Abstract
We describe two dogs with persistent visual impairment after initially mild intoxication signs following ingestion of Ornithogalum arabicum plant material. Additionally, a 12-year analysis of the Dutch Poisons Information Centre database additionally reveals that ingestion of Ornithogalum plant material can be potentially life-threatening to companion animals. Further studies are necessary to confirm the involvement of cardiac glycoside-like toxins present in Ornithogalum arabicum and the toxicity of these substances to the retina.
Keywords: Ornithogalum arabicum, Cardiac glycosides, Retinal degeneration, Blindness
Highlights
-
•
Intoxication with Ornithogalum arabicum leads to visual impairment and irreversible blindness in dogs.
-
•
Intoxication with Ornithogalum arabicum may be life-threatening in companion animals.
-
•
Ornithogalum arabicum and other species are suspected to contain heart glycosides.
1. Introduction
The genus Ornithogalum native to Africa, Europe and Asia, comprises approximately 150–200 species(Plančić et al., 2014). Several Southern African species are considered extremely toxic and are known to cause poisoning of livestock.(Wink and Van Wyk, 2008) Acute poisoning is characterized by cardiac arrhythmia, runs of tachycardia, heart-block, dyspnoea, posterior paresis, and diarrhoea (Botha et al., 2000). Involvement of cardiac glycoside-like substances is suspected, as two toxicological important Southern African species, O. nanodes and O. toxicarium, cross-reacted with digoxin antibodies when tested for cardiac glycosides by fluorescence polarization immune-assay(Bamhare, 1998, Botha et al., 2000). Also several cardiac glycosides of the cardenolide type, e.g. convallatoxin and convallosid, have been identified in O. nutans (Ferth et al., 1992), O. boucheanum (Ghannamy et al., 1987) and O. umbellatum (Ferth and Kopp, 1992), most notably in the bulb. Additionally, in the 1950s, the digitalis-like action of O. umbellatum on the heart was investigated, (Waud, 1954) leading to a limited clinical trial involving two patients with tablets prepared from O. umbellatum. (Vogelsang, 1955) Some species are known to contain phytotoxins other than cardiac glycosides that are responsible for severe intoxications. For example, O. thyrsoides (Fig. 1A) contains cholestane glycosides and saponins(Plančić et al., 2014). After ingestion, this species induces gastro-enteritis with severe persistent watery diarrhoea and liver degeneration in sheep.(Breukink, 1963) Calcium oxalate crystals are held responsible for contact dermatitis seen in O. caudatum.(Pohl, 1965) Nowadays several Ornithogalum species are held for ornamental purposes, as garden plants and/or cut flowers. Intoxications in humans and companion animals (i.e. dogs and cats) are rare but do occur. Here we present two suspected Ornithogalum arabicum (Fig. 1B) intoxications in dogs resulting in minor gastro-intestinal symptoms and subacute blindness. Additionally, we provide a 12-year retrospective overview of all veterinary information requests concerning Ornithogalum species to the Dutch Poisons Information Centre (DPIC).
Fig. 1.
1A: Ornithogalum thyroides. 1B: Ornithogalum arabicum.
2. Case descriptions and DPIC overview
Case 1: A 4-month-old otherwise healthy female Labrador Retriever pup (9 kg) was referred to the Ophthalmology service of the Faculty of Veterinary Medicine in The Netherlands after the owners noticed a sudden loss of vision starting less than one day ago.
Medical history revealed that the dog was seen playing with Ornithogalum arabicum bulbs. Three weeks prior to the loss of vision one bulb had been retrieved out of the pup's mouth. After this incident it vomited once. Two weeks later the owners noticed some redness and discharge in both eyes. These signs resolved spontaneously a day later. Repeated ingestion of bulbs was suspected as compost containing many O. arabicum bulbs was used as fertilizer on several gardens in the neighbourhood, including the garden of the dog owner. The owner mentioned that other people in his neighbourhood also reported functional vision losses in their dogs. The previous Labrador retriever pup (10 weeks old) of the same owners was euthanised two months earlier because of its poor clinical condition due to severe haemorrhagic diarrhoea. According to the owners, this pup had acutely gone blind as well and had access to O. arabicum bulbs.
Upon ocular examination of the presented pup, both pupils were dilated. Direct and consensual pupillary light reflexes, menace response and dazzle reflex were absent in both eyes. Ophthalmoscopy revealed moderate diffuse hyperreflectivity of the tapetal area of the fundus. The retinal changes were not bilaterally symmetrical. The optic nerve head in both eyes was slightly elevated and had a normal pinkish white colour. Diameters of the retinal blood vessels seemed normal. Intraocular pressures were below reference range (right eye: 4 mmHg, left eye: 3 mmHg)(Knollinger et al., 2005). No treatment was started. At follow up by telephone consultation the owners stated that the puppy had been unable to adjust to the blindness and was therefore euthanised two months after visiting the Ophthalmology service.
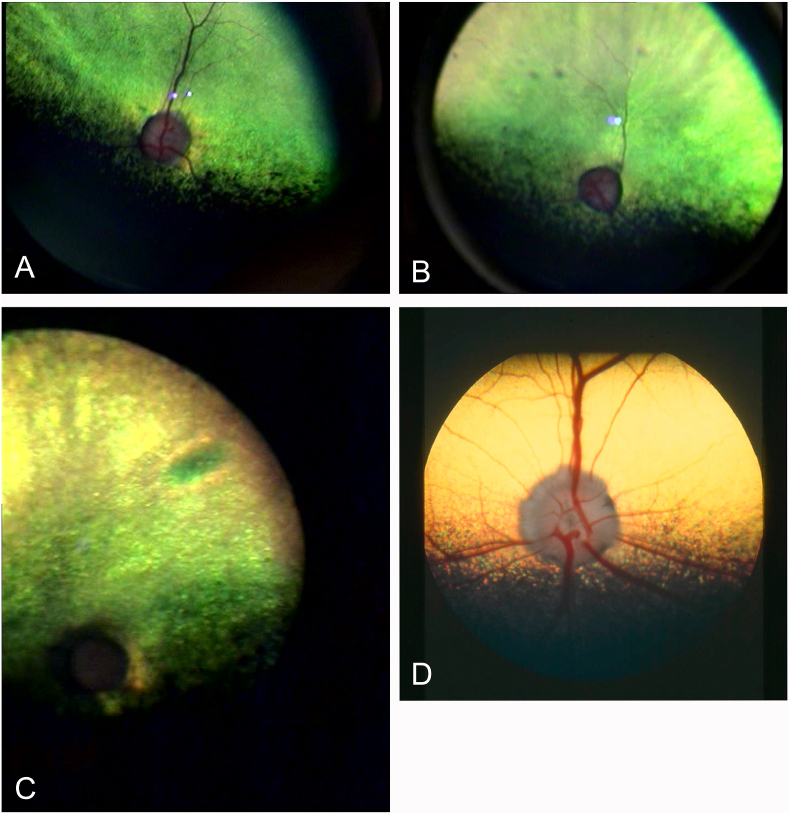
Case 2: A two-year-old otherwise healthy female Dachshund (5.8 kg) was referred to the Ophthalmology service after the owners noticed a sudden onset of blindness starting approximately one week earlier. After careful history taking, the owners remembered that the dog had been playing with an Ornithogalum arabicum plant approximately three weeks earlier (two weeks prior to the observed loss of vision). Immediately after plant exposure, the dog had vomited once and had watery diarrhoea for the following three days. Ocular examination revealed fully dilated pupils. Menace response and dazzle reflex were absent, while the direct pupillary light reflex was absent in the left eye and sluggish and incomplete in the right eye. Ophthalmoscopy revealed diffuse hyperreflectivity of the tapetal area, pallor of the optic nerve head and attenuation of the retinal blood vessels. The retinal changes were not bilaterally symmetrical, the left eye was more severely affected than the right eye (Fig. 2A–B). Asymmetry was also noticed during chromatic pupillary light reflex testing for evaluation of the contributions to the pupillary light reflex of photoreceptor cells (sensitive to red light) and intrinsically photosensitive retinal ganglion cells (sensitive to blue light)(Grozdanic et al., 2013). On exposure to red light there was no pupillary constriction in the left eye and only a minor pupillary constriction in the right eye. In response to blue light stimulation, however, the pupils of both eyes constricted briskly. Intraocular pressures were within reference range (right eye: 14 mmHg, left eye: 16 mmHg) (Knollinger et al., 2005). No treatment was started. At 6-month follow-up the owners had noticed improvement in the visual behaviour of the dog in the home environment. Outside, however, the dog was still bumping into things. Functional vision was tested in an obstacle course in bright as well as dimmed light. In both light conditions the dog bumped into the obstacles. Ophthalmoscopic examination of the left eye revealed advanced retinal degeneration, with a pale and flattened optic nerve head, indiscernible retinal blood vessels and severe diffuse hyperreflectivity of the tapetal area (Fig. 2C). The retinal blood vessels of the right eye were severely attenuated but still distinguishable. Chromatic pupillary light testing revealed absent responses to red light and positive responses to blue light in both eyes.
Fig. 2.
A: Right eye of case 2 at initial presentation. B: Left eye of case 2 at initial presentation. Note the attenuation of retinal blood vessels and changes in reflectivity (hyperreflectivity) of the tapetal area (yellow-green area). C: Left eye of case 2 at 6-month follow-up. Note the complete absence of retinal blood vessels and marked hyperreflectivity of the tapetal area. D: Normal canine retina as reference image. The yellow colour of the tapetum is a normal variation in palette. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The DPIC database was searched retrospectively from 2007 to 2018 using the search term “Ornithogalum” combined with “acute or chronic exposure”. The available information, (audiotape and/or written) of each case was analysed. Only veterinary cases with witnessed exposure were included. A total of 13 information requests involving 12 dogs (including case 1), and two cats were found. In 38% of the requests the Ornithogalum species was specified; O. arabicum (one dog), and O. saundersiae (four dogs). Cats were predominantly exposed to the upper ground parts (stems, leaves, and flowers) while dogs were apparently more attracted to the bulbs. Predominantly young animals (n = 7; 0–1 year, all dogs) were exposed. Veterinary consultations to the DPIC were mostly made by the time the animals were symptomatic (12 out of 14). Time between exposure and consultation was known in 11 cases with a median delay of 12 h (range: 4–504). Many animals experienced severe gastrointestinal signs and some were lethargic. Visual dysfunction was noted in two dogs (including case 1) and one cat. An irregular heartbeat was observed in one dog (Table 1). Information on the clinical outcome was available for cases 12, 13, and 14. These dogs died shortly after the DPIC had been consulted by the veterinarian.
Table 1.
Retrospective overview of veterinary information requests to the Dutch Poisons Information Centre (DPIC) concerning exposure to Ornithogalum species, 2007–2018 (n = 14).
| No. | Animal species | Ornithogalum species | Time after exposure | Signs |
|---|---|---|---|---|
| 1 | Dog | unknown | unknown | no signs mentioned |
| 2 | Cat | unknown | unknown | Signs not specified |
| 3 | Cat | unknown | 24 h | weakness, nystagmus, abnormal pupillary light reflex, blindness |
| 4 | Dog | O. saundersiae | 24 h | (persistent) vomiting |
| 5 | Dog | unknown | ±7 h | (persistent) vomiting, anorexia |
| 6 | Dog | unknown | ±12 h | (persistent) vomiting, depression |
| 7 | Dog | unknown | 48 h | abdominal pain, diarrhoea, lethargy, irregular heartbeat, bradycardia, mydriasis |
| 8 | Dog | O. saundersiae | 4 h | (persistent) vomiting, anorexia |
| 9 | Dog | O. saundersiae | 24 h | hematemesis, bloody diarrhoea, unspecified liver and kidney dysfunction |
| 10 | Dog | O. saundersiae | 10 min. | no clinical signs mentioned |
| 11 | Dog (case 1) | O. arabicum | unknown | vomiting, diarrhoea |
| 3 weeks | blindness | |||
| 12 | Doga | unknown | ±12 h | (persistent) vomiting, bloody diarrhoea, lethargy |
| 13 | Doga | unknown | ±12 h | (persistent) vomiting, bloody diarrhoea, lethargy |
| 14 | Doga | unknown | ±12 h | (persistent) vomiting, diarrhoea, melena, lethargy, weakness, hypotension |
Information on clinical outcome was present; these dogs died soon after the information request.
3. Discussion
Transient to permanent blindness due to Ornithogalum intoxication has been previously reported in cattle in southern Africa.(Botha and Penrith, 2008) This is, as far as the authors know, the first description of retinal degeneration and blindness following Ornithogalum arabicum ingestion in dogs. Additional reports of visual disturbances were found in the DPIC database with one other dog and one cat exposed to unspecified Ornithogalum species. Other reported clinical signs were primarily severe gastro-intestinal disturbances, as well as an irregular heartbeat. At least three dogs died shortly after ingestion of Ornithogalum plant material.
As for many Ornithogalum species, there is no information available concerning toxins present in the Eurasian Ornithogalum arabicum. Some Ornithogalum species contain cardiac glycosides of the cardenolide type. Next to the well-known effects on the heart, cardiac glycosides also affect other organ systems such as the gastrointestinal, neurological and visual system. They inhibit the Na+,K+-ATPase ion pump found in the cell membrane of all mammalian cells, including retinal cells and the non-pigmented epithelial cells of the ciliary body(Katz et al., 2015). The presence of cardiac glycosides could explain the visual impairment seen in the presented cases and the low intraocular pressures in case number 1. For example digoxin, a well-known cardiac glycoside, induces cone dysfunction in canines at therapeutic dosages (Maehara et al., 2005, Landfried et al., 2017), and causes retinal degeneration and loss of vision in mice at high doses(Hinshaw et al., 2016). It induces cell death specifically in photoreceptor cells, but has no or only minor effects on other retinal cell types(Landfried et al., 2017). The documented digoxin-induced retinal degeneration corresponds well with the retinal degeneration found in the ophthalmic examination of case 1 and 2. In addition, the abnormal chromatic pupillary light reflex in case 2 indicates a defect at the level of the photoreceptor cells (i.e., rods and cones) and a normal function of the intrinsically photosensitive retinal ganglion cells(Grozdanic et al., 2013). Humans experiencing visual disturbances in response to therapeutic oral digoxin saw a (partial) reversal of their visual deficits when they decreased their dose or discontinued the medication altogether(Renard et al., 2015). In case 2, however, vision was not restored at 6-month follow-up at all. Moreover, indirect ophthalmoscopy revealed that the retinal degeneration had severely progressed. This difference might be explained by different cardiac glycosides having different binding affinities for the various isoforms of Na+,K+-ATPase(Katz et al., 2010). Ornithogalum arabicum might contain cardiac glycosides with a high affinity for retinal Na+,K+-ATPases, resulting in more devastating effects on the retina compared to other cardiac glycosides, as well as a selectivity for the isoform present in the ciliary body. The effect on the intraocular pressure might be transient as the second case presented with normal intraocular pressures at the time of presentation at the clinic.
A different or additional contributing factor to the possible pathogenesis of Ornithogalum induced vision loss, might be the presence of cholestane glycosides, another group of glycosides that, similarly like the cardiac glycosides, belong to the steroidal glycosides (Tang et al., 2013). These cytotoxic glycosides are present in for example O. caudatum, O. saundersaie, and O. thyroides and have a reported inhibitory activity on cAMP phosphodiesterase (Kubo et al., 1992, Plančić et al., 2014) and a potential similar effect on cGMP phosphodiesterase (Chen et al., 2019, Laties, 2009). cGMP phosphodiesterase is important in the phototransduction process and decreased enzyme concentration can result in blindness (Kolandaivelu et al., 2009). In the two described cases, the association of the gastrointestinal and visual disturbances with Ornithogalum ingestion were highly suggestive of an intoxication, but other causes of the blindness could not be completely ruled out. For example, an acute-onset, rapidly progressive retinal degeneration in conjunction with an absent pupillary light reflex on stimulation with a bright red light may also be found in cases of Sudden Acquired Retinal Degeneration syndrome (SARDs). However, the age and sex of the dogs and the absence of other commonly co-presenting signs, such as polyuria and polydipsia (Komáromy et al., 2016), made SARDs a less likely diagnosis. In the acute phase, electroretinography might have aided in differentiating SARDs from a toxic insult, but unfortunately both owners declined further diagnostic testing.
The time delay between acute intoxication symptoms (gastrointestinal disturbances) and the onset of vision loss in our two canine cases corresponds with reports in human literature (Piltz et al., 1993, Renard et al., 2015). However, early signs of visual impairment might have been missed, as owners of dogs often only notice changes in the behaviour of their pet by the time the vision deficit is substantial. Time to vision loss in the cat (DPIC database) was substantially shorter (24 h), possibly related to species differences (e.g. retinal isoforms of Na+,K + -ATPase) or a possible difference in Ornithogalum species as the species was unknown.
In conclusion, ingestion of plants belonging to the genus Ornithogalum can be potentially life-threatening to companion animals. Animals surviving intoxications by Ornithogalum arabicum may suffer from retinal degeneration and associated permanent visual impairment. Further studies are necessary to confirm the suggested involvement of cardiac glycoside-like toxins present in Ornithogalum arabicum and the toxicity of these substances to the retina.
Declarations of interest
The authors have no competing interests to declare.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Bamhare C. Suspected cardiac glycoside intoxication in sheep and goats in Namibia due to Ornithogalum nanodes (Leighton) Onderstepoort J. Vet. Res. 1998;65(1):25–30. [PubMed] [Google Scholar]
- Botha C.J., Penrith M.L. Poisonous plants of veterinary and human importance in southern Africa. J. Ethnopharmacol. 2008;119(3):549–558. doi: 10.1016/j.jep.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Botha C.J., Schultz R.A., van der Lugt J.J., Archer C. A krimpsiekte-like syndrome in small stock poisoned by Ornithogalum toxicarium Archer & Archer. J. S. Afr. Vet. Assoc. 2000;71(1):6–9. doi: 10.4102/jsava.v71i1.668. [DOI] [PubMed] [Google Scholar]
- Breukink H.J. Ornithogalosis of Zuidenwindlelie-vergiftiging bij schapen (Ornithogalosis in sheep) Tijdschr. Diergeneeskd. 1963;88(10):65–658. [Google Scholar]
- Chen Q., Zhang X., Gong T., Gao W., Yuan S., Zhang P. Structure and bioactivity of cholestane glycosides from the bulbs of Ornithogalum saundersiae Baker. Phytochemistry. 2019;164:206–214. doi: 10.1016/j.phytochem.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Ferth R., Kopp B. Cardenolides from Ornithogalum umbellatum. Die Pharmazie. 1992;47:626–629. [Google Scholar]
- Ferth R., Baumann A., Mayer K.K., Robien W., Kopp B. Cardenolides from Ornithogalum nutans (2n=30), Part 2. Z. Naturforschung. 1992;47b:1459–1468. [Google Scholar]
- Ghannamy U., Kopp B., Robien W., Kubelka W. Cardenolides from Ornithogalum boucheanum. Planta Med. 1987;53(2):172–178. doi: 10.1055/s-2006-962666. [DOI] [PubMed] [Google Scholar]
- Grozdanic S.D., Kecova H., Lazic T. Rapid diagnosis of retina and optic nerve abnormalities in canine patients with and without cataracts using chromatic pupil light reflex testing. Vet. Ophthalmol. 2013;16(5):329–340. doi: 10.1111/vop.12003. [DOI] [PubMed] [Google Scholar]
- Hinshaw S.J., Ogbeifun O., Wandu W.S., Lyu C., Shi G., Li Y., Qian H., Gery I. Digoxin inhibits induction of experimental autoimmune uveitis in mice, but causes severe retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2016;57(3):1441–1447. doi: 10.1167/iovs.15-19040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Lifshitz Y., Bab-Dinitz E., Kapri-Pardes E., Goldshleger R., Tal D.M., Karlish S.J. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem. 2010;285(25):19582–19592. doi: 10.1074/jbc.M110.119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Tal D.M., Heller D., Habeck M., Ben Zeev E., Rabah B., Bar Kana Y., Marcovich A.L., Karlish S.J. Digoxin derivatives with selectivity for the α2β3 isoform of Na,K-ATPase potently reduce intraocular pressure. Proc. Natl. Acad. Sci. U. S. A. 2015;112(44):13723–13728. doi: 10.1073/pnas.1514569112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollinger A.M., La Croix N.C., Barrett P.M., Miller P.E. Evaluation of a rebound tonometer for measuring intraocular pressure in dogs and horses. J. Am. Vet. Med. Assoc. 2005;227(2):244–248. doi: 10.2460/javma.2005.227.244. [DOI] [PubMed] [Google Scholar]
- Kolandaivelu S., Huang J., Hurley J.B., Ramamurthy V. AIPL1, a protein associated with childhood blindness, interacts with alpha-subunit of rod phosphodiesterase (PDE6) and is essential for its proper assembly. J. Biol. Chem. 2009;284:30853–30861. doi: 10.1074/jbc.M109.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komáromy A.M., Abrams K.L., Heckenlively J.R., Lundy S.K., Maggs D.J., Leeth C.M., Mohan Kumar P.S., Peterson-Jones S.M., Serreze D.V., Van der Woerdt A. Sudden acquired retinal degeneration syndrome (SARDS) - a review and proposed strategies toward a better understanding of pathogenesis, early diagnosis, and therapy. Vet. Ophthalmol. 2016;19(4):319–331. doi: 10.1111/vop.12291. [DOI] [PubMed] [Google Scholar]
- Kubo S., Mimaki Y., Terao M., Sashida Y., Nikaido T., Ohmoto T. Acylated cholestane glycosides from the bulbs of Ornithogalum saundersiae. Phytochemistry. 1992;31(11):3969–3973. [Google Scholar]
- Landfried B., Samardzija M., Barben M., Schori C., Klee K., Storti F., Grimm C. Digoxin-induced retinal degeneration depends on rhodopsin. Cell Death Dis. 2017;8(3) doi: 10.1038/cddis.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties A.M. Vision disorders and phosphodiesterase type 5 inhibitors: a review of the evidence to date. Drug Saf. 2009;32:1–18. doi: 10.2165/00002018-200932010-00001. [DOI] [PubMed] [Google Scholar]
- Maehara S., Osawa A., Itoh N., Wakaiki S., Tsuzuki K., Seno T., Kushiro T., Yamashita K., Izumisawa Y., Kotani T. Detection of cone dysfunction induced by digoxin in dogs by multicolor electroretinography. Vet. Ophthalmol. 2005;8(6):407–413. doi: 10.1111/j.1463-5224.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- Piltz J.R., Wertenbaker C., Lance S.E., Slamovits T., Leeper H.F. Digoxin toxicity. Recognizing the varied visual presentations. J. Clin. Neuro Ophthalmol. 1993;13:275–280. [PubMed] [Google Scholar]
- Plančić M., Božin B., Kladar N., Rat M., Srđenović M. Phytochemical profile and biological activities of the genus Ornithogalum L. (Hyacinthaceae) Biologica Serbica. 2014;36(1–2):3–17. [Google Scholar]
- Pohl R.W. Contact dermatitis from the juice of Ornithogalum caudatum. Toxicon. 1965;3(2):167–168. doi: 10.1016/0041-0101(65)90011-5. [DOI] [PubMed] [Google Scholar]
- Renard D., Rubli E., Voide N., Borruat F.X., Rothuizen L.E. Spectrum of digoxin-induced ocular toxicity: a case report and literature review. BMC Res. Notes. 2015;8:368. doi: 10.1186/s13104-015-1367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Li N., Duan J.A., Tao W. Structure, bioactivity, and chemical synthesis of OSW-1 and other steroidal glycosides in the genus Ornithogalum. Chem. Rev. 2013;113:5480–5514. doi: 10.1021/cr300072s. [DOI] [PubMed] [Google Scholar]
- Vogelsang A. Clinical trial of Ornithogalum umbellatum on the human heart; preliminary report. Can. Med. Assoc. J. 1955;73(4):295–296. [PMC free article] [PubMed] [Google Scholar]
- Waud R.A. The action of Ornithogalum umbellatum on the heart. J. Pharmacol. Exp. Ther. 1954;111(2):147–151. [PubMed] [Google Scholar]
- Wink M., Van Wyk B.E. Timber press; London: 2008. Handbook Mind-Altering and Poisonous Plants of the World; p. 178. [Google Scholar]