Abstract
Background:
Off-label drug use in children is common and potentially harmful. In most previous off-label use research, authors studied hospitalized children, specific drug classes, or non-US settings. We characterized frequencies, trends, and reasons for off-label systemic drug orders for children in ambulatory US settings.
Methods:
Using national surveys of office-based physicians (National Ambulatory Medical Care Surveys, 2006–2015), we studied off-label orders of systemic drugs for children age <18 based on US Food and Drug Administration–approved labeling for age, weight, and indication. We characterized the top classes and diagnoses with off-label orders and analyzed factors and trends of off-label orders using logistic regression.
Results:
Physicians ordered ≥1 off-label drug at 18.5% (95% confidence interval: 17.7%–19.3%) of visits, usually (74.6%) because of unapproved conditions. Off-label ordering was most common proportionally in neonates (83%) and in absolute terms among adolescents (322 orders out of 1000 visits). Off-label ordering was also associated with female sex, subspecialists, polypharmacy, and chronic conditions. Rates and reasons for off-label orders varied considerably by age. Relative and absolute rates of off-label orders rose over time. Among common off label classes, rates of antihistamines and several psychotropic drugs increased over time, whereas off-label orders for several classes of antibiotics were stable or declined.
Conclusions:
US office-based physicians have ordered systemic drugs off label for children at increasing rates, most often for unapproved conditions, despite recent efforts to increase evidence and drug approvals for children. These findings can help inform education, research, and policies around effective, safe use of medications in children.
Table of Contents Summary:
Using nationally representative survey data from 2006–2015, this study examined patterns of off-label systemic drug orders for children in ambulatory United States settings.
INTRODUCTION:
Children often take drugs off-label, outside of an approved age, indication, weight, dose, formulation, or route of administration.1–3 Off-label prescribing has been associated with higher rates of adverse effects in children.4 However, off-label prescribing is legal and can represent best practice based on extensive clinical experience and supporting evidence of efficacy and safety, particularly when no labeled alternatives exist.1, 5 Laws and policies including the Best Pharmaceutical for Children Act (BPCA, 2002), Pediatric Research Equity Act (PREA, 2003), and the European Pediatric Regulation (2007) have incentivized or mandated pediatric clinical trials, intending to increase the quality of evidence and number of drugs approved for children.6–8 Nonetheless, many drugs remain off-label for children, and legislative efforts to stimulate clinical trials for both new and off-patent drugs may not yet have realized their potential.9, 10
Reported rates of off-label drug use in children vary widely across studies, owing to differences in definitions of off-label usage, methodology and sampling, composition of the study population (e.g., age range), number of drugs considered, geography, and settings of care (e.g., inpatient vs. ambulatory).3 Particularly high rates of off-label pediatric drug use have been reported in inpatient settings (36–92%), especially neonatal and pediatric intensive care units (80–97%).3, 4, 11 Nonetheless, the vast majority of children receive care and medicines exclusively in the outpatient setting.12 Studies of off-label prescribing in outpatient settings have often focused on select drugs or drug classes (e.g., antidepressants).13, 14 A more comprehensive study of pediatric drug utilization in ambulatory US settings was limited to 4 years of data through 2004.15 More recent studies on outpatient off-label pediatric drug utilization have come predominantly from European countries, which have different systems of care, prescribing practices, regulations, and populations than the US.16–21 We sought to describe recent patterns of drugs ordered off-label in a US-representative ambulatory pediatric population, including time trends and common diagnoses with off-label orders, focusing on systemic drugs because of their greater potential for drug toxicity.
METHODS:
Study Design and Setting:
We conducted a retrospective utilization study of serial, cross-sectional data from the National Ambulatory Medical Care Surveys (NAMCS, 2006–2015).22 This annual survey collects anonymous, visit-level data from US office-based physicians, including demographics, reasons for visit, diagnoses, and drugs provided or ordered at the visit, including recommended over-the-counter (OTC) drugs. NAMCS data are collected through a probability-based, complex sampling design that allows researchers to produce nationally representative estimates. This study was determined by the Rutgers IRB to be non-human subjects research (Pro20170000577).
Study Population:
We included all visits for children under 18 years old. We focused on the 141 drugs predominantly or exclusively used in systemic formulations and ordered at least 30 times in the data set (Supplemental Methods). We used the Anatomical Therapeutic Chemical (ATC) classification to present data based on broad categories (level 1) and drug classes (level 3) corresponding to the predominant systemic use (Supplemental Methods). We excluded from consideration vaccines, vitamins, and drugs no longer FDA-approved because of withdrawal of market authorization (mostly cough medicines).
Off-label definitions:
Our main outcomes of interest were absolute and relative prevalence rates of off-label orders of systemic drugs. Absolute prevalence was defined as the number of drugs ordered per 1000 visits; relative prevalence was defined as the percentage of off-label orders among all ordered drugs. We defined off-label usage based on US drug labeling as recorded in the Prescribers’ Digital Reference and the Food and Drug Administration (FDA) website in 2018.23, 24 Off-label status was determined using the following criteria (see also Supplemental Methods, Off-Label Definitions):
Age: drugs were considered off-label by age when ordered for children younger than the approved age for any indication.
Weight: drugs were considered off-label by weight when weight was specified in the product labeling and drugs were ordered for a child weighing less than the approved weight for any age or indication. Drugs were considered possibly off-label when weight was missing.
Indication: drugs were considered off-label by indication when ordered in visits without a documented condition corresponding to an FDA-approved indication. For each ordered drug at each visit, FDA-approved indications were compared to recorded diagnoses (up to 5 International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes), chronic disease indicators (checkboxes for asthma, depression, etc.), and reasons for visit (up to 5 additional symptoms, diagnoses, or other reasons recorded in NAMCS). Each unmatched drug-visit observation was manually reviewed by two individuals, including a practicing pediatrician (DBH). Drugs were considered possibly off-label when ordered for nonspecific diagnoses or symptoms broader than the listed FDA-approved indication.
Overall: a drug was considered off-label overall when it was off-label by age, weight, or indication, or was ordered for an approved indication at an unapproved age or (where applicable) weight. A drug was considered possibly off-label overall when deemed to be possibly off-label by indication or weight but not off-label by age.
Dose information was unavailable and not considered for off-label status determination.
Independent variables:
Independent variables of interest included age subgroups, sex, race/ethnicity, US geographic region, insurance status, physician specialty, number of systemic drugs ordered, presence of a chronic condition, and calendar year (Supplemental Methods).
Data Analysis:
We estimated absolute prevalence of systemic and off-label systemic drug orders with 95% confidence intervals (CI) by taking the mean of orders-per-visit across visits and multiplying by 1000 to produce rates per 1000 visits. We estimated relative prevalence of off-label drug orders with 95% CI by tabulating the percentage of off-label orders across all visits and visits with systemic drug orders. All estimates were repeated for drug categories, drug classes, drugs, calendar year, and age subgroups. We presented results for the drug classes most commonly ordered off-label, including the most common drugs within each class and the most common diagnoses for off-label orders at the level of 3-digit ICD-9-CM code, excluding nonspecific codes (V20 health supervision child and V67 follow-up examination). We estimated the association of calendar time and other covariates with off-label orders among visits with ≥1 systemic drug order using multivariable logistic regression. We used model-based predictive margins to estimate covariate-specific probabilities of off-label orders with 95% CI. We also used logistic regression to evaluate the relative change in off-label orders over time for more common drug classes. We performed sensitivity analyses classifying possibly off-label orders as off-label.
Analyses were conducted on Stata/MP 14 and 15.1, accounting for the complex sampling design to produce nationally representative estimates. All p-values were two-sided using alpha of 0.05.
RESULTS:
Prevalence of Off-Label Orders
Of the 1.74 billion ambulatory pediatric visits estimated over the 10 years of NAMCS data used in this study, 41.5% (95% CI 40.5–42.6%) of these visits resulted in ≥1 order of the 141 systemic drugs studied, totaling 108.0 (95% CI 105.2–110.9) million orders per year. 18.5% of visits (44.5% of visits with systemic drugs) included ≥1 off-label systemic drug order, or 41.2 million off-label orders per year (Table 1). An additional 1.6% of visits had possibly off-label orders due to nonspecific documentation. Of drugs considered off-label or possibly off-label, 74.6% were off-label by indication, 17.6% were off-label by age, 0.6% were off-label by weight, and 4.7% were off-label based on the combination of age, indication, and (where applicable) weight (Table 1).
Table 1.
Prevalence rates of off-label systemic drug orders in ambulatory pediatric visits in the United States (2006–2015)1
| Off-label characteristic | Sample N | % of total visits (95% CI) | % of visits with systemic drugs (95% CI) | % of off-label or possibly off-label drugs (95% CI) | Estimated orders, millions/year (95% CI) |
|---|---|---|---|---|---|
| Off-label for any reason | 17,064 | 18.5 (17.7–19.3) | 44.5 (43.3, 45.8) | 90.6 (89.8, 91.4) | 41.2 (39.9, 42.4) |
| Possibly off-label for any reason | 1,801 | 1.6 (1.5, 1.8) | 3.9 (3.5, 4.4) | 9.4 (8.6, 10.2) | 4.3 (3.9, 4.7) |
| Off-label by indication | 13,914 | 15.6 (15.0, 16.3) | 37.6 (36.4, 38.8) | 74.6 (73.2, 75.9) | 33.9 (32.7, 35.1) |
| Possibly off-label by indication | 2,079 | 1.8 (1.7, 2.0) | 4.4 (4.0, 4.9) | 10.2 (9.5, 11.1) | 4.6 (4.3, 5.1) |
| Off-label by age | 3,501 | 4.3 (3.9, 4.6) | 10.3 (9.6, 11.0) | 17.6 (16.5, 18.7) | 8.0 (7.4, 8.6) |
| Off-label by weight | 131 | 0.2 (0.2, 0.3) | 0.6 (0.4, 0.8) | 0.6 (0.4, 0.8) | 0.4 (0.3, 0.6) |
| Possibly off-label by weight | 419 | 0.5 (0.4, 0.6) | 1.2 (1.0, 1.5) | 1.2 (0.9, 1.5) | 0.9 (0.7, 1.1) |
| Off-label by combination of age, indication, and (where applicable) weight2 | 893 | 1.2 (1.0, 1.4) | 2.8 (2.5, 3.3) | 4.6 (4.1, 5.3) | 2.1 (1.8, 2.4) |
All rates account for sampling, clustering, and strata, reflecting nationally representative estimates
Drugs that were ordered for an approved indication, an approved age (and, where applicable, weight) for a different indication, but an unapproved age or weight for the documented diagnoses and symptoms
In absolute terms, off-label orders were most common among adolescents (321.5 orders/1000 visits) and least common among neonates (52.0 orders/1000 visits), reflecting to some degree the overall prevalence of systemic drug orders (Table 2). In relative terms, the youngest age groups were most likely to receive medications off-label: approximately 83% of all neonatal visits and 49% of infant visits with ≥1 systemic drug included ≥1 off-label drug order, compared to 39–44% of visits for other age groups (Table 3). Additionally, visits for girls, offices in the South, subspecialists, and presence of polypharmacy or a chronic condition, but not race, ethnicity, or insurance type, were associated with increased relative rates of off-label orders (Table 3).
Table 2.
Absolute prevalence of systemic drug orders for children in US ambulatory settings (2006–2015)
| Orders per 1000 ambulatory visits (95% confidence intervals) | ||||||
|---|---|---|---|---|---|---|
| Category | Age <1m | Age 1m-<2y | Age 2–5y | Age 6–11y | Age 12–17y | All children |
| Any drug | 64.1 (45.3, 83.0) | 406.1 (380.2, 432.0) | 635.8 (605.3, 666.3) | 731.7 (698.1, 765.3) | 759.1 (722.9, 795.2) | 620.7 (597.2, 644.2) |
| Off-label, overall | 52.0 (35.7, 68.4) | 164.0 (149.3, 178.7) | 218.7 (205.0, 232.5) | 253.7 (238.2, 269.3) | 321.5 (299.9, 343.1) | 236.5 (225.1, 247.8) |
| Off-label by indication | 28.21 (17.5, 39.0) | 112.4 (102.1, 122.8) | 190.0 (177.7, 202.3) | 219.3 (204.8, 233.7) | 268.0 (249.0, 287.0) | 194.6 (185.0, 204.2) |
| Off-label by age | 40.8 (26.1, 55.5) | 61.4 (53.3, 69.6) | 33.5 (28.3, 38.6) | 37.6 (32.7, 42.5) | 51.2 (44.8, 57.7) | 45.9 (42.1, 49.8) |
Values based on <30 observations
Table 3.
Characteristics of ambulatory pediatric visits in the United States (2006–2015) with or without off-label systemic drug orders
| Characteristic | No off-label drug order, % (95% CI) (N=14,772) | Off-label drug order, % (95% CI) (N=12,833) | OR | aOR1 (95% CI) | P-value | % of visits with off-label orders2, (95% CI) |
|---|---|---|---|---|---|---|
| Age group | ||||||
| <1m | 0.1 (0.1–0.3) | 0.7 (0.5–1.0) | 6.1 | 9.1 (4.5–18.3) | <0.001 | 83 (73–92) |
| 1m-<2y | 16.3 (15.4–17.4) | 17.5 (16.2–18.8) | 1.3 | 1.6 (1.4–1.8) | <0.001 | 48.7 (46.4–51.1) |
| 2–5y | 25.1 (24.0–26.3) | 22.4 (21.2–23.6) | 1.05 | 1.2 (1.05–1.3) | 0.005 | 42.4 (40.5–44.3) |
| 6–11y | 31.4 (30.3–32.6) | 26.7 (25.5–28.0) | Reference | Reference | - | 39.2 (37.4–41.0) |
| 12–17y | 27.0 (25.8–28.2) | 32.7 (31.2–34.3) | 1.4 | 1.3 (1.1–1.4) | <0.001 | 44.1 (42.4–45.9) |
| Sex | ||||||
| Male | 46.8 (45.6–47.9) | 49.3 (47.9–50.7) | Reference | Reference | - | 43.1 (41.6–44.5) |
| Female | 53.2 (52.1–54.4) | 50.7 (49.3–52.1) | 1.1 | 1.2 (1.1–1.3) | <0.001 | 46.3 (44.7–47.9) |
| Race/ethnicity | ||||||
| White, Non-Hispanic | 64.2 (61.6–66.7) | 64.4 (61.6–67.2) | Reference | Reference | - | 45.0 (43.6–46.4) |
| Black, Non-Hispanic | 11.1 (9.7–12.7) | 11.6 (10.1–13.2) | 1.04 | 0.95 (0.8–1.1) | 0.51 | 44.0 (41.0–46.9) |
| Hispanic | 18.8 (16.3–21.5) | 18.2 (15.7–21.1) | 0.97 | 0.9 (0.8–1.1) | 0.26 | 43.4 (40.6–46.1) |
| Other | 5.9 (5.1–6.9) | 5.8 (5.0–6.6) | 0.96 | 1.03 (0.9–1.2) | 0.72 | 45.6 (42.2–49.1) |
| Region | ||||||
| Northeast | 19.0 (16.8–21.5) | 16.6 (14.3–19.1) | Reference | Reference | - | 42.2 (39.4–45.1) |
| Midwest | 21.3 (18.3–24.7) | 19.4 (16.8–22.2) | 1.04 | 1.01 (0.9–1.2) | 0.91 | 42.4 (40.1–44.8) |
| South | 39.5 (35.7–43.5) | 44.7 (40.8–48.6) | 1.3 | 1.3 (1.1–1.5) | 0.002 | 47.4 (45.6–49.2) |
| West | 20.1 (17.0–23.7) | 19.3 (16.4–22.6) | 1.1 | 1.1 (0.9–1.3) | 0.49 | 43.6 (40.9–46.3) |
| Insurance | ||||||
| Private | 57.8 (55.0–60.7) | 55.2 (52.3–58.0) | Reference | Reference | - | 44.6 (43.1–46.0) |
| Public | 33.5 (30.7–36.5) | 35.3 (32.5–38.2) | 1.1 | 1.00 (0.9–1.1) | 0.97 | 44.5 (42.3–46.7) |
| Other/none | 8.6 (7.6–9.8) | 9.6 (8.3–11.0) | 1.2 | 1.04 (0.9–1.2) | 0.58 | 45.4 (42.5–48.4) |
| Clinical Specialty | ||||||
| Primary care | 84.8 (83.1–86.4) | 77.5 (74.7–80.1) | Reference | Reference | - | 43.0 (41.6–44.5) |
| Medical subspecialty | 11.9 (10.4–13.6) | 17.0 (14.5–19.8) | 1.6 | 1.2 (1.1–1.4) | 0.007 | 47.4 (44.4–50.3) |
| Surgical subspecialty | 3.3 (2.8–3.8) | 5.5 (4.8–6.3) | 1.8 | 1.9 (1.6–2.2) | <0.001 | 57.2 (53.8–60.7) |
| Total number of systemic drugs at visit | ||||||
| 1 | 77.6 (76.2–79.1) | 51.2 (49.3–53.2) | Reference | Reference | - | 35.7 (34.4–37.0) |
| 2 | 18.0 (16.9–19.2) | 30.1 (28.8–31.5) | 2.5 | 2.5 (2.3–2.8) | <0.001 | 57.7 (55.5–59.9) |
| ≥3 | 4.3 (3.6–5.1) | 18.7 (17.1–20.3) | 6.6 | 6.3 (5.3–7.6) | <0.001 | 76.9 (73.6–80.2) |
| Presence of chronic disease | ||||||
| Not present | 65.5 (63.9–67.0) | 56.8 (55.0–58.6) | Reference | Reference | - | 43.6 (42.0–45.1) |
| Present | 34.5 (33.0–36.1) | 43.2 (41.4–45.0) | 1.4 | 1.3 (1.1–1.4) | <0.001 | 48.7 (46.9–50.5) |
| Year | ||||||
| 2006–2008 | 34.3 (31.5–37.2) | 28.6 (25.8–31.6) | Reference | Reference | - | 41.9 (39.9–44.0) |
| 2009–2011 | 34.1 (31.5–36.9) | 33.5 (30.3–36.8) | 1.2 | 1.1 (0.98–1.3) | 0.11 | 44.3 (42.1–46.6) |
| 2012–2015 | 31.6 (28.8–34.5) | 37.9 (34.7–41.3) | 1.4 | 1.3 (1.1–1.4) | <0.001 | 47.2 (45.3–49.0) |
Multivariable logistic regression model adjusted for all covariates shown, accounting for sampling, clustering, and strata to produce nationally representative estimates
Percentages based on model-based predictive margins, accounting for all other covariates
Among drug categories (ATC level 1), the absolute prevalence of off-label orders was highest for anti-infectives (75 orders/1000 visits), followed by respiratory drugs and nervous system drugs (each ~54 orders/1000 visits) (Table 4). The absolute prevalence of off-label orders for all other drug categories was less than 30 orders/1000 visits. Considerable age-related variation in rates of off-label orders existed for certain drug categories, including anti-infectives, respiratory, nervous system, and genitourinary drugs, reflecting underlying differences in overall orders by age (Table 4). Of all drug categories and all age groups, absolute rates of off-label orders were highest in nervous system drugs for adolescents (123 orders/1000 visits). The relative prevalence of off-label orders was variable across drug categories (highest overall for alimentary and genitourinary drugs) and age groups (generally highest for neonates) (Table 5).
Table 4.
Absolute prevalence of off-label systemic drug orders by drug class and age for children in US ambulatory settings (2006–2015)
| Off-label orders per 1000 ambulatory visits (95% confidence intervals) | ||||||
|---|---|---|---|---|---|---|
| Drug class1 | Age <1m | Age 1m-<2y | Age 2–5y | Age 6–11y | Age 12–17y | All children |
| Anti-infectives | 13.72 (6.8, 20.5) | 60.1 (52.9, 67.2) | 88.6 (80.7, 96.6) | 78.3 (70.3, 86.2) | 79.8 (70.1, 89.5) | 74.8 (69.6, 79.9) |
| Respiratory | 0.52 (0, 1.6) | 48.0 (40.6, 55.4) | 63.5 (56.5, 70.6) | 61.0 (53.8, 68.2) | 50.1 (43.8, 56.5) | 53.8 (49.8, 57.9) |
| Nervous | 9.02 (1.2, 16.7) | 8.6 (4.3, 12.9) | 16.0 (12.8, 19.1) | 62.6 (54.2, 71.0) | 123.0 (108.9, 137.2) | 53.7 (47.8, 59.5) |
| Alimentary | 27.5 (15.8, 39.2) | 26.3 (21.9, 30.8) | 26.9 (22.1, 31.8) | 27.8 (23.5, 32.1) | 22.1 (18.8, 25.5) | 25.8 (23.2, 28.4) |
| Hormonal | ND2 | 13.4 (10.5, 16.3) | 15.8 (12.8, 18.7) | 12.2 (9.1, 15.2) | 10.3 (7.8, 12.9) | 12.4 (10.9, 13.9) |
| Cardiovascular | 02 (0, 0.1) | 1.62 (0.8, 2.4) | 3.4 (2.2, 4.6) | 6.4 (4.5, 8.4) | 8.3 (6.2, 10.4) | 4.9 (4.0, 5.9) |
| Musculoskeletal | 1.32 (0, 3.4) | 3.8 (2.5, 5.1) | 3.3 (1.8, 4.8) | 3.4 (2.2, 4.5) | 8.5 (6.4, 10.5) | 4.7 (3.9, 5.6) |
| Genitourinary | ND2 | 0.22 (0, 0.5) | 0.32 (0, 0.5) | 0.62 (0.1, 1.2) | 15.7 (12.8, 18.6) | 4.4 (3.6, 5.2) |
ND not defined
Drug class according to Anatomical Therapeutic Chemical level 1 classification
Values based on <30 observations
Table 5.
Relative prevalence of off-label systemic drug orders by drug class and age for children in US ambulatory settings (2006–2015)
| Percentage of orders that were off-label (95% confidence intervals) | ||||||
|---|---|---|---|---|---|---|
| Drug class1 | Age <1m | Age 1m-<2y | Age 2–5y | Age 6–11y | Age 12–17y | All children |
| Anti-infectives | 86.32 (57.2, 96.7) | 33.9 (30.6, 37.2) | 33.2 (31.0, 35.5) | 36.9 (34.1, 39.7) | 43.6 (40.5, 46.8) | 36.9 (35.2, 38.6) |
| Respiratory | 1002 (ND) | 85.2 (80.9, 88.7) | 41.0 (37.6, 44.5) | 36.7 (33.5, 40.1) | 40.8 (37.7, 44.0) | 44.3 (42.3, 46.4) |
| Nervous | 50.52 (24.1, 76.6) | 11.4 (7.3, 17.4) | 19.3 (16.0, 23.1) | 31.2 (28.6, 34.0) | 49.2 (46.8, 51.6) | 35.2 (33.3, 37.2) |
| Alimentary | 98.8 (91.5, 99.8) | 72.9 (67.3, 77.8) | 84.1 (78.6, 88.5) | 73.8 (67.7, 79.1) | 52.9 (46.0, 59.7) | 70.0 (65.9, 73.7) |
| Hormonal | ND2 | 64.9 (56.7, 72.2) | 47.4 (40.6, 54.3) | 34.7 (28.6, 41.4) | 36.1 (28.2, 44.9) | 43.5 (39.5, 47.6) |
| Cardiovascular | 1002 (ND) | 11.12 (8.3, 14.8) | 5.7 (3.7, 8.7) | 6.2 (4.6, 8.5) | 11.0 (8.8, 13.6) | 8.7 (7.4, 10.1) |
| Musculoskeletal | 2.72 (0.1, 35.6) | 42.5 (24.1, 63.3) | 86.1 (69.5, 94.4) | 30.9 (23.4, 39.5) | 38.7 (30.3, 47.7) | 39.0 (32.8, 45.6) |
| Genitourinary | ND2 | 1002 (ND) | 86.52 (41.7, 98.3) | 42.52 (19.2, 69.7) | 68.8 (61.9, 74.9) | 67.7 (61.3, 73.5) |
ND not defined
Drug class according to Anatomical Therapeutic Chemical level 1 classification
Values based on <30 observations
According to drug class (ATC level 3), antihistamines were the drug class most commonly ordered off-label, followed by penicillins; macrolides plus clindamycin; antidepressants; and cephalosporins (Supplemental Table 2). Within each drug class, the drugs most commonly ordered off-label tended to reflect the prevalence of orders overall; however, the relative prevalence of off-label orders occasionally diverged for drugs within the same class (e.g., antidepressants: sertraline 93%, fluoxetine 37%; nonsteroidal anti-inflammatory drugs (NSAIDs): ibuprofen 5%; diclofenac 100%) (Supplemental Table 3). The most common diagnoses in visits with off-label orders generally corresponded to off-label indications (e.g., antihistamines for upper respiratory tract infections, antidepressants for attention deficit/hyperactivity disorder [ADHD]) (Supplemental Table 2). However, certain more common diagnoses corresponded to indications approved for some but not all drugs within the class (e.g., amoxicillin-clavulanate, not approved for pharyngitis) or drugs ordered off-label by age (e.g., stimulants for young children with ADHD, laxatives for children with constipation). Some diagnoses for visits with off-label drugs were related to approved indications (e.g., antihistamines for asthma) yet without documentation of the related conditions (e.g., allergic rhinitis). Age groups differed in the drug classes most commonly ordered off-label (Supplemental Tables 4–8) and the most common diagnoses for off-label ordering (Supplemental Table 9).
Trends of Off-Label Orders
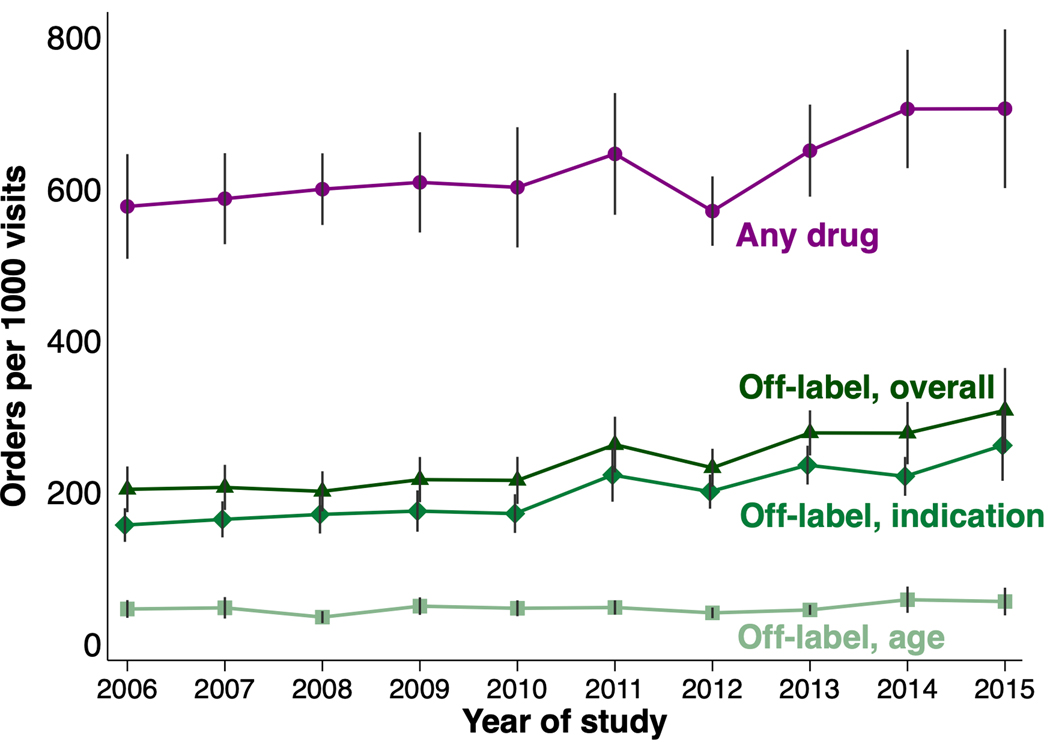
Absolute rates of off-label drug orders increased throughout the study period, predominantly reflecting a rise in off-label orders by indication (Figure). After adjusting for other factors, relative rates of off-label ordering were also higher in later years (47.2% in 2012–2015 vs. 41.9% in 2006–2008) (Table 3). In analyses by drug class, absolute rates of off-label orders rose over time for numerous classes, including antihistamines, several classes of psychotropic drugs (antidepressants, stimulants, antiepileptics, antiadrenergics), anti-inflammatory drugs (corticosteroids, NSAIDs), and certain GI drugs (reflux drugs, antiemetics) (Supplemental Figures 1–3). Relative changes over time in class-specific off-label orders generally paralleled the observed changes in absolute rates; relative declines were seen for penicillins and antipsychotics along with a marked increase for antiemetics (Supplemental Figures 4–6).
Figure.
Yearly absolute prevalence of systemic drug orders for children in US ambulatory settings (2006–2015)
Yearly national rates of orders per 1000 ambulatory visits between 2006 and 2015 for any systemic drug (purple) and systemic drugs ordered off-label (based on age [light green], indication [medium green], or age, indication, and/or weight [dark green]). Error bars represent 95% confidence intervals.
Sensitivity Analyses
In sensitivity analyses, a broader definition of off-label drugs (including possibly off-label orders) produced slightly higher estimates of absolute off-label prevalence and modestly changed the order of the most commonly off-label drug classes; overall the findings were highly consistent (Supplemental Figure 7, Supplemental Tables 10–14).
DISCUSSION:
Across 10 years of national US data, office-based physicians ordered ≥1 systemic off-label drug in nearly 1 in 5 of all pediatric visits, most commonly for an unapproved condition. In absolute terms, visits for adolescents most commonly resulted in off-label orders, but in relative terms, off-label ordering was highest among neonates, for whom roughly 5 of 6 systemic drugs ordered were off-label. Relative rates of off-label orders were also higher in visits for girls, for children with a chronic condition and multiple drug orders, for subspecialists, and in the US South. The drug classes most commonly ordered off-label were antihistamines (especially for respiratory infections and conditions), several classes of antibiotics (especially for viral infections), and antidepressants (especially for ADHD). Gastrointestinal drugs were more commonly ordered off-label for infants, while off-label psychotropic drug ordering was highest for adolescents. In both absolute and relative terms, off-label ordering has risen over time, most notably for unapproved conditions. Rates of off-label ordering have increased for antihistamines and several class of psychotropic, anti-inflammatory, and GI drugs, while off-label orders for certain antibiotic classes and antipsychotics have declined.
An older study examining pediatric off-label drug orders using the same data source (NAMCS, 2001–2004) found that over 60% of visits with prescribed drugs included off-label orders, significantly higher than our estimated 45%.15 Despite the common data source, that study differed from ours in its inclusion of non-systemic drugs and exclusion of OTC drugs. Our study also used additional information (i.e., chronic disease checkboxes and reasons for visit) to establish concordance with labeled indications, which may have also contributed to the lower estimates of off-label orders. Other pediatric studies from US ambulatory settings focusing on antidepressants and antipsychotics have reported similar (and high) relative rates and reasons for off-label prescribing.25, 26
Our findings were also consistent with declining trends in antibiotic prescribing over the last 20 years, particularly for certain drug classes, including penicillins and cephalosporins.27, 28 Our study also corroborates a recent US antibiotic utilization study showing that approximately 1/3 of antibiotics dispensed for commercially insured children were either clinically inappropriate or without documented diagnoses, while nearly half of all fills were of questionable appropriateness.29 On the other hand, certain class-specific changes in off-label orders (e.g., for antihistamines, psychotropics) contrast with overall pediatric prescribing trends reported elsewhere.28 These discrepancies may relate to our focus on off-label orders as well as differences in data source and exposure definitions: our study in NAMCS captured physician-reported data on prescribed and recommended OTC drugs, while a study using NHANES focused on self-reported data on prescribed (not OTC) drugs taken (not ordered) in the prior month.
Our findings show lower rates of off-label orders than in other studies from acute and critical care settings.30 While hospitalized children are a high-risk group for drug-related adverse effects, the vast majority of children are treated exclusively in outpatient settings, where tolerance of risk is also much lower.
It is more difficult to compare our findings with pediatric studies of outpatient off-label drugs from other countries, given the differences in pediatric populations (including age ranges considered), prescribing practices, definitions of off-label usage (age alone, consideration of dose, etc.), and number and types of drugs considered.3, 11, 17, 21, 31, 32 A recent utilization study from southwestern France also showed that indication was a more common reason for off-label prescribing than age or dose, with relatively high rates of off-label prescribing of antihistamines, antibiotics, and among children prescribed multiple drugs.16 Overall rates of off-label prescribing were lower overall (as with multiple other European studies), but this may relate in part to their consideration of non-systemic drugs and to their assumption that antibiotics prescribed for viral infections were not off-label, along with geographic differences in prescribing.3, 11, 16
While prescribing and use of medicines off-label is common in pediatrics, off-label drug use is not always off-evidence.1, 5 Certain more common indications we identified as off-label were, in fact, supported by high-quality evidence, e.g., glucocorticoids for croup and ondansetron for vomiting.33, 34 Indeed, frequency of off-label use is only one of several aspects that should be considered when prioritizing pediatric drug research and policies, including the potential for benefit (e.g., common diseases as well as rare diseases with high morbidity or mortality), risk of adverse effects (including from drug-drug interactions), level of uncertainty about relative risks and benefits, and availability of therapeutic alternatives.35–37 While legislation in the US and Europe has stimulated research and approvals of drugs for children, recent studies have shown that completion of required pediatric trials have been delayed beyond the FDA-mandated times.38–40 Additionally, the reporting of results from some required studies in trial registries (e.g., ClinicalTrials.gov) and peer-reviewed journals has been incomplete (e.g., 15–24% of completed studies without reported results) and suboptimal (e.g., missing elements about study design and reasons for study discontinuation).38, 39 Furthermore, efforts to advance research about off-patent drugs, including the BPCA priority list and the European Pediatric Use Marketing Authorization, may be insufficient in producing all of the necessary evidence for safe and effective use of off-patent medicines in children.10, 36, 41, 42 Our study’s focus on systemic drugs (given their greater potential for toxicity) and enumeration of the most common indications with off-label orders, complement other efforts to prioritize research and policies for off-label, off-evidence medicines in children.
Our study had several strengths, including its long study period, large and nationally representative study population, and comprehensive evaluation of over 140 of the most common systemic drugs ordered for children in clinics across the US. These data enabled us to identify recent increases in off-label ordering, highlight the drugs and conditions with the most off-label orders, and identify factors associated with off-label drugs that have not be reported in smaller studies.
This study also had limitations. The cross-sectional data within NAMCS may have limited ascertainment of all relevant indications for drug orders, including historical diagnoses. We excluded less commonly ordered drugs, whose concordance with labeling may have been different than included medications. We were also unable to ascertain drug formulation or dosage, which is a common reason for off-label usage in some settings, thus resulting in a systematic underestimation in overall off-label use.30, 43, 44 NAMCS provides data on medicines ordered by physicians, which include medicines not dispensed to or consumed by patients and do not include OTC medicines not ordered by physicians or received in other settings (e.g., inpatient). Finally, we did not formally evaluate the evidence behind off-label drugs and indications reported in this study.
CONCLUSION:
In summary, office-based physicians commonly order systemic drugs for children off-label, particularly for unapproved conditions. Despite legislation to generate more data on the effects of drugs in children, off-label orders have risen in recent years, most notably of antihistamines and psychotropic drugs, such as antidepressants. The rates and reasons for off-label orders vary by age, with more off-label orders for GI conditions in the youngest age groups, for psychiatric conditions in older age groups, and for infections and respiratory conditions across age groups. These results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children.
Supplementary Material
What’s Known on This Subject:
In past studies, pediatric off-label use was shown to be common and potentially harmful, but researchers have focused predominantly on acute care settings, specific classes of drugs, outpatient prescribing outside the United States, or older data sources.
What This Study Adds:
In 2006–2015, US office-based physicians ordered systemic drugs off label for children at rising rates, particularly for unapproved conditions. Updated data on common off-label drugs, classes, and unapproved conditions treated in children will help inform education, research, and policies.
Acknowledgment:
Brian Cao and Matthew Del Signore assisted with manual reviews of visit data, and Edward Nonnenmacher assisted with data collection and presentation. Research reported in this publication was supported by the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers K23-AR070286 and L40-AR070497. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosure: Dr. Gerhard has received grant funding on unrelated matters from BMS and has consulted on unrelated matters for BMS and Eli Lilly. Dr. Strom has consulted on unrelated matters for AstraZeneca, Bayer, Celgene, Janssen, Lundbeck, Innovative Science Solutions LLC, and McKesson Specialty Arizona Inc. Dr. Horton has received grant funding on unrelated matters from BMS. Ms. Hoon, Ms. Kapadia, and Mr. Taylor have no financial relationships relative to this article to disclose.
Funding Source: Funding was provided by National Institutes of Health/NIAMS and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship.
Abbreviations:
- ADHD
attention deficit/hyperactivity disorder
- ATC
Anatomical Therapeutic Chemical
- BPCA
Best Pharmaceuticals for Children Act
- CI
confidence intervals
- FDA
Food and Drug Administration
- ICD-9
International Classification of Diseases 9th Edition
- NAMCS
National Ambulatory Medical Care Surveys
- OTC
over-the-counter
- PREA
Pediatric Research Equity Act
Footnotes
Potential Conflicts of Interest: The authors have no potential conflicts to disclose.
References:
- 1.Neville KA, Frattarelli DAC, Galinkin JL, et al. Off-label use of drugs in children. 2014;133(3):563–567. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre J, Conroy S, Avery A, Corns H, Choonara I. Unlicensed and off label prescribing of drugs in general practice. Arch Dis Child. 2000;83(6):498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–558. [DOI] [PubMed] [Google Scholar]
- 4.Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88(9):965–968. [DOI] [PubMed] [Google Scholar]
- 5.Ito S. Drugs for Children. Clin Pharmacol Ther. 2017;101(6):704–706. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez W, Selen A, Avant D, et al. Improving pediatric dosing through pediatric initiatives: what we have learned. Pediatrics. 2008;121(3):530–539. [DOI] [PubMed] [Google Scholar]
- 7.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290(7):905–911. [DOI] [PubMed] [Google Scholar]
- 8.Bucci-Rechtweg C. Enhancing the Pediatric Drug Development Framework to Deliver Better Pediatric Therapies Tomorrow. Clin Ther. 2017;39(10):1920–1932. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois FT, Hwang TJ. The Pediatric Research Equity Act Moves Into Adolescence. JAMA. 2017;317(3):259–260. [DOI] [PubMed] [Google Scholar]
- 10.European Commission. State of Paediatric Medicines in the EU: 10 years of the EU Paediatric Regulation. https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf. Published 2017. Accessed April 18, 2018.
- 11.Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf. 2006;5(5):703–718. [DOI] [PubMed] [Google Scholar]
- 12.Mohr JJ, Lannon CM, Thoma KA, et al. Learning from Errors in Ambulatory Pediatrics In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in Patient Safety: From Research to Implementation (Volume 1: Research Findings; ). Rockville (MD)2005. [Google Scholar]
- 13.Schroder C, Dorks M, Kollhorst B, et al. Outpatient antidepressant drug use in children and adolescents in Germany between 2004 and 2011. Pharmacoepidemiol Drug Saf. 2017;26(2):170–179. [DOI] [PubMed] [Google Scholar]
- 14.Lai LL, Koh L, Ho JA, Ting A, Obi A. Off-Label Prescribing for Children with Migraines in U.S. Ambulatory Care Settings. J Manag Care Spec Pharm. 2017;23(3):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazzano AT, Mangione-Smith R, Schonlau M, Suttorp MJ, Brook RH. Off-label prescribing to children in the United States outpatient setting. Acad Pediatr. 2009;9(2):81–88. [DOI] [PubMed] [Google Scholar]
- 16.Palmaro A, Bissuel R, Renaud N, et al. Off-label prescribing in pediatric outpatients. Pediatrics. 2015;135(1):49–58. [DOI] [PubMed] [Google Scholar]
- 17.Sturkenboom MC, Verhamme KM, Nicolosi A, et al. Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schirm E, Tobi H, de Jong-van den Berg LT. Unlicensed and off label drug use by children in the community: cross sectional study. BMJ. 2002;324(7349):1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schirm E, Tobi H, de Jong-van den Berg LT. Risk factors for unlicensed and off-label drug use in children outside the hospital. Pediatrics. 2003;111(2):291–295. [DOI] [PubMed] [Google Scholar]
- 20.Carnovale C, Conti V, Perrone V, et al. Paediatric drug use with focus on off-label prescriptions in Lombardy and implications for therapeutic approaches. Eur J Pediatr. 2013;172(12):1679–1685. [DOI] [PubMed] [Google Scholar]
- 21.Olsson J, Kimland E, Pettersson S, Odlind V. Paediatric drug use with focus on off-label prescriptions in Swedish outpatient care--a nationwide study. Acta Paediatr. 2011;100(9):1272–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics, Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey. https://www.cdc.gov/nchs/ahcd/about_ahcd.htm. Published 2017. Accessed February 27, 2017.
- 23.Prescribers’ Digital Reference. http://www.pdr.net. Published 2018. Accessed January 2, 2018.
- 24.U.S. Food and Drug Administration. Drugs. https://www.fda.gov/Drugs/default.htm. Published 2018. Accessed January 3, 2018.
- 25.Lee E, Teschemaker AR, Johann-Liang R, et al. Off-label prescribing patterns of antidepressants in children and adolescents. Pharmacoepidemiol Drug Saf. 2012;21(2):137–144. [DOI] [PubMed] [Google Scholar]
- 26.Sohn M, Moga DC, Blumenschein K, Talbert J. National trends in off-label use of atypical antipsychotics in children and adolescents in the United States. Medicine (Baltimore). 2016;95(23):e3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frenk SM, Kit BK, Lukacs SL, Hicks LA, Gu Q. Trends in the use of prescription antibiotics: NHANES 1999–2012. J Antimicrob Chemother. 2016;71(1):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hales CM, Kit BK, Gu Q, Ogden CL. Trends in Prescription Medication Use Among Children and Adolescents-United States, 1999–2014. JAMA. 2018;319(19):2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chua KP, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patients: ICD-10-CM based cross sectional study. BMJ. 2019;364:k5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magalhaes J, Rodrigues AT, Roque F, Figueiras A, Falcao A, Herdeiro MT. Use of off-label and unlicenced drugs in hospitalised paediatric patients: a systematic review. Eur J Clin Pharmacol. 2015;71(1):1–13. [DOI] [PubMed] [Google Scholar]
- 31.Lass J, Irs A, Pisarev H, Leinemann T, Lutsar I. Off label use of prescription medicines in children in outpatient setting in Estonia is common. Pharmacoepidemiol Drug Saf. 2011;20(5):474–481. [DOI] [PubMed] [Google Scholar]
- 32.Langerova P, Vrtal J, Urbanek K. Incidence of unlicensed and off-label prescription in children. Ital J Pediatr. 2014;40:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8:CD001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchetti F, Bonati M, Maestro A, et al. Oral Ondansetron versus Domperidone for Acute Gastroenteritis in Pediatric Emergency Departments: Multicenter Double Blind Randomized Controlled Trial. PLoS ONE. 2016;11(11):e0165441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazarian M. Off-label use of medicines in the paediatric populations: recommendations for assessing appropriateness. World Health Organization; 2007:25. [Google Scholar]
- 36.Czaja AS, Fiks AG, Wasserman RC, Valuck RJ, Comparative Effectiveness Research Through Collaborative Electronic Reporting C. Beyond the Label: Steering the Focus Toward Safe and Effective Prescribing. Pediatrics. 2017;139(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinstein JA, Morrato EH, Feudtner C. Prioritizing Pediatric Drug Research Using Population-Level Health Data. JAMA Pediatr. 2017;171(1):7–8. [DOI] [PubMed] [Google Scholar]
- 38.Hwang TJ, Tomasi PA, Bourgeois FT. Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study. PLoS Med. 2018;15(3):e1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion Rate and Reporting of Mandatory Pediatric Postmarketing Studies Under the US Pediatric Research Equity Act. JAMA Pediatr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudgins JD, Bacho MA, Olsen KL, Bourgeois FT. Pediatric drug information available at the time of new drug approvals: A cross-sectional analysis. Pharmacoepidemiol Drug Saf. 2018;27(2):161–167. [DOI] [PubMed] [Google Scholar]
- 41.Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Best Pharmaceuticals for Children Act Priority List. https://bpca.nichd.nih.gov/prioritization/priority_lists/Pages/priority_list.aspx. Published 2018. Accessed March 3, 2019.
- 42.Wimmer S, Rascher W, McCarthy S, Neubert A. The EU paediatric regulation: still a large discrepancy between therapeutic needs and approved paediatric investigation plans. Paediatr Drugs. 2014;16(5):397–406. [DOI] [PubMed] [Google Scholar]
- 43.Ballard CD, Peterson GM, Thompson AJ, Beggs SA. Off-label use of medicines in paediatric inpatients at an Australian teaching hospital. J Paediatr Child Health. 2013;49(1):38–42. [DOI] [PubMed] [Google Scholar]
- 44.Czarniak P, Bint L, Favie L, Parsons R, Hughes J, Sunderland B. Clinical setting influences off-label and unlicensed prescribing in a paediatric teaching hospital. PLoS ONE. 2015;10(3):e0120630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.