Abstract
New strategies are needed to mitigate the mycotoxin deoxynivalenol (DON) in feed and food products. Microbial DNA fragments were generated from a library of DON-tolerant microorganisms. These fragments were screened in DON-sensitive yeast strains for their ability to modify or transport DON. Fragments were cloned into a PCR8/TOPO vector, and recombined into the yeast vector, pYES-DEST52. Resulting yeast transformants were screened in the presence of 100 ppm DON. Transformants that were able to grow in the presence of DON were plated on a selective medium, and the cloned microbial DNA fragments were sequenced. BLAST queries of one microbial DNA fragment (4D) showed a high degree of similarity to an ABC transporter. A series of screening and inhibition assays were conducted with a transport inhibitor (propanol), to test the hypothesis that 4D is a mycotoxin transporter. DON concentrations did not change for yeast transformants expressing 4D. The ability of yeast transformants expressing 4D to transport DON was inhibited by the addition of propanol. Moreover, yeast transformants expressing a known efflux pump (PDR5) showed similar trends in propanol transport inhibition compared to 4D. Future work should consider mycotoxin transporters such as 4D to the development of transgenic plants to limit DON accumulation in seeds.
Keywords: Deoxynivalenol, Transporter, Mycotoxin, Yeast, Microbial DNA fragment
Highlights
-
•
Microbial DNA fragments were generated from a library of DON-tolerant microorganisms.
-
•
Fragments were screened in DON-sensitive yeast strains for their ability to modify or transport DON.
-
•
BLAST queries of one microbial fragment (4D) showed a high degree of similarity to an ABC transporter.
-
•
Transformants expressing a known efflux pump (PDR5) showed similar trends in propanol transport inhibition compared to 4D.
-
•
Future work should consider mycotoxin transporters such as 4D to the development of transgenic plants.
1. Introduction
Food and feed products can be contaminated with toxic metabolites produced by fungi called mycotoxins. Deoxynivalenol (DON) is a mycotoxin produced by the fungus Fusarium graminearum. DON is a contaminant of staple crops such as wheat, barley, and maize (Binder et al., 2007). DON causes deleterious effects when consumed by domestic animals including feed refusal, vomiting, reduced weight, and even death (Rotter et al., 1996). The mode of action for DON occurs via interfering with initiation and elongation of protein synthesis (Pestka, 2010, Ueno et al., 1973). Traditional breeding and fungicide applications have been the main means to mitigate DON in crops (Khatibi et al., 2011). However, new strategies are needed to mitigate DON.
The epoxide ring present on C-12, 13 of DON is critical for toxicity (Karlovsky, 2011). Since the 1990s, there have been reports of microorganisms able to either modify or detoxify DON (McCormick, 2013). Microorganisms able to oxidize DON to the less toxic metabolite 3-keto-4-deoxynivalenol (3-keto-DON) have been reported in Shima et al. (1997) (Shima et al., 1997) and Völkl et al. (2004) (Völkl et al., 2004). Ikunaga et al. (2011) (Ikunaga et al., 2011) reported a species in the genus Nocardioides that was able to epimerize DON to 3-epimer-deoxynivalenol (3-epi-DON). More recently, a mixed culture of bacteria discovered by He et al. (2016) (He et al., 2016) was reported to convert DON to the less toxic de-epoxy-DON (DED). The modification of DON by acetylation (Kimura et al., 1998a, Kimura et al., 1998b), epoxidation (Binder et al., 2007, Fuchs et al., 2002, Fuchs et al., 2000), or glucosylation (Poppenberger et al., 2003) produced secondary metabolites of DON that are reduced in their toxicity. Other microorganisms, including Aspergillus tubingensis (He et al., 2008), Nocardioides sp. (Ikunaga et al., 2011), and Devosia sp. (Sato et al., 2012) have been reported to be effective in the microbial degradation of DON. A recent study described the ability of the bacterium Devosia insulae to transform DON (Wang et al., 2019).
An alternative strategy for mitigating DON is to increase the transport of the mycotoxin out of cells. DON has been found to be transported by two of the main known groups of efflux pumps, ATP-binding cassette (ABC) transporters and major facilitator superfamily (MSF) transporters. The ABC transporter Pdr5p was shown to be an exporter of DON and 15-ADON in Saccharomyces cerevisae (Gunter et al., 2016, Mitterbauer and Adam, 2002, Suzuki and Iwahashi, 2012). The MFS transporter TRI12 was shown to be trichothecene efflux pump (Alexander et al., 1999).
Wilson et al. (2017) showed that microorganisms isolated from environmental samples could modify DON to less toxic products. Here, we report the identity of a unique DON transporter (4D) from a library of microbial DNA fragments generated from the assemblages of microbes collected by Wilson et al. (2017) (Wilson et al., 2017). The microbial DNA fragments were generated using a technique known as oligonucleotide primed polymerase chain reaction (DOP-PCR) (Freedman, 2014). This form of PCR eliminates blunt-end cloning making for an easier transition into a plasmid. DOP-PCR uses degenerate PCR primers that bind randomly to genomic DNA and amplify different fragments during a PCR run. The Taq polymerase used with this procedure adds a poly “A” tail to PCR ends allowing for easy cloning into an entry vector and then a destination vector. We hypothesized that enzymes for modifying and/or transporting DON could be discovered from a library of microbial DNA fragments expressed in a DON-sensitive yeast system. To test this hypothesis, fragments were cloned into a PCR8/TOPO vector, and recombined into the yeast vector, pYES-DEST52. Resulting yeast transformants were screened in the presence of 100 ppm DON. Transformants that were able to grow in the presence of DON were plated on a selective medium, and the cloned microbial DNA fragments were sequenced. BLAST queries of one microbial DNA fragment (4D) showed a high degree of similarity to an ABC transporter. A series of screening and inhibition assays were conducted with a transport inhibitor (propanol), to test the hypothesis that 4D is a DON transporter. The specific objectives of this study were to: (1) generate a library of microbial DNA fragments from DON-tolerant microorganisms described in Wilson et al. (2017) using degenerate oligonucleotide primed polymerase chain reaction (DOP-PCR); (2) screen the library of microbial DNA fragments in a DON-sensitive yeast strain for their ability of provide resistance to DON, either by modification or transport from the cell; (3) identify fragments capable of modifying or transporting DON; and (4) conduct experiments to demonstrate the role of a putative transporter (4D) to transport DON out of yeast cells.
2. Materials and methods
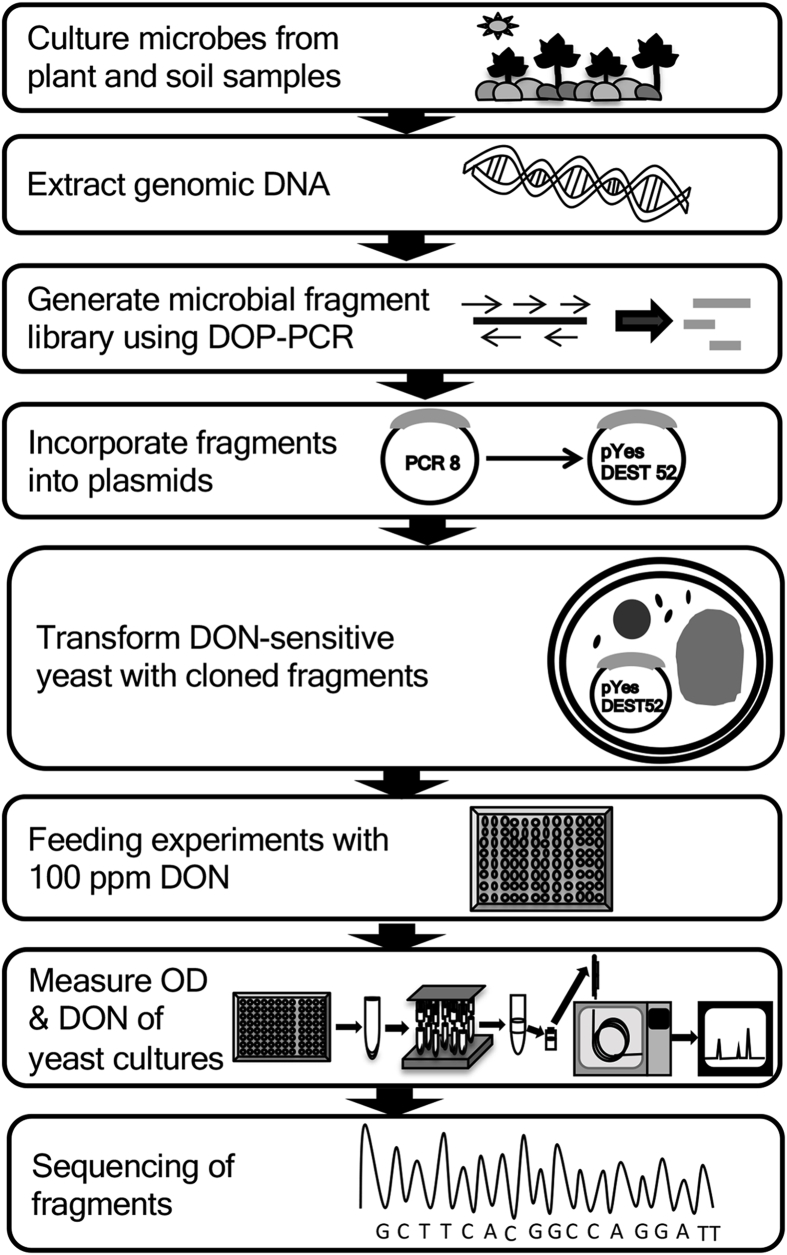
2.1. Generation of microbial library fragments
Microbial library fragments were generated from one pure culture of bacteria (Pure Culture 1) and three mixed cultures (Mixed Culture 1, Mixed Culture 2, and Mixed Culture 3) from Wilson et al. (2017). These samples came from a series of plant and soil collections, and were processed following the schematic shown in Fig. 1. A 50 μl sample from the glycerol stock was taken and cultured in 2 mL of Reasoner's 2A (R2A, VWR, Radnor, PA) broth. DNA was extracted from the four cultures using a DNeasy PowerSoil Kit (Mo Bio Laboratories, Carlsbad, CA). Primers from the M set (M1, M2, M4 and M5) and Rand3 (Freedman, 2014) were used to generate library fragments via DOP-PCR from Pure Culture 1, Mixed Culture 1, Mixed Culture 2, and Mixed Culture 3 from Wilson et al. (2017). The DOP-PCR parameters involved 10 cycles of low stringency amplification with a 5 min denaturation at 95 °C followed by 10 cycles of: 94 °C for 1 min, 30 °C for 2 min, ramp to 68 °C for 3 min, and amplification at 68 °C for 8 min. Following this program, a high stringency program of 25 cycles was implemented. Each cycle consisted of: 94 °C for 1 min, 55 °C for 2 min, and 68 °C for 8 min, with 5 s added for each cycle. The DOP-PCR products were then held at 68 °C for 7 min. The low stringency cycles were designed to encourage random priming, and the high stringency cycles were designed to further amplify those randomly produced DNA fragments. A 50 μl DOP-PCR reaction was prepared with 2x GoTaq Green master mix (Promega, Madison, WI) so that resulting fragments had a poly A tail and were easy to clone into an entry vector. Fragments were visualized on a 0.8% agarose gel. All microbial library fragments from the four microbial cultures tested that produced a wide spread band on the gel were combined. Library fragments were generated with the M set primers (M1, M2, M4 and M5) and Rand3 primer as described by Freedman (2014). Pure Culture 1 DOP-PCR reactions using M1, M2, and M3 primers were combined for cloning into the DON sensitive yeast strains. Mixed Culture 1 DOP-PCR reactions using all primers were combined for cloning into the DON sensitive yeast strains. Mixed Culture 2 DOP-PCR reactions using M1 and M2 primers were combined for cloning into the DON sensitive yeast strains. Mixed Culture 3 DOP-PCR reactions using M1, M2, M5, and Rand3 primers were combined for cloning into the DON sensitive yeast strains.
Fig. 1.
Schematic of sample collection, selection, library generation, screening assays, and sequencing to identify the mycotoxin transporter, 4D.
2.2. Yeast strains
Saccharomyces cerevisiae strain R2802 (PDR5 leu2 ura3-52 met5) (Meyers et al., 1992) was used as the parental yeast strain to create a DON sensitive yeast strain as a tool to screen for microbial DNA fragments containing functional DON detoxifying/modifying enzymes or proteins. S. cerevisiae strain R2802 was kindly provided by J. Golin, The Catholic University, Washington, DC. A DON sensitive yeast strain (DSY) was created as described in Abolmaali et al. (2008). Briefly, PDR5 and PDR10 were deleted from wild-type RW2802 (these genes encode for ATP-binding cassette (ABC) multidrug resistance transporter proteins). This strain was then tested for sensitivity to 100 μg/mL DON. DSY5 as well as the parental yeast strain RW2802 were cultured in 2 mL of yeast peptone dextrose (YPD) media for 24 h at 28 °C. 10 μl samples of DSY5 and RW2802 were assayed in triplicate in a 96 well plate in 200 μl of YPD media supplemented with 100 μg/mL DON, 100 μg/mL de-epoxy-DON (DED), 50 μg/mL DON and 50 μg/mL DED, and YPD media without any toxins. The yeast was incubated for 24 h at 28 °C with shaking. Then, optical density (OD600) measurements were taken with a plate reader to measure growth.
2.3. Cloning into DON sensitive yeast plasmid
Vector pCR8/GW/TOPO TA (Life Technologies, Carlsbad, CA) was combined with 5 μl of the microbial library fragments (described previously) and incubated for 30 min at room temperature before transforming into 50 μl of Clonetech® stellar competent cells (Clonetech Laboratories, Mountain View, CA). Cells were pooled and grown in 2 mL of lysogeny broth (LB) media with 50 μg/L spectinomycin. Plasmid DNA in pCR8 was recombined with pYesDEST-52 by combining 150 ng of plasmid with 50 ng of the pooled pCR8 library DNA using the Invitrogen Gateway LR Clonase II kit (Thermo Fisher Scientific, Waltham, MA) and incubated overnight at 25 °C. Aliquots of 5 μl of the transformation mixture were used to transform E. coli ccdB competent cells. Transformed cells were grown in 2 mL of LB media with ampicillin (100 μg/L). Plasmid DNA was purified using a GeneJet miniprep kit (Thermo Fisher Scientific, Waltham, MA) for transformation into DSY5. We also included a transformation in the DSY5 with the empty plasmid pYesDEST-52. This yeast strain served as a control in the screening.
To create the positive control, Pdr5 was amplified from the extracted DNA from the parental yeast RW2808 with designed primers Pdr5_full_For (5′ TCC GCT CGT TCG AAA GAC TT 3′) and Pdr5_full_Rev (5′ ACG CAC CTA TAT GTA GTG ATT ATG T 3′). Hot Start Q5 High Fidelity Master Mix 2X (BioLabs) was used with 2 μL of template (DNA extracted from parental yeast) in a 50 μL PCR reaction. The parameters used in the thermal cycler were: 1 × cycle denaturation 98 °C for 30 s, 35 x cycle denaturation 98 °C for 10 s, 35 x cycle annealing 53 °C for 30 s, 35 x cycle extension 72 °C for 2 min 30 s, 1 × cycle 72 °C for 2 min, hold 4 °C). The amplified Pdr5 gene was then purified with Monarch PCR & DNA Cleanup Kit (BioLabs #T1030G) and treated to have incorporated a poly A tail, in order to be used in TOPO TA cloning (Life Technologies, Carlsbad, CA). The addition of the poly A tail was made by Taq Polymerase 5 U/uL (BioLabs) in a 5 mL reaction of: 1 μL DNA template (purified Pdr5 gene) + 0.2 μL + 0.03 μL dATP + 0.5 μL standard Taq buffer 10x + at 72 °C for 15 min in thermal cycler. TOPO vector (with Pdr5) was recombined with pYesDEST-52 using the Invitrogen Gateway LR Clonase II kit (Thermo Fisher Scientific, Waltham, MA) and incubated 3 h at 25 °C. Aliquots of 5 μl transformation mix were used to transform into 50 μl vials of E. coli TOP 10 competent cells and grown in 2 mL of LB media with ampicillin (150 μg/L) selection. Pdr5 gene was identified inside the PyesDEST52 by sequencing the plasmids with universal primer T7 Promotor (5′ TAA TAC GAC TCA CTA TAG GG 3′ and the designed primers Pdr5-350bp-Rev (5′ACC ACA GTT GAC TGA TAG GCG AC 3′) and Pdr5-350bp-For (5′ CGC GTC TTC TTC TAC TGA AAA CGC 3′).
2.4. Screening for DON transporters
A screen of the pool of microbial library fragments was performed to find candidate transporters of DON. Two different collections of microbial DNA fragments were used, S3I and S3B. DSY5 was transformed with the pyesDEST52 containing the pool of microbial DNA fragments, S3I and S3B. Screens were prepared in 96-well plates with 200 μl of selective media, synthetic dropout media without uracil (SD-U; Sigma), and 100 μg/mL DON. Pure DON was obtained from S. McCormick, USDA-ARS. 10 μL of a diluted yeast suspension in SD-U was inoculated on the 96 well plate and incubated at 28 °C with shaking (140 rpm). After 48 h, OD600 measurements were taken to determine growth of yeast cells able to grow in the presence of 100 μg/mL DON. Wells with noticeable growth compared to controls were plated on SD-U media for 2–3 days at 28 °C until colonies were clearly visible. For each plate, 6 yeast colonies were selected for a second and third screen in DON 100 ppm, with the goal of identifying a single fragment containing a putative transporter.
Microbial DNA fragments were identified by sequencing the PCR product of the plasmids harboring the fragments, with universal primer T7 Promotor (5′ TAA TAC GAC TCA CTA TAG GG 3′ and the designed Pyes Primer Reverse 5′ TAG GGA TAG GCT TAC CTT CG 3’. GoTaqR Green Master Mix (Promega M712) was used with 0.5 μL of plasmid in a 20 μL PCR reaction. The parameters used in the thermal cycler were: 1 × cycle denaturation 95 °C for 2 min, 35 x cycle denaturation 95 °C for 45 s, 35 x cycle annealing 54 °C for 45 s, 35 x cycle extension 72 °C for 3 min, 1 × cycle 72 °C for 5 min, hold 4 °C). The fragment sequences then were queried against the NCBI nr (non-redundant) database to identify similar structures in the database. Accession numbers were obtained from GenBank following submission using the BankIt submission tool at NCBI (ncbi.nlm.nih.gov).
DON concentrations following the screen were measured by GC/MS following standard laboratory protocols (Wilson et al., 2017). Aliquots of 50 μL from each culture were dried down in a glass tube using compressed air in a nitrogen evaporator set at 55 °C. Dried samples were then derivatized at room temperature with a mixture of 99 μL of N-trimethylsilylimidazole (TMSI) and 1 μL of trimethylchlorosilane (TMCS) for 10 min. Then, 500 μL of isooctane containing 0.5 μg/g of mirex (Sigma-Aldrich, St. Louis, MO, USA) was added to the glass tube, followed by the addition of 500 μL of water to quench the reaction. Samples were vortexed thoroughly. From the top organic layer, 150 μL was transferred to chromatography vials for GC/MS analysis.
An Agilent 6890/5975 system was used for GC/MS analysis operating in selected ion monitoring (SIM) mode. An autosampler in split less mode injected 1 μL of each sample onto an HP-5MS column (0.25 mm inner-diameter, 0.25 μm film thickness, 30 m length) to detect DON. The inlet temperature was set at 280 °C with a column flow rate of 1.2 mL/min using helium. The initial column temperature was held at 150 °C for 1 min, increased to 280 °C at a rate of 30 °C/min, and held constant for 3.5 min. A post-run of 325 °C for 2.5 min was used to clean the column. DON was detected in SIM mode at a mass/charge ratio of 512.3 and had reference ions at 422.4 and 497.3. Mirex (hexachloropentadiene dimer) was used as an internal standard to check the quantitative precision of the instrument and was detected in SIM mode at a mass/charge ratio of 271.8 and had a reference ion of 275.8 (Mirocha et al., 1998). A quadratic regression model was used to quantify DON with standards (Romer Labs, Austria and Sigma-Aldrich, St. Louis, MO, USA) at concentrations of 0.05, 0.10, 0.25, 0.5, 1.0, 2.50, 5.0 and 10.0 μg/mL.
2.5. Inhibition experiments
A series of inhibition experiments were conducted to compare OD600 measurements of the DSY5 transformed with the fragments encoding putative transporters, and DSY5 transformed with Pdr5 (as a positive control). Propanol (1.5%) was used as an efflux pump inhibitor for DON (Gášková et al., 2013). These experiments were performed on 96 well-plates with the yeast growing on induction media (Khatibi et al., 2011) supplemented with 40 ppm DON. Yeast was harvested in exponential phase in SD-U, to ensure the expression of Pdr5 (Mamnun et al., 2004) and other putative transporters. Yeast cultures were then diluted in selective media to give an OD of approximately 0.05, and then 10 μL of this yeast suspension were inoculated in the 96 well-plate to a final volume of 200 μL per well. The inhibition experiments on the plates were incubated for 20 h at 28 °C and 140 rpm.
2.6. Statistical analyses
An analysis of variance (ANOVA) was used to test for significant differences between treatments (selective media, DON, Propanol, DON and propanol) in the same yeast; and between yeast strains (DSY5_emptyPyesDEST52, DSY5_Pdr5 and DSY5_4D) concerning a single treatment (the growth in DON compared to the growth in media only). The ANOVA test was running as a one-way analysis of variance using the software Minitab 19 (Minitab, Inc.).
3. Results
3.1. Screening of yeast transformants in the presence of 100 ppm DON
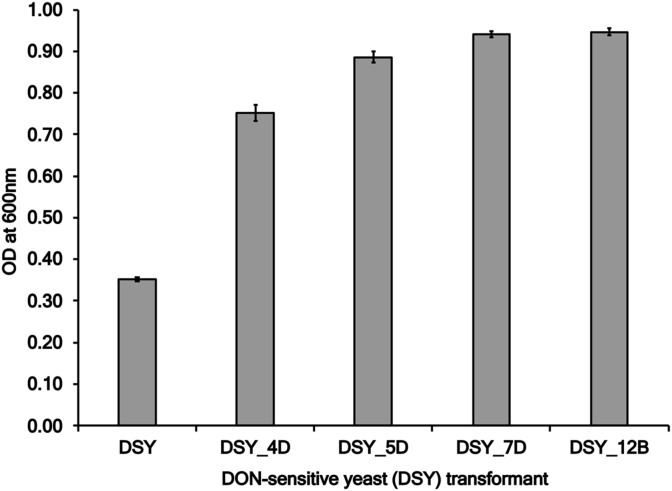
Four yeast transformants (4D, 5D, 7D and 12B) were able to grow significantly better than the DON-sensitive parent strain in the presence of 100 ppm DON (Fig. 2) (P < 0.05). DON concentrations of the yeast transformants were similar to the parent yeast sensitive strain (98.9% of the total DON for DSY, 89% for DSY_4D, 91% for DSY_5D, 90% for DSY_7D, and 86% for DSY_12B), suggesting that the fragments represented potential transporters that were enabling the yeast to survive in the presence of the toxin.
Fig. 2.
Growth of DON-sensitive yeast strains with or without microbial DNA fragments in the presence of 100 ppm DON. Data shown from six replicates. Error bars represent standard error of the mean.
3.2. Sequencing of microbial DNA fragments
Transformants that were able to grow in the presence of DON were plated on a selective medium, and the cloned microbial DNA fragments were sequenced. BLAST queries of one microbial DNA fragment (4D) showed a high degree of similarity to an ABC transporter. New primers were designed in order to get the full sequence (4D-1-For: 5′ TGCTGGGCATTACCAAGCGC 3’; 4D-2-For: 5′ GTCGAAGGCGCCATGAACGC3’; 4D-1-Rev: 5′ ACGCGACAGCACTTCCAGGG 3’; 4D-2-Rev: 5′ TGCCGTTATAGGCGGCCTGC 3’). The sequence of 4D has been deposited in GenBank, accession #MN536485.4D contains 13 open reading frames (ORFs) and, although the majority of them correspond to hypothetical proteins, one has 84% identity with an ABC transporter from Bordetella bronquiseptica strain FDAARGOS_176 (sequence ID in NCBI: CP014013.2).
3.3. Inhibition experiments
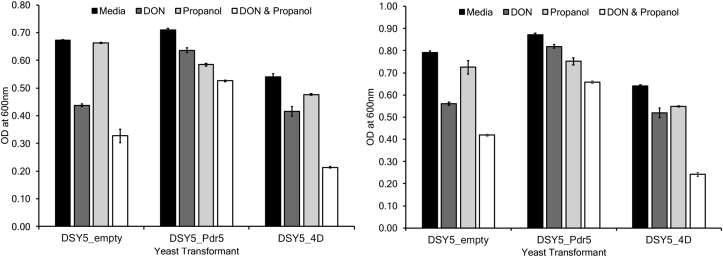
A series of screening and inhibition assays were conducted with a transport inhibitor (propanol), to test the hypothesis that 4D is a mycotoxin transporter. The ability of yeast transformants expressing 4D to transport DON was inhibited by the addition of propanol for two independent experiments (Fig. 3). Moreover, yeast transformants expressing a known efflux pump (PDR5) showed similar trends in propanol transport inhibition compared to 4D for two independent experiments (Fig. 3).
Fig. 3.
Growth of DON-sensitive yeast strains with or without Pdr5 or 4D in the presence of induction medium, DON (40 ppm), propanol (1.5%), and DON (40 ppm) plus propanol (1.5%). Data shown from three replicates for experiment 1 (left) and experiment 2 (right). Error bars represent standard error of the mean.
3.4. DON measurements following inhibition experiments
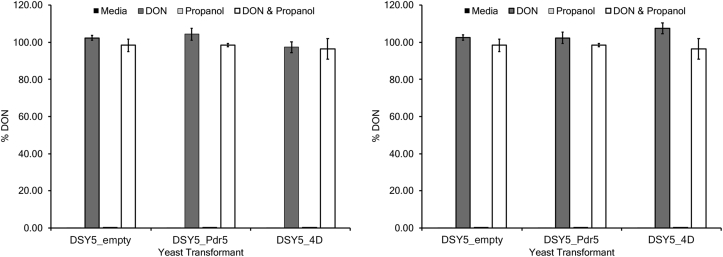
DON concentrations did not change for yeast transformants expressing 4D for two independent experiments. No DON was found in the treatments without DON (media and propanol) (Fig. 4), and DON concentrations did not change significantly in any treatment containing DON (e.g. DON, DON + propanol) for two independent experiments (P < 0.01).
Fig. 4.
Percentage of DON following screening assays of DON-sensitive yeast strains with or without Pdr5 or 4D in the presence of induction medium, DON (40 ppm), propanol (1.5%), and DON (40 ppm) plus propanol (1.5%). Data shown from three replicates for experiment 1 (left) and experiment 2 (right). Error bars represent standard error of the mean. No DON was found in the treatments without DON (media and propanol), and DON did not change significantly in the treatments containing DON (DON and DON + propanol).
4. Discussion
New approaches are needed to detoxify or remove the mycotoxin deoxynivalenol in feed and food. In this study, microbial DNA fragments were generated from a library of DON-tolerant microorganisms described in Wilson et al. (2017) using DOP-PCR. These fragments were screened in DON-sensitive yeast strains for their ability to modify or transport DON. BLAST queries of one microbial DNA fragment (4D) showed a high degree of similarity to an ABC transporter. To our knowledge, this is the first report of a mycotoxin transporter being discovered from a pool of unknown microbial DNA fragments. This work has important implications for the bioprospecting of unique enzymes from unknown pools of microorganisms isolated from the environment.
To test the hypothesis that 4D is a mycotoxin transporter, a series of screening and inhibition assays were conducted with a transport inhibitor (propanol). DON concentrations did not change when transformants expressing 4D were grown in the presence of DON. The ability of yeast transformants expressing 4D to transport DON was inhibited by the addition of propanol. Moreover, yeast transformants expressing a known efflux pump (PDR5) showed similar trends in propanol transport inhibition compared to 4D. ABC transporters, such as PDR5, are ubiquitous in many different groups of microorganisms (Taglicht and Michaelis, 1998), and some act as efflux pumps for a wide spectrum of substances (Taglicht and Michaelis, 1998). PDR5 has been recently found to be actively produced even under conditions of nutrient limitation, because it is important for the survival in the presence of toxic compounds that can be pumped out of cells (Rahman et al., 2018). The relative expression of 4D under nutrient-limiting conditions is unknown, and was beyond the scope of the present study. Further research is warranted to examine the potential for 4D to transport mycotoxins other than deoxynivalenol. We don't currently know the level of specificity of 4D for DON, and comparisons with the parent DON-sensitive yeast strain in the screening experiments suggest that there may be some cost to expressing the transporter. Our work extends previous studies that have demonstrated the potential of microbial strategies in DON reduction. The transporter 4D, along with other candidates from our library of microbial DNA fragments, could be used to develop transgenic plants to limit DON accumulations in seeds. For example, resistance of tobacco plants to Fusarium was increased by the insertion of PDR5 (Mitterbauer and Adam, 2002).
CRediT authorship contribution statement
Celia Jimenez-Sanchez: Writing - review & editing. Nina Wilson: Writing - review & editing. Nicole McMaster: Writing - review & editing. Dash Gantulga: Writing - review & editing. Benjamin G. Freedman: Writing - review & editing. Ryan Senger: Writing - review & editing. David G. Schmale: Writing - review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported in part by grants to D. G. Schmale III from the Virginia Small Grains Board (VT PANs #449102, #449282, #449426, #449552, #449719, and #449873) and the U.S. Wheat and Barley Scab Initiative (VT PANs #422288 #422533). This material is based upon work supported by the U.S. Department of Agriculture and the Virginia Small Grains Board. This is a cooperative project with the U.S. Wheat & Barley Scab Initiative and the Virginia Small Grains Board. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the sponsors.
Abbreviations
- GC/MS
Gas Chromatography Mass Spectrometery
- DOP-PCR
Oligonucleotide Primed Polymerase Chain Reaction
References
- Abolmaali S., Mitterbauer R., Spadiut O., Peruci M., Weindorfer H., Lucyshyn D., Ellersdorfer G., Lemmens M., Moll W.-D., Adam G. Engineered bakers yeast as a sensitive bioassay indicator organism for the trichothecene toxin deoxynivalenol. J. Microbiol. Methods. 2008;72:306–312. doi: 10.1016/j.mimet.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Alexander N.J., McCormick S.P., Hohn T.M. TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: gene isolation and expression in yeast. Mol. Gen. Genet. 1999;261:977–984. doi: 10.1007/s004380051046. [DOI] [PubMed] [Google Scholar]
- Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. vol. 137. 2007. pp. 265–282. (Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal Feed Science and Technology, Fusarium and their toxins: Mycology, occurrence, toxicity, control and economic impact). [DOI] [Google Scholar]
- Freedman B.G. 2014. Degenerate Oligonucleotide Primed Amplification of Genomic DNA for Combinatorial Screening Libraries and Strain Enrichment. [Google Scholar]
- Fuchs E., Binder E., Heidler D., Krska R. Characterisation of metabolites after the microbial degradation of A- and B-trichothecenes by BBSH 797. Mycotoxin Res. 2000;16(1):66–69. doi: 10.1007/BF02942984. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Binder E.M., Heidler D., Krska R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 2002;19:379–386. doi: 10.1080/02652030110091154. [DOI] [PubMed] [Google Scholar]
- Gášková D., Plášek J., Zahumenský J., Benešová I., Buriánková L., Sigler K. Alcohols are inhibitors of Saccharomyces cerevisiae multidrug-resistance pumps Pdr5p and Snq2p. FEMS Yeast Res. 2013;13:782–795. doi: 10.1111/1567-1364.12088. [DOI] [PubMed] [Google Scholar]
- Gunter A.B., Hermans A., Bosnich W., Johnson D.A., Harris L.J., Gleddie S. Protein engineering of Saccharomyces cerevisiae transporter Pdr5p identifies key residues that impact Fusarium mycotoxin export and resistance to inhibition. Microbiologyopen. 2016;5:979–991. doi: 10.1002/mbo3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Fan Y., Liu G., Zhang H. Isolation and identification of a strain of Aspergillus tubingensis with deoxynivalenol biotransformation capability. Int. J. Mol. Sci. 2008;9:2366–2375. doi: 10.3390/ijms9122366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W.J., Yuan Q.S., Zhang Y.B., Guo M.W., Gong A.D., Zhang J.B., Wu A.B., Huang T., Qu B., Li H.-P., Liao Y.C. Aerobic de-epoxydation of trichothecene mycotoxins by a soil bacterial consortium isolated using in situ soil enrichment. Toxins. 2016;8:277. doi: 10.3390/toxins8100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikunaga Y., Sato I., Grond S., Numaziri N., Yoshida S., Yamaya H., Hiradate S., Hasegawa M., Toshima H., Koitabashi M., Ito M., Karlovsky P., Tsushima S. Nocardioides sp. strain WSN05-2, isolated from a wheat field, degrades deoxynivalenol, producing the novel intermediate 3-epi-deoxynivalenol. Appl. Microbiol. Biotechnol. 2011;89:419–427. doi: 10.1007/s00253-010-2857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlovsky P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 2011;91:491–504. doi: 10.1007/s00253-011-3401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatibi P.A., Montanti J., Nghiem N.P., Hicks K.B., Berger G., Brooks W.S., Griffey C.A., Schmale D.G. Conversion of deoxynivalenol to 3-acetyldeoxynivalenol in barley-derived fuel ethanol co-products with yeast expressing trichothecene 3-O-acetyltransferases. Biotechnol. Biofuels. 2011;4:26. doi: 10.1186/1754-6834-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Kaneko I., Komiyama M., Takatsuki A., Koshino H., Yoneyama K., Yamaguchi I. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J. Biol. Chem. 1998;273:1654–1661. doi: 10.1074/jbc.273.3.1654. [DOI] [PubMed] [Google Scholar]
- Kimura M., Shingu Y., Yoneyama K., Yamaguchi I. Features of Tri101, the trichothecene 3-O-acetyltransferase gene, related to the self-defense mechanism in Fusarium graminearum. Biosci. Biotechnol. Biochem. 1998;62:1033–1036. doi: 10.1271/bbb.62.1033. [DOI] [PubMed] [Google Scholar]
- Mamnun Y.M., Schüller C., Kuchler K. Expression regulation of the yeast PDR5 ATP-binding cassette (ABC) transporter suggests a role in cellular detoxification during the exponential growth phase. FEBS Lett. 2004;559:111–117. doi: 10.1016/S0014-5793(04)00046-8. [DOI] [PubMed] [Google Scholar]
- McCormick S.P. Microbial detoxification of mycotoxins. J. Chem. Ecol. 2013;39:907–918. doi: 10.1007/s10886-013-0321-0. [DOI] [PubMed] [Google Scholar]
- Meyers S., Schauer W., Balzi E., Wagner M., Goffeau A., Golin J. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr. Genet. 1992;21:431–436. doi: 10.1007/bf00351651. [DOI] [PubMed] [Google Scholar]
- Mirocha C.J., Kolaczkowski E., Xie W., Yu H., Jelen H. Analysis of deoxynivalenol and its derivatives (batch and single kernel) using Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 1998;46:1414–1418. doi: 10.1021/jf970857o. [DOI] [Google Scholar]
- Mitterbauer R., Adam G. Saccharomyces cerevisae and Arabidopsis thaliana: useful model systems for the identification of molecular mechanisms involved in resistance of plants to toxins. Eur. J. Plant Pathol. 2002;108:699–703. doi: 10.1023/A:1020666627267. [DOI] [Google Scholar]
- Pestka J.J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- Poppenberger B., Berthiller F., Lucyshyn D., Sieberer T., Schuhmacher R., Krska R., Kuchler K., Glössl J., Luschnig C., Adam G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003;278:47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- Rahman H., Carneglia J., Lausten M., Robertello M., Choy J., Golin J. Robust, pleiotropic drug resistance 5 (Pdr5)-mediated multidrug resistance is vigorously maintained in Saccharomyces cerevisiae cells during glucose and nitrogen limitation. FEMS Yeast Res. 2018;18 doi: 10.1093/femsyr/foy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter B.A., Prelusky D.B., Pestka J.J. Toxicology of deoxynivalenol (vomitoxin) J. Toxicol. Environ. Health. 1996;48:1–34. doi: 10.1080/713851046. [DOI] [PubMed] [Google Scholar]
- Sato I., Ito M., Ishizaka M., Ikunaga Y., Sato Y., Yoshida S., Koitabashi M., Tsushima S. Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms. FEMS Microbiol. Lett. 2012;327:110–117. doi: 10.1111/j.1574-6968.2011.02461.x. [DOI] [PubMed] [Google Scholar]
- Shima J., Takase S., Takahashi Y., Iwai Y., Fujimoto H., Yamazaki M., Ochi K. Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl. Environ. Microbiol. 1997;63:3825–3830. doi: 10.1128/aem.63.10.3825-3830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Iwahashi Y. Comprehensive gene expression analysis of type B trichothecenes. J. Agric. Food Chem. 2012;60:9519–9527. doi: 10.1021/jf3020975. [DOI] [PubMed] [Google Scholar]
- Taglicht D., Michaelis S. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 1998;292:130–162. doi: 10.1016/s0076-6879(98)92012-2. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Nakajima M., Sakai K., Ishii K., Sato N. Comparative toxicology of trichothecene mycotoxins: inhibition of protein synthesis in animal cells. J. Biochem. 1973;74:285–296. doi: 10.1093/oxfordjournals.jbchem.a130246. [DOI] [PubMed] [Google Scholar]
- Völkl A., Vogler B., Schollenberger M., Karlovsky P. Microbial detoxification of mycotoxin deoxynivalenol. J. Basic Microbiol. 2004;44:147–156. doi: 10.1002/jobm.200310353. [DOI] [PubMed] [Google Scholar]
- Wang G., Wang Y., Ji F., Xu L., Yu M., Shi J., Xu J. Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 2019;276:436–442. doi: 10.1016/j.foodchem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Wilson N.M., McMaster N., Gantulga D., Soyars C., McCormick S.P., Knott K., Senger R.S., Schmale D.G. Modification of the mycotoxin deoxynivalenol using microorganisms isolated from environmental samples. Toxins. 2017;9 doi: 10.3390/toxins9040141. [DOI] [PMC free article] [PubMed] [Google Scholar]