and current smoking status, in women and men respectively. †P values are for comparing risk factor burden associated differences between women and men.
In summary, we observed that a greater cardiometabolic risk factor burden was associated with more accentuated BP elevation and greater overall BP load in women than in men throughout the adult life course. Given that BP is one of the most accessible indices of vascular function, these results suggest possibly greater sensitivity of the arterial vasculature in women than men to risk exposures – particularly earlier rather than later in life – potentially due to intrinsic baseline differences in arterial anatomy or physiology or both. Our findings may also be related to previously reported sex variation in the manifestation and effects of cardiometabolic risk traits, particularly early in life, including but not limited to differences in the prevalence of dysglycemia (i.e. more impaired fasting glucose in women), metabolic syndrome subtype distribution (i.e. more clustering of lipid and anthropometric abnormalities in women), fat partitioning (i.e. greater subcutaneous adipose tissue in women), and adipocyte biology.14,15 As commonly seen in longer term cohort studies, a survival bias that generally favors healthier males to survive into older age may have influenced a slowing of the BP elevation rate in men with advancing age; notably, we did observe attenuation of the BP increase in older age in both sexes. Notwithstanding potential limitations for interpreting data available from our cohort in the later decades of life, we observed sex differences that were pronounced beginning in early life and persistent through at least middle age. Further studies are needed to determine whether more tailored interventions targeting modifiable risk factors could effectively alter sex-specific trajectories in BP elevation, particularly for young women at risk.
Supplementary Material
Acknowledgments:
This study was conducted using ARIC, FHS, CARDIA, and MESA Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of each cohort studies or the NHLBI. We thank all the participants of the NHLBI cohort studies for their invaluable contributions to this work.
Sources of Funding: This study was funded in part by an unrestricted Gilead Sciences research grant, National Institutes of Health contracts N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164, National Institutes of Health grants U01–64829, U01-HL649141, U01-HL649241, R01-HL090957, R01-HL134168, R01-HL131532, R01-HL143227, R03AG032631, the National Center for Research Resources GCRC grant MO1-RR00425, the National Center for Advancing Translational Sciences Grant UL1TR000124, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, the Barbra Streisand Women’s Cardiovascular Research and Education Program, The Society for Women’s Health Research (SWHR), the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation. Dr. Niiranen was funded by grants from the Academy of Finland (Grant 321351), the Emil Aaltonen Foundation, the Paavo Nurmi Foundation, and the Finnish Medical Foundation. Dr. Ji was funded by grants from the China Scholarship Council (201806260086).
Footnotes
Disclosures: None reported.
REFERENCES
- 1.Merz AA and Cheng S. Sex differences in cardiovascular ageing. Heart. 2016;102:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G and Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, Fernandez C, Bazzano L, He J and Chen W. Race and Sex Differences of Long-Term Blood Pressure Profiles From Childhood and Adult Hypertension: The Bogalusa Heart Study. Hypertension. 2017;70:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S, Xanthakis V, Sullivan LM and Vasan RS. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60:1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen NB, Lloyd-Jones D, Hwang SJ, Rasmussen-Torvik L, Fornage M, Morrison AC, Baldridge AS, Boerwinkle E, Levy D, Cupples LA, Fox CS, Thanassoulis G, Dufresne L, Daviglus M, Johnson AD, Reis J, Rotter J, Palmas W, Allison M, Pankow JS and O’Donnell CJ. Genetic loci associated with ideal cardiovascular health: A meta-analysis of genome-wide association studies. Am Heart J. 2016;175:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien E, Mee F, Atkins N and O’Malley K. Inaccuracy of the Hawksley random zero sphygmomanometer. Lancet. 1990;336:1465–8. [DOI] [PubMed] [Google Scholar]
- 7.Ni H, Wu C, Prineas R, Shea S, Liu K, Kronmal R and Bild D. Comparison of Dinamap PRO-100 and mercury sphygmomanometer blood pressure measurements in a population-based study. Am J Hypertens. 2006;19:353–60. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs DR Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RGand Duprez DA Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobin MD, Sheehan NA, Scurrah KJ and Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–35. [DOI] [PubMed] [Google Scholar]
- 10.Wald DS, Law M, Morris JK, Bestwick JP and Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. [DOI] [PubMed] [Google Scholar]
- 11.Niiranen TJ, Henglin M, Claggett B, Muggeo VMR, McCabe E, Jain M, Vasan RS, Larson MG and Cheng S. Trajectories of Blood Pressure Elevation Preceding Hypertension Onset: An Analysis of the Framingham Heart Study Original Cohort. JAMA Cardiol. 2018;3:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN and Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ. 2007;31:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chella Krishnan K, Mehrabian M and Lusis AJ. Sex differences in metabolism and cardiometabolic disorders. Curr Opin Lipidol. 2018;29:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem. 2014;60:44–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


