Abstract
Assessing the efficacy of botulinum neurotoxin (BoNT) in vivo is essential given the growing number of BoNT products used in the clinic. Here, we evaluated the dynamic weight bearing (DWB) test for sensitivity to paralytic effects of BoNT-A following intramuscular administration. The toxin was administered into the gastrocnemius lateralis as a single bolus or into the gastrocnemius lateralis and medialis as two boluses. The effects of BoNT-A in DWB were compared to those in the compound muscle action potential (CMAP) and the Digit Abduction Score (DAS) tests. Female Sprague-Dawley rats received an acute, intramuscular (i.m.) injection of BoNT-A1 (0.1, 1, 10 pg/rat) into the right gastrocnemius muscle, while the left received vehicle. The DWB and CMAP tests were performed one-two days after the injection in order to detect the onset of sub-maximal BoNT-A activity. Both tests were preceded by the DAS test. BoNT-A produced dose-related reductions in both the weight-bearing and surface-bearing outcomes of up to 60% while showing moderate activity in the DAS. BoNT-A effects in the DWB test were well-aligned with those in the CMAP test, which showed dose-dependent reductions in CMAP amplitude and the area under the curve (AUC; up to 100%) as well as increases in latency (up to 130%). The efficacy of BoNT-A in DWB and CMAP was more pronounced with two boluses. Thus, the DWB test can be used to assess the properties of BoNTs following i.m. administration. It can be used to assess the candidate therapies and is more ethical than the mouse lethality assay.
Keywords: Botulinum neurotoxin, In vivo, Rat, Dynamic weight bearing, Digit abduction, Muscle action potential
Highlights
-
•
The dynamic weight bearing test measures activity of botulinum neurotoxin in vivo.
-
•
The weight bearing test can be combined with other in vivo tests.
-
•
Botulinum neurotoxin is more active when injected as two boluses than as one.
1. Introduction
Botulinum neurotoxins (BoNTs) are a diverse group of proteins produced by the gram-positive anaerobic bacterium Clostridium botulinum and related species. The group includes at least seven immunologically distinct BoNT serotypes (termed A to G) and 40 subtypes (Peck et al., 2017). All BoNTs are 150 kDa proteins consisting of a 100 kDa heavy chain (HC) and a 50 kDa light chain (LC) connected by a disulfide bond (Rossetto et al., 2014). BoNT toxicity is typically expressed as persistent muscle paralysis, caused by the interruption of the presynaptic release of acetylcholine at the neuromuscular junction (Rossetto et al., 2014). In order to enter the neurons and exert its action, the BoNT HC binds to dual-protein and ganglioside cell surface receptors, leading to endocytosis (Rummel, 2015). The LC is then translocated into the cell's cytosol where the disulfide bond between the HC and LC is cleaved, releasing the LC (Fischer and Montal, 2007). Once free in the cytosol, the LC cleaves a member of the SNARE protein family, the protein required for the vesicle release, thereby blocking neurotransmission and causing flaccid paralysis (Pantano and Montecucco, 2014).
Currently, BoNT serotype A1 and B1 products are used as a therapeutic tool in a range of neurological conditions characterized by muscle or glandular hyperactivity and pain, such as cervical dystonia, spasticity, blepharospasm, sialorrhea, hyperhidrosis, neurogenic detrusor overactivity, and chronic migraine (Dressler, 2012, Dressler, 2016; Fonfria et al., 2018). Investigating the biological properties of BoNTs in a skeletal muscle in vivo allows better understanding of the mechanisms responsible for the therapeutic effects of current BoNT products, as well as for testing novel therapies based on the recombinant production of modified neurotoxins.
Several in vivo assays are used to measure the local muscle flaccidity-inducing effects of BoNTs. One group of assays performed in conscious animals includes the mouse LD50 bioassay (Schantz and Kautter, 1978); the Digit Abduction Score (DAS; Aoki, 1999, Aoki, 2001, Broide et al., 2013, Pellett et al., 2015; Moritz et al., 2019; Cornet et al., 2020); grip strength (Yoneda et al., 2005; Torii et al., 2011, Torii et al., 2011); wheel or rotarod running (Keller, 2006; Pellett et al., 2015; Kutschenko et al., 2016; Moritz et al., 2019); and the CatWalk assays (Moritz et al., 2019), whereas another group is based on electrophysiological readouts, such as the compound muscle action potential (CMAP) performed in anaesthetized animals (Sakamoto et al., 2009; Torii et al., 2010).
The mouse LD50 test has historically been used to measure BoNT potency (Pearce et al., 1995). However, its clinical relevance and ethics are questionable, as it relies on the intraperitoneal route of administration and assesses the lethality induced by BoNT intoxication. In all other in vivo assays, in alignment with its common therapeutic application, BoNT is administered via the intramuscular (i.m.) route with the aim of characterizing its local muscle flaccidity-inducing effects in terms of potency (ED50) and onset and duration of action. Concomitant assessment of body weight in these assays allows the identification of minimal doses of BoNT that impact body weight gain. A reduction in body weight gain in animals locally injected with BoNT is believed to be indicative of the systemic spread of the toxin and therefore represents an important tolerability readout (Broide et al., 2013).
The DAS assay is based on the reflexive spread of digits in rodents and is used to measure local paralysis after i.m. BoNT administration (Aoki, 1999, Aoki, 2001, Broide et al., 2013, Cornet et al., 2020). In the original design of the DAS assay, BoNT is administered into the gastrocnemius complex of the hind paw of female mice and the degree of digit abduction inhibition is scored on a five-point scale (Aoki, 1999, Aoki, 2001). In a recent rat DAS study, BoNT-A injections localized at either of the two hind limb muscles (the gastrocnemius lateralis or peronei) resulted in a dose-dependent reduction of digit abduction, albeit with differences in potency and distinct body weight activity profile (Cornet et al., 2020). The DAS assay is relatively easy to perform and does not require specialized equipment; however, the subjective scoring carries a risk of experimenter bias. The assay also relies on a narrow five-point scale, which reduces the statistical power.
The grip strength assay aims to measure the local flaccid paralysis after i.m. administration of BoNT and to provide more objective data than the DAS assay. It requires a grip strength meter and is based on the method described previously by Meyer et al. (1979). According to Torii et al. (2011a) a dose-dependent reduction in the grip strength of the injected/ipsilateral paw in animals treated with BoNT serotypes A1 or A2 was indicative of local muscle flaccidity, whereas a reduction in the grip strength of the non-injected/contralateral forelimb was indicative of BoNT diffusion from the site of injection. In a follow-up study, the authors replicated these effects in mice and showed that the axonal transport mechanisms (both retrograde and anterograde) play an important role in mediating the transfer of BoNT from the ipsilateral to the contralateral forelimb (Torii et al., 2011b).
While both the DAS and grip strength assays aim to measure the local muscle flaccidity-inducing effects of BoNT, the wheel running assays evaluate BoNT effects on the whole animal (Pellett et al., 2015). The voluntary wheel running assay is typically performed in mice and requires cages equipped with running wheels (Allen et al., 2001; Turner et al., 2005). The BoNT-A activity profile, expressed as a dose-dependent reduction in daily running distance, follows a pattern also seen in other assays and characterized by rapid onset, peak activity around Day 3, and gradual recovery to baseline (Stone et al., 2011). However, the doses of BoNTs that inhibit running distance by more than 50% also impact body weight gain (Stone et al., 2011). Effects of BoNTs on running activity in the accelerated rotarod follow the same pattern (Pellett et al., 2015). Here, reductions in time spent on the rotarod appear to be related to the systemic spread of the toxin rather than its local effects (Pellett et al., 2015).
The CatWalk system was recently evaluated to measure the local effects of BoNT serotypes A1, A2, A6, and B1 on mouse locomotion and gait (Moritz et al., 2019). The CatWalk system is an advanced, quantitative locomotor analysis tool capable of measuring over 50 static and dynamic parameters related to gait (Koopmans et al., 2007). Unilateral i.m. administration of any of these serotypes into the right gastrocnemius complex produced significant dose-dependent and overall similar changes in several static and dynamic parameters. For example, toxin-injected animals showed reductions in maximum contact area, print width/area and swing speed, which was accompanied by increases in the stand index and swing duration of the injected/ipsilateral hind limb (Moritz et al., 2019). While the non-injected/contralateral limb showed milder changes in those parameters, they were in the opposite direction from those of the ipsilateral limb, indicative of possible functional compensation (Moritz et al., 2019). The lack of muscle flaccidity in the contralateral limb in this study is noteworthy, in the light of the growing evidence that BoNT can diffuse to the contralateral side via axonal transport mechanism (Antonucci et al., 2008; Torii et al., 2011b; Matak et al., 2012; Koizumi et al., 2014; Cai et al., 2017).
The CMAP assay directly measures the inhibition of neuromuscular transmission after i.m. administration of the toxin (Sakamoto et al., 2009; Torii et al., 2010). A dose-dependent reduction in CMAP amplitude in the injected muscle is used to characterize BoNT activity in terms of onset, time to peak, potency, and duration (Sakamoto et al., 2009; Torii et al., 2010). The CMAP assay is one of the most sensitive in vivo assays for the evaluation of BoNT efficacy, even though its functional significance and therapeutic relevance remain unclear.
Here, we investigated the dynamic weight bearing (DWB) test in rats with the aim of obtaining objective and quantitative measures of BoNT activity following i.m. administration. The DWB test belongs to a growing category of rodent gait analysis methods used in rodent models of various neurological conditions (Lakes and Allen, 2016; Moritz et al., 2019). In the DWB test, a freely moving animal is introduced into a chamber equipped with floor sensors, allowing automatic assessment of weight and surface bearing for each hind paw (Tétreault et al., 2011; Robinson et al., 2012; Ängeby Möller et al., 2018). We hypothesized that muscle flaccidity induced by BoNT can be detected as a reduction in weight and/or surface bearing of the toxin-injected limb. We also hypothesized that the effects of BoNT-A in DWB are more pronounced when it is injected as two boluses into two adjacent muscles than into one. Therefore, BoNT-A was administered into the head of the gastrocnemius lateralis either as a single 30 μL bolus or into the heads of the gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses. In order to evaluate the relative sensitivity of DWB to the paralytic effects of BoNTs, animals were also tested in the DAS assay and in the CMAP test. The CMAP offers high sensitivity and specificity, as it measures BoNT activity in a skeletal muscle following i.m. administration (Torii et al., 2010). The method is based on the assessment of amplified micro potentials generated by the contraction of muscle fibers and is performed in anaesthetized animals.
2. Materials and methods
2.1. Ethics
All experimental procedures were approved by the Ethics Committee of Ipsen Innovation (C2EA; registration number 32) and were performed in full compliance with the ARRIVE guidelines, EU Directive 2010/63/EU for animal experiments, and the 2013 French Regulatory Decree. Animals were closely monitored for clinical signs throughout the experiment. The range of BoNT-A doses used in this study were significantly lower comped with those causing early signs of intoxication, such as marked reduction in body weight gain or body weight loss. Also, the injection volumes used in the current study were within the recommended range for i.m. injections in rats (up to 50 μL per injection site; see Turner et al., 2011). We did not observe any signs of pain following the injection or throughout the experiment.
2.2. Animals
Fifty-four adult, female, Sprague-Dawley rats were purchased from Janvier Labs (Saint-Berthevin, France). Animals were housed 3–4 per cage and maintained on a 12 h light/dark cycle (lights on from 07:00 to 19:00 h) under a constant temperature (22 ± 2 °C) and humidity (55 ± 5%) with food and water available ad libitum. Animals were acclimatized for at least 7 days prior to experimentation. Animals weighed 170–210 g on the day of injection and were in free estrous cycle when tested.
2.3. Study design
Animals that received acute i.m. injection of BoNT-A or its vehicle (Gelatin Phosphate Buffer; GPB) were tested in a DWB test 24 h later and in a CMAP test 48 h later. These tests were performed 24 h apart in order to avoid potential impact of DWB testing on CMAP activity and for reasons of practicality. Both tests were preceded by the DAS assay. We performed these tests only at early time-points (24 and 48 h post-injection), as we aimed to investigate the onset of sub-maximal BoNT-A activity using DWB, CMAP and DAS tests. As the later time-points were not included, the kinetics of the BoNT-A-induced paralysis was not characterized here, which is a limitation of the study. The study involved testing two cohorts of 27 animals, injected 2 days apart.
2.4. Botulinum neurotoxin and its administration
Research-grade, purified, native BoNT serotype A1 (BoNT-A; 150 kDa) was purchased from List Biological Laboratories (Campbell, CA, USA). It was received as a lyophilized powder, which was reconstituted in 1 mg/mL phosphate buffer saline/bovine serum albumin (0.1%) to obtain a stock solution (0.1 mg/mL), which was aliquoted and kept frozen at −80 °C. Dilutions of aliquots were performed with 0.2% GPB to obtain the final concentrations (see below).
BoNT-A1 activity was previously confirmed in a cell-free assay using Botulinum Neurotoxin Detection Kit (BoTest®; BioSentinel, Madison, WI, USA; Piazza et al., 2011, Dunning et al., 2012), as well as in a cell-based assay using rat embryonic spinal cord neuronal (eSCN) cultures as described previously (Fonfria et al., 2016, Donald et al., 2018).
In the BoTest, the activity readout was LC potency (EC50; pM), calculated using the fluorescence emission ratio of cleaved (470 nm) and uncleaved (528 nM) BoTest Reporter kit. BoNT-A was tested using an eight-point concentration-response curve: 1250 pM, 250 pM, 75 pM, 25 pM, 10 pM, 4 pM, 0.5 pM, and a control (0 pM). The activity (EC50) of the current batch of BoNT-A1 across seven experiments was calculated as 22.16 ± 2.72 (pM).
In the rat eSCN culture, the activity readout was potency (pEC50) in the SNAP-25 cleavage assay, using a Western blot determination of percentage of cleaved SNAP-25 versus total SNAP-25 (eight-point concentration-response curve). The concentrations of BoNT-A1 used for the curve were 1 nM, 100 pM, 10 pM, 1 pM, 100 fM, 10 fM, 1 fm, and a control (0 fM). In the eSCN assay, BoNT-A activity was used as its own internal standard. Following assay development, acceptance criteria for native BoNT-A1 in the eSCN SNAP-25 assay was set at pEC50 of 12 ± 0.5 log M (pEC50 ~1 pM). The activity (pEC50) of the current batch of BoNT-A1 in the eSCN assay, across six experiments (each performed in triplicate), was calculated as 11.84 ± 0.07 (Donald et al., 2018).
On Day 0 (D0), animals were weighed, pre-screened for a normal digit abduction response (DAS 0), and randomized to obtain the comparable mean body weight in each group. To perform the i.m. BoNT-A injection, animals were anaesthetized with 3% isoflurane-oxygen mixture, placed on a surgical table in a ventral position, and had the distal part of their left and right hind legs shaved. Animals received an i.m. injection of BoNT-A (0.1, 1, or 10 pg/rat) into the right gastrocnemius lateralis muscle, while the left gastrocnemius lateralis received vehicle (GPB). BoNT-A or GPB were administered into the head of the gastrocnemius lateralis muscle as a single bolus (30 μL), or into the heads of the gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses (n = 8/dose/injection; Fig. 1).
Fig. 1.
Schematic representation of BoNT-A injection sites in the gastrocnemius muscle of the rat hind paw. BoNT-A was administered into the head of the gastrocnemius lateralis as a single 30 μL bolus (right blue cross), or into the heads of gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses (shown with right and left crosses, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Control animals received GPB in the right leg, administered into the head of gastrocnemius lateralis as a single 30 μL bolus or into the heads of the gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses (n = 3/injection). The left leg remained intact (injection-free) in control animals. In total, six groups of animals received BoNT-A (n = 8/group) and two groups of animals received GPB (n = 3/group). All injections were made using a 30-gauge needle attached to a 100 μL syringe (SGE Analytical Science, Interchim; Montluçon, France). To inject, the needle was introduced perpendicularly into the center of the head of the gastrocnemius muscles. Experimenters were blinded to the treatment.
2.5. DWB
The DWB system (BioSeb; Vitrolles, France) includes a Plexiglas chamber (24 × 24 × 39 cm), a floor equipped with 44 × 44 captors (10.89 mm2 per sensor) to detect pressure variations, and a camera positioned above the chamber to record and validate the animal's posture during analysis (Robinson et al., 2012, Tétreault et al., 2011). During the test, animals moved freely in the chamber for 5 min. There was no acclimatization to the chamber before the test. The pressure applied by each animal's paw on the floor's sensors was recorded at a 10 Hz frequency with the aid of the DWB software v1.4.1.23 (Bioseb; Vitrolles, France). The software algorithm automatically extracted the weight and surface bearing for each hind paw in a sequential time segment. Each segment was considered stable and valid if ≥ 3 identical pictures (frames) were detected. The software automatically discarded sequences in which excessive movement made the reading of the paw pressure unstable. The position of the paws in each time segment was then checked, corrected where applicable, and validated by an operator according to the video recording. The operator was blind to treatment. At the end of the analysis, the mean over the time of the weight (or surface) value applied by each paw on the floor was extracted. The mean and standard deviation obtained present a mean of all measured prints for all animals.
Specific filters were used to trim the data obtained during the two-paw/rearing posture (when the animal is on its hind paws), the three-paw posture (when the animal is on two hind paws and one front paw), and four-paw posture (when the animal is standing on all four paws). The DWB data are expressed as a ratio of weight (or surface) bearing between the right and the left hind paws. The DWB experiment was performed on day 1 (D1). As in our experience, the weight and surface bearing in the right and in the left hind paws in intact animals is always identical (the ratio approaching 1), the assessment of the baseline before BoNT-A treatment was considered unnecessary in this experiment. A good alignment with the historical baseline was confirmed by the weight and surface bearing values of vehicle-treated animals.
2.6. DAS assay
The rat DAS assay was performed according to the protocol developed and validated in our laboratory (Cornet et al., 2020). It is an optimized version of the rat DAS study protocol used previously by other investigators (Adler et al., 2001, Rosales et al., 2006, Torii et al., 2010, Broide et al., 2013). The hind limb digit abduction reflex was induced by grasping the animal lightly around the torso and lifting it swiftly into the air or by lifting it with the nose pointing downwards. Animals were pre-screened for a normal digit abduction response (i.e. DAS 0, considered as the baseline) before the experiment and those showing abnormal digit abduction responses (>DAS 0) or hind paw deformities were excluded from the study. The digit abduction response of each rat was scored on a five-point scale, from normal reflex/no inhibition (DAS 0) to full inhibition of the digit abduction reflex (DAS 4). The DAS assay was performed on D1 and D2, preceding the DWB and CMAP tests. All scoring was done by the same experimenter, who was blind to treatment.
2.7. CMAP
Recording for the CMAP test was performed with a Keypoint electromyograph (Medtronic; Boulogne-Billancourt, France). Subcutaneous monopolar needle electrodes (Medtronic, 9013R0312) were used for both stimulation and recording. A ground electrode was placed in the paw pad of the recorded hind paw. The sciatic nerve was stimulated with a single pulse (12.8 mA of 0.2 ms duration) using a monopolar needle placed at the sciatic notch. The CMAP was recorded using needle electrodes placed into the center of the lateral gastrocnemius muscle. Animals were anaesthetized using a 2–3% isoflurane-oxygen mixture and placed in the prone position. Once the electrodes were implanted, the sciatic nerve was stimulated and CMAP was recorded in the gastrocnemius muscle. After recording, the animal was returned to its home cage. CMAP parameters measured in the study include amplitude (mV), onset latency (ms), duration (ms) and the area under the curve (AUC; mV x ms). The CMAP latency is determined by the delay from the stimulation to the onset of the CMAP waveform response. The CMAP latency reflects the speed of the fastest conducting motor fibers. The CMAP amplitude is the voltage/height response measured from the negative to the positive peak of the waveform and represents the summation of the action potential from each activated individual muscle fibers. The CMAP duration is the time measured from the initial deflection from baseline to the terminal deflection back to baseline. Duration reflects the synchrony of the activated muscle fibers. The CMAP AUC is a computerized electronic integration of the area under the negative and positive waveforms. It reflects the number and synchrony of the activated muscle fibers. In the current experiment the CMAP test was performed on D2. As there is a wealth of historical baseline data involving all CMAP measures from intact animals in our laboratory, a dedicated baseline test was not performed in this study. A good alignment of the readouts with historical baseline data was confirmed with the vehicle-treated group.
2.8. Statistical analysis
At each time point, mean DAS was calculated for each treatment group. Both DWB and CMAP measurements were analyzed by analysis of variance (ANOVA) followed by Fisher's least significant difference (LSD) post-hoc test.
3. Results
3.1. DWB test
Control animals that received vehicle either in the ipsilateral/right gastrocnemius muscle as a single bolus, or in the gastrocnemius lateralis and gastrocnemius medialis as two boluses, showed no change in weight distribution between the right and left hind paws (i.e. ratio close to 1) across all postures (Fig. 2 A–C). Therefore, the two groups of control animals were combined into a single control group.
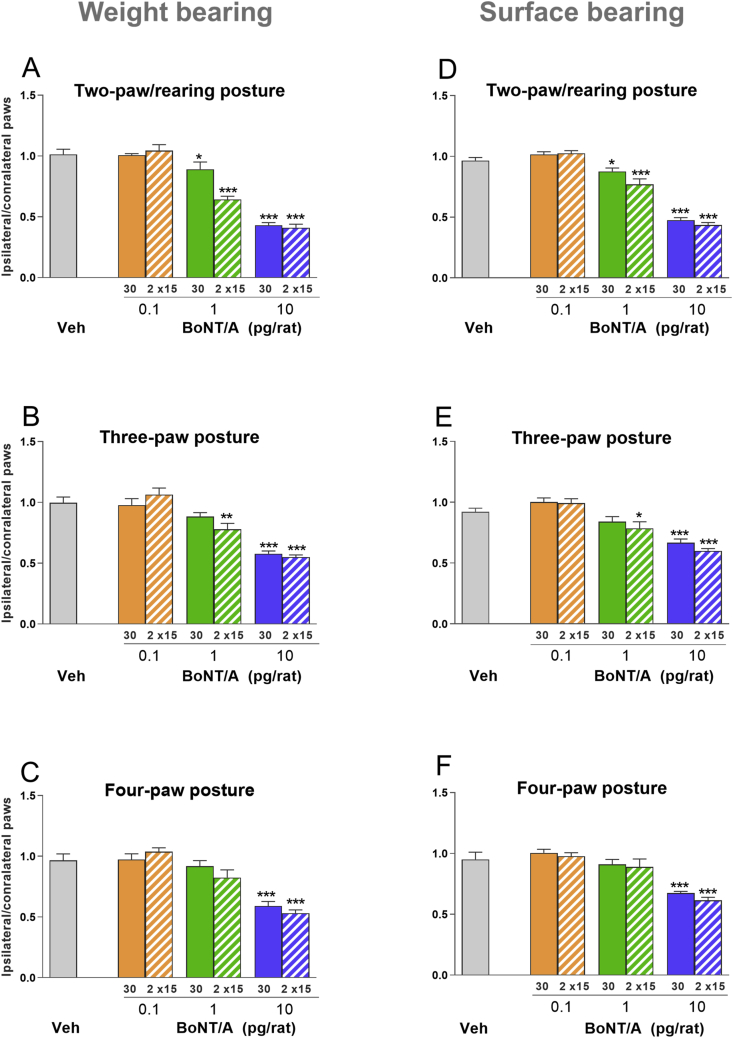
Fig. 2.
Effects of i.m. administered BoNT-A in the rat DWB test (see Methods). Animals received an i.m. injection of BoNT-A (0.1, 1, or 10 pg/rat) into the right gastrocnemius lateralis, while the left gastrocnemius lateralis received vehicle (GPB). BoNT-A or GPB were administered into the head of the gastrocnemius lateralis muscle as a single bolus (30 μL), or into the heads of the gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses (n = 8/dose/injection). Control animals received vehicle (GPB) into the head of the gastrocnemius lateralis or the heads of the gastrocnemius lateralis and gastrocnemius medialis, while the left gastrocnemius remained injection-free (n = 6). Figures show the ratio of weight (A–C) and surface (D–F) distribution on toxin-injected/right versus vehicle-injected/left hind paws at 1 day following injection of BoNT-A and vehicle (GPB). The measurements were taken when the animal was rearing (standing on two hind paws; A, D), standing on three paws (two hind paws and one forepaw; B, E), or standing on all four paws (C, F). Each point represents the observed mean (+SEM). *p < 0.05, **p < 0.01, ***p < 0.001.
BoNT-A injected as a single bolus into the head of the right gastrocnemius lateralis produced a dose-dependent reduction in the weight-bearing ratios between the right and left hind paws, detected across all three postures (Fig. 2 A–C). Specifically, while, the lowest dose (0.1 pg/rat) did not affect the weight-bearing performance regardless of the posture, the intermediate dose (1 pg/rat), resulted in weight-bearing deficits across all postures. One bolus produced 12% (p < 0.05) reductions in weight bearing under the rearing posture, while under three- and four-paw postures these reductions were present only as trends (Fig. 2A-C). Two boluses produced 35% reductions (p < 0.001) in weight-bearing ratios under the rearing posture, approximately 20% (p < 0.01) reductions under the three-paw posture and only a trend of reduction under the four-paw posture (Fig. 2A-C). At the high dose (10 pg/rat), weight-bearing deficit (p < 0.001) under the rearing posture was comparable (~60% reduction) between groups treated with one or two boluses (Fig. 2 A), followed by similar deficit (~40% reduction; p < 0.001) under three- and four-paw postures, while in the latter the effect was slightly more pronounced for two bolus injections (Fig. 2 B, C).
The patterns of BoNT-A activity on surface bearing were similar to those observed in weight bearing, as the most pronounced effects were seen under the two-paw/rearing posture (Fig. 2 D). Specifically, while the low dose (0.1 pg/rat) of BoNT-A had no effect on surface bearing, the intermediate dose (1 pg/rat) produced 10 (p < 0.05) to 20% (p < 0.001) reductions in surface bearing ratio, when given as one and two boluses, respectively (Fig. 2 D). The high dose (10 pg/rat) produced similar, 60% (p < 0.001) reductions in surface bearing, when injected as one or two boluses (Fig. 2 D). Under the two- and three-paw postures, there was a small effect (15%; p < 0.05) of the intermediate dose only when given as two boluses (Fig. 2 E). The effects of the highe dose (10 pg/rat) of BoNT-A on surface bearing ratio (35%; p < 0.001) were similar when injected as one or two boluses, and when the animal was under the three- or four-paw posture (Fig. 2 E, F).
3.2. DAS assay
The administration of vehicle (GPB) into the right gastrocnemius lateralis as either a single bolus or two boluses had no effect on the DAS response of the right paw on both days of testing (Table 1).
Table 1.
Effects of i.m. administered BoNT-A in the rat DAS test (see Methods). BoNT-A (0.1, 1, 10 pg/rat) or vehicle (GPB) were injected into the right and left gastrocnemius muscles, respectively, using one or two boluses (n = 8/group). Control animals received vehicle (GPB) as one or two boluses, into the right gastrocnemius muscle, while the left gastrocnemius remained injection-free (n = 3/group). The table shows the number of animals with DAS scores 0 to 2 on post-injections Days 1 and 2.
| Dose (pg/rat) |
0 |
0.1 |
1 |
10 |
DAS score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of boluses/volume (μL) | 1 × 30 | 2 × 15 | 1 × 30 | 2 × 15 | 1 × 30 | 2 × 15 | 1 × 30 | 2 × 15 | |
|
Number of animals Day 1 |
3 | 3 | 7 | 8 | 6 | 8 | 3 | 2 | 0 |
| 0 | 0 | 1 | 0 | 2 | 0 | 4 | 5 | 1 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | |
|
Number of animals Day 2 |
3 | 3 | 8 | 8 | 6 | 8 | 1 | 2 | 0 |
| 0 | 0 | 0 | 0 | 2 | 0 | 5 | 5 | 1 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | |
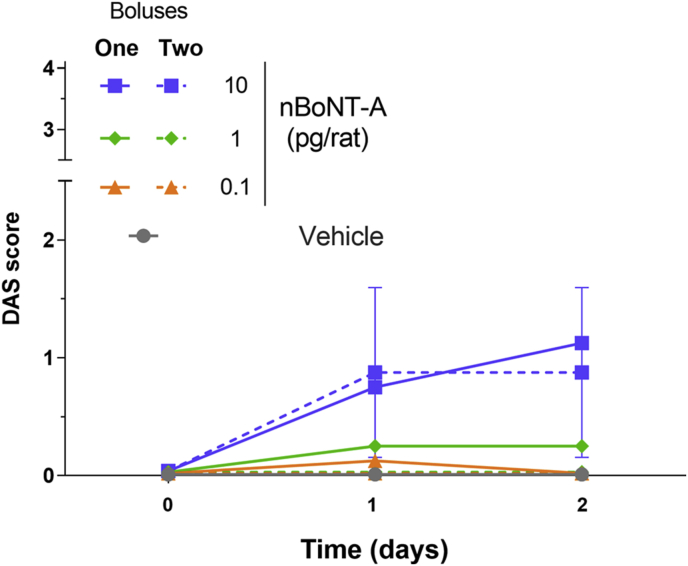
The administration of 0.1 and 1 pg/rat BoNT-A into the right gastrocnemius lateralis had only mild effects on the digit abduction responses, as only one of eight (0.1 pg/rat) or two of eight (1 pg/rat) animals exhibited a DAS 1 response (Table 1). While there seems to be a difference in DAS scores between one or two bolus injections for animals treated at 0.1 and 1 pg/rat, the values are too low to be considered biologically meaningful. In response to 10 pg/rat BoNT-A, four to five animals exhibited DAS 1 per day, while one to two animals exhibited DAS 2 (Table 1). There were no differences in DAS responses across the groups on D1 and D2 and between one or two bolus-injected groups (Fig. 3). Body weight assessment on D1 and D2 showed no difference between BoNT-A- and vehicle-treated groups (data not included).
Fig. 3.
DAS measured on days 1 and 2 after animals received an i.m. injection of BoNT-A (0.1, 1, or 10 pg/rat) into the right gastrocnemius lateralis, while the left gastrocnemius lateralis received vehicle (GPB). BoNT-A or GPB were administered into the head of the gastrocnemius lateralis muscle as a single bolus (30 μL), or into the heads of the gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses (n = 8/dose/injection). Control animals received vehicle (GPB) into the head of the gastrocnemius lateralis or the heads of the gastrocnemius lateralis and gastrocnemius medialis, while the left gastrocnemius remained injection-free (n = 6).
3.3. CMAP assay
Administration of the vehicle control (GPB) as one (30 μL) or two (15 μL) boluses had no effect on any of the CMAP measures taken from the injected ipsilateral/right muscle. Therefore, the two groups of control animals were combined into a single control group.
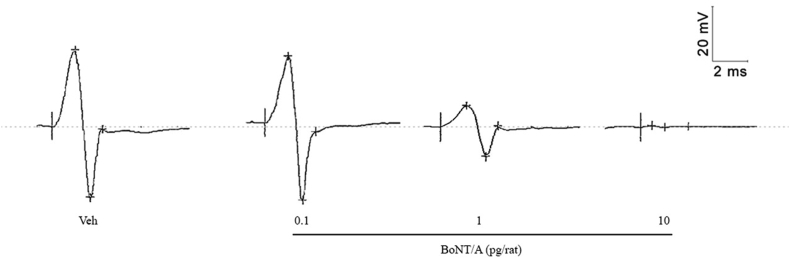
BoNT-A produced dose-dependent reductions in the CMAP amplitude in the toxin-injected, ipsilateral muscle (Fig. 4). Reductions in CMAP amplitude were markedly more pronounced when the toxin was given as two boluses rather than one (Fig. 5 A). Specifically, BoNT-A injections as one versus two boluses resulted in 15 (n.s.) versus 30% (p < 0.001) and 60 versus 90% reductions (both p < 0.01) in CMAP amplitude at 0.1 and 1 pg/rat, respectively (Fig. 5 A). The high dose (10 pg/rat) of BoNT-A resulted in full suppression of CMAP amplitudes in both groups (Fig. 5 A).
Fig. 4.
Representative CMAP responses obtained from the right gastrocnemius muscle 2 days after receiving i.m. administration of BoNT-A (0.1, 1, 10 pg/rat) or vehicle (GPB). The stimulation occurred on the sciatic nerve, and the recording on the gastrocnemius lateralis muscle (see Methods).
Fig. 5.
Effects of i.m. administered BoNT-A in the rat CMAP test (see Methods). Animals received an i.m. injection of BoNT-A (0.1, 1, or 10 pg/rat) into the right gastrocnemius lateralis, while the left gastrocnemius lateralis received vehicle (GPB). BoNT-A or GPB were administered into the head of the gastrocnemius lateralis muscle as a single bolus (30 μL), or into the heads of the gastrocnemius lateralis and gastrocnemius medialis as two 15 μL boluses (n = 8/dose/injection). Control animals received vehicle (GPB) into the head of the gastrocnemius lateralis or the heads of the gastrocnemius lateralis and gastrocnemius medialis, while the left gastrocnemius remained injection-free (n = 6). Figures show the amplitude (A, E), AUC (B, F), latency (C, G), and duration (D, H) of CMAP measured in the ipsilateral/right and contralateral/left gastrocnemius muscles 2 days after receiving BoNT-A and vehicle (GPB), respectively. Each point represents the observed mean (+SEM). *p < 0.05, ***p < 0.001 compared to the vehicle group.
BoNT-A also produced dose-dependent reductions in the CMAP AUC (Fig. 5 B), with injections of two boluses producing more pronounced effects. Specifically, there were 20 (n.s.) versus 30% (p < 0.001) and 70 versus 90% reductions (both p < 0.01) of CMAP AUC at 0.1 and 1 pg/rat, respectively, when they were given as one versus two boluses (Fig. 5 B). At the high dose (10 pg/rat), CMAP AUC was fully suppressed regardless of the number of boluses (Fig. 5 B). BoNT-A also produced dose-dependent increases in the CMAP latency (Fig. 5 C). Specifically, while trends of increases were seen at the low dose (0.1 pg/rat), the intermediate dose (1 pg/rat), resulted in 30 (n.s.) and more than 40% (p < 0.05) increases when administered as one versus two boluses, respectively, followed by 70 versus 130% (both p < 0.001) increases, respectively, at the high dose (10 pg/rat; Fig. 5 C). BoNT-A injection had no effect on CMAP duration in the injected, ipsilateral/right muscle (Fig. 5 D).
The administration of BoNT-A into the ipsilateral/right paw had a minor effect on CMAP measures taken from the vehicle-injected, contralateral/left muscle (Fig. 5 E–H). Specifically, the intermediate dose (1 pg/rat), when given as two boluses, produced small reductions (13–15%; p < 0.05) in the CMAP amplitude and AUC (Fig. 5E–F). No other change was detected in CMAP measures taken from the vehicle-injected contralateral/left muscle (Fig. 5 E–H).
4. Discussion
The growing interest in the therapeutic potential of native and modified BoNTs means there is a need for in vivo assays that can provide objective, quantitative, and reproducible assessment of the biological properties of these proteins following i.m. administration. With the aim of providing such characterization of activity of BoNT-A, the effects of local i.m. administration of the toxin into the gastrocnemius were evaluated in the DWB test in rats. The test was shown to be highly sensitive to BoNT-A since pg amounts of the toxin were detected by muscle flaccidity after i.m. administration in the rat. In agreement with our hypothesis, dose-dependent reductions were seen within 24 h post-administration of BoNT-A. Animals were also tested in the DAS and CMAP assays to evaluate relative sensitivity of the assays to muscle flaccidity-inducing effects of BoNT-A. These assays are more ethical and clinically relevant than the mouse lethality assay. The current study was performed in female Sprague-Dawley rats according to the requirements of the rat DAS study design recently optimized in our laboratory (Cornet et al., 2020; see below). However, we can speculate that the DWB test, when used on its own, can be performed in either male or female rats. While we tested animals only at one time point, we can also speculate that, as the test measures spontaneous activity of conscious and unrestrained rats, it is also suitable for longitudinal analysis.
We found that BoNT-A-injected rats showed dose-dependent reductions in the weight and surface-bearing ratios when tested 1 day after toxin administration. The effect was most robust when body weight was distributed between two hind paws when animal was rearing and least pronounced when body weight was distributed across all four paws. Additionally, BoNT-A activity in weight bearing was virtually identical to that in surface bearing. Thus, the evaluation of weight bearing while the animal is rearing appears to represent the most sensitive DWB readouts, sufficient for the analysis of BoNT activity in this assay.
The effects of BoNT-A in the DWB obtained in the current study agree with the reductions in paw print length, width, and area, as well as in the duration of stand assessed in BoNT-injected mice tested in the CatWalk assay (Moritz et al., 2019). While we did not measure the nerve functional indices in this study, based on similarity in the injection site and readouts between the DWB and the CatWalk assays, we can speculate that the DWB was affected by peroneal nerve activity (Moritz et al., 2019). This should be taken with the caveat that the nerve functional indices can be BoNT-dose-dependent and factors such as dilutions could impact the results (Moritz et al., 2019).
In the current study, BoNT-A was evaluated in three doses, each increasing 10-fold from 0.1 to 1 and 10 pg/rat. As the lowest dose (0.1 pg/rat) was without any effect, while the highest dose (10 pg/rat) produced approximately 60% reduction in weight or surface bearing, the full dose range of BoNT-A in this assay was not determined, which is a limitation of the current study. The effects of BoNT-A in the DWB were not accompanied by changes in body weight gain, suggesting that the effects of BoNT-A at up to 10 pg/rat in the assay were due to flaccid paralysis of the injected muscle.
In the current study, BoNT-A injection was performed either as a single 30 μL bolus administered into the head of the gastrocnemius lateralis or as two 15 μL boluses administered into the heads of the gastrocnemius lateralis and gastrocnemius medialis, the two largest adjacent muscles of the rat hind limb (Wang and Kernell, 2001). At the intermediate dose (1 pg/rat), the effect of two boluses on weight bearing was more pronounced than the effect of a single bolus, suggesting that increasing the number of affected muscles of the hind limb increases the effect of BoNT on weight bearing. The higher efficacy of the two boluses in comparison to one was even more pronounced in effects of BoNT-A on CMAP amplitude and AUC. These effects are in line with CMAP studies in humans, where experimental conditions with BoNT-A administered at multiple sites facilitate the diffusion of the toxin within the muscle and into adjacent muscles, thus increasing its efficacy (Wohlfarth et al., 2008).
In this study, we showed that BoNT-A injected i.m. into the gastrocnemius of female Sprague-Dawley rats results in dose-dependent reductions in the amplitude and AUC of the CMAP, confirming previous findings also obtained in female Sprague-Dawley rats (Sakamoto et al., 2009; Torii et al., 2010, 2014). We compared the magnitude of the effects of two BoNT in the CMAP, native BoNT-A (in the current study) and onabotulinumtoxinA (onaBoNT-A) described by Sakamoto et al. (2009). In order to overcome the difficulty of comparison of the doses of two forms of BoNT-A toxins, we used the conversion of onaBoNT-A units to pg recently reported by Field et al., (2018). Specifically, as 100 U vial of onaBoNT-A contains 0.90 ng of 150 kDa BoNT-A, there are 9 pg of BoNT-A in 1 U of onaBoNT-A (Field et al., 2018). Using this conversion, we confirmed that reductions in CMAP amplitude 2 days after the injection of BoNT-A (0.1, 1, and 10 pg/rat) in the current study and the closest doses of onaBoNT-A (0.01 U/0.09 pg/rat, 0.1 U/0.9 pg/rat, and 1 U/9 pg/rat) reported by Sakamoto et al. (2009) were overall very similar.
In the current study, dose-related reductions in the CMAP amplitude and AUC were accompanied by dose-related increases in CMAP latency, especially at intermediate (1 pg/rat) and high doses (10 pg/rat) of BoNT-A. These increases in CMAP latency are most probably related to the delay in neuromuscular junction transmission, which is a consequence of the alteration of the release of acetylcholine from the endings of the motor nerves (Sakamoto et al., 2009; Torii et al., 2010, 2014). Despite marked changes in the CMAP amplitude, AUC, and latency, we did not see any changes in the duration of the CMAP, even at the high dose. This suggests that the relative synchrony of the muscle fiber response remains unchanged. The neuromuscular junction structures may remain fully functional in BoNT-treated animals or compensatory mechanisms may mask the effects of a reduced release of acetylcholine.
In the current study, assessment of CMAP activity in the contralateral, vehicle-injected gastrocnemius provided an opportunity to evaluate signs of the potential spread of the toxin to the contralateral muscle, either through retrograde transport or via systemic spread (Torii et al., 2011). Two days after the i.m. administration of BoNT-A, see only very mild changes in any CMAP measures taken from the contralateral, vehicle-injected gastrocnemius lateralis, even when the ipsilateral muscle was injected with the highest dose. While there were small (~15%; p < 0.05) reductions in contralateral CMAP amplitude and AUC in animals receiving intermediate dose (1 pg/rat) of BoNT-A injected at two sites, they lacked dose-dependency. Indeed, according to Robinson and Snyder-Mackler (Robinson and Snyder-Mackler, 1988), reductions in CMAP amplitude that are less than 20% in magnitude may lack clinical relevance. Thus, we can conclude that in the current study, 2 days following administration of BoNT-A, at up to 10 pg/rat, there are no clear signs of its spread either via retrograde transport or by systemic circulation. However, this conclusion needs to be taken with caution, as detection of contralateral effects may depend on time. Thus, additional studies with longer time points are needed to evaluate contralateral effects of BoNT-A in CMAP.
In the current study, animals were tested in the DAS assay just before they were tested in the DWB and CMAP on post-injection Days 1 and 2, respectively. The choice of female rats in the current study was linked to the optimized rat DAS study design, where we identified few advantages of using female animals instead of males (Cornet et al., 2020). Briefly, while there is no difference between male and female rats in sensitivity to BoNT-A, longitudinal studies involving DAS readout are easier to perform with females than with males. In our experience, as male rats grow older and heavier, the digit abduction reflex is more difficult to elicit, while it remains stable in female rats (Cornet et al., 2020).
We confirmed the lower sensitivity of the DAS test after i.m. administration of the BoNT-A into the gastrocnemius muscle. Specifically, the lack of effect in response to low doses of BoNT-A (0.1 and 1 pg/rat) in the DAS was followed by relatively moderate effects of DAS 1 (six rats) or DAS 2 (two rats) at 10 pg/rat two days post-injection. Previously, in female rats injected in the gastrocnemius lateralis, the dose of native BoNT-A associated with half-maximal inhibition of the digit abduction response (i.e. DAS 2) was 10 pg/rat and with maximal inhibition (i.e. DAS 4), 20 pg/rat, obtained between 2 and 3 days post-injection (Cornet et al., 2020). Thus, in the current study, the peak effect in the DAS may not have been reached. In contrast to both studies, onaBoNT-A injected in the gastrocnemius of male rats appears significantly less potent, as a markedly higher dose (38.2 U/kg) is required to obtain the maximal inhibition of digit abduction (Broide et al., 2013). If we perform the unit to pg conversion in onaBoNT-A proposed by Field et al., (2018), 38.2 U/kg (in 250 g rats) is equivalent to nearly 86 pg/rat of BoNT-A. We can speculate on specific experimental factors or their combination that can explain this discrepancy in potency of native BoNT-A and onaBoNT-A in the DAS assay between these two studies. Methodological differences, including the use of males versus females or potential differences in exact site of the injection (undefined by Broide et al., 2013), may have contributed to this discrepancy.
5. Conclusions
In conclusion, we showed for the first time that the DWB test in the rat is highly sensitive to pg amounts of BoNT-A being detected by muscle flaccidity when the toxin is injected i.m. into the gastrocnemius muscle. BoNT-A-injected animals showed dose-related reductions in the weight and surface bearing, which were most pronounced when the animal was rearing. Changes in weight and surface bearing in BoNT-A-injected animals was accompanied by a marked dose-dependent reduction in CMAP amplitude and moderate changes in the DAS assay. The activity of BoNT-A in the DWB and CMAP tests was more robust when the toxin was administered as two boluses aimed at two adjacent muscles rather than one bolus. A follow-up longitudinal experiment is needed in order to evaluate kinetics of the paralysis induced by BoNT-A in the DWB. Thus, the DWB test can provide objective and quantitative measures that are influenced by acute, i.m. administration of BoNT.
CRediT authorship contribution statement
Sylvie Cornet: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing - original draft. Cindy Périer: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing - original draft. Stéphanie Wagner: Conceptualization, Methodology, Investigation, Validation, Formal analysis. Emile Andriambeloson: Conceptualization, Methodology, Investigation, Validation, Formal analysis. Bruno Pouzet: Conceptualization, Methodology, Writing - original draft. Mikhail Kalinichev: Visualization, Supervision, Writing - original draft, Writing - review & editing.
Acknowledgments
The authors thank Drs. Johannes Krupp and Philippe Picaut for their insightful comments on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2020.100041.
Ethical statement
All experimental procedures were approved by the Ethics Committee of Ipsen Innovation (C2EA; registration number 32) and were performed in full compliance with the ARRIVE guidelines, EU Directive 2010/63/EU for animal experiments and the 2013 French Regulatory Decree.
Disclosures
SC, CP, and MK are employees of Ipsen. EA and SW are employees of NEUROFIT. BP is an employee of BeVivo.
Funding
This study was sponsored by Ipsen.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adler M., Keller J., Sheridan R.E., Deshpande S.S. Persistence of botulinum neurotoxin A demonstrated by sequential administration of serotypes A and E in rat EDL muscle. Toxicon. 2001;39(2–3):233–243. doi: 10.1016/s0041-0101(00)00120-3. [DOI] [PubMed] [Google Scholar]
- Allen D.L., Harrison B.C., Maass A., Bell M.L., Byrnes W.C., Leinwand L.A. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 2001;90(5):1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- Ängeby Möller K., Svärd H., Suominen A., Immonen J., Holappa J., Stenfors C. Gait analysis and weight bearing in pre-clinical joint pain research. J. Neurosci. Methods. 2018;300:92–102. doi: 10.1016/j.jneumeth.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Antonucci F., Rossi C., Gianfranceschi L., Rossetto O., Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008;28(14):3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K.R. Preclinical update on BOTOX (botulinum toxin type A)-purified neurotoxin complex relative to other botulinum neurotoxin preparations. Eur. J. Neurol. 1999;6(4):S3–S10. doi: 10.1111/j.1468-1331.1999.tb00032.x. [DOI] [Google Scholar]
- Aoki K.R. A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon. 2001;39(12):1815–1820. doi: 10.1016/s0041-0101(01)00101-5. [DOI] [PubMed] [Google Scholar]
- Broide R.S., Rubino J., Nicholson G.S., Ardila M.C., Brown M.S., Aoki K.R., Francis J. The rat Digit Abduction Score (DAS) assay: a physiological model for assessing botulinum neurotoxin-induced skeletal muscle paralysis. Toxicon. 2013;71:18–24. doi: 10.1016/j.toxicon.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Cai B.B., Francis J., Brin M.F., Broide R.S. Botulinum neurotoxin type A-cleaved SNAP25 is confirmed to primary motor neurons andlocalized on the plasma membrane following intramuscular toxin injection. Neuroscience. 2017;352:155–169. doi: 10.1016/j.neuroscience.2017.03.049. [DOI] [PubMed] [Google Scholar]
- Cornet S., Périer C., Kalinichev M. Optimization of the rat digit abduction score (DAS) assay: evaluation of botulinum neurotoxin activity in the gastrocnemius lateralis, peronei and extensor digitorum longus. Toxicon X. 2020;6 doi: 10.1016/j.toxcx.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald S., Elliott M., Gray B., Hornby F., Lewandowska A., Marlin S., Favre-Guilmard C., Périer C., Cornet S., Kalinichev M., Krupp J., Fonfria E. A comparison of biological activity of naturally occurring botulinum neurotoxin serotypes A to F in vitro, ex vivo and in vivo. Pharmacol. Res. Perspect. 2018;6(6):e00446. doi: 10.1002/prp2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D. Clinical applications of botulinum toxin. Curr. Opin. Microbiol. 2012;15:325–336. doi: 10.1016/j.mib.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Dressler D. Botulinum toxin drugs: brief history and outlook. J. Neural Transm. 2016;123(3):277–279. doi: 10.1007/s00702-015-1478-1. [DOI] [PubMed] [Google Scholar]
- Dunning F.M., Ruge D.R., Piazza T.M., Stanker L.H., Zeytin F.N., Tucker W.C. Detection of botulinum neurotoxin serotype A, B, and F proteolytic activity in complex matrices with picomolar to femtomolar sensitivity. Appl. Environ. Microbiol. 2012;78(21):7687–7697. doi: 10.1128/AEM.01664-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Splevins A., Picaut P., Van der Schans M., Langenberg J., Noort D., Snyder D., Foster K. AbobotulinumtoxinA (Dysport®), onabotulinumtoxinA (Botox®), and incobotulinumtoxinA (Xeomin®), neurotoxin content and potential implications for durations of response in patients. Toxins. 2018;10(12):535. doi: 10.3390/toxins10120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Montal M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J. Biol. Chem. 2007;282:29504–29611. doi: 10.1074/jbc.m703619200. [DOI] [PubMed] [Google Scholar]
- Fonfria E., Donald S., Cadd V.A. Botulinum neurotoxin A and an engineered derivate targeted secretion inhibitor (TSI) A enter cells via different vesicular compartments. J. Recept. Signal Transduct. Res. 2016;36(1):79–88. doi: 10.3109/10799893.2015.1049359. [DOI] [PubMed] [Google Scholar]
- Fonfria E., Maignel J., Lezmi S., Martin V., Splevins A., Shubber S., Kalinichev M., Foster K., Picaut P., Krupp J. The expanding therapeutic utility of botulinum neurotoxins. Toxins. 2018;10(5):E208. doi: 10.3390/toxins10050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J.E. Recovery from botulinum neurotoxin poisoning in vivo. Neuroscience. 2006;139(2):629–637. doi: 10.1016/j.neuroscience.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Koizumi H., Goto S., Okita S., Morigaki R., Akaike N., Torii Y., Harakawa T., Ginnaga A., Kaji R. Spinal central effects of peripherally applied botulinum neurotoxin A in comparison between its subtypes A1 and A2. Front. Neurol. 2014;5:98. doi: 10.3389/fneur.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans G.C., Deumens R., Brook G., Gerver J., Honig W.M., Harners F.P., Joosten E.A. Strain and locomotor speed affect overground locomotion in intact rats. Physiol. Behav. 2007;92:993–1001. doi: 10.1016/j.physbeh.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Kutschenko A., Manig A., Reinert M-C., Mönnich A., Liebetanz D. In-vivo comparison of the neurotoxic potencies of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA. Neurosci. Lett. 2016;627:216–221. doi: 10.1016/j.neulet.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Lakes E.H., Allen K. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthr. Cartilage. 2016;24:1837–1849. doi: 10.1016/j.joca.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matak I., Riederer P., Lacković Z. Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem. Int. 2012;61:236–239. doi: 10.1016/j.neuint.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer O.A., Tilson H.A., Byrd W.C., Riley M.T. A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav. Toxicol. 1979;1(3):233–236. [PubMed] [Google Scholar]
- Moritz M.S., Tepp W.H., Inzalaco H.N., Johnson E.A., Pellett S. Comparative functional analysis of mice after local injection with botulinum neurotoxin A1, A2, A6, and B1 by catwalk analysis. Toxicon. 2019;167:20–28. doi: 10.1016/j.toxicon.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano S., Montecucco C. The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell Mol. Life Sci. 2014;71(5):793–811. doi: 10.1007/s00018-013-1380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L.B., Borodic G.E., Johnson E.A., First E.R., MacCallum R. The median paralysis unit: a more pharmacologically relevant unit of biologic activity for botulinum toxin. Toxicon. 1995;33(2):217–227. doi: 10.1016/0041-0101(94)00137-w. [DOI] [PubMed] [Google Scholar]
- Pellett S., Tepp W.H., Whitemarsh R.C.M., Bradshaw M., Johnson E.A. In vivo onset and duration of action varies for Botulinum neurotoxin A subtypes 1-5. Toxicon. 2015;107:37–42. doi: 10.1016/j.toxicon.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza T.M., Blehert D.S., Dunning F.M., Berlowski-Zier B.M., Zeytin F.N., Samuel M.D., Tucker W.C. In vitro detection and quantification of botulinum neurotoxin type E in avian blood. Appl. Environ. Microbiol. 2011;77(21):7815–7822. doi: 10.1128/AEM.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A.J., Snyder-Mackler L. Clinical application of electrotherapeutic modalities. Phys. Ther. 1988;68(8):1235–1238. [PubMed] [Google Scholar]
- Robinson I., Sargent B., Hatcher J.P. Use of dynamic weight bearing as a novel end-point for the assessment of Freund's Complete Adjuvant induced hypersensitivity in mice. Neurosci. Lett. 2012;524(2):107–110. doi: 10.1016/j.neulet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Rosales R.L., Bigalke H., Dressler D. Pharmacology of botulinum toxin: differences between type A preparations. Eur. J. Neurol. 2006;13(Suppl. 1):2–10. doi: 10.1111/j.1468-1331.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- Rossetto O., Pirazzini M., Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014;12(8):535–549. doi: 10.1016/j.toxicon.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Rummel A. The long journey of botulinum neurotoxins into the synapse. Toxicon. 2015;107:9–24. doi: 10.1016/j.toxicon.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Torii Y., Takahashi M. Quantitative determination of the biological activity of botulinum toxin type A by measuring the compound muscle action potential (CMAP) in rats. Toxicon. 2009;54(6):857–861. doi: 10.1016/j.toxicon.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Schantz E.J., Kautter D.A. Standardized assay for Clostridium botulinum toxins. J. Assoc. Off. Analyt. Chem. 1978;61(1):96–99. doi: 10.1093/jaoac/61.1.96. [DOI] [Google Scholar]
- Stone H.F., Zhu Z., Thach T.Q., Ruegg C.L. Characterization of diffusion and duration of action of a new botulinum toxin type A formulation. Toxicon. 2011;58(2):159–167. doi: 10.1016/j.toxicon.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Torii Y., Goto Y., Takahashi M., Takahashi M., Ishida S., Harakawa T., Sakamoto T., Kaji R., Kozaki S., Ginnaga A. Quantitative determination of biological activity of botulinum toxins utilizing compound muscle action potentials (CMAP), and comparison of neuromuscular transmission blockage and muscle flaccidity among toxins. Toxicon. 2010;55(2–3):407–414. doi: 10.1016/j.toxicon.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Tétreault P., Dansereau M.A., Doré-Savard L., Beaudet N., Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol. Behav. 2011;104(3):495–502. doi: 10.1016/j.physbeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Torii Y., Akaike N., Harakawa T., Kato K., Sugimoto N., Goto Y., Nakahira S., Kohda T., Kozaki S., Ryuji Kaji R., Akihiro Ginnaga A., Möller J. Type A1 but not type A2 botulinum toxin decreases the grip strength of the contralateral foreleg through axonal transport from the toxin-treated foreleg of rats. Pharmacol. Sci. 2011;117(4):275–285. doi: 10.1254/jphs.11121fp. [DOI] [PubMed] [Google Scholar]
- Torii Y., Kiyota N., Sugimoto N., Mori Y., Goto Y., Harakawa T., Nakahira S., Kaji R., Kozaki S., Ginnaga A. Comparison of effects of botulinum toxin subtype A1 and A2 using twitch tension assay and rat grip strength test. Toxicon. 2011;57:93–99. doi: 10.1016/j.toxicon.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab Sci. 2011;50(5):600–613. [PMC free article] [PubMed] [Google Scholar]
- Turner M.J., Kleeberger S.R., Lightfoot J.T. Influence of genetic background on daily running-wheel activity differs with aging. Physiol. Genom. 2005;22:76–85. doi: 10.1152/physiolgenomics.00243.2004. [DOI] [PubMed] [Google Scholar]
- Wang L.C., Kernell D. Quantification of the fibre type regionalization: an analysis of lower hindlimb muscles in the rat. J. Anat. 2001;198(Pt 3):295–308. doi: 10.1046/j.1469-7580.2001.19830295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth K., Schwandt I., Wegner F., Jürgens T., Gelbrich G., Wagner A., Bogdahn U., Schulte-Mattler W. Biological activity of two botulinum toxin type A complexes (Dysport and Botox) in volunteers: a double-blind, randomized, dose-ranging study. J. Neurol. 2008;255(12) doi: 10.1007/s00415-008-0031-7. 1932–1239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.