Abstract
In Portugal, the potent paralytic shellfish toxins (PSTs) have appeared irregularly since the onset of a national monitoring program for marine biotoxins in 1986. In years where high contamination levels were attained in bivalves, sporadic cases of human poisonings have been recorded, as in 1994 and 2007. The reappearance of high contamination levels led to the appearance of new cases during the autumn of 2018. This study details toxin ingestion, symptomatology and toxin elimination and metabolization in the fluids of two patients, who ingested mussels from the Portuguese southwest coast and required hospitalization due to the severity of symptoms. Toxin elimination was confirmed by ELISA in plasma and urine samples. In mussel samples, the toxin profile obtained by HPLC-FLD displayed a wide diversity of toxins, typical of Gymnodinum catenatum ingestion. However, in the urine samples, the toxin profile was reduced to B1 and dcSTX. Abundant compounds in mussels having an O-sulphate at C11, such as C1+2 and dcGTX2+3, were absent in urine. In plasma, PSTs were not detected by HPLC-FLD. Calculated toxin ingestion, resulting from consumption of an estimated 200-g portion, was in the range of 104–120 μg STX eq./kg b. w.
Keywords: Paralytic shellfish poisoning, Saxitoxin, Seafood poisoning, Human samples, HPLC, ELISA, Portugal
Graphical abstract

Highlights
-
•
Since 1986 Gymnodinium catenatum contaminates irregularly bivalves in Portugal with PSTs.
-
•
Acute neurological symptomology, such as paraesthesias, has often required hospitalization.
-
•
During an episode in October 2018, toxins in fluids of two victims and toxin ingestion were studied.
-
•
Preliminary confirmation was done by ELISA in serum and urine.
-
•
PSP toxins with an O-sulphate at C11, abundant in mussels, were absent in urine.
1. Introduction
A few species of marine microalgae can produce toxins that enter the marine food web. In some occasions, seafood contamination can provoke acute syndromes in human consumers, mainly through vectors as fish or shellfish. The most reputed syndrome originating from bivalve molluscs is paralytic shellfish poisoning (PSP), mainly due to its distinct neurological symptomatology and fatal outcome. Symptoms of human PSP intoxication vary from a slight tingling or numbness to complete respiratory paralysis. In fatal cases, respiratory paralysis occurs within 2–12 h of consumption of the PSP contaminated food (FAO, 2004). The pharmacological action of PSP toxins is via blockade of the voltage-gated sodium channel in a selective manner and with high affinity (FAO, 2004).
Harmful algal blooms (HABs) of genus Alexandrium, and the species Pyrodinium bahamense and Gymnodinum catenatum are responsible for producing and contaminating seafood resources with paralytic shellfish poisoning toxins (PSTs) in several discrete world regions (Bolch and de Salas, 2007; Orr et al., 2013). PSTs comprise saxitoxin (STX) and several of its analogues (Vale, 2010).
Worldwide ongoing national monitoring programmes and international trade codes, such as the Codex Alimentarius Standard for Live and Raw Bivalve Molluscs (FAO, 2015), include provisions for marine biotoxins in order to minimize the risk of human poisonings. However, even after implementation of national monitoring programmes, occasional human outbreaks still take place worldwide. Sometimes these are due to the vastness and complexity of some coastlines, which prevent an effective monitoring coverage, such as in the case of the Patagonian Chilean fjords (García et al., 2004) or the Alaskan coast (Knaack et al., 2016). In others, this occurs due to recreational harvest practices, which intentionally or unintentionally do not comply with the harvest bans in force (Rodrigues et al., 2012, Turnbull et al., 2013).
In the West Iberian coast (Portugal and Spain), several registered PSP outbreaks have taken place in the past: a) 1946 and 1955 in Óbidos (Correia, 1946, Pinto and Silva, 1956); b) 1976 in Galicia (Lüthy, 1979), c) 1994 in Ericeira (Carvalho et al., 1998) and d) 2007 in Óbidos (Rodrigues et al., 2012). The first ones (a and b) were due to the lack of suitable monitoring programmes at the time. Except for the first Óbidos outbreaks, the remaining outbreaks (1976–2007) were due to contamination originated by the microalgae Gymnodinum catenatum (Estrada et al., 1984, Sampayo et al., 1997, Pazos et al., 2006).
In Portugal, after the 2007 outbreak, severe contamination with PSTs occurred again in 2008 and 2009, followed by the absence or weak short-lived contamination episodes during the following years (Vale, 2013, Rodrigues et al., 2017). In early autumn 2018, contamination with PSTs increased in the centre and southwest of the Portuguese coastline, and a very sharp increase in just a couple of weeks was observed in some cases (IPMA, 2019). The high toxin levels attained in some commercial bivalve species from the Lisbon and Setubal coasts, originated prolonged harvest bans which lasted until December 2018 or later, such as the bans applied to clams (Callista chione, Donax spp.) or blue mussels (Mytilus spp.) (IPMA, 2019).
Despite the harvest bans in force, recreational harvest originated a few suspected human poisonings during October 2018, attributed to blue mussels collected south of Lisbon, at Caparica's beach. The present study report two human cases where it was possible to detect the presence of toxins and confirm the diagnosis of PSP.
2. Materials & methods
2.1. Sample collection
On October 11th, two patients that were hospitalized at Garcia da Orta Hospital in Almada (south of Lisbon, Fig. 1), were suspected of presenting the PSP syndrome. The two patients provided urine and blood specimens for detection of STX within 48 h of shellfish consumption, and during the next seven days after the hospitalization. All blood samples were centrifuged to obtain the plasma. The urine and plasma samples from the two patients did not have any previous preparation for ELISA tests.
Fig. 1.
Location of main Portuguese production areas affected by PSP contamination during October–November 2018. Red areas were more severely affected than yellow areas (geographical details available in http://www.ipma.pt/pt/bivalves/index.jsp). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
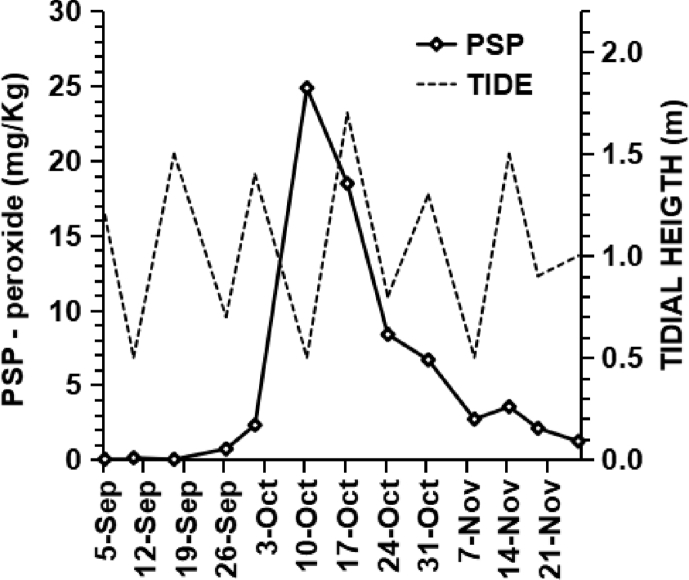
During 2018, blue mussels were collected weekly at Caparica's beach (L5b production area) by hand picking during low tide, as part of the ongoing national monitoring programme for bivalve molluscs (SNMB; 2019). At the time of the event, samples had been collected on September 26, October 1 and 10, respectively (Fig. 2).
Fig. 2.
PSP levels increased sharply over a short two-week period in Caparica's mussels. The poisoning outbreak coincided with extremely low-tide levels: 0.6 m or below after sun rise, lasting from 8 until 11 October.
2.2. ELISA assays
The saxitoxin test used for ELISA is a competitive enzyme immunoassay for the screening and quantitative analysis of saxitoxin in various matrices. This test is based on rabbit polyclonal antibodies against saxitoxin (Europroxima, The Netherlands).
All reagents of the ELISA kits were brought to room temperature before starting the procedure. The assay procedure was done as described by Campbell et al. (2009). Antibody, conjugate, standards and the samples were pipetted into the wells and incubated for 30 min at 20 °C–25 °C. After a washing procedure ready-to-use substrate was added and incubated for 15 min at 20 °C–25 °C. The reaction was stopped and the absorbance read in a spectrophotometer at 450 nm. The urine samples collected from 11th until 18th of October and the plasma sample from the first day from both patients were tested. All these samples were tested in duplicate.
2.3. Extraction and clean-up procedure for shellfish samples
Shellfish samples were opened, soft tissues were removed from the shell, rinsed with water and drained. The extraction and cleanup were performed according to HPLC pre-column oxidation reference method for PSTs following the AOAC Official Method 2005.06 (Anon, 2005, Anonymous, 2017). Briefly, 5 g of shellfish homogenate were double extracted with 3 mL 1% acetic acid (first extraction with heating) and adjusted to 10 mL. 1 mL of this extract was cleaned in a 3 mL SPE C18 cartridge (Supelclean Supelco, USA). In this C18 fraction, peroxide reaction was performed to determine dcGTX2+3, C1+2, dcSTX, GTX2+3, B1 and STX.
A second column purification and partitioning was performed with a 3 mL SPE carboxylic acid (COOH) ion–exchange cartridge (Bakerbond, J.T. Baker, USA) for determination of N-1-hydroxylated toxins (C3+4, GTX6, dcNEO). These toxins could only be detected using periodate oxidation (Lawrence et al., 2005).
2.4. Extraction and cleanup procedures for human samples
Urine and plasma samples collected from the first and fourth day of hospitalization (October 11th and 14th, respectively) were chosen for chromatographic analysis. 10 mL of urine were extracted with 50 μL glacial acetic acid, mixed by vortexing and centrifuged at 4000 rpm for 10 min. 2.0 mL supernatant were loaded into an SPE C18 cartridge previously conditioned with 10 mL methanol followed by 10 mL of water. The eluate was collected with 2.0 mL water and the pH was adjusted to 6.2 with 0.2 M NaOH. Further purification was achieved on an SPE carboxylic acid (COOH) ion–exchange. After pre-conditioning with 0.01 M ammonium acetate, 4.0 mL were loaded, washed with 4.0 mL water and eluted with 5.0 mL 2.0 M NaCl.
For the plasma samples, 5 mL were extracted with 25 μL glacial acetic acid, mixed by vortexing and centrifuged at 4000 rpm for 10 min. 1.0 mL supernatant was loaded into SPE C18 cartridges previously pre-conditioned, and the eluate collected with 2.0 mL water, the pH adjusted to 6.2 with 0.2 M NaOH, and volume adjusted to 4.0 mL.
2.5. HPLC-FLD analysis
Oxidation products from PSP toxins were obtained as described in Lawrence et al. (2005). The HPLC analysis were carried out using an Agilent system comprising: a quaternary pump with built-in degasser and a refrigerated autosampler (1290 Infinity series); a column oven and a fluorescence detector (1260 Infinity series). The software OpenLAB (Rev. C) performed data acquisition and peak integration.
Separation was performed on a Supelcosil LC-18, 150 × 4.6 mm, 5 μm column (Supelco, USA) equipped with a C18 guard column (SecurityGuard™, 4 × 3 mm, Luna, Phenomenex, USA). Mobile phase A was made of 0.1 M ammonium formate, pH = 6 and mobile phase B was made of 0.1 M ammonium formate in 5% acetonitrile, pH = 6. The flow rate was 1 mL/min at 100% A, with the following gradient during run: 5% B at 0.25 min, 10% B at 4.0 min, 90% B at 9.0 min, 10% B at 11 min, 0% B at 13.0 min. Run time was 13 min, with 1.5 min post-run. For GC toxins, gradient was prolonged after 9.0 min up to 60% B and 40% acetonitrile at 18.0 min.
The column was maintained at 30 °C, and 40 μL were injected for peroxide reactions or 80 μL for periodate reactions. Detection wavelengths were set at 340 nm for excitation, 395 nm for quantitation and 430 nm for confirmation. Toxins were identified by comparison of retention times with standards and wavelength ratios.
For toxin quantification in mussels, several matrix-matched calibration curves were prepared in oyster extracts (C18 or COOH) using NRC's Certified Reference Materials: CRM-C1&2, CRM-dcGTX2&3, CRM-dcSTX, CRM-dcNEO, CRM-GTX2&3, CRM-GTX5, CRM-GTX6 and CRM-STX. For toxin quantification in human samples, a matrix-matched calibration curve was prepared in patient's A C18 urine extract containing CRM-C1&2, dcGTX2&3, CRM-dcSTX, CRM-GTX2&3, CRM-GTX5 and NRC CRM-STX.
Individual toxin levels were converted into equivalents of saxitoxin using the toxicity equivalency factors (TEFs) of STX-group toxins proposed by the EFSA panel (EFSA, 2009). The EU Reference Laboratory adopted this approach for Marine Biotoxins who recommends it to be used routinely by monitoring laboratories.
3. Results
3.1. Description of the case
Throughout September 2018, the proliferation of Gymnodinium catenatum contaminated bivalves above the regulatory levels mainly between Peniche on the west coast to Sagres in the southwest coast (Fig. 1). The most severely affected places and species were in production areas L5b (Caparica coast) and L6 (Setúbal coast) with toxins levels in several commercial bivalves repeatedly surpassing three times the regulatory limit in force of 0.8 mg STXequiv./Kg during October and November 2018 (IPMA, 2019).
In the contaminated production areas, it is possible to gather manually intertidal blue mussels and donax clams on certain beaches. Other infralittoral and circumlittoral bivalves require dredge, mainly in L5b, L6 and LAL (Albufeira's lagoon). In several species contamination levels increased rapidly between the last week of September and the second week of October (Fig. 2; data not shown). On October 2, the maximal cell concentration of Gymnodinium catenatum at L5b of 18 × 103 cells/L was attained (IPMA, 2019). On October 10, total PSTs attained 38 mg STXequiv./Kg in Caparica's blue mussels and 32 mg STXequiv./Kg in donax clams. These levels were 47 and 40 times, respectively, above the regulatory limit in force.
The second week of October coincided with extreme low tide levels between October 8th and 11th due to the new moon on October 9th (Fig. 2; de Lisboa, 2018). Low tide levels allow easier hand-gathering of larger bivalve specimens. Although extremely toxic, intertidal donax clams present a reduced food poisoning risk in relation to blue mussels, due to their smaller size: average body size of 1.0 g and 2.9 g, respectively. Exceptionally, during new moons, picking only the biggest mussels, an average individual size of 5.1 g can be attained (Fig. S1).
Blue mussels collected on October 9th originated severe neurological symptoms in two consumers. These were later admitted into the Garcia da Orta Hospital on October 11th: one was a 68-year male patient (patient A), and the other a 62-year female patient (patient B). The two patients reported acute pain of general malaise, dizziness, paraesthesias in the perioral region and hands, generalized myalgias, alteration of verbal articulation and a generalized decrease of muscle strength, with gait difficult, some hours after mussel consumption.
The association between the recent consumption of toxic shellfish (as confirmed by IPMA's ongoing monitoring programme) and the acute neurological symptomatology, led to suspect of paralytic shellfish poisoning syndrome. The hospitalization took place between 11 and 26 October and both had 5 days in the intermediate care of the intensive care unit, with the remainder being admitted to the neurology service, without the need for invasive ventilation. There was complete remission of signs and symptoms and patients were discharged on October 26th.
3.2. ELISA assays on human samples
The Emergency Response and Biopreparedness Unit (UREB) of the National Institute of Health Dr. Ricardo Jorge (INSA) is the reference laboratory for response to biological events. The UREB has available rapid diagnostic methods for microorganisms and biological toxins, which can be used as bioterrorism agents, e.g. saxitoxin. At this unit, the ELISA for diagnosis of PSP is one of the techniques available and was used to confirm the clinical diagnosis of these patients.
In patient A, in October 11th, the plasma and urine samples had concentrations of STX of 4.7 ng/mL and 7.42 ng/mL respectively. In patient B, the concentrations of STX were 4.41 in plasma and 4.47 ng/mL in urine in the same day (Fig. 3). After day 1, we could observe the decreasing of the STX in both patients. The small variations in concentration were possibly due to the error inherent in the execution of the technique.
Fig. 3.
Data of the urine samples collected from the two positive patients during seven days and data of plasma samples collected on the first day of hospitalization by a competitive enzyme immunoassay for quantitative analysis of saxitoxin (ELISA). a) Patient A and b) Patient B.
3.3. Toxin confirmation by HPLC-FLD
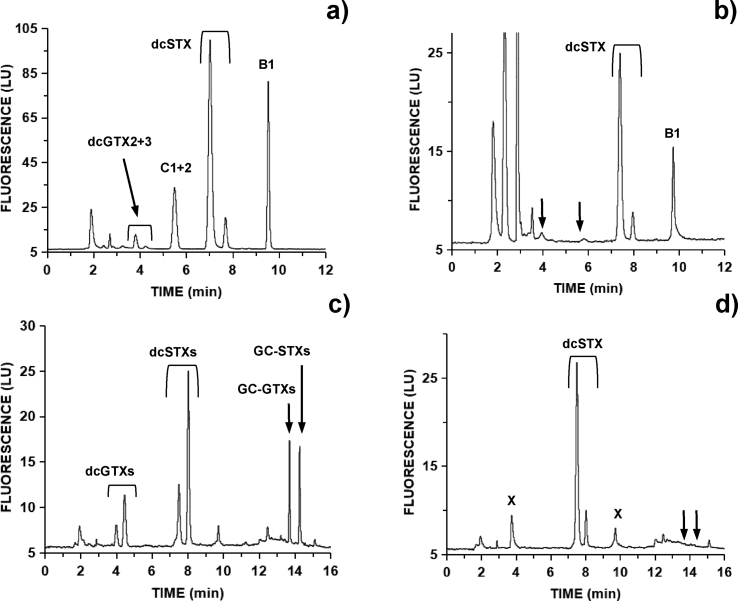
Toxin profiles in mussels contaminated by G. catenatum contained several N-sulfocarbamoyl and decarbamoyl toxins. PSP toxins present high fluorescence response after peroxide oxidation. This reaction allows determination only of N-1-H containing toxins. After peroxide oxidation, mussels collected on October 10 contained dcGTX2+3, C1+2, dcSTX and B1 (Fig. 4a).
Fig. 4.
HPLC-FLD chromatograms of PSP toxins using pre-column peroxide oxidation from: a) aqueous SPE fraction of blue mussel from Caparica on 2018-10-10; b) urine sample from patient A on 2018-10-11; c) methanolic SPE fraction of same blue mussel with modified gradient separation; d) methanolic fraction after hot alkaline hydrolysis. X denotes interfering compounds; arrows denote compounds altered biologically (b) or chemically (d).
Either patient A and B urine samples from October 11 or 14 were dominated only by dcSTX and B1 toxins (Fig. 4b; Table 1). In blood samples from day one, no toxins were detected probably due to the prolonged time these samples were kept refrigerated but not frozen, before the HPLC analysis. In plasma samples from October 14, also only dcSTX and B1 toxins were found (Table 1).
Table 1.
Toxin concentration (ng STXequiv./mL) in fluids collected one and four days after ingestion. LC data was presented discriminated by toxin found and total toxin corrected by using the antibody cross-reactivity. ELISA data was calculated directly from the STX calibration curve. * overestimation due to interfering compounds overlapping toxin peak; nd = not detected; np = not performed.
| DATE |
Oct-11 |
Oct-14 |
||||||
|---|---|---|---|---|---|---|---|---|
| Toxin | dcSTX | B1 | LC-total | ELISA | dcSTX | B1 | LC-total | ELISA |
| Patient A | ||||||||
| Urine | 41.5 | 12.7 | 11.2 | 7.4 | 10.7 | 5.5 | 3.5 | 1.9 |
| Plasma | nd | nd | nd | 4.7 | 9.6 | 4.3 | 3.0 | np |
| Patient B | ||||||||
| Urine | 10.4 | 24.1* | 8.2 | 4.5 | 5.0 | 5.7* | 2.4 | 1.7 |
| plasma | nd | nd | nd | 4.4 | 23.5 | 8.1 | 6.6 | np |
Fluorimetric confirmation of toxins was determined for patient A on Oct 11 resorting to dual wavelength detection (Fig. S2a). Wavelength ratios determined by peak height matched those of individual toxins standards for dcSTX and B1 used in routine monitoring analysis (Table 2). These compounds were not auto-fluorescent peaks, as determined by the null oxidation step (Fig. S2b). In the case of B1, the baseline was contaminated with interfering compounds, in particular in the case of patient B (Fig. S3a). Further purification on a COOH cartridge allowed improved confirmation for the presence of B1 toxin (Fig. S3b).
Table 2.
Toxin emission wavelength ratios at 395/430 nm as measured by peak height. Monitoring calibration standards of dcSTX and B1 were spiked in toxin-free oyster C18 extract at 0.26 and 0.51 μM, respectively. Except for cleaned samples, values represent X ± SD from four separate oxidation rounds.
| Toxin | dcSTX | B1 |
|---|---|---|
| Standard in oyster | 2.87 ± 0.02 | 2.09 ± 0.01 |
| Patient A urine (Oct-11) | 2.87 ± 0.02 | 2.07 ± 0.01 |
| Patient B urine cleaned (Oct-14) | * | 2.07 |
| Patient B plasma cleaned (Oct-14) | * | 2.07 |
Oxidation with periodate allowed determination of N-1-OH containing toxins. Mussels contaminated by G. catenatum contained several N1–OH toxins: C3+4, B2 and dcNEO. In human samples, evidence for the presence of N1–OH toxins was scarce due to the low toxin levels present. Toxin products after periodate oxidation from patient A one day after ingestion were mostly accounted by the single presence of dcSTX, not rendering evidence for simultaneous contamination with dcNEO (oxidation product 1) or B2 (oxidation product 2) (Fig. 5).
Fig. 5.
HPLC-FLD chromatograms after periodate oxidation from: a) overlaid toxins standards of dcNEO (0.37 μM) and dcSTX (0.70 μM), both producing two oxidation products coded ‘1’ and ‘2’; b) urine sample from patient A on 2018-10-11 after C18 cleanup dominated by dcSTX oxidation products.
Summarising, profiles in the human samples contained a diversity of toxins narrower than present in blue mussels, due to loss of the C-11-ortho-sulphate group or the N-1-OH (Fig. 6).
Fig. 6.
Molar profiles of PSP toxins in a) blue mussel on 10-October; b) patient A urine on 11-October. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Toxin levels determined by HPLC were higher than those found with ELISA in the same human fluids (Table 1). Individual toxins determined by LC were then multiplied by the respective cross-reactivity factors reported by the kit manufacturer, and a total toxin equivalent was calculated. This corrected LC levels approached then those found by ELISA (Table 1). Due to the dominance of dcSTX, the TEF of 0.5 determined by Oshima (1995) and previous authors was used instead of the precautionary TEF of 1.0 proposed by EFSA (2009).
3.4. Calculation of toxin ingestion
Besides the common STX analogues determined by the Lawrence method, other analogues are produced by G. catenatum, namely the hydroxybenzoate analogues or GC toxins.
These were found only in trace levels in Caparica's mussels (Fig. 4c). Due to the absence of analytical standards, this trace contamination was chemically converted into decarbamoyl analogues (dcSTX) to search for its potential role in cryptic toxicity (Fig. 4d). A minor 2.1% increase in toxicity was obtained: from 38.1 to 38.9 mg STXequiv./Kg. In the case of donax clams harvested at L5b, a 4.6% increase was obtained: from 31.7 to 33.1 mg STXequiv./Kg.
By description from the patients, circa 2 kg of mussels were cooked by the victims. Splitting between both of them would provide ingestion of circa a 200 g flesh portion for each one, which would supply an equivalent of 7.8 mg of saxitoxin equivalents. For individuals between 65 and 75 Kg bodyweight, this translates into ingestion of 104–120 μg STX eq./kg b. w.
4. Discussion
Human exposure to PSP toxins can occur through a variety of mechanisms including consumption of contaminated shellfish, intentional poisoning, or as a part of medical treatment (Mons et al., 1998). It is important to rapidly identify exposures to PSTs for appropriate management of patients. Identification of exposures based on symptoms alone can be complicated by non-specific symptoms of PSP such as nausea and vomiting (FAO, 2004), though dysphagia and dysarthria have been identified as more specific indicators of potential exposure (NSSP, 2015).
The diagnosis of PSP based only on the recent consumption of shellfish and the development of clinical manifestations is difficult and can be inaccurate because these findings are nonspecific and similar to other diseases. In such cases, laboratory diagnosis using human samples can provide crucial information. Enzyme-linked immunosorbent assays (ELISA) are a preferred screening tools because these assays can be performed rapidly, are accurate and sensitive. Findings indicate that the detection of STX in urine can confirm the diagnosis of STX-PSP in patients with suspected PSP. PSTs levels in urine were within the low range found in previous studies (Knaack et al., 2016), mostly due to the delay (48 h) in collecting the samples.
From the most recent model developed by Arnich and Thébault (2018), the critical minimal dose with a probability higher than 10% of showing symptoms is 0.88 μg STX eq./kg b. w. This means that 10% of the individuals exposed to this dose would have symptoms (without consideration for the severity of the symptoms). An acute dose around 102 μg STX eq./kg b. w will translate in a 50% chance of severe symptoms and both a 20% chance of either mild or moderate symptoms (Arnich and Thébault, 2018).
The patients might have ingested doses between 104 and 120 μg STX eq./kg b. w that could translate into a moderate to severe intoxication scenario, or levels 2–3 in a 4-level scale. Moderate symptoms include incoherent speech, general weakness, slight respiratory difficulty, and rapid pulse; while severe symptoms include muscular paralysis and pronounced respiratory difficulty (EFSA, 2009).
For attaining an average consumption of 100 g of meat would require ingestion of only 20 large mussels (when caught during full/new moon tides), 34 median mussels (when caught during intermediate tides), while for donax clams 99 individuals would have been required. Given free accessibility of these two intertidal bivalves, donax clams are more cumbersome to catch than mussels (the artisanal way is by manual sand digging, assisted sometimes by a hand-dredge) and also demand a prolonged leisure time to attain consumption of a large portion size. Although donax clams are then less likely to originate severe human poisoning outbreaks than blue mussels, unreported or misdiagnosed cases could have occurred on that particular week of October 2018. Even a modest 20-g consumption (or circa 20 individuals) could have supplied 9–10 μg STX eq./kg b. w. This is enough for a 30% chance of a level 1 (mild symptoms) scenario, according to Arnich and Thébault (2018). Mild symptoms include tingling sensation or numbness around the lips gradually spreading to the face and neck, a prickly sensation in fingertips and toes, headache, dizziness, and nausea. (EFSA, 2009).
High consumption of 200 g was more easily attained in the present case report because larger wild mussels can be hand-picked during extremely low tides. This pernicious coincidence, favouring high levels of marine biotoxin's ingestion, has been observed before for traditional hand-picking of mussels by a population during the common ‘live’ tides occurring at the end of summer. In September 2002, more than a dozen people were admitted into a hospital with diarrhoea symptoms at the Portuguese northwest coast, a time-window that was coincidental with both high diarrhetic shellfish poisoning toxin levels and very low full moon tides (Vale et al., 2003).
Different microalgae species produce different STX analogues worldwide. G. catenatum strains isolated from Portugal present dominance of N-sulfocarbamoyl toxins (C1+2, C3+4, B1 and B2) (Silva et al., 2015). In bivalves, a higher proportion of decarbamoyl toxins is additionally present. These can result from biotransformation of N-sulfocarbamoyl toxins (Artigas et al., 2007), as well as from cleavage of hydroxybenzoate (GC) toxins (Vale, 2008). The measurement of cryptic toxicity posed by hydroxybenzoate (GC) toxins in extremely contaminated samples involved in human poisonings was performed here for the first time. These added only a small contribution to the total toxin burden in Caparica's mussels. Preliminary research on GC toxins showed that although these were very abundant in microalgae, only trace levels were present on several commercial bivalve species (Vale, 2008).
In human samples, such as serum and urine, a wide diversity of toxins has been found when collection times were very short, such as 6 h or less post-ingestion (Gessner et al., 1997). Elimination rates are toxin dependent, and in the case of the 11-O-sulphated congeners GTX1/4 and GTX2/3, these displayed shorter half-lives compared to NEO and STX (DeGrasse et al., 2014). This differential elimination will reduce the diversity of toxins than can be detectable by HPLC when collection times extends past 24 h. In the present case, C1+2 and dcGTX2+3 were not detectable, and only their respective non-11-O-sulphated congeners (B1 and dcSTX) remained at detectable levels.
This case took place in October just south of Lisbon. Historical PSP maxima in shellfish from the Portuguese coast have been observed recurrently in autumn since 1986, with the highest toxic levels being registered in October–November (Vale et al., 2008, Vale, 2013). All past PSP events requiring hospitalization have also been attributed to shellfish collected in October-November (Carvalho et al., 1998, Rodrigues et al., 2012). The respective collection sites were located between Carvoeira and Espichel Capes, were Caparica is also located.
PSP outbreaks studied previously have been mostly attributed to ingestion of mussels, and less often to other bivalves (Carvalho et al., 1998, Rodrigues et al., 2012). Mussel is more prone to accumulate toxins for long periods and thus attaining higher contamination levels, than for example cockles (Artigas et al., 2007, Vale et al., 2008, Vale, 2011), representing a very high-risk transfer vector for humans. The toxic level found in mussel was 47 times the regulatory limit (RL), which was of similar magnitude to the levels reported in 1994 resorting to the mouse bioassay: 50 times the RL (Carvalho et al., 1998).
For several decades, the occurrence of high contamination levels with PSP biotoxins in bivalves of the Iberian Atlantic coast has occurred in decadal-like intervals. These years of very high toxicity have coincided with years in which solar activity (derived from the 11-year sunspot cycle) was at its minimum (Vale, 2013, Vale, 2019). The large time lags of the phenomena, as well as rotation of hospital emergency and/or internal teams along the years, lead clinicians to oblivion the knowledge about this syndrome. This study alerts to the need of continuously maintaining costly monitoring programmes, adequate surveillance of shellfish collection areas, and the importance of physicians to be aware of this human syndrome with only rare occurrence in Portugal.
Ethical statement
This study was carried out in accordance with the recommendations of the guidelines of the Helsinki Committee.
Informed consent was obtained from the patients for publication of this case report by the Local Health Authority. The case report was also approved by the National Institute of Health Dr. Ricardo Jorge's Ethical Committee.
Privacy of patients was respected at all times.
Author contributions
ILC and PV designed the study and analysed the results; IR performed the HPLC analysis; AP and RC performed the ELISA tests; MSN made the first approach to the case.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Appreciation is due to Dr. Miguel Grunho, from Garcia da Orta Hospital, neurology service, for informing about the cases and sample collection. We would like also to acknowledge Dr. João Vieira Martins from the local Health authority for the support in the collection of information details of the patients. Appreciation is due to the National Bivalve Monitoring System (SNMB) teams involved in year-round field sampling and laboratory analysis. Funding was obtained from INSA and from IPMA’s ‘SNMB-MONITOR’ (FEAMP–2020) projects. A grant to IR from ‘SNMB-MONITOR’ (FEAMP–2020) is acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2019.100017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anon . AOAC International; Gaithersburg, MD, USA: 2005. AOAC Official Method 2005.06 Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection. [PubMed] [Google Scholar]
- Anonymous Commission Regulation (EU) 2017/1980 of 31 October 2017 amending Annex III to Regulation (EC) No 2074/2005 as regards paralytic shellfish poison (PSP) detection method. Off.J.Eur.Union. 2017;L285:8–9. [Google Scholar]
- Arnich N., Thébault A. Dose-response modelling of paralytic shellfish poisoning (PSP) in humans. Toxins. 2018;10:141. doi: 10.3390/toxins10040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas M.L., Vale P., Gomes S.S., Botelho M.J., Rodrigues S.M., Amorim A. Profiles of PSP toxins in shellfish from Portugal explained by carbamoylase activity. J. Chromatogr. A. 2007;1160(1–2):99–105. doi: 10.1016/j.chroma.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Bolch C.J.S., de Salas M.F. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia. Harmful Algae. 2007;6:465–485. [Google Scholar]
- Campbell K., Huet A.C., Charlier C., Higgins C., Delahaut P., Elliott C.T. Comparison of ELISA and SPR biosensor technology for the detection of paralytic shellfish poisoning toxins. J. Chromatogr. B. 2009;877(32):4079–4089. doi: 10.1016/j.jchromb.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Carvalho M., Jacinto J., Ramos N. Paralytic shellfish poisoning: clinical and electrophysiological observations. J. Neurol. 1998;245(8):551–554. doi: 10.1007/s004150050241. [DOI] [PubMed] [Google Scholar]
- Correia F.S. Um caso raro de intoxicação alimentar colectiva. Bol. Inst. Sup. Higiene Dr. Ricardo Jorge. 1946;3:216–221. [Google Scholar]
- de Lisboa Porto. 2018. http://www.portodelisboa.pt/portal/page/portal/PORTAL_PORTO_LISBOA/HIDROGRAFIA/TABELA_MARES
- DeGrasse Stacey, Rivera Victor, Roach John, White Kevin, Callahan John, Couture Darcie, Simone Karen, Peredy Tamas, Poli Mark. Paralytic shellfish toxins in clinical matrices: extension of AOAC official method 2005.06 to human urine and serum and application to a 2007 case study in Maine. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014;103:368–375. [Google Scholar]
- EFSA Scientific opinion of the panel on contaminants in the food chain on a request from the European commission on marine biotoxins in shellfish – saxitoxin group. EFSA.J. 2009;1019:1–76. [Google Scholar]
- Estrada M., Sánchez F.J., Fraga S. Gymnodinium catenatum (Graham) en las rias gallegas (NO de España) Investig. Pesq. 1984;48(1):31–40. [Google Scholar]
- FAO . Food and Agriculture Organization of the United Nations; Rome: 2004. Marine Biotoxins, FAO Food and Nutrition Paper, 80; p. 278. [Google Scholar]
- FAO . 292–2008. 2015. Codex Alimentarius: standard for live and raw bivalve molluscs, CODEX STAN; p. 9. [Google Scholar]
- García C., Carmen Bravo M., del Lagos M., Lagos N. Paralytic shellfish poisoning: post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon. 2004;43:149–158. doi: 10.1016/j.toxicon.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Gessner B.D., Bell P., Doucette G.J., Moczydlowski E., Poli M.A., Van Dolah F., Hall S. Hypertension and identification of toxin in human urine and serum following a cluster of mussel-associated paralytic shellfish poisoning outbreaks. Toxicon. 1997;35(5):711–722. doi: 10.1016/s0041-0101(96)00154-7. [DOI] [PubMed] [Google Scholar]
- IPMA 2019. http://www.ipma.pt/pt/bivalves/biotox/index.jsp
- Knaack J.S., Porter K.A., Jacob J.T. Case diagnosis and characterization of suspected paralytic shellfish poisoning in Alaska. Harmful Algae. 2016;57(B):45–50. doi: 10.1016/j.hal.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Lawrence J.F., Niedzwiadek B., Menard C. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: collaborative study. J. AOAC Int. 2005;88:1714–1732. [PubMed] [Google Scholar]
- Lüthy J. Epidemic paralytic shellfish poisoning in western Europe, 1976. In: Taylor D.L., Seliger H.H., editors. Toxic Dinoflagellate Blooms. Elsevier; Amsterdam: 1979. pp. 15–22. [Google Scholar]
- Mons M.N., van Egmond H.P., Speijers G.J.A. The Netherlands: National Institute of Public Health and the Environment; Bithloven: 1998. Paralytic Shellfish Poisoning, a Review; p. 47. [Google Scholar]
- NSSP . US Department of Health and Human Services, Public Health service; 2015. National Shellfish Sanitation Program (NSSP): Guide for the Control of Molluscan Shellfish, 2015 Revision. Silver Spring, MD. (Food and Drug Administration) [Google Scholar]
- Orr R.J.S., Stüken A., Murray S.A., Kjetill J.S. Evolution and distribution of saxitoxin biosynthesis in dinoflagellates. Mar. Drugs. 2013;11(8):2814–2828. doi: 10.3390/md11082814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y. Postcolumn derivatization liquid-chromatographic method for paralytic shellfish toxins. J. AOAC Int. 1995;78(2):528–532. [Google Scholar]
- Pazos Y., Moroño A., Triñanes J., Doval M., Montero P., Vilarinho M.G., Moita M.T. 12th International Conference on Harmful Algae. Programme and Abstracts; Copenhagen, Denmark: 2006. Early detection and intensive monitoring during an unusual toxic bloom of Gymnodinium catenatum advected into the Galician Rías (NW Spain) p. 259. [Google Scholar]
- Pinto J.S., e Silva E.S. The toxicity of Cardium edule L. and its possible relation to the dinoflagellate Prorocentrum micans Ehr. Notas e Estudos Inst.Biol.Marít. 1956;12:21. [Google Scholar]
- Rodrigues S.M., de Carvalho M., Mestre T., Ferreira J.F., Coelho M., Peralta R., Vale P. Paralytic shellfish poisoning due to ingestion of Gymnodinium catenatum contaminated cockles – application of the AOAC HPLC Official Method. Toxicon. 2012;59(5):558–566. doi: 10.1016/j.toxicon.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Rodrigues S.M., Botelho M.J., Costa P.R., Vale P., Ribeiro I., Costa S.T. 10a Reunião Anual PortFIR. INSA; Lisboa: 2017. Contaminação de moluscos bivalves com biotoxinas marinhas na costa portuguesa de 2014 a 2016. 27/Oct//2017. [Google Scholar]
- Sampayo M.A.M., Franca S., Sousa I., Alvito P., Vale P., Botelho M.J., Rodrigues S., Vieira A. Dez anos de monitorização de biotoxinas marinhas em Portugal (1986-1996) Arq. Inst. Nacional Saúde. 1997;23:187–194. [Google Scholar]
- Silva T., Caeiro M.F., Costa P.R.C., Amorim A. Gymnodinium catenatum Graham isolated from the Portuguese coast: toxin content and genetic characterization. Harmful Algae. 2015;48:94–104. doi: 10.1016/j.hal.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Turnbull A., Harrison R., McKeown S. Paralytic shellfish poisoning in south Eastern Tasmania. Commun. Dis. Intell. Q. Rep. 2013;37:52–54. doi: 10.33321/cdi.2013.37.5. [DOI] [PubMed] [Google Scholar]
- Vale P. Fate of benzoate paralytic shellfish poisoning toxins from Gymnodinium catenatum in shellfish and fish detected by pre-column oxidation and liquid chromatography with fluorescence detection. J. Chromatogr. A. 2008;1190(1–2):191–197. doi: 10.1016/j.chroma.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vale P. Saxitoxin analogues: developments in toxin chemistry, detection and biotransformation during the 2000's. Phytochem. Rev. 2010;9(4):525–535. [Google Scholar]
- Vale P. Marine biotoxins and blue mussel: one of the most troublesome species during harmful algal blooms. In: McGevin L.E., editor. Mussels: Anatomy, Habitat and Environmental Impact. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2011. pp. 413–428. [Google Scholar]
- Vale P. Can solar/geomagnetic activity restrict the occurrence of some shellfish poisoning outbreaks? The example of PSP caused by Gymnodinium catenatum at the Atlantic Portuguese coast. Biophysics. 2013;58(4):554–567. [PubMed] [Google Scholar]
- Vale P. 26 (3) Medicina Interna; 2019. Intoxicação paralisante por marisco (PSP): uma síndroma rara com recorrência decadal? In press. [Google Scholar]
- Vale P., Maia A.J., Correia A., Rodrigues S.M., Botelho M.J., Casanova G., Silva A., Vilarinho M.G., Silva A.D. An outbreak of Diarrhetic Shellfish Poisoning after ingestion of wild mussels at the northern coast in summer 2002. Electron. J. Environ. Agric. Food Chem. 2003;2(4):449–452. [Google Scholar]
- Vale P., Botelho M.J., Rodrigues S.M., Gomes S.S., Sampayo M.A.M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986-2006): a review of exposure assessment. Harmful Algae. 2008;7(1):11–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.