Abstract
Bothrops envenomation is associated with a cellular inflammatory response, characterized by pronounced neutrophil infiltration at the site of injury. Neutrophils act as the first line of defence, owing to their ability to migrate to the infected tissue, promoting an acute inflammatory response. At the site of inflammation, neutrophils perform defence functions such as phagocytosis, release of proteolytic enzymes, generation of reactive oxygen species (ROS), and synthesis of inflammatory mediators such as cytokines and lipid mediators. Neutrophils can also form neutrophil extracellular nets (NETs), webs composed of chromatin and granule proteins. This occurs after neutrophil activation and delivers high concentrations of anti-microbial molecules to the site of injury. This study evaluated the impact of BaTX-II, an Asp49 phospholipase A2 (PLA2) isolated from Bothrops atrox snake venom on human neutrophils in vitro. At non-toxic concentrations, BaTX-II induced hydrogen peroxide production by neutrophils, and this was reduced by wortmannin, a PI3K inhibitor. BaTX-II stimulated IL-1β, IL-8, LTB4, myeloperoxidase (MPO), and DNA content release, consistent with NET formation. This is the first study to show the triggering of relevant pro-inflammatory events by PLA2 Asp49 isolated from secretory venom.
Keywords: Bothrops atrox, Phospholipase A2, Neutrophils, Reactive oxygen species, Cytokines
1. Introduction
Phospholipases A2s (PLA2s) are enzymes found abundantly in nature, including in mammalian fluids and tissues, as well as in high concentrations in snake, bee, and wasp venom (Harris, 1985). Although mammalian PLA2 is non-toxic and is found in the pancreas, whereas venomous PLA2 is highly active and toxic, they have common catalytic properties and are considerably homologous in primary, secondary, and tertiary structure (Dufton et al., 1983, Verheij et al., 1981). In addition to their role in prey digestion, venom PLA2s have a variety of other functions, which include indirect haemolytic action, neurotoxicity, cardiotoxicity, platelet aggregation, myotoxicity, as well as anticoagulant, oedematogenic, bactericidal, and inflammatory activities (Kini and Evans, 1989, Teixeira et al., 2003).
There are two types of myotoxic PLA2s, ‘classical’ and ‘variant’. ‘Classical’ PLA2s contain an aspartate, at position 49 (Asp49) and catalyse, in a Ca2+-dependent manner, the hydrolysis of the ester linkage at the sn-2 position of glycerophospholipids. ‘Variant’ PLA2s have no, or low, catalytic activity and contain a lysine at position 49 (Lys49) (Gutiérrez and Lomonte, 1997). Since PLA2s present in snake venoms are homologous to these mammalian inflammatory PLA2s, snake venom PLA2s serve as an important tool for pharmacological studies.
Neutrophils represent the largest fraction of leukocytes in the peripheral blood and are also called polymorphonuclear granulocytes (PMNs), as they have a segmented nucleus and different types of granules. These granules are classified according to their protein content, consisting of primary (azurophiles), secondary (specific), and tertiary granules (Mantovani et al., 2011). Primary granules contain myeloperoxidase (MPO), cathepsin G, defensins, elastase, and proteinase 3, among many other proteins. Secondary granules contain collagenase, gelatinase, lactoferrin, and sialidase. Tertiary granules include gelatinase and β2-microglobulin (Borregaard et al., 1993; Mantovani et al., 2011).
Neutrophils have a short life span, ranging from 8 to 12 h in the bloodstream (Summers et al., 2010). However, this life span can be prolonged by several stimuli, such as cytokines and bacterial products (Colotta et al., 1992). Neutrophils represent an important line of defence for an organism, as they are attracted to infected tissue by chemokines, promoting an acute inflammatory response (Nathan, 2006). At the site of inflammation, neutrophils perform phagocytosis, release proteolytic enzymes, generate reactive oxygen species (ROS), and synthesize inflammatory mediators such as cytokines and lipid mediators (Cassatella, 1995, Timár et al., 2013). Although neutrophils are considered an important effector cell in the innate immune response, they also have a function in the acquired immune response, where they are progenitor cells of antigens, capable of priming Th1 and Th17 lymphocytes (Abi Abdallah et al., 2011, Mantovani et al., 2011). This demonstrates the importance of neutrophils as a link between the innate and acquired immune responses. Neutrophils can also form neutrophil extracellular traps (NETs), composed of webs of chromatin and granular proteins. This occurs after neutrophil activation, providing the site of injury with high concentrations of microbicidal molecules (Brinkmann and Zychlinsky, 2007, Brinkmann et al., 2004).
Given these diverse functions, neutrophils are an important cell type to study when investigating the pro-inflammatory actions of venoms and their isolated fractions. The objective of this work was to evaluate the effect of BaTX-II, isolated from Bothrops atrox snake venom, on isolated human neutrophil function. We identified hydrogen peroxide production by these neutrophils and studied the role of PI3K in this process. We also showed release of IL-1β, IL-8, LTB4, MPO, as well as DNA content in response to BaTX-II.
2. Materials and methods
2.1. Venom
The venoms of Bothrops atrox specimens were from Porto Velho, Rondônia, Brazil (SisGen authorization protocol n° AFCAB61). The venom was extracted, pooled and lyophilized for storage biochemical procedures.
2.2. Phospholipases A2 isolation
2.2.1. Cation exchange chromatography
The cation exchange chromatography was performed according to the method previously described by Andrião-Escarso et al. (2000) with adaptations. Approximately 40 mg of B. atrox venom was suspended in 1 mL of 50 mM ammonium bicarbonate buffer (NH4HCO3 - AMBIC) pH 8.0 and centrifuged at 3500×g for 5 min. To remove insoluble material the venom was fractionated on a CM-Sepharose FF® column (10 × 30 cm), with matrix composed of carboxymethyl (OCH2COO) functional group. The column was previously equilibrated with the same buffer used to solubilize the venom and the sample eluted under a gradient of 0–100% AMBIC 500 mM pH 8.0, in 5 column volumes, under a flow of 1 mL/min, in a chromatography Akta Purifier 10 (GE) system. Elution was monitored at 280 nm and fractions were collected manually (Supplementary Fig. 1A).
2.2.2. Reverse phase chromatography
Reverse phase chromatography was performed according to the method previously described by Stábeli et al. (2012) with adaptations. Fractions F7 and F9 from cation exchange chromatography were lyophilized and solubilized in 0.1% TFA (solution A) and subjected to high performance liquid chromatography (HPLC) in column C-18 (25 mm × 4.6 mm, Supelco), previously equilibrated with solution A and eluted under gradient 0–70% of solution B (acetonitrile 99.9% and TFA 0.1%) in 5 column volumes, under flow of 1 mL/min, in a chromatography Akta Purifier 10 (GE) system. Elution was monitored at 280 nm (Supplementary Fig. 1B).
2.2.3. SDS-PAGE
Electrophoresis on 12.5% (w/v) polyacrylamide gel in the presence of SDS (SDS-PAGE), was performed in a discontinuous pH system, in reducing conditions, previously described by Laemmli (1970). Electrophoretic separation was performed at 100 V, until the bromophenol blue reached the forehead. The gel was fixed in a 40% aqueous solution of methanol (v/v) and acetic acid 7% (v/v) for 30 min. The protein bands were evidenced by immersion in a solution containing Coomassie Brillant Blue G-250® 0.08% (m/v), aluminum sulfate 8.0% (w/v), 1.6% o-phosphoric acid (m/v) and 20.0% (v/v) methanol for 2 h. The dye excess was removed by immersion in a bleach solution containing 4.0% ethanol and 7.0% (v/v) acetic acid in water. Several changes of this solution were carried out until obtaining a gel with adequate color. The image of the gels was obtained using an Image scanner® equipment (GE Healthcare LifeSc.) and the relative molecular mass (Mr) determined by comparing the relative migration distances of the samples and the molecular mass standards (Supplementary Fig. 1A and 1C).
2.2.4. Phospholipase activity
The procedure was performed as described by Petrovic et al. (2001), with modifications. For the experiment, 5 mg of 4N3OBA were diluted in 5.4 mL of acetonitrile (ACN). 0.1 mL aliquots were dried and maintained at −20 °C. Each tube containing the 4N3OBA aliquot was diluted in 1.2 mL of sample buffer (10 mM Tris-HCl at pH 8.0, 10 mM CaCl2 and 100 mM NaCl) and kept on ice. To determine phospholipase activity, 190 μL of 4N3OBA reagent was combined with 10 μL of sample in triplicate. The samples used were: B. atrox venom, a basic phospholipase Lys49 (BaTX-I) and an Asp49 (BaTX-II). After adding PLA2, absorbance was determined at 425 nm using an Eon microplate spectrophotometer (Biotek), after 30 min of incubation at 37 °C (Supplementary Fig. 1C).
2.3. Neutrophil isolation
Peripheral blood neutrophils were obtained from self-reportedly healthy donators (18–40 years old). Informed consents were obtained at the time of the blood draw. All participants gave informed consent prior to their inclusion in the study, and the Brazilian IRB (Institutio-nal Review Board) of the Center of Tropical Medicine Research (CEPEM, Rondônia, Brazil - approval number 108/2010) approved it. In brief, according to Setúbal et al. (2013a) blood was collected in vacuum tubes containing heparin and diluted in phosphate buffered saline (PBS, 14 mM NaCl, 2 mM NaH2PO4H2O, 7mMNa2HPO412H2O), pH 7.4, after local asepsis. For the separation of leukocytes, Histopaque 1077 was added to the tubes and then the diluted blood was carefully added over the reagent. After centrifugation at 400×g for 30 min, neutrophils were collected from the bottom of the tube, along with the erythrocytes and were transferred to another tube. Lysis of red blood cells was performed using lysis buffer (0.15 M NH4Cl; 0.01 M KHCO3; 0.0001 M Na2 EDTA), homogenized and subjected to a temperature of −8 °C for 5 min, and then centrifuged. Neutrophils were washed with PBS and an aliquot of isolated neutrophils was used for determining the total number of neutrophils in a Neubauer's chamber after cell staining (1:20, v/v) with Turk solution (violet crystal 0.2% in acetic acid 30%). The purity of the isolated cell population was determined by Panotic staining of cytospin preparations and by flow cytometry analysis with anti-human CD-66b-FITC for 30 min as a granulocyte marker analyses (Setubal et al., 2013a) in Accuri C6 cytometer (BD, USA) (Supplementary Fig. 2).
2.4. Determination of cell viability by MTT
Thus, 2 × 105 neutrophils were cultured in 96-well plates with 200 μL of RPMI supple-mented and incubated with the BaTX-II at concentrations of 1.5, 3, 6, 12.5 and 25 μg/mL for 6 h at 37 °C in a humidified atmosphere (5% CO2). After this time, the samples were centrifuged for 5 min at 400×g and the supernatant removed. Then 10 μL of MTT (5 mg/mL in PBS) and 90 μL of RPMI/well were added, followed by incubation at 37 °C and 5% CO2 for 2 h. After this time, the cells were centrifuged again and the supernatant containing unreduced MTT was removed and then 100 μL of DMSO was added to solubilize the formazan crystals. The degree of reduction of MTT to formazan was quantified by the optical density (OD) measurement at 570 nm in a spectrophotometer. The results were expressed as OD.
2.5. Determination of hydrogen peroxide (H2O2) production by human neutrophils
The method used was described by Pick and Keisari (1980), adapted for microassay by Pick and Mizel (1981), and with modifications proposed by Russo et al. (1989). In brief, neutrophils (2 × 105/50 μL) were resuspended in 1.0 mL of phenol red solution (140 mM NaCl, 10 mM potassium phosphate buffer, pH 7.0, 0.56 mM phenol red) containing 0.05 mg/mL of horseradish peroxidase. Then the cells were incubated with an BaTX-II at several concentrations (1.5, 3, 6, 12.5 and 25 μg/mL) for 1:30 h at 37 °C in humidified atmosphere (5% CO2). Cells were maintained simultaneously with or without stimulation by phorbol myristate acetate (PMA, 50 ng/mL). After this time, the reaction was stopped by addition of 1 N sodium hydroxide (10 μL). The absorbance was measured spectrophotometrically at 620 nm against blank constituted of phenol red medium. Generated results were compared to a standard curve conducted for each test. The results were expressed as μM of H2O2 produced.
To evaluate the participation of PI3K in the production of hydrogen peroxide by human neutrophils stimulated with BaTX-II, the cells were incubated with 50 μL of the Wortmannin, a PI3K inhibitor (500 nM), for 15 min prior to addition of the toxin at different concentrations, and hydrogen peroxide production was determined as previously described. The inhibitor concentration employed in this study was based on concentrations described as effective in the literature (Wortmannin - 500 nM, 15 min; Ingvar et al., 1996) and did not cause adverse effects on cell viability during the assay. In the different experimental protocols, the control cells were incubated with the same concentration of vehicle used to dissolve the inhibitor.
2.6. DNA content release
Neutrophils (2 × 105/100 μL) were incubated with different concentrations of BaTX-II at different concentrations (1.5, 3, 6, 12.5 and 25 μg/mL) or RPMI (negative control) or PMA (500 ng/mL, positive control) for 4 h at 37 °C in humidified atmosphere (5% CO2) as described by Setubal et al. (2013a). After centrifugation the supernatant were used for determination of DNA content (suggesting NETs release) accordingly to procedure described in kit Quant-iT™ Picogreen dsDNA (Invitrogen). Briefly, 50 μL of samples were incubated with 100 μL of PI (Quant-iT) and 50 μL of PE buffer in a 96-well dark plate. After 15 min incubation absorbances at 520 nm emission and 480 nm excitation were recorded and DNA content release were estimated from standard curve. The results were expressed as ng/mL of DNA.
2.7. Myeloperoxidase assay
For this assay, 1 × 106 neutrophils were resuspended in RPMI culture medium, plated and incubated with RPMI (negative control), LPS (1 μg/mL; positive control), PMA (500 ng/mL; positive control) or different concentrations of BaTX-II (6, 12.5 and 25 μg/mL), for 4 h at 37 °C, in a humidified atmosphere (5% CO2) according to Pontes et al. (2016). Then, the plate was centrifuged at 400×g for 5 min and the supernatant was collected for MPO level determination. For this purpose, 50 μL of each sample was added to 100 μL of TMB (Suzuki et al., 1983). After 5 min, the reaction was interrupted with 2N sulfuric acid. MPO levels were measured with the Bio-Tek Synergy HT Multi-Detection (Winooski, VT) at absorbance wavelengths of 450 nm. Results were estimated through a standard curve prepared with recombinant MPO and expressed by ng/mL of MPO.
2.8. Interleukin-1β (IL-1β) and Interleukin-8 (IL-8) quantifications
For this assay, 2 × 105 neutrophil suspensions resuspended in assay medium were plated and incubated with RPMI (negative control), PMA (500 ng/mL; positive control) or different concentrations of BaTX-II (1.5, 3, 6, 12.5 and 25 μg/mL), at 37 °C, in a humidified atmosphere (5% CO2) for 4 h. After centrifugation, the supernatant was used for quantification of IL-1β and IL-8 levels by specific EIA, according to Pontes et al. (2014). The results were expressed in pg/mL of each cytokine.
2.9. Leukotriene B4 (LTB4) assay
LTB4 concentrations were measured in the supernatant of neutrophils (2 × 105 cells/mL) suspended in assay medium. Briefly, neutrophils were incubated with assay medium (negative control), PMA (500 ng/mL; positive control) or BaTX-II in different concentrations (1.5, 3, 6, 12.5 and 25 μg/mL) diluted in assay medium for 4 h at 37 °C in a humidified atmosphere (5% CO2) according to Pontes et al. (2016). LTB4 concentrations in the supernatant were determined by a specific enzymatic immunoassay (EIA) using a commercial kit (Cayman Chemicals, MI, USA).
2.10. Statistical analysis
The means and S.E.M. of all data were obtained and compared by one-way ANOVA, followed by Tukey test with significance probability levels less than 0.05.
3. Results
3.1. Effect of BaTX-II, isolated from the B. atrox snake venom, on human neutrophils viability
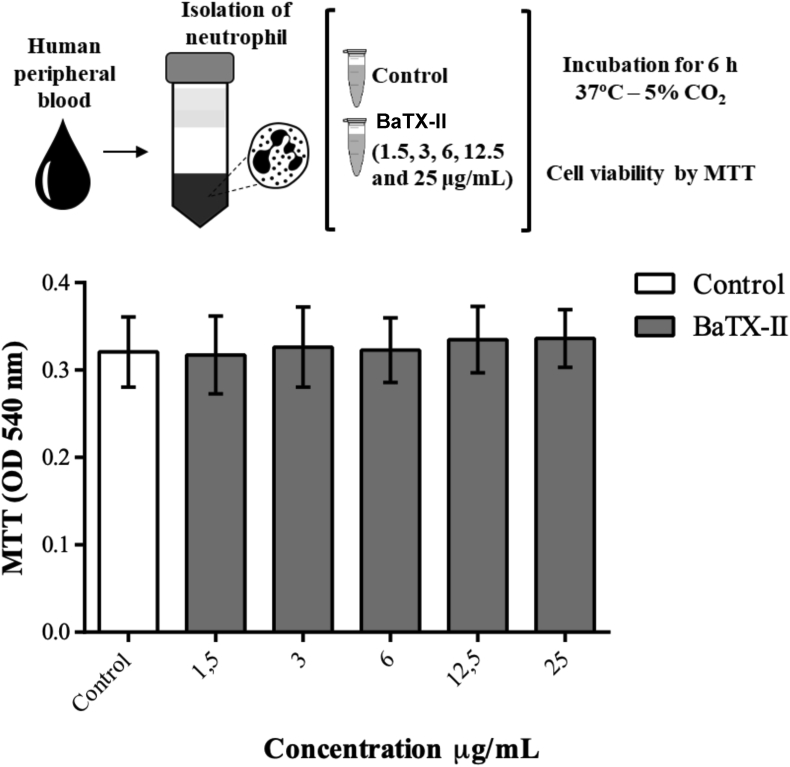
Neutrophil viability was assessed 6 h after incubation with different concentrations of the BaTX-II. The viability determination was performed by the MTT reduction method. The re-sults showed that the toxin at all different concentrations (1.5, 3, 6, 12.5 and 25 μg/mL) did not affect neutrophil viability in the evaluated period of time (Fig. 1).
Fig. 1.
Effect BaTX-II, isolated from the B. atrox snake venom on human neutrophils viability. 2 × 105 neutrophils were incubated for 6 h with BaTX-II at concentrations of 1.5, 3, 6, 12.5 and 25 μg/mL or RPMI (control) at 37 °C and 5% CO2. Neutrophil viability was assessed by the reduction of MTT. The data represent the mean +SEM of 5 independent donors.
3.2. Effect of BaTX-II on human neutrophils hydrogen peroxide production
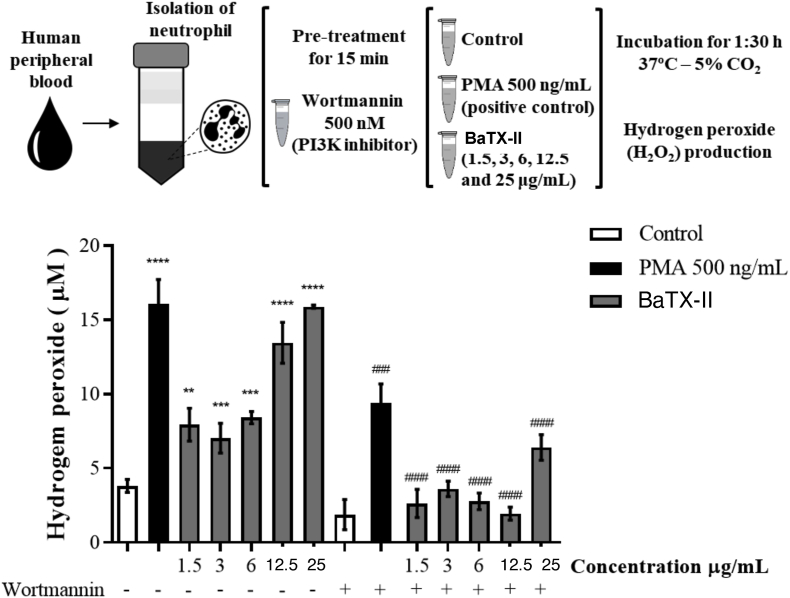
To verify the ability of BatX-II to induce the hydrogen peroxide production by human neutrophils, cells were incubated with the toxin with non-toxic concentrations, PMA (positive control) or RPMI (negative control). As shown in Fig. 2, incubation of neutrophils with the BaTX-II resulted in a significant increase in hydrogen peroxide production compared to the ne-gative control, at all concentrations (1.5–25 μg/mL) studied. The toxin effect at the highest concentrations was not statistically different from the positive control, PMA.
Fig. 2.
Effect of BaTX-II, isolated from B. atrox snake venom on human neutrophils hydrogen peroxide production. 2 × 105 neutrophils were incubated for 1:30 h with BaTX-II at concentrations (1.5, 3, 6, 12.5 and 25 μg/mL) or RPMI (negative control) or PMA (500 ng/mL; positive control) at 37 °C and 5% of CO2. After incubation, the hydrogen peroxide production was determined in the presence or absence of wortmannin, a PI3K inhibitor, after reading the optical density at 620 nm. The results were expressed as μM H2O2. Data represent the mean ± SEM of 5 independent donors. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to negative control. ###P < 0.001, ####P < 0.0001 compared to respective experimental without inhibitor (ANOVA).
To assess the participation of PI3K in the venom Asp-49 PLA2-stimulated hydrogen peroxide production, neutrophils were incubated with a specific inhibitor of PI3K, Wortmannin, at the concentration of 500 nM. Neutrophils were maintained for 15 min with the inhibitor and stimulated for 1:30 h with the BaTX-II. As shown in Fig. 2, when neutrophils were incubated with the BaTX-II, in the presence of Wortmannin, there was a significant decrease in the hydrogen peroxide production compared to the controls.
3.3. Effect of BaTX-II on DNA release by human neutrophils
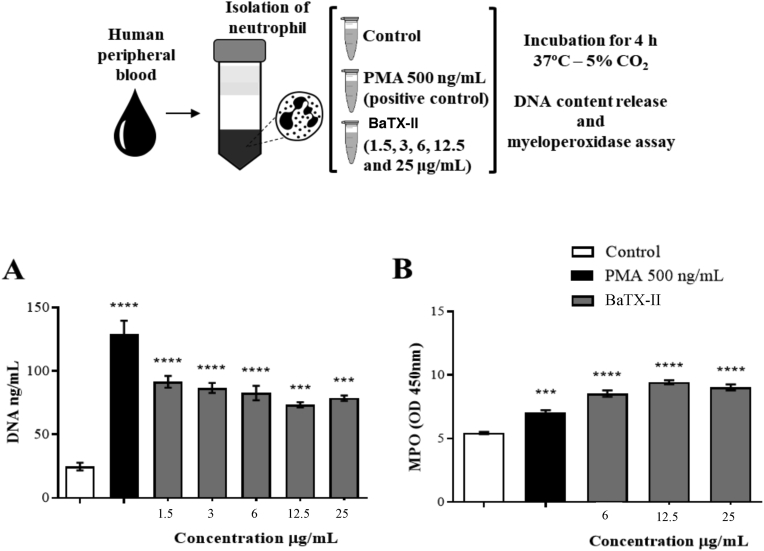
To assess the ability of the BaTX-II to induce the release of DNA content suggesting NETs release, neutrophils were incubated with BaTX-II in non-cytotoxic concentrations, RPMI (negative control) or PMA (positive control). As shown in Fig. 3A the toxin induced an increased in DNA release compared to the negative control after 4 h of incubation. These results evidence the ability of the toxin to activate human neutrophils and stimulate the NETs release.
Fig. 3.
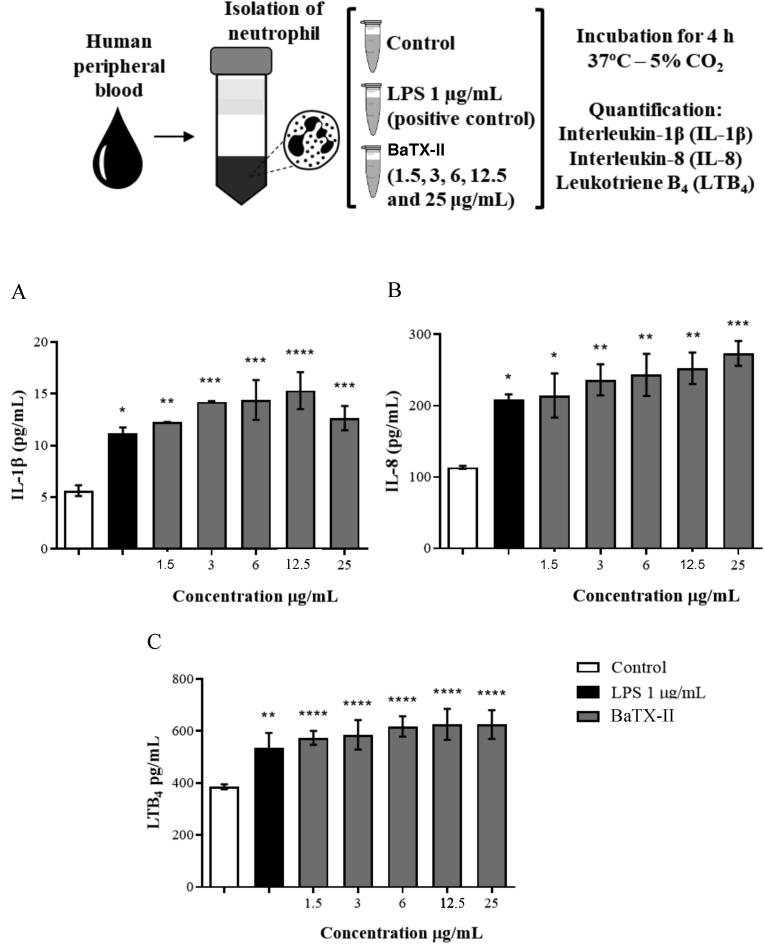
Effect of BaTX-II, isolated from B. atrox venom on IL-1β, IL-8 and LTB4 release by human neutrophils. 2 × 105 neutrophils were incubated with BaTX-II (1.5, 3, 6, 12.5 and 25 μg/mL), RPMI (negative control) or LPS (1 μg/mL; positive control) for 4 h at 37 °C and 5% CO2. The cytokines and LTB4 concentrations were determined by specific enzymatic immunoassay (EIA). (A) IL-1β, (B) LTB4 and (C) IL-8. Data represent the mean ± SEM of 5 independent donors. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to negative control (ANOVA).
3.4. Effect of BaTX-II on MPO release by human neutrophils
To determine the ability of the BaTX-II to induce MPO release, neutrophils were incubated with BaTX-II in non-cytotoxic concentrations, RPMI (negative control) or PMA (positive control). As shown in Fig. 3B BaTX-II induced an increase in MPO release to the supernatant compared to the negative control. These results evidenced the ability of BaTX-II to activate human neutrophils and probably causing degranulation, resulting in the MPO release.
3.5. Effect of BaTX-II on human neutrophils IL-1β, IL-8 and LTB4 liberation
IL-1β and IL-8 concentrations in the neutrophil supernatant was evaluated 4 h after incubation of cells with the BaTX-II (1.5, 3, 6, 12.5 and 25 μg/mL), RPMI (negative control) or LPS (positive control). Fig. 4A and B shows that in the time period studied there was a significant increase in IL-1β and IL-8 concentrations in the neutrophil supernatant after incubation with the venom BaTX-II compared to the control.
Fig. 4.
Effect of BaTX-II, isolated from B. atrox venom on DNA and MPO release by human neutrophils. 2 × 105 neutrophils were incubated with BaTX-II (1.5, 3, 6, 12.5 and 25 μg/mL) or RPMI (negative control) or PMA (positive control; 500 ng/mL) for 4 h at 37 °C and 5% of CO2. (A) After incubation the DNA content release was determined by picogreen dsDNA kit (Invitrogen). Results were compared to a standard curve of DNA supplied by the kit. (B) The MPO concentration was obtained by the peroxide oxidation dependent on the TMB and determined in a spectrophotometer at 450 nm. Data were compared to a standard MPO curve. Data represent the mean ± SEM of 5 independent donors. ***P < 0.001, ****P < 0.0001 compared to negative control (ANOVA).
The ability of the BaTX-II to induce LTB4 production by human neutrophils was assessed by determining the concentration of this lipid mediator in the neutrophil supernatant incubated with the BaTX-II at different concentrations (1.5, 3, 6, 12.5 and 25 μg/mL), PMA (positive control; 500 ng/mL) or LPS (positive control; 1 μg/mL) or RPMI (negative control). Incubation of neutrophils with the BaTX-II for 4 h induced a significant increase in LTB4 levels at all concentrations used compared to the control cells (Fig. 4C) suggesting that this lipid metabolite may represent an important mediator resulting from the mechanism of action triggered by the BaT-II on the neutrophil.
4. Discussion
Previous studies have demonstrated that B. atrox venom induces an increase in vascular permeability and an in vivo leukocyte influx (Barros et al., 1998, Moreira et al., 2012), consisting mainly of MNs initially and PMNs during the later stages, mediated by lipids and cytokines. This occurs directly via the action of venom-associated molecular patterns (VAMPs), activating TLR2 (Moreira et al., 2016) and MyD88 signalling (Moreira et al., 2013). Few studies have addressed the role of B. atrox PLA2 in this process (Furtado et al., 2014, Menaldo et al., 2017), and little is known about the effect of this isolated PLA2 on neutrophil function. Therefore, here we evaluated the effect of BaTX-II, isolated from B. atrox snake venom, on isolated human neutrophil function.
First, the cytotoxic activity of BaTX-II on neutrophils was evaluated. An MTT assay demonstrated that at concentrations of 1, 5, 3, 6, 12.5, and 25 μg/mL, the toxin did not alter the viability of neutrophils. Therefore, these concentrations were used in subsequent experiments. Similarly, Zuliani et al. (2005) showed that at concentrations less than 25 μg/mL, MTX-III isolated from B. asper was not toxic to mice peritoneal macrophages. Other studies demonstrated that two phospholipases isolated from B. asper and B. atrox exerted toxic effects on several cell types only at high concentrations (Bultrón et al., 1993, Furtado et al., 2014, Lomonte et al., 1994, Setúbal et al., 2013b). These results show that the cytotoxic potential of BaTX-II from B. atrox is similar to that of the PLA2s from snake species of the genus Bothrops.
Neutrophils are the first cells recruited to inflamed tissue, and after activation play a key role in innate immunity, phagocytosing and destroying microbial pathogens. This occurs through a series of fast and coordinated responses such as adhesion, migration, degranulation, and release of inflammatory mediators, proteolytic enzymes and reactive oxygen species (ROS) (Fialkow et al., 2007, Hampton et al., 1998, Witko-Sarsat et al., 2000). The increase in ROS production occurs by a phenomenon known as the “respiratory burst” that is mediated by a NADPH oxidase complex, promoting high oxygen consumption with subsequent production of superoxide anion and hydrogen peroxide (Groemping and Rittinger, 2005). These reactive species derived from oxygen result in microbial death and tissue damage by oxidizing a wide range of target biological molecules, including carbohydrates (Schiller et al., 1996), nucleotides, DNA (Hawkins et al., 2002), lipids (Panasenko et al., 2007, Skaff et al., 2007), and proteins (Hawkins et al., 2003). However, excessive release of hydrogen peroxide can cause tissue damage, contributing to an increase in the inflammatory reaction.
Thus, we performed experiments to verify the effect of BaTX-II on hydrogen peroxide production by human neutrophils. After 90 min of incubation, the toxin significantly stimulated neutrophils to produce hydrogen peroxide compared to a negative control; however, there was no difference when compared to PMA (a positive control). This venom PLA2 induced the release of hydrogen peroxide, indicating that the toxin is capable of stimulating neutrophils to activate the respiratory burst. These results are in accordance with the findings of Zuliani et al. (2005), who showed that MT-II (PLA2 Lys-49) and MT-III (PLA2 Asp49) isolated from B. asper venom induced the release of hydrogen peroxide, by macrophages.
Phosphatidylinositol 3-kinase (PI3K) is a heterodimeric enzyme whose main product is phosphatidylinositol (3, 4, 5) triphosphate. Among other functions, it can act as a secondary modulator of ROS production in multiple cell types (Korbecki et al., 2013). Therefore, we evaluated the role of PI3K in modulating ROS production by neutrophils stimulated with BaTX_II. To determine whether the PI3K signalling pathway is involved in BaTX-II-induced ROS production by neutrophils, a PI3K inhibitor, wortmannin, was used. This is a fungal metabolite that blocks PI3K signalling (Powis et al., 1994). When treated with wortmannin, as well as PMA (a positive control), we observed a significant decrease in the amount of hydrogen peroxide produced by neutrophils stimulated by the venom PLA2. This suggests a role for PI3K in the signalling cascade triggered by the toxin.
In addition to the well-known microbicidal capacity of neutrophils, they can also capture and kill extracellular pathogens through the release of neutrophil extracellular traps (NETs). Furthermore, ROS released by the NADPH oxidase complex can activate granular proteases and induce NET formation (Nguyen et al., 2017). ROS can cross membranes and damage nucleic acids, proteins, and cell membranes. Several enzymes located in NETs have pro-inflammatory function, such as elastase and MPO, suggesting a role for the formation of NETs in inflammation (Manfredi et al., 2018). Thus, we conducted experiments to evaluate the effect of venom BaTX-II on this important neutrophil function. Results showed that the toxin, BaTX-II, induced the release of DNA and MPO, suggesting that it induces NET formation. These observations are congruent with literature demonstrating that ROS derived from NADPH oxidase activity, as well as MPO liberation, are key signals for the generation of NETs (Rada et al., 2013). This strongly suggests a direct effect of toxin-induced ROS and MPO on the formation and release of NETs.
Further experiments were carried out to assess the capacity of the venom BaTX-II to induce the release of IL-1β, IL-8 and LTB4 by human neutrophils. Results showed that the toxin induced the release of both IL-1β and IL-8. This is the first demonstration of the effect of PLA2 on cytokine production by human neutrophils. Moura et al. (2014) described an increase in IL-1β concentration in thioglycollate mouse-derived neutrophils after 12 h of incubation with PLA2s isolated from B. mattogrossensis venom. Corasolla Carregari et al. (2013) showed an increase in IL-6, IL-1 and TNF-α serum levels using a BaTX-II from B. bilineata venom, in in vivo experiments. Menaldo et al. (2017) showed that an acidic PLA2 from B. atrox, BatroxPLA2, induced IL-6, PGE2 and LTB4 release from mouse macrophages.
IL-1β is a pro-inflammatory cytokine produced by many cell types including macrophages, NK cells, monocytes, and neutrophils (Cassatella, 1999). This cytokine has important homeostatic functions in the body, including in regulating sleep and temperature (Dinarello, 1996). However, the overproduction of IL-1β is involved in physiopathological changes which occur in inflammatory diseases (Ren and Torres, 2009). In addition, literature suggests that the release of IL-1β is correlated with the generation of NETs by neutrophils derived from the inflammatory synovial fluid (Mitroulis et al., 2011).
As a powerful chemokine that plays a key role in innate immunity, IL-8 production could contribute to the maintenance of chemotaxis, attracting more neutrophils to the site of inflammation (Baggiolini et al., 1994). In addition, PLA2s from B. jararacussu (Bothropstoxin-I and Bothropstoxin-II) and B. pirajai (Piratoxin-I) can induce neutrophil migration in a concentration-dependent manner (Gambero et al., 2002). However, IL-8 also contributes to tissue damage and organ failure in conditions such as acute respiratory stress syndrome, reperfusion injury, and septic shock (Feuerstein and Rabinovici, 1994). Moreover, IL-8 release, as well as ROS production, stimulates the release of NETs (Fuchs et al., 2007), suggesting that IL-8 and ROS production induced by BaTX-II could contribute to the release of NETs.
LTB4 is a metabolite resulting from the activity of 5-lipoxygenase (5-LO) and is considered a potent lipid mediator with chemoattractant properties. It plays an essential role in the inflammatory response and is a key player in the initiation of inflammation as a lipid mediator (Henderson, 1994, Peters-Golden and Henderson, 2007). LTB4 stimulation leads to neutrophil functional responses important to host defence, such as the secretion of lysosomal enzymes, the activation of NADPH oxidase activity, NO formation, and phagocytosis. In addition, LTB4 stimulates the expression of the β2-integrin (CD11b/CD18), an effect likely related to its ability to stimulate leukocyte migration and phagocytosis (Flamand et al., 2007). Our results showed that the venom PLA2 induced a significant increase in LTB4 release by neutrophils. This suggests that, along with IL-8, this plays a key role in neutrophil chemotaxis induced by the toxin, as has been described by Gambero et al. (2002).
Given the important role of neutrophils as front-line cells in innate immunity, as well as the importance of MPO in neutrophil functional responses, we investigated the effect of the venom PLA2 on MPO production by this cell type. MPO, a haeme-protein, is the principal enzyme released from the neutrophil's azurophilic granules upon neutrophil activation (Witko-Sarsat et al., 2000) when stimulated by ROS and cytokines (Arimura et al., 1993). Here, we showed that BaTX-II induced the release of IL-8, as well as the production of ROS. We also showed BaTX-II-induced MPO release, suggesting that this enzyme leads to the degranulation of human neutrophils.
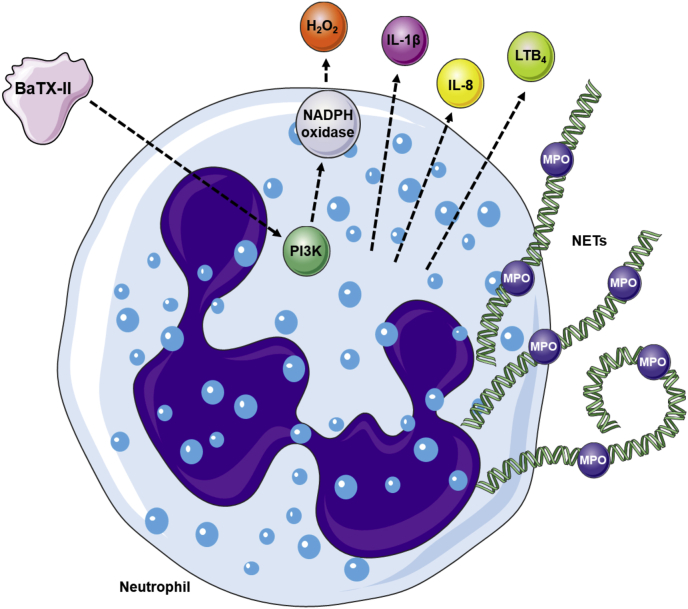
Taken together, our data show that the BaTX-II has the capacity to induce the activation of neutrophils (Fig. 5). The stimulation of hydrogen peroxide production involves a signalling event dependent on PI3K. In addition, BaTX-II induces the release of inflammatory mediators IL-1β, IL-8, LTB4, MPO, and DNA, contributing to the formation of NETs. Significantly, this is the first description of the ability of BaTX-II to stimulate neutrophil functions. Our findings help contribute to a better understanding of the role of phospholipases at the site of inflammation, as well as the role of these enzymes in the inflammatory response triggered by snake venom.
Fig. 5.
Proposed mechanism of action of BaTX-II, isolated from B. atrox venom on human neutrophils. The representative scheme shows BaTX-II acting on neutrophils to produce H2O2, IL-1β, LTB4 and IL-8. Firstly, BaTX-II interacts with the cellular membrane by an unknown mechanism, leading to PI3K stimulation and NADPH oxidase activation, culminating in H2O2 liberation. BaTX-II also stimulates neutrophil to secrete pro-inflammatory mediators such as cytokines IL-1β and IL-8 and the lipid mediator LTB4 and to liberate MPO and NETs. The illustration was made using the resources of “SMART - Servier Medical Art”.
Authorship
J.P.Z. and S.S.S. designed the study; S.S.S., A.S.P., N.M.N., H.M.S. and M.V.P. performed the experiments; A.M.S. provided venom; C.M.A.R. and C.N.B performed and supervised the flow cytometer studies; A.M.L. and A.M.S. performed the toxin isolation and characterization; J.P.Z, S.S.S and A.S.P., N.M.N., H.M.S., C.M.A.R., C.N.B, A.M.L., M.V.P. and A.M.S collected and analyzed the data; J.P.Z and A.M.S. provided reagents; J.P.Z., S.S.S. and A.M.S. wrote the manuscript and M.V.P. performed the figures. All of the authors discussed the results and implications and commented on the manuscript at all stages.
Declaration of competing interest
There is no conflict of interest statement.
Acknowledgments
The authors express their gratitude to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Rondônia (FAPERO) for the financial support. The authors thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for their facilities use. This study was supported by grants (479316-2013-6) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Juliana Pavan Zuliani was a recipient of productivity grant 306672/2014-6 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Sulamita Setubal was the beneficiary of CAPES by Doctoral's fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2020.100032.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abi Abdallah D.S., Egan C.E., Butcher B.A., Denkers E.Y. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrião-Escarso S.H., Soares A.M., Rodrigues V.M., Angulo Y., Diaz C., Lomonte B., Giglio J.R. Myotoxic phospholipases A(2) in bothrops snake venoms: effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie. 2000;82(8):755–763. doi: 10.1016/s0300-9084(00)01150-0. [DOI] [PubMed] [Google Scholar]
- Arimura Y., Minoshima S., Kamiya Y., Tanaka U., Nakabayashi K., Kitamoto K., Naga- sawa T., Sasaki T., Suzuki K. Serum myeloperoxidase and serum cytokines in anti- myeloperoxidase antibody-associated glomerulonephritis. Clin. Nephrol. 1993;40:256–264. [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines - CXC and CC chemokines. Adv. Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Barros S.F., Friedlanskaia I., Petricevich V.L., Kipnis T.L. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediat. Inflamm. 1998;7:339–346. doi: 10.1080/09629359890866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Lollike K., Kjeldsen L., Sengelcw H., Bastholm L., Nielsen M.H., Bain- ton D.F. Human neutrophil granules and secretory vesicles. Eur. J. Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrau- ch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- Bultrón E., Gutiérrez J.M., Thelestam M. Effects of Bothrops asper (terciopelo) myotoxin III, a basic phospholipase A2, on liposomes and mouse gastrocnemius muscle. Toxicon. 1993;31:217–222. doi: 10.1016/0041-0101(93)90289-u. [DOI] [PubMed] [Google Scholar]
- Cassatella M.A. The production of cytokines by polymorphonuclear neutrophils. Im- munol. Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Cassatella M.A. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granu- locyte survival na programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- Corasolla Carregari V., Stuani Floriano R., Rodrigues-Simioni L., Winck F.V., Baldasso P.A., Ponce-Soto L.A., Marangoni S. Biochemical, pharmacological, and structural characterization of new basic PLA 2 Bbil-TX from Bothriopsis bilineata snake venom. BioMed Res. Int. 2013;2013:1–12. doi: 10.1155/2013/612649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Thermoregulation and the pathogenesis of fever. Infect. Dis. Clin. 1996;10:433–449. doi: 10.1016/s0891-5520(05)70306-8. [DOI] [PubMed] [Google Scholar]
- Dufton M.J., Eaker D., Hider R.C. Conformational properties of phospholipases A2. Secondary-structure prediction, circular dichroism and relative interface hydrophobicity. Eur. J. Biochem. 1983;137:537–544. doi: 10.1111/j.1432-1033.1983.tb07859.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Rabinovici R. Importance of interleukin-8 and chemokines in organ injury and shock. Crit. Care Med. 1994;4:550–551. doi: 10.1097/00003246-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Fialkow L., Wang Y., Downey G.P. Reactive oxygen and nitrogen species as signa- ling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Flamand N., Mancuso P., Serezani C.H., Brock T.G. Leukotrienes: mediators that have been typecast as villains. Cell. Mol. Life Sci. 2007;64(19–20):2657–2670. doi: 10.1007/s00018-007-7228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado J.L., Oliveira A.G., Pontes A.S., Setúbal S.S., Xavier C.V., Lacouth-Silva F., Lima B.F., Zaqueo K.D., Kayano A.M., Calderon L.A., Stábeli R.G., Soares A.M., Zuliani J.P. Activation of J77A.1 macrophages by three phospholipases A2 isolated from Bothrops atrox snake venom. BioMed Res. Int. 2014;2014:1–13. doi: 10.1155/2014/683123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambero A., Landucci E.C., Toyama M.H., Marangoni S., Giglio J.R., Nader H.B., Die- trich C.P., De Nucci G., Antunes E. Human neutrophil migration in vitro induced by secretory phospholipases A2: a role for cell surface glycosaminoglycans. Biochem. Pharmacol. 2002;63:65–72. doi: 10.1016/s0006-2952(01)00841-3. [DOI] [PubMed] [Google Scholar]
- Groemping Y., Rittinger K. Activation and assembly of the NADPH-oxidase: a struc- tural perspective. Biochem. J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Lomonte B. Wiley; New York: 1997. Phospholipase A2 myotoxins from Bothrops snake ve- noms, Venom Phospholipase A2 Enzymes, Structure, Function and Mechanisms; pp. 321–352. [Google Scholar]
- Hampton M.B., Kettle A.J., Winterbourn C.C. Inside the neutrophil phagosome: oxi- dants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Harris J.B. Phospholipases in snake venoms and their effects on nerve and muscle. Pharmacol. Ther. 1985;31:79–102. doi: 10.1016/0163-7258(85)90038-5. [DOI] [PubMed] [Google Scholar]
- Hawkins C.L., Pattison D.I., Davies M.J. Reaction of protein chloramines with DNA and nucleosides: evidence for the formation of radicals, protein-DNA cross-links and DNA fragmentation. Biochem. J. 2002;365:605–615. doi: 10.1042/BJ20020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C.L., Pattison D.I., Davies M.J. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- Henderson W.R., Jr. The role of leukotrienes in inflammation. Ann. Intern. Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- Ingvar M., Ferby I.W., Mitsunobu H.K.K., Takao S. Wortmannin inhibits mitogen- activated protein kinase activation by platelet-activating factor through a mechanism independent of p85/p110-type phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:11684–11688. doi: 10.1074/jbc.271.20.11684. [DOI] [PubMed] [Google Scholar]
- Kini R.M., Evans H.J. A model to explain the pharmacological effects of snake venom phospholipase A2. Toxicon. 1989;27:613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Korbecki J., Baranowska-Bosiacka I., Gutowska I., Chlubek D. The effect of reacti- ve oxygen species on the synthesis of prostanoids from arachidonic acid. J. Physiol. Pharmacol. 2013;64:409–421. [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bac- teriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomonte B., Lundgren J., Johansson B., Bagge U. The dynamics of local tissue da- mage induced by Bothrops asper snake venom and myotoxin II on the mouse cremaster mus- cle: an intravital and electron microscopic study. Toxicon. 1994;32:41–55. doi: 10.1016/0041-0101(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Manfredi A.A., Ramirez G.A., Rovere-Querini P., Maugeri N. The neutrophil's choice: phagocytose vs make neutrophil extracellular traps. Front. Immunol. 2018;9:288. doi: 10.3389/fimmu.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Constantini C., Jaillon S. Neutrophils in the activa- tion and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Menaldo D.L., Bernardes C.P., Zoccal K.F., Jacob-Ferreira A.L., Costa T.R., Del Lama M.P., Naal R.M., Frantz F.G., Faccioli L.H., Sampaio S.V. Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A2 from Bothrops atrox venom. Mol. Immunol. 2017;85:238–247. doi: 10.1016/j.molimm.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Mitroulis I., Kambas K., Chrysanthopoulou A., Skendros P., Apostolidou E., Kourtzelis I., Drosos G.I., Boumpas D.T., Ritis K. Neutrophil extracellular trap formation is as- sociated with IL-1β and autophagy-related signaling in gout. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira V., Dos-Santos M.C., Nascimento N.G., Borges Da Silva H., Fernandes C.M., D'império Lima M.R., Teixeira C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon. 2012;60:12–20. doi: 10.1016/j.toxicon.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Moreira V., Teixeira C., Borges Da Silva H., D'império Lima M.R., Dos-Santos M.C. The crucial role of the MyD88 adaptor protein in the inflammatory response induced by Bothrops atrox venom. Toxicon. 2013;67:37–46. doi: 10.1016/j.toxicon.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Moreira V., Teixeira C., Borges Da Silva H., D'império Lima M.R., Dos-Santos M.C. The role of TLR2 in the acute inflammatory response induced by Bothrops atrox snake venom. Toxicon. 2016;118:121–128. doi: 10.1016/j.toxicon.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Moura A.A., Kayano A.M., Oliveira A.O., Setúbal S.S., Ribeiro J.G., Barros N.B., Nico-lete R., Moura L.A., Fuly A.L., Nomizo A., Silva S.L., Fernandes C.F.C., Zuliani J.P., Stábeli R.G., Soares A.M., Calderon L.A. Purification and biochemical characterization of three myotoxins from Bothrops mattogrossensis snake venom with toxicity against leishmania and tumor cells. BioMed Res. Internat. 2014:13. doi: 10.1155/2014/195356. Article ID 195356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nguyen G.T., Green E.R., Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko O.M., Vakhrusheva T.V., Vlasova I.I., Chekanov A.V., Baranov Y.V., Sergienko V.I. Role of myeloperoxidase-mediated modification of human blood lipoproteins in atherosclerosis development. Bull. Exp. Biol. Med. 2007;144:428–431. doi: 10.1007/s10517-007-0346-x. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M., Henderson W.R., Jr. Leukotrienes. N. Engl. J. Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Petrovic N., Grove C., Langton P.E., Misso N.L., Thompson P.J. A simple assay for a human serum phospholipase A2 that is associated with high-density lipoproteins. J. Lipid Res. 2001;42:1706–1713. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassay for the measurement of peroxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay re- ader. J. Immunol. Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Pontes A.S., Setúbal S. Da S., Nery N.M., Da Silva F.S., Da Silva S.D., Fernandes C.F., Stábeli R.G., Soares A.M., Zuliani J.P. p38 MAPK is involved in human neutrophil chemotaxis induced by L-amino acid oxidase from Calloselasma rhodosthoma. Toxicon. 2016;119:106–116. doi: 10.1016/j.toxicon.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Pontes A.S., Setúbal S.S., Xavier C.V., Lacouth-Silva F., Kayano A.M., Pires W.L., Nery N.M., Boeri De Castro O., Da Silva S.D., Calderon L.A., Stábeli R.G., Soares A.M., Zuli- ani J.P. Effect of l-amino acid oxidase from Calloselasma rhodosthoma snake venom on human neutrophils. Toxicon. 2014;80:27–37. doi: 10.1016/j.toxicon.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Powis G., Bonjouklian R., Berggren M.M., Gallegos A., Abraham R., Ashendel C., Zal- kow L., Matter W.F., Dodge J., Grindey G. Wortmannin, a potent and selective inhi- bitor of phosphatidylinositol-3-kinase. Canc. Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- Rada B., Jendrysik M.A., Pang L., Hayes C.P., Yoo D.G., Park J.J., Moskowitz S.M., Malech H.L., Leto T.L. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PloS One. 2013;8 doi: 10.1371/journal.pone.0054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K., Torres R. Role of interleukin-1β during pain and inflammation. Nat Inst He- alth. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M., Teixeira H.C., Marcondes M.C.G., Barbutto J.A.M. Superoxide-indepen- dent hydrogen release by active macrophages. Braz. J. Med. Biol. Res. 1989;22:1271–1273. [PubMed] [Google Scholar]
- Schiller J., Arnhold J., Sonntag K., Arnol K. NMR studies on human, pathologically changed synovial fluids: role of hypochlorosus acid. Magn. Reson. Med. 1996;35:848–853. doi: 10.1002/mrm.1910350610. [DOI] [PubMed] [Google Scholar]
- Stábeli R.G., Simões-Silva R., Kayano A.M., Gimenez G.S., Moura A.A., Caldeira C.A.S., Coutinho-Neto A., Zaqueo K.D., Zuliani J.P., Calderon L.A., Soares A.M. Chromatography – the Most Versatile Method of Chemical Analysis. IntechOpen; 2012. Purification of phospholipases A2 from American snake venoms. [DOI] [Google Scholar]
- Setúbal S.S., Pontes A.S., Furtado J.L., Xavier C.V., Silva F.L., Kayano A.M., Izidoro L.F., Calderon L.A., Soares A.M., Calderon L.A., Stábeli R.G., Zuliani J.P. Action of two phospholipases A2 purified from Bothrops alternatus snake venom on macrophages. Biochemistry. 2013;78:194–203. doi: 10.1134/S0006297913020089. [DOI] [PubMed] [Google Scholar]
- Setubal S.S., Pontes A.S., Nery N.M., Bastos J.S., Castro O.B., Pires W.L., Zaqueo K.D., Stábeli R.G., Zuliani J.P. Effect of Bothrops bilineata snake venom on neutrophil function. Toxicon. 2013;76:143–149. doi: 10.1016/j.toxicon.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Skaff O., Pattison D.I., Davies M.J. Kinetics of hypobromous acid-mediated oxidation of lipid components and antioxidants. Chem. Res. Toxicol. 2007;20:1980–1988. doi: 10.1021/tx7003097. [DOI] [PubMed] [Google Scholar]
- Summers C., Rankin S.M., Condliffe A.M., Singh N., Peters A.M., Chilvers E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikur T. Assay method for myelope- roxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Teixeira C.F., Landucci E.C., Antunes E., Chacur M., Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42:947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Timár C.I., Lorincz A.M., Ligeti E. Changing world of neutrophils. Pflügers Archiv. 2013;465:1521–1533. doi: 10.1007/s00424-013-1285-1. [DOI] [PubMed] [Google Scholar]
- Verheij H.M., Egmond M.R., De Haas G.H. Chemical modification of the alpha- amino group in snake venom phospholipases A2. A comparison of the interaction of pancreatic and venom phospholipases with lipid-water interfaces. Biochemistry. 1981;20:94–99. doi: 10.1021/bi00504a016. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- Zuliani J.P., Gutierrez J.M., Casais E Silva L.L., Sampaio S.C., Teixeira C.F.P. Acti- vation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 Phospho- lipases A2. Toxicon. 2005;46:523–532. doi: 10.1016/j.toxicon.2005.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.