Abstract
Viral emergence is an unpredictable but obvious event, particularly in the era of climate change and globalization. Efficient management of viral outbreaks depends on pre-existing knowledge and alertness. The potential hotspots of viral emergence often remain neglected and the information related to them is insufficient, particularly for emerging viruses. Viral replication and transmission rely upon usurping the host metabolic machineries. So altered host metabolic pathways can be exploited for containment of these viruses. Metabolomics provides the insight for tracing out such checkpoints. Consequently introspection of metabolic alteration at virus-host interface has evolved as prime area in current virology research. Chromatographic separation followed by mass spectrometry has been used as the predominant analytical platform in bulk of the analyses followed by nuclear magnetic resonance (NMR) and fluorescence based techniques. Although valuable information regarding viral replication and modulation of host metabolic pathways have been extracted but ambiguity often superseded the real events due to population effect over the infected cells. Exploration of cellular heterogeneity and differentiation of infected cells from the nearby healthy ones has become essential. Single cell metabolomics (SCM) emerges as necessity to explore such minute details. Mass spectrometry imaging (MSI) coupled with several soft ionization techniques such as electrospray ionization (ESI), laser ablation electrospray ionization (LAESI), matrix assisted laser desorption/ionization (MALDI), matrix-free laser desorption ionization (LDI) have evolved as the best suited platforms for SCM analyses. The potential of SCM has already been exploited to resolve several biological conundrums. Thus SCM is knocking at the door of virus-host interface.
Keywords: single cell metabolomics, virus-host interaction, emerging viruses, mass spectrometry, imaging
Introduction
“The risk from viruses is an unanswered question.” is such a meaningful quote with multi-directional implications. The enormous genetic diversity of existing viruses and unpredictability of viral behavior complemented by inadequacy of knowledge about global virome database always poses potential threats over the entire living world to acquire infection from any emerging or re-emerging viruses. Rapid climate change, increased traveling in the era of globalization and frequent mingling of sylvatic and urban lifecycle have also potentiated the viruses for genetic undulation, emergence and re-emergence to become pandemic at occasions (Devaux, 2012). Keen introspection regarding the virus-host interaction is a primordial aspect to reduce the knowledge gap and prepare the answer for the upcoming viral outbreaks. Exploitation of the highly specific nature of the virus-host interaction as well as the virus-cell interaction which are key for completion of viral life cycle and their transmission can offer crucial information to combat emerging viral infections. The fundamental cellular components required for viral replication and the host restriction factors to counter them can be of immense value to decide the checkpoints and therapeutic targets for customizing effective anti-viral mechanisms (Lassen, 2011). Single cell omics tools although in its budding age, still have enough penetration and resolution to introspect at the cellular and sub-cellular level for elucidation of such useful information. Progress in this arena is going on apace which will enable to explore the changing pattern of the viral interactions in cellular heterogeneity under variable ecosystem and host subjects. Suitable theranostics and effective vaccine of broad host specificity by unveiling the common pathways of viral interaction are the need of the hour to counter the ever-ending viral heterogeneity and future pandemics. The security of “One Health” concept in current scenario depends immensely on symbiotic development and convergence of several high throughput techniques such as but not limited to single cell omics, nanotechnology, artificial intelligence and various computational platforms (Minakshi et al., 2019a). The present review will focus toward various developments in single cell metabolomics tools and their few existing and mostly the probable future applications in unveiling the host virus interactions to acquire new armory in the arsenal for combating the emerging viral diseases.
Single Cell Metabolomics (SCM)
“Omics” technologies pertain to the entire sets of molecules expressed at cellular or tissue level, or within an organism under specific set of condition. However cellular heterogeneity in multicellular organisms is often masked in pooled analyses. There the importance of single cell omics can be apprehended because it holds the potential to identify several targets expressed within a single individual cell or a cell population of interest individually at a specified time, or analyze handful of parameters in the same cells over time (Minakshi et al., 2019a). The enormous utility and popularity of omics tools have left almost no stone untouched in any of the biological research arena; the case is also not unlike in unveiling virus host interaction. As single cell omics tools are mostly in its naïve stage, thus ample evidences of their application at virus host interface is currently lacking but surely not too far in future which can be easily predicted from the bulk of omics interventions in terms of genomics, transcriptomics, proteomics, metabolomics and interactomics in the current perspective. Other single cell omics techniques have been employed to explore virus-host interaction in recent years (Dhillon and Li, 2015; Labonté et al., 2015; Drayman et al., 2017, 2019; Zanini et al., 2018; Rosenwasser et al., 2019; Russell et al., 2019; Wang S. et al., 2019). However SCM techniques are yet to start contributing significantly in understanding virus-host interaction.
Metabolites are smaller molecules, usually of lesser than 1.5 kD in size reflecting various cellular pathways which include sugar, lipids, glycolytic products (pyruvate, lactate, etc.), phosphate compounds (AMP, ADP, and ATP etc.), xenobiotics etc., but generally excludes nucleic acids, minerals, and salts (Minakshi et al., 2019a, b). They are arguably the terminal product of the basic central dogma process and provide the most nascent information about the phenotype. Metabolomics refers to the study of entire set of metabolites at a specific time-point. It is advantageous in understanding host-virus interaction in terms of generating immediate information regarding the exact downstream effects and ultimate fates of the analytes. It can yield a snap shot of dynamic and vulnerable host metabolites in response to its viral counterpart modulating each pathways at a particular infection stage. A recent flush of literature in metabolomics targeting virus-host interaction vividly justifies the utility of such analysis in current context (Table 1). Precise sampling methods accompanied by robust and high throughput analytical platforms such as nuclear magnetic resonance (NMR), receptive separation-based methods such as capillary electrophoresis (CE) or liquid chromatography (LC) and gas chromatography (GC) coupled with mass spectrometry (MS), and fluorescence-based techniques have delivered significant precision to “metabolomics.” Parallel progress in metabolite databases and bioinformatics programs have also assisted to generate an array of valuable information in a single go to match the expectations (Minakshi et al., 2019a). However single cell metabolomics (SCM) is obviously beneficial over population study alike other omics techniques to obtain specific temporal information regarding cell differentiation and division, communication, interaction with surroundings, stress responses including stage-specific metabolomic changes during viral infection (Strzelecka et al., 2018).
TABLE 1.
List of application and salient findings of metabolomics techniques in emerging viral diseases.
| Sl. no | Virus | Sample | Technique | Finding | References |
| 1. | DENV | Plasma | 1H nuclear magnetic resonance (NMR) | Low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) decreased in all patient was same for lipoprotein in severe cases indicating importance of lipid metabolism in progression, Further Level of glutamine can have prognosis value | El-Bacha et al., 2016 |
| 2. | Early febrile, defervescence, and convalescent stages DENV | Serum | Liquid chromatography- mass spectrometry (LCMS) | Sixty differential metabolites, mainly affecting pathways of fatty acid biosynthesis and β-oxidation, phospholipid catabolism, steroid hormone pathway had been identified. further study revealed most metabolome changes are reversible and highest change occurs at early febrile including increase of serum inosine, cortisol, GlcCer | Cui et al., 2013 |
| 3. | H1N1 virus | Serum | UPLC - LCMS | Array of metabolites were present differentially pre and post treatment regime including 30 metabolites which can be potential biomarkers affecting multi-metabolic pathways including immune system arachidonic acid | Chuanjian et al., 2012 |
| 4. | DEN-1, DEN-2, DEN-3, and DEN-4 | EA.hy926 cell line | H NMR and MS | Found alteration in metabolites like amino acids, dicarboxylic acids, fatty acids, and organic acids at significant level related to the tricarboxylic acid (TCA) cycle | Grace et al., 2010 |
| 5. | Dengue | Urine | 1H NMR | Various compounds like organic acids, betaine, valeryl glycine, myo-inositol and glycine and amino acids were distinctly varied in patient while reduction in N-acetylglutamic acid, creatinine, myo-inositol, cre-atine phosphate, succinic acid, citrate, and 3-hydroxy-3- methylglutarate was also reported indicating amino acid metabolism, tricarboxylic acid intermediates cycle and β-oxidation of fatty acids were altered hence proposed highly predictive model for diagnosis by urine | Nurul et al., 2017 |
| 6. | Chikungunya and dengue | Serum | NMR spectroscopy | Fever, inflammation, energy deprivation and joint pain pathways were effected indicated by study | Shrinet et al., 2016 |
| 7. | Hepatitis C virus | Urine samples | NMR | 66 patient study revealed metabolic fingerprint’ with 94% sensitivity and specificity of 97% with accuracy of 95% for non-invasive diagnosis | Godoy et al., 2010 |
| 8. | Hepatitis B | Serum | GC-MS | In a study on 47 patient a total of 25 metabolites identified were glyceric acid, cis-aconitic acid and citric acid were diagnosed with 93.62% predictive accuracy for diagnosis | Mao et al., 2008 |
| 9. | Hepatitis E | Plasma and urine | H NMR | Used for diagnosis 99.1 and 92.9% accuracy with reported various significant changes of metabolites indicating alteration of glycolysis, tricarboxylic acid cycle, urea cycle, and amino acid metabolism pathways more particularly amino acid metabolism, as aromatic amino acid (AAAs) | Munshi et al., 2011 |
| 10. | HIV/AIDS patients | Plasma, urine, and saliva | NMR | Twenty-six metabolites expressed differentially between ART-naïve patients and those receiving therapy leading to significant alternations in 12 metabolic pathways were have at least 2 metabolites are differentially regulated further proposed urinary Neopterin, and plasma Choline and Sarcosine could be used as metabolic biomarkers of HIV/AIDS | Munshi et al., 2013 |
| 11. | HIV-1 | Serum | H NMR | 34 HIV-1 positive 29 with treatment and 5 no treatment, techniques could distinguish between HIV-1 positive/AIDS patients | Hewer et al., 2006 |
| 12. | HIV | Oral ringe | LC/MS/MS and GC/MS | In study on 12 HIV-infected and 12 healthy individual, 98 identifiable and 85 non-identifiable metabolites identified of which 12 increased and 15 decreased altering carbohydrate biosynthesis and degradation pathways | Mahmoud et al., 2013 |
| 13. | HIV | Serum | Fourier transform Infrared (FTIR) spectroscopy | Quick method to diagnose | Lungile et al., 2014 |
| 14. | DENV-4 | Serum | LC MS/MS | Three precursors of Platelet Activation Factor (PAF), three Phosphatidylcholine derivatives, four triglycerides as characteristic for DENV infection was identified affecting synthesis of PAF, remodeling inflammatory pathways, immune response autophagy process | Melo et al., 2018 |
| 15. | H1N1 influenza virus | Lung tissue samples from mice | LCMS | Total of 4,552 m/z ions (metabolic features) having 549 metabolites of 25 different pathways detected of which 49 features vary differentially including 10 decrease including methylniacinamide (metabolic product of niacin and Trp), pyrroline-5-carboxylate, amino acid asparagine, and hexaprenyl hydroxybenzoic acid and 39 increases includes cytidine, cytosine), a dipeptide breakdown product of protein cross-linking (_-glutamyl-lysine), kynurenic acid (Trp metabolite), and the essential amino acid, threonine in disease condition | Joshua et al., 2016 |
| 16. | Influenza | Serum, lung tissue and bronchoalveolar lavage fluid (BALF) of t 0, 6, 10, 14, 21, and 28 days post infection (dpi) | LC MS | More than 100 differential metabolites recorded, some indicating role of surfactant system in etiology, growth and control of virus | Cui et al., 2016b |
| 17. | Influenza A virus | A549 and AGS cell line | GC/MS | AGS cell line might be more susceptible, Fatty acid biosynthesis and cholesterol metabolism as the target as emphasized by 12 and 10 metabolites differential expression. | Lin et al., 2010 |
| 18. | Nfluenza-associated encephalopath | Cerebrospinal fluid (CSF) | FT-ICMS | Two molecular weights, 246.0092 and 204.061 can be used for diagnose influenza with convulsion. It may be C6H13NaO6, C6H14O6(possibly mannitol), or C12H12OS | Kawashima et al., 2006 |
| 19. | H3N2 influenza infection or dengue fever | Serum | LC-MS | Comparison between two virus metabolome revealed 26 differential metabolites involved in purine metabolism, fatty acid biosynthesis and β-oxidation, tryptophan metabolism, phospholipid catabolism and steroid hormone pathway | Cui et al., 2017a |
| 20. | DENV serotype 2 infection at 0, 3, 7, 14, and 28 days postinfection | Humanized mice serum | LC-MS | Forty-eight differential metabolites were identified altered significantly indicating changes various pathways in pyrimidine metabolism, fatty acid β-oxidation, phospholipid catabolism, arachidonic acid and linoleic acid metabolism, sphingolipid metabolism, tryptophan metabolism, phenylalanine metabolism, lysine biosynthesis and degradation, and bile acid biosynthesis | Cui et al., 2017b |
| 21. | Chronic hepatitis B | Serum | Targeted LCMS | In study n Sixty-nine chronic HBV patients and 19 healthy controls given 314 metabolites where decrease hijacking of the glycerol-3-phosphate–NADH shuttle occurs while Oxylipins were increased | Schoeman et al., 2016 |
| 22. | Human cytomegalovirus (HCMV) and herpes simplex virus type-1 | Fibroblast and epithelial host cells | LC-MS | 80 metabolites detected reveled metabolic changes were conserved in different type of host cell | Vastag et al., 2011 |
| 23. | Hepatitis C | Huh-7.5 cells (human hepatoma cell line) | LC-MS | Identify 272 lipids at different concentration and time variations, necessary for virus replication, downstream assembly and secretion | Diamond et al., 2010 |
| 24. | Influenza vaccine strain | MDCK cells | LCMS | 0–12 h post infection study average time of metabolic switch for all 29 metabolites included was 11.6 h | Ritter et al., 2010 |
| 25. | Human cytomegalovirus (HCMV) | Human fibroblasts | LCMS | 63 different intracellular metabolites at multiple times of infection were identified | Munger et al., 2008 |
| 26. | Adenovirus | Breast epithelial cell line MCF10A | Product E4ORF1 localizes to the nucleus and binds the transcription factor glutaminase critical enzyme for HIV, Influenza & adenovirus replication | Thai et al., 2014 | |
| 27. | Rhinovirus (RV) | HeLa and Fibroblasts Female C57BL/6 J mice | UPLC-MS/MS | Showed metabolites associated with glycogenolysas glucose deprivation from medium and via glycolysis inhibition by 2-deoxyglucose (2-DG) potently impairs viral replication, glucose and fatty acid uptake were up-regulated after 1.5 h in primary human fibroblasts. Further revealed a specific fingerprint of RV infection can be use for diagnosis | Guido et al., 2018 |
| 28. | HIV | CD4+ T cells and macrophages U937 cell line | FACS sorting and LC- MS/MS | A total of 66 metabolite identified, infected CD4+ T cells In macrophages substantial reductions in glucose uptake and steady state glycolytic intermediate, Showed cell types exhibit very different metabolic outcomes | Hollenbaugh et al., 2011 |
| 29. | HIV-1 and HIV-2 | Monocyte-derived macrophages (MDMs) | LC–MS/MS | Macrophage infected with both type of virus have similar metabolic profiles in relation to glycolysis and TCA cycle | Hollenbaugh et al., 2016 |
| 30. | Hepatitis C virus | Whole cell extracts and subcellular compartments of HEK293T cells | LC-MS/MS | Membrane lipids, especially cholesterol and phospholipids, accumulated in the microsomal fraction in HCV-infected cells, phosphatidylcholines and triglycerides with longer fatty acyl chains were higher so the utilization of C18 fatty acids | Hofmann et al., 2018 |
| 31. | Zika virus (ZIKV) | Serum | ESI-MSMS | 79 metabolites analysis revealed Glycosphingolipids Ganglioside GM2, in the nervous tissue was elevated and proposed as marker so was series of phosphatidylinositols. Study also revealed upregulation of Angiotensin and Angiotensin I., Furthermore it also described first time that lipids for ZIKV infection are described | Melo et al., 2017 |
| 32. | Respiratory Syncytial Virus (RSV) is | Lung epithelial cellsA549 cells, human alveolar epithelial like cells | LCMS | 122 oxylipins were identified and analyzed | Schoeman, 2020 |
| 33. | Respiratory Syncytial Virus (RSV) is | Lung epithelial cells A549 cells, human alveolar epithelial like cells, BALB/c mice bronchoalveolar lavage and infant nasopharyngeal secretions | LCMS | A total of 74 amine metabolites were identified indicating down-regulate key anti-oxidant enzymes reveled by the fact compromised sulfur metabolism, glutathione and taurine while increased cysteine and cystathionine | Schoeman, 2020 |
| 34. | Hepatitis B virus (HBV) | Targeted metabolomics approach on serum | Reveled reduced glycerophospholipids and increased plasmalogen Species in immune tolerant phase indicating viral hijacking of the glycerol-3-phosphate-NADH shuttle while in chronic condition, increased very long chain triglycerides, citrulline and ornithine was observed in study over 88 samples where a total 92 significant metabolites were identified | Schoeman, 2020 | |
| 35. | HIV-infected women | Effect on infant, cord blood plasma | LC-MS targeted metabolomics analyses | Increased triglyceride, oxidized lipids, pro-inflammatory lysophospholipids, 2 diacylglycerols and 32 triglycerides and decreased phospholipid was reveled on analysis of 12 positive HEU infants and 15 HUU infants negative infant | Schoeman, 2020 |
| 36. | Human cytomegalovirus (HCMV) | Human fibroblasts, microarray | LC-MS/MS | Several different metabolites were identified at various time intervals and related with glycolysis, the citric acid cycle | Munger et al., 2008 |
| 37. | Dengu | Blood plasma | GCMS | Profiling of esterified fatty acids as biomarkers revealed saturated fatty acids, C14:0, C15:0, C16:0 and C18:0 decrease significantly | Khedra et al., 2014 |
| 38. | Dengue, hepatitis B and hepatitis C | Serum | LC-TQ-MS | Thirty-five phospholipids were identified and characterized in lipomoic study and 28 PLs were determined to be significantly different in DF, HBV, and HCV | Khedr et al., 2016 |
| 39. | Infectious spleen and kidney necrosis virus (ISKNV) | Chinese perch brain cells (CPB) | UHPLC-Q-TOF/MS | Metabolic Shift from Glucose to Glutamine was observed | Fu et al., 2019 |
| 40. | DENV | Blood | GCMS | Sixty differential metabolites involved in pathways included fatty acid biosynthesis and b-oxidation, phospholipid catabolism, steroid hormone pathway with temporal change with phospholipids and sphingolipids as prognosis marker since showing association with lymphocytes and platelets numbers were identified | Cui et al., 2013 |
| 41. | Dengu | Serum samples from dengue patients from Nicaragua and Mexico | HILIC-MS | 306 metabolites differentiated between DF from ND37DHF/DSS and DF outcomes from Mexican, and 191 metabolites differentiated DF from ND outcomes and 83 differentiated DHF/DSS and DF outcomes were identified further data analysis revealed that 65 metabolites panel can predicted dengue disease outcomes | Voge et al., 2016 |
| 42. | Dengu | 116 dengue patients serum | LCMS | 20 metabolites differentially Enriched while further analysis revealed that Serotonin can indicate Severe Dengue in the Early Phase, while Combining serotonin and IFN-γ improved the prognosis | Cui et al., 2016a |
| 43. | Newcastle disease virus | DF-1 cells | UHPLC-QTOF-MS | Identified 305 metabolites with significant changes mostly belonging to belong to the amino acid and nucleotide metabolic pathway | Liu et al., 2019 |
| 44. | Ebola | Plasma lipidome | LC-MS/MS | EVD survivors and fatalities from Sierra Leone, Samples reveled insight on role of lipome profile in viremia, liver dysfunction, apoptosis, autophagy, and general critical illness where a total of 423 lipid were identified, further data study indicates significant differences of 30.6% & 59.4% between fatalities vs. initial diagnosis survivor and recovered survivor. Further deep digging between healthy and survivor indicates 50.7% altered lipid profiles | Kylea et al., 2018 |
| 45. | Ebola virus | Plasma of healthy, survivor and fatal | LCMS | Acute reduction in plasma free amino acids (PFAAs), in EVD fatalities was observed, plasma lipid species was also significantly altered | Eisfeld et al., 2017 |
| 46. | Ebola Virus and Marburg Virus | Licorice root | Mass spectrometry HRMS and MS/MS analysis | Identified two compounds 18β-glycyrrhetinic acid and licochalcone A, can bind with virus nucleoprotein | Fu et al., 2016 |
| 47. | Lassa virus (LASV) | Serum from febrile patients of 28 female and 21 male patient | LCMS | 3100 and 6900 different spectral features were obtained, in fatal cases analysis indicates nearly all PAFs or PAF-like molecules were present in lower amounts same was true for D-urobilinogen and I-urobilin a hem breakdown product, whereas 7-methylinosine was elevated out of 153 lipid compound identified, half of them were in lower amount | Gale et al., 2017 |
| 48. | Vesicular stomatitis virus (VSV) | BHK cells | Single-Cell Kinetics in presence of Defective interfering particles (DIPs) | Akpinar et al., 2015 | |
| 49. | Hepatitis B virus | Serum | Both GC-MS and LC-MS analysis | A total 427, 382, and 243 mass feature were identified changing significantly between different group HBV-cirrhosis and control, HCC and control as well as HCC and HBV respectively, of which 14 metabolites consistently altered in HBV-cirrhosis and HCC patients | Schoeman, 2020 |
| 50. | HIV-1 | Macrophages | LCMS | Virus – macrophage interaction reveled increase in macrophage fumarate, glucose uptake | Datta et al., 2016 |
| 51. | Influenza A virus (IAV) | A549 Cells | UHPLC–Q-TOF MS | In study on first cycle, replication the metabolic profile which includes 50 differentially expressed metabolites at different time-lag, indicate hijacking of cell metabolism for its own replication, and affecting innate immunity and affecting nearly 30 pathways | Tian et al., 2019 |
| 52. | Classical swine fever (CSF) | Serum | UPLC/ESI-Q-TOF/MS | Ten Virulent form infected days 3 and 7 post-infected piglet and 10 healthy study revealed disturbance in tryptophan catabolism and the kynurenine pathway | Gong et al., 2017 |
| 53. | HIV | Cerebrospinal fluid of rhesus macaques | LCMS | 3,687 features were observed of which twelve metabolites were elevated | William et al., 2008 |
| 54. | Norovirus | RAW 264.7 cells | LCMS | Assay reveled glycolysis, OXPHOS, glucuronic acid, pentose phosphate pathway (PPP), adenosine catabolism increased, was supported by enhanced fructose-bisphosphate, 2- and 3-phosphoglycerate, dihydroxyacetone-phosphate, 6-phosphogluconate, citrate/isocitrate, malate inosine-monophosphate (IMP), hypoxanthine and xanthine, uridine tri-phosphate (UTP), UDP-glucose, and UDP-D glucuronate | Karla et al., 2018 |
| 55. | HIV-1 | CD4 + T cells | LC-MS/MS, NMR analysis, Flow cytometry | Glutamine concentrations are elevated, glutamic acid secretion increase, Intracellular amino acid content was also changing | Hegedus et al., 2017 |
| 56. | Murine cytomegalovirus (MCMV) | Bone marrow derived macrophages (BMDM) | Level | Glycolysis, TCA cycle and urea cycle and fatty acid metabolism alterations was evidenced by metabolomics study indicating modulation of host environment and immunity | Konstantinos, 2017 |
| 57. | MERS-CoV | Calu-3 cells | LC-MS | Lipid metabolism chemical most effected | Yuan et al., 2019 |
| 58. | Ebola viruses | Compound of Piper nigrum | 7 compound including HJ-1, HJ-4 and HJ-6) strongly promoted formation of large NP oligomers and reduced protein thermal stability | Wang Z. et al., 2019 | |
| 59. | H9N2 (avian), H6N2 (avian), and H1N1 (human | B lymphoblastoid cells | GC/MS | Volatile organic compounds analysis revealed production of esters and other oxygenated compounds | Aksenov et al., 2014 |
| 60. | Influenza | Male ferrets nasal washes | GC-MS | Oseltamivir treatment showed different metabolome behavior in cell | David et al., 2019 |
| 61. | H1N1 influenza | Mice lung | LC MS | 396 metabolites significantly associated with inflammatory Cytokines were identified, Further study of data indicates associations with vitamin D, E and purine, metabolism with inflammatory molecule IL-1_, TNF-α, and IL-10. Anti-inflammatory cytokine IL-10 | Chandler et al., 2016. |
| 62. | H1N1 pneumonia | Plasma, culture | 1H nuclear magnetic resonance spectroscopy and gas chromatography-mass spectrometry | 42 patients and control metabolome revealed various molecules for diagnosis and Prognosis where more than 88 metabolites had been observed significantly altered between, healthy, survivor, non-survivor patient | Banoei et al., 2017 |
| 63. | H7N9 Infection | Serum samples | UPLC-MS | In 33 sample of healthy and diseased study Metabolome study revealed that disease severity and metabolism change of fatty acid are related | Sun et al., 2018 |
| 64. | Hepatitis B virus | Serum | Untargeted metabolomics (UPLC-MS) | 33 potential biomarkers were identified of which 25 regulated by syringing were involved in 8 metabolic pathways, the data further suggest a potential of Amino acid metabolism as target for the treatment | Jiang et al., 2020 |
Research related to virus host interaction and information regarding susceptibility, virulence, mechanisms of gene regulation and metabolic pathway modulation under variable environmental factors such as temperature, humidity, light color, intensity and also radiation etc. are mostly lacking that may be useful to combat the incidences of outbreaks from novel viral emergence. Although metabolomics has found its value in virology research but mostly in population study whereas SCM intervention is almost yet to be started to introspect virus-host interaction and is reached upto the level of cell lines only, may be due to the hardship of SCM in terms of single cell isolation and handling, structural diversity and rapid metabolite turnover, lack of amplification opportunity, sensitivity and repeatability of the analytical platforms etc. (Table 1; Minakshi et al., 2019a). However it’s not tough predictions that no longer will SCM be left incumbent considering the worth of information it generates and its ample application in the current context will be reflected in near future.
Sample Preparation Methods for Single Cell Isolation for SCM Analysis
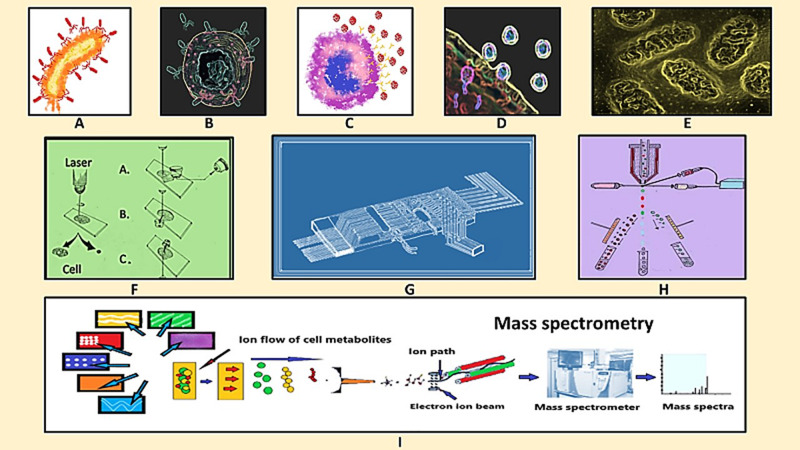
The most common hurdle in SCM analyses is the isolation of single target cell followed by its careful handling and delivery to the downstream analytical platforms because it is directly reflected over the precision of SCM data. Limiting dilutions, manual cell-picking by micromanipulator and laser micro dissection are the common manual methods having relatively low throughput yet applied for single cell analyses where as fluorescence-based cell sorting, high-density microarrays and microfluidics are the most popular automatic high throughput single-cell isolation techniques employed for SCM (Gross et al., 2015; Minakshi et al., 2019b; Figure 1 and Table 2). High cost involvement is a major limitation associated with all of these high end techniques.
FIGURE 1.
Schematic diagram of virus-host interaction, single cell isolation and single cell metabolomics analysis. (A) Virus infecting the host cell, (B) virus-macrophage interaction, (C) virus encounters immune cell, (D) virus-host cell interaction, (E) metabolic changes at sub-cellular level, (F) Single cell isolation by laser microdisection, (G) microfluidic chip for single cell isolation, (H) fluorescence based cell sorting, and (I) mass spectrometry for single cell metabolomics analysis.
TABLE 2.
Single cell isolation Techniques and platforms for Single cell metabolomics analysis to elucidate Virus-host interaction.
| Techniques/Platform | Advantage | Limitations |
| CELL ISOLATION TECHNIQUES | ||
| Limiting dilutions, manual cell-picking | Very simple, no advance instrumentation, inexpensive | Low throughput, Issue of multi-step handling |
| Laser capture micro dissection | No marker required | Low throughput, cost-intensive, laser-induced quenching issue |
| Fluorescence-based cell sorting (FACS) & Magnetic -based cell sorting (MACS) | High throughput, multiplexing is possible | Need specific marker for sorting target cell, preferred for cells suspended in liquid media, Laser or Magnetic field may affect cellular metabolites, cost-intensive |
| Microfluidics | High throughput, minimum sample handling, all steps from quenching to isolation in one go, compatible with multiple analytical platform | Need to design specifically as per requirement i.e., custom made, cost-intensive |
| Aptamer-based | Living cells can be isolated | Need knowledge of structural conformation |
| Single-cell printing technique | Living cells can be isolated | Still in initial stage of development, cost-intensive |
| Direct sampling by electrospray ionization (ESI) Tip | Minimum sample handling, Living cell and its organelle can be imaged, high throughput | Costly, skill-intensive |
| ANALYSIS PLATFORM | ||
| Mass Spectrometry like Capillary electrophoresis-coupled-mass spectrometry (CE-μESI-MS), High-Resolution Mass Spectrometry (CE-ESI-HRMS), Matrix assisted laser desorption/ionization (MALDI) MS | Highly sensitive, Label free, for targeted and untargeted metabolites, both qualitative and quantitative metabolomics can be performed | Cost-intensive, advanced instrumentation required still cannot map all metabolites in one go |
| Gas chromatography Time-of-Flight mass spectrometry (GC-TOF-MS) | Highly accurate, sensitive | Mostly for volatile compounds |
| Mass Spectrometry Imaging: Matrix-Assisted Laser Desorption Ionization (MALDI)-MSI and Electrospray Ionization (ESI)-MSI | Highly sensitive, minimum sample handling, imaging of even sub cellular component is possible | Resource intensive, still in native stage of development |
| NMR | Highly accurate and robust than LC-MS | Low sensitivity to achieve penetration upto single cell level |
| Surface Enhanced Raman Scattering (SERS)/Vibrational Spectroscopy | Fast, label-free non-destructive method | specific spectra for metabolite are not reveled but only for spectroscopically active and concentrated metabolites |
| Fluorescence-based detection | For targeted metabolites | Limited multiplexing option, cannot cover all range; very few metabolites are autofluorescent and nanosensor probes exist for only a handful of specific small mole |
| Matrix-free laser desorption ionization (LDI) mass spectrometry imaging methods: Desorption Ionization on Silicon (DIOS)-MSI, surface-assisted laser desorption ionization (SALDI) | Highly sensitive, excellent coverage | Cost and Resource intensive, still at early age of development |
Fluorescence-activated cell sorting isolates the target cells specifically labeled with antibody coupled with a fluorescent tags. This technique prefers the samples that are naturally suspended in liquid media, such as blood, bone marrow cells, semen, ovum, secretions, yeast, protoplast, bacteria, and viruses (Ciuffi et al., 2016; Khalil et al., 2017; Janssens et al., 2018; Singh, 2019). Time-lapse fluorescence imaging is a similar type of fluorescence based single cell analysis technique used for monitoring of cell virus interaction (Drayman et al., 2017).
Immune-magnetic separation using antibody-coated magnetic beads or attached with other ligands for capturing the target cells is also an important single cell isolation method. Dynabeads or MACS (Dynabeads Magnetic Separation Technology) employs such isolation principle (Miltenyi et al., 1990).
Aptamer-based cell isolation relies upon the precise binding of aptamers (single-stranded oligonucleotides, DNA or RNA) to its target using structural conformation and ease the isolation of single cells (Guo et al., 2006; Hu et al., 2016; Delley et al., 2018; Chen, 2019; Kacherovsky et al., 2019). Fluorescent nanodiamonds (FNDs) are diamond nano-crystals which have also been used for single cell labeling for cell tracking and illuminating the cellular processes under different conditions (Prabhakar et al., 2017; Claveau et al., 2018).
Single-cell printing technique like “Block-Cell-Printing” is another example of high throughput single cell isolation technique yet to find ample application (Gross et al., 2013; Zhang et al., 2014; Schoendube et al., 2015).
Microfluidics or Lab-on-a-Chip devices provide excellent opportunity for simultaneous isolation of target single cells along with its handling and manipulation followed by SCM suitable platforms with high end automation (Warrick et al., 2016). Several analytical platforms such as electrophoresis, optical spectroscopy, fluorescence based imaging, or mass spectrometry are compatible with microfluidics based cell isolation. Soft lithographic fabrication onto polydimethylsiloxane (PDMS), glass or silicon chips provides the base of microfluidic devices and hydrodynamic or gravity-driven flow of cells are used to capture the target cells. Microfluidics device installed on a fluorescence microscope has yielded valuable information regarding the kinetics of viral infection in single cells and variability of cellular factors regulating the viral replication (Guo et al., 2017). Hybrid microfluidics using either active or passive approaches or their combination, integration of nano- and microfluidics, digital or virtual microfluidics are the emerging microfluidic platforms which are being recently introduced for high throughput and continuous single cell isolation avoiding the general limitations of the existing technique (Xu et al., 2016; Yan et al., 2016; Wang et al., 2017; Vanderpoorten et al., 2019).
Direct sampling through nano-electrospray ionization tips followed by mass spectrometric analyses method is gaining popularity in recent times due to its minimum sample requirement and processing along with high throughput data generation. Pipette tip column electrospray ionization (PTC-ESI) or ballpoint electrospray ionization (BP-ESI) followed by simultaneous mass spectrometry or live single-cell video-mass spectrometry are the SCM platforms where the technique has been used (Tsuyama et al., 2008; Huang et al., 2012; Ji et al., 2016; Pu et al., 2018).
Analytical Platforms for Single Cell Metabolomics
The precision and coverage of SCM analysis depends upon the “resolution” and “sensitivity” of the analytical platform. Although theoretically SCM analyze the entire set of cellular metabolites but more often it is restricted to qualitative or quantitative analysis of a cluster of metabolites under certain condition. “Targeted” for acquiring information regarding specific metabolite(s) or “untargeted” SCM approach for obtaining the maximum coverage can be chosen according to the requirement and selection of the analytical platform should be done accordingly. Mass Spectrometry Imaging (MSI)-based methods are arguably the most sensitive and thus preferred SCM platform. However, the compromised reliability, restricted quantitation option, and poor resolution for structural characterization are the certain limitations need to be taken care of. Other SCM platforms include but not limited to separation-based methods like capillary electrophoresis (CE), liquid chromatography (LC), or gas chromatography (GC) combined with MS or fluorescent tagging approach which are mostly used for “targeted SCM” due to the sensitivity issue (Minakshi et al., 2019a; Table 2).
Separation-Based Methods Coupled With Mass Spectrometry
Capillary electrophoresis coupled with electrospray ionization and mass spectrometry (CE-μESI-MS) has been employed to analyze single 8-cell embryo of Xenopus laevis to depict more than 80 metabolites (Onjiko et al., 2015). Similarly CE-ESI-TOF-MS has explored over 300 metabolites from the neuronal cell of sea slug Aplysia californica (Nemes et al., 2013). CE-ESI-MS has quantitatively characterized over 15 anionic metabolites (nucleotides and derivatives) from Aplysia sensory neuron with the detection limits in <22 nM range (Liu et al., 2014). High-Resolution Mass Spectrometry (CE-ESI-HRMS) analyses of a single 16-cell stage embryo of Xenopuslaevishasrevealed438 non-redundant protein groups by label-free quantification with a detection limit of ∼75 attomol (∼11 nm) (Lombard-Banek et al., 2016). Targeted automated SCM analysis of neuronal cell by a microchip electrophoresis-mass spectrometric method (MCE-MS) has quantitatively measured dopamine and glutamic acid content within individual cell with detection limits 8.3 and 15.6 nM for the metabolites, respectively. Although the introduction of this platform in virus-host interaction arena is pending but the mentioned evidences has clearly suggested about its potential in SCM analysis.
Nano-flow liquid chromatography-electro spray ionization mass spectrometry (LC-ESI-MS) based analysis has yielded 5 anthocyanins from 2-4picolitervolume of individual Torenia hybrida petal cell (Kajiyama et al., 2006). Gas chromatography Time-of-Flight mass spectrometry (GC-TOF-MS) analyses of Mesembryanthemum crystallinum epidermal bladder cells has identified 194 known and 722 overall molecular features which generate significant pathway information regarding metabolic alterations in salty environment (Barkla and Vera-Estrella, 2015). Thus chromatographic methods have great potential for SCM analyses in combination with MS platforms. Although not in single cell level but GC/MS based metabolomics technique has already been applied for introspection of fatty acid biosynthesis and cholesterol metabolism in A549 and AGS cell lines infected with influenza A virus yielding valuable information regarding susceptibility and cell differentiation in association with viral replication (Lin et al., 2010). Ultra-high-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry (UHPLC-QTOF-MS) analyses has depicted significant changes in 305 metabolites during infection of DF-1 cells with the Herts/33 strain of New Castle Disease Virus (Liu et al., 2019). Gut metabolomic analyses of rhesus monkeys by GC-MS and LC-MS during gut virome alteration has been correlated with gut microbiome and the underlying role of metabolites like tryptophan, arginine, and quinine (Li et al., 2019). Although the penetration upto the single cell level is yet to be reached but the evidences are enough to ensure the utility and future of this platform for metabolomic analyses in virology research.
Mass Spectrometry Imaging
Extreme sensitivity, vast coverage and the opportunity of label-free analysis has rendered MSI as one of the most preferred method of SCM analysis, particularly for untargeted approach. Improvement in resolution is going on in terms of reducing the laser spot area by lowering the diameter of optical fiber along with providing multiplexing option by integration of multiple mass analyzers. Matrix-Assisted Laser Desorption Ionization (MALDI)-MSI and Electrospray Ionization (ESI)-MSI are the two conventional platforms applied for SCM analyses (Figure 1).
Polarity-switching option is lacking in MALDI-MSI, it can be run in either ion mode at a time, multiplexing can be added subsequently for increasing the coverage. However, introduction of advanced matrices, such as 9-AA or silver, gold, or titanium and grapheme oxide nanoparticles, have increased the sensitivity by reducing background noise. Multiplex MSI platforms are equipped to directly explore individual intracellular metabolites, even their sub-cellular location irrespective of chromatographic separation. SCM analysis of by MALDI-MS operated in negative ion mode has revealed several metabolites such as ADP, ATP, GTP, and UDP-Glucose etc. from Closterium acerosum (Amantonico et al., 2010). Metabolic analysis of single Yeast cell by coupling microscale sampling and MALDI-MS at negative ion mode detection has elucidated information regarding some valuable metabolites such as ADP, GDP, ATP, GTP, and acetyl-CoA. The detection limit ranged from 5 to 12 attomoles (Amantonico et al., 2008). Pressure probe sampling (in picoliters) coupled with UV-MALDI MS has been applied for shotgun metabolite profiling of organic compounds live single plant cell (Gholipour et al., 2012). High spatial resolution MSI analysis of Maize root epidermal and cortex cell has elucidated several TCA cycle metabolites including their localization with spatial resolution improved to ∼5 μm (Hansen and Lee, 2018). Turkey gut microbiome has been introspected using MALDI-MS platform operated in positive ion mode with nanoparticle microarray and organic matrices (Hansen et al., 2018). Metabolomics variability during virus host interaction in bloom-forming alga Emiliania huxleyi has been evaluated by Plaque assay combined with several MSI techniques (Flow-probe-MSI, MALDI-MSI, UPLC-q-TOF MS, and LC-MS/MC for lipidomics, GC-MS for fatty acid methyl esters) (Schleyer et al., 2019). Fluorescence in situ hybridization (FISH) microscopy combined with high-resolution atmospheric pressure-MALDI-MSI of Bathymodiolus puteoserpensis epithelial cells has also been used to understand host-microbe symbiosis (Geier et al., 2019). Lipidomics profile of infected Aedes albopictus cells during Zika virus infection has been investigated using MALDI-MS platform to identify thirteen infection-specific lipid markers in the mosquitoes (Melo et al., 2016). Thus the mentioned evidences easily suggest about the utility and potential of MALDI-MSI based platform for SCM analyses and can be predicted more often application to evaluate host-virus interaction at single cell level.
Alike MALDI, Electrospray Ionization (ESI) is another important soft ionization technique where the analytes after ionization, evaluated by MS to facilitate metabolite characterization. ESI-MS is equipped with simultaneous detection at both positive and negative ion mode yielding enormous sensitivity. Live nano-ESI-LTQ-MS analyses of Pelargonium zonale leaf cell has depicted over16 metabolites from 1to 5pL of sample (Tejedor et al., 2009). Probe ESI mass spectrometry (PESI-MS) with tungsten probe of 1 μM diameter has detected several metabolites including 6 fructans, 4 lipids and 8 flavone derivatives in single Allium cepa cell (Gong et al., 2014). ESI-Q-TOF-MS operated in either positive or negative-ion mode has yielded status regarding several metabolites such as tryptophan, creatinine, dopamine, and glycine, and cofactors such as lactate and pyruvate to link energy metabolism and immunodeficiency in microgravity (Chakraborty et al., 2018).
Laser ablation electrospray ionization (LAESI) employs mid-infrared (mid-IR) laser to generate gas phase metabolites. Large plant cells or cluster of animal cells are preferred subjects for this platform but high sensitivity, little chemical background and a direct sample delivery to MS platform has made it a desired choice for SCM analysis. Metabolites from single cells or cluster of Allium cepa and Narcissus pseudonarcissus bulb epidermis and single eggs from Lytechinus pictus has been evaluated through LAESI to detect 35 prominent metabolites including anthocyanidins, flavonoids, and their glucosides depicting its potential in SCM analysis (Shrestha and Vertes, 2009). LAESI-MS coupled with ion mobility separation (IMS) has been described as capable of direct profiling and imaging of several biomolecules including metabolites from biological tissues and single cells (Etalo et al., 2018; Paglia and Astarita, 2020). LAESI coupled with a 21 tesla Fourier transform ion cyclotron resonance (21T-FTICR) has capable of elucidating isotopic fine structure of the biomolecules by direct MS analysis and imaging (Stopka et al., 2019).
Live Single-Cell Video-Mass Spectrometry using micro sampling method by video microscopy and micromanipulator guided gold-coated glass capillary nano-ESI tip along with Nano-ESI-Q-TOF platform has demonstrated good potential for SCM analysis (Lapainis et al., 2009; Hiyama et al., 2015).
Non-Mass Spectrometric Methods
Next to mass spectrometry, NMR is a major tool for metabolite analyses. It is even robust and requires less sample processing than the other metabolomics tools. However requirement of large sample volume and the issue of sensitivity render it imperfect for SCM analyses rather it is preferred for tissue sample and biofluid introspection. For example continuous in vivo metabolism has been followed by using NMR (CIVM-NMR) coupled with high-resolution-magic angle spinning to elucidate branched-chain amino acid metabolism in human chronic lymphoid leukemia cells (Judge et al., 2019).
Fluorescence-based sensors are also being applied in targeted SCM analyses. CE with laser-induced fluorescence (LIF) detection has been employed for detection of chiral amino acids and neurotransmitters from mouse brain and neuron cells (Jakó et al., 2014; Qi et al., 2017). Capillary micro sampling MS with fluorescence microscopy has successfully identified 29 metabolites and 54 lipids from Human HepG2/C3Acancer cells (Zhang et al., 2018). Microchip electrophoresis coupled with LIF detection have been employed for identifying ethanol induced metabolite change in mice liver cells to depict elevated hydrogen peroxide along with depleted glutathione and cysteine level in the target cells (Li et al., 2016). However limited multiplexing option has restricted its application mostly within targeted approach.
Surface Enhanced Raman Scattering (SERS) employs laser in the visible, near-infrared, or near-ultraviolet range for label free detection of the metabolites within the cells (Shalabaeva et al., 2017). SERS has been applied for fast, label-free detection of Chlamydia trahomatis and Neisseria gonorrheoae along with associated extra-cellular metabolite changes (Chen et al., 2018). Raman spectroscopy is a non-destructive method for metabolite analysis but only few functional groups emit detectable Raman signals reducing the applicability of the method for SCM analyses (Smith et al., 2016). Recently, Raman micro-spectroscopy using graded X-ray doses has successfully uncovered several nucleus and cytoplasmic specific metabolic features from single SH-SY5Y human cancer cells (Delfino et al., 2019).
Future SCM Techniques
Secondary Ion Mass Spectrometry Imaging with spatial resolution capacity at the range of 50–100 nm is a potential future tool for SCM as well as sub-cellular metabolite analyses (Svatos, 2011; Kollmer et al., 2012; Gilmore et al., 2019). The matrix-free laser desorption ionization (LDI) methods include Desorption Ionization on Silicon (DIOS)-MSI which is a variant of surface-assisted laser desorption ionization (SALDI), both use soft ionization of analytes followed by MS analyses. LDI-MS platform employing HR-MS along with Orbitrap FT-MS has been used for SCM analysis of microalgae, marine diatoms and freshwater chlorophytes (Baumeister et al., 2019). Matrix-free nanophotonic ionization of analytes on Silicon Nanopost Array chip followed by MSI offers an effective platform for detection of small metabolites at single cell level (Walker et al., 2012; Korte et al., 2016). Desorption Electrospray Ionization is another soft ionization technique having capability to ionize wide variable molecules however poor spatial resolution is a limitation of this technique which can be improved further for SCM analyses. MS coupled with Microfluidic chips or microarray systems and 3D MALDI Mass Spectrometry Imaging are the other potential SCM techniques which can be used for analyzing virus-host interaction in near future. Single-Molecule Array which resembles the digital ELISA format may also be employed for targeted SCM by using specific tagged antibody against the target metabolite(s) (Minakshi et al., 2019a).
Metabolomic Insight at Virus-Host Interface to Combat Emerging Viruses
Zika virus, Dengu virus, Chikungunya virus, HIV, avian influenza, Nipah (NiV) viruses, Ebola and Marburg filoviruses, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS CoV) are the emerging viruses as evidenced in recent times (Afrough et al., 2019). Several of them are having zoonotic importance and emerge from viral spill over from the sylvatic cycle infecting multiple hosts. Metabolomic intervention can trace out some common metabolic pathway(s) in different host range which are also essential for viral replication (Passalacqua et al., 2018; Mayer et al., 2019). For Example, Progression of human cytomegalovirus (HCMV) infection has increased the metabolite flux through glycolysis, citric acid cycle, and pyrimidine nucleotide biosynthesis in human fibroblasts, probably to meet the energy demand for viral replication and supply of macromolecular precursors (Munger et al., 2008). HCMV mediated metabolic reprogramming also includes enhanced lipogenesis through mTOR-sterol regulatory element-binding protein (SREBP)-regulated and other pathways inducing several enzymes such as acetyl-CoA carboxylase, fatty acid synthase, fatty acid elongases etc. The synthesized very long chain and long chain fatty acids are essential for viral envelope production, because inhibition of such pathways result into impaired viral replication. Similarly other DNA viruses such as herpes simplex virus-1 (HSV-1), Kaposi’s sarcoma-associated herpes virus (KSHV), vaccinia virus (VACV) are also found to modulate glycolysis, glutamine metabolism and fatty acid synthesis as common pathways. Thus any substrate analog (e.g., glucose analog 2-deoxyglucose) or enzyme inhibitor which prevents such virus-induced host metabolic reprogramming can be effective against multiple viruses. Although RNA viruses like rhinovirus, hepatitis C virus and several emerging arboviruses such as Zika virus, Dengu virus, Chikungunya virus etc. modulate host metabolic pathways in a little different manner but glycolysis, glutamine metabolism and fatty acid synthesis remain as the prime targets. Similar host enzyme machineries are being exploited by these RNA viruses too. These common metabolic pathway nodes can be exploited for preventive, diagnostic and therapeutic management of emerging viral infections. Further, such exploration can be of interest for therapeutic repurposing of existing drugs to combat any emerging viral outbreak. For example, Remdesivir (GS-5734) is a novel nucleoside analog antiviral prodrug, actually developed for the treatment of single stranded RNA containing Ebola virus disease and Marburg virus infections, has now been suggested as therapeutic option against recent SARS-CoV-2 pandemic. SARS-CoV-2 is also having positive-sense single-stranded RNA genome and Remdesivir gets incorporated into the nascent viral RNA chains leading to inhibition of viral replication through premature termination of RNA transcription (Centers for Disease Control and Prevention, 2020; Liu et al., 2020). However, the toxic effects of such substrate analogs or pathway inhibitors on host cells should be critically analyzed prior to their extensive application. Recently, traditional anti-parasitic drug ivermectin has also found to exhibit in vitro antiviral activity against SARS-CoV-2 infection and warrants rapid further in vivo introspection to combat current COVID-19 pandemic. This is also an example of drug repurposing relying upon the virus-exploited common pathways. Because in earlier research, ivermectin has depicted efficacy against multiple RNA viruses such as DENV 1-4, West Nile Virus, influenza virus, Venezuelan equine encephalitis virus (VEEV) and human immunodeficiency virus-1 (HIV-1) by destabilizing the importin (IMP) α/β1 heterodimer and blocking IMP α/β1-mediated nuclear import of viral proteins (Caly et al., 2020).
Metabolite profiling can also provide an idea about the disease severity and predictive outcome of the viral infection. Serum metabolite analyses of H7N9 infected subjects have revealed that increase in palmitic acid, erucic acid, and phytal level is related to virus-induced repression of fatty acid metabolism and negatively correlated with the disease outcome (Sun et al., 2018).
In arboviral infections, metabolic reprogramming in arthropod vector and vertebrate host takes place in different manner. Zika virus (ZIKV) induced metabolic reprogramming of human foreskin fibroblast cells (HFF-1) shapes differently from C6/36 cells of Aedes albopictus mosquito which serves as the vector of ZIKV infection. Glucose influx has been increased through glycolysis in both the cases, however, it is mainly diverted toward tricarboxylic acid (TCA) cycle and amino acid synthesis in human cells whereas glucose contribution to TCA cycle gets reduced to augment the intermediates of pentose phosphate pathway in mosquito cells. Further, ZIKV infection depletes ATP level in HFF-1 cells leading to elevated AMPK phosphorylation and increased caspase-mediated cell death whereas neither such increase in AMP/ATP and ADP/ATP ratios as well as AMPK phosphorylation nor decreased cell viability has been evidenced in infected C6/36 mosquito cells. Such difference in cell viability may be contributed by both altered pentose phosphate pathway and AMPK activation (Thaker et al., 2019). So metabolic introspection is not only capable of elucidating virus-host interaction but also provides information regarding maintenance and propagation of virus through their arthropod vectors. Understanding of lifecycle of such viruses exploiting metabolic pathways within arthropod vectors can assist to design strategies for preventing their transmission in vertebrate hosts.
Considerable numbers of researches have followed such path providing metabolomic insight at virus-host interface. Chromatography (LC/GC) coupled with tandem mass spectrometry (MS/MS) is the most common method employed for metabolomics introspection followed by NMR, FTIR and fluorescence based methods (Table 1). Most of these experiments have reached upto the level of cell line but significant penetration at single cell level is yet to be achieved in terms of SCM analysis in the context of virus-host interaction. But single-cell analysis may elucidate far precise information regarding viral replication and infection kinetics which is beyond the capacity of population study (Guo et al., 2017). A high-throughput, microfluidics-based platform has been devised to perform kinetic analysis of green fluorescence protein (GFP) tagged polio virus (PV) infections in individual HeLa S3 cells. Monitoring of fluorescence intensity has revealed marked variation in viral replication and infection cycle in each of the GFP-PV infected cells which are uniquely and independently controlled by viral as well as the cellular factors. The time of onset of viral replication varied significantly among the individual infected cells. Similar cellular-factor regulated variable pattern has continued to follow throughout the viral infection cycle upto the cell lysis. The analysis at single cell level has unearthed plethora of valuable information regarding virulence determinants and mechanisms of drug action which have been escaped in population methods (Guo et al., 2017). The mentioned evidence is extremely useful to justify the value of single cell techniques over population measurements and several such SCM introspection at virus-host interface may follow the path in near future. It will not only surmount the heterogeneity issue of samples otherwise succumb to superimposed results but also help to identify the effect of mutation both in virus as well as host, virus homing affinity toward cell types, post-infection survival, progression of disease etc. Although SCM analyses have its own advantage but it must be complemented by the bulk metabolomic analyses of the infected tissue or organ because cell-to-cell communication may have crucial effect in virus infection cycle which may not be reflected in SCM analyses. Thus a combinatorial approach is more beneficial over a standalone SCM introspection so that the real reflection of cellular heterogeneity at virus-host interface as well as the influence of cell communication can be elucidated precisely. The specific information extracted from such analyses like mutation study along with computational modeling may assist in future vaccine formulation, mapping of virus evolution strategy to identify the potential hot-spots of novel viral emergence, their virulence and putative contentment recipes.
However, the inherent challenges of SCM techniques require to be surmounted with diligence and passion for proper utilization of the potential of single-cell analyses. The difficulty in single cell isolation along with rapid turnover, transport and degradation of the cellular metabolites are the key challenges of SCM analyses. Rapid metabolite turnover can produce random noise which may create analytical error and difficulty in differentiating infected cells from the healthy one. Thus normalization of such random error must be performed during the experimental set-up for SCM data acquisition to differentiate the deterministic effects from the stochastic one. Similar type of random fluorescence noise normalization has been performed by Guo et al. (2017) during GFP-PV infected single-cell analyses. Further dealing with minute sample volume, diverse types of metabolites and their femto-molar range concentration demands high sensitivity and throughput of the analytical platform which is essential particularly for SCM analyses. For example, NMR is extremely robust, reliable, requiring minimal sample processing and commonly employed metabolomics analysis platform for population study but low sensitivity has limited the utility of the technique for SCM analyses. Proper analyses of SCM data is another crucial challenge which has been eased out significantly with recent advances in bioinformatics tools and growing metabolite database (Minakshi et al., 2019a). Moreover, integration of SCM with single-cell proteomics, transcriptomics, genomics and interactomics can provide a whole-some image of the life processes which may assist to explore the biological missing links regarding virus infection and transmission cycle.
Conclusion
Rapid development in single cell isolation and handling methods, persistent improvement in sensitivity and resolution of the analytical platforms along with growing database and data interpretation programs are smoothening the hurdles of SCM to introduce it at the virus-host interface as early as possible. The SCM derived precise information regarding the altered metabolism of infected cell over the healthy cells may assist to investigate the virulence, survival, and progression of diseases. The exploration of cellular heterogeneity can illuminate the aspects which remain concealed in population study. Thus SCM holds the promise as a key tool of future to introspect virus-host interaction and assist in efficient management of emerging viral disease.
Author Contributions
MP and MG conceptualized the manuscript. RK and SK wrote the manuscript. MP critically analyzed and improved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Afrough B., Dowall S., Hewson R. (2019). Emerging viruses and current strategies for vaccine intervention. ClinExpImmunol 196 157–166. 10.1111/cei.13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpinar F., Timm A., Yin J. (2015). High-throughput single-cell kinetics of virus infections in the presence of defective interfering particles. J. Virol. 90 1599–1612. 10.1128/jvi.02190-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov A. A., Sandrock C. E., Zhao W., Sankaran S., Schivo M., Harper R., et al. (2014). Cellular scent of influenza virus infection. Chem. Bio. Chem. 15 1040–1048. 10.1002/cbic.201300695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantonico A., Oh J. Y., Sobek J., Heinemann M., Zenobi R. (2008). Mass spectrometric methodfor analyzing metabolites in yeast with single cell sensitivity. Angew. Chem. Int. Ed. Engl. 47 5382–5385. 10.1002/anie.200705923 [DOI] [PubMed] [Google Scholar]
- Amantonico A., Urban P. L., Fagerer S. R., Balabin R. M., Zenobi R. (2010). Single-cell MALDI-MS as an analytical tool for studying intrapopulation metabolic heterogeneity of unicellular organisms. Anal. Chem. 82 7394–7400. 10.1021/ac1015326 [DOI] [PubMed] [Google Scholar]
- Banoei M. M., Vogel H. J., Weljie A. M., Kumar A., Yende S., Winston B. W. (2017). Plasma metabolomics for the diagnosis and prognosis of H1N1 influenza pneumonia. Crit. Care 21:97. 10.1186/s13054-017-1672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla B. J., Vera-Estrella R. (2015). Single cell-type comparative metabolomics of epidermal bladder cells from the halophyte Mesembryanthemum crystallinum. Front. Plant Sci. 6:435. 10.3389/fpls.2015.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister T. U. H., Vallet M., Kaftan F., Svatoš A., Pohnert G. (2019). Live single-cell metabolomics with matrix-free laser/desorption ionization mass spectrometry to address microalgal physiology. Front. Plant Sci. 10:172. 10.3389/fpls.2019.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J. D., Catton M. G., Jans D. A., Wagstaff K. M. (2020). The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178:104787. 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2020). Coronavirus Disease 2019 (COVID-19). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html (accessed April 5, 2020). [Google Scholar]
- Chakraborty N., Cheema A., Gautam A., Donohue D., Hoke A., Conley C., et al. (2018). Gene-metabolite profile integration to understand the cause of spaceflight induced immunodeficiency. NPJ Microgravity 4:4. 10.1038/s41526-017-0038-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. D., Hu X., Ko E. J., Park S., Lee Y. T., Orr M., et al. (2016). Metabolic pathways of lung inflammation revealed by high-resolution metabolomics (HRM) of H1N1 influenza virus infection in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311 R906–R916. 10.1152/ajpregu.00298.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Y. (2019). Cell fishing with DNA aptamers. Nat. Biomed. Eng. 3 757–758. [DOI] [PubMed] [Google Scholar]
- Chen Y., Premasiri W. R., Ziegler L. D. (2018). Surface enhanced Raman spectroscopy of Chlamydia trachomatis and Neisseria gonorrhoeae for diagnostics, and extra-cellular metabolomics and biochemical monitoring. Sci. Rep. 8:5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuanjian L., Zhiting J., Xuemei F., Guiya L., Shasha L., Chunxia H., et al. (2012). A metabonomic approach to the effect evaluation of treatment in patients infected with influenza a (H1N1). Talanta 100 51–56. 10.1016/j.talanta.2012.07.076 [DOI] [PubMed] [Google Scholar]
- Ciuffi A., Rato S., Telenti A. (2016). Single-cell genomics for virology. Viruses 8:123. 10.3390/v8050123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claveau S., Bertrand J.-R., Treussart F. (2018). Fluorescent nanodiamond applications for cellular process sensing and cell tracking. Micromachines 9:247. 10.3390/mi9050247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Fang J., Ooi E. E., Lee Y. H. (2017a). Serial metabolome changes in a prospective cohort of subjects with influenza viral infection and comparison with dengue fever. J. Proteome Res. 16 2614–2622. 10.1021/acs.jproteome.7b00173 [DOI] [PubMed] [Google Scholar]
- Cui L., Hou J., Fang J., Lee Y. H., Costa V. V., Wong L. H., et al. (2017b). Serum metabolomics investigation of humanized mouse model of dengue virus infection. J. Virol. 91:e00386-17. 10.1128/JVI.00386-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Lee Y. H., Kumar Y., Xu F., Lu K., Ooi E. E., et al. (2013). Serum metabolome and lipidome changes in adult patients with primary dengue infection. PLoS Negl. Trop. Dis. 7:e2373. 10.1371/journal.pntd.0002373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Lee Y. H., Thein T. L., Fang J., Pang J., Ooi E. E., et al. (2016a). Serum metabolomics reveals serotonin as a predictor of severe dengue in the early phase of dengue fever. PLoS Negl. Trop. Dis. 10:e0004607. 10.1371/journal.pntd.0004607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Zheng D., Lee Y., Chan T. K., Kumar Y., Ho W. E., et al. (2016b). Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci. Rep. 6:26076. 10.1038/srep26076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P. K., Deshmane S., Khalili K., Merali S., Gordon J. C., Fecchio C., et al. (2016). Glutamate metabolism in HIV-1 infected macrophages: role of HIV-1 Vpr. Cell Cycle 15 2288–2298. 10.1080/15384101.2016.1190054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. B., Ding Y. O., Avinash V. K., Celeste T., Michael S., Dunn D. T., et al. (2019). Untargeted metabolomics analysis of the upper respiratory tract of ferrets following influenza A virus infection and oseltamivir treatment. Metabolomics 15:33. 10.1007/s11306-019-1499-0 [DOI] [PubMed] [Google Scholar]
- Delfino I., Ricciardi V., Manti L., Lasalvia M., Lepore M. (2019). Multivariate analysis of difference raman spectra of the irradiated nucleus and cytoplasm region of SH-SY5Y human neuroblastoma cells. Sensors 19:3971. 10.3390/s19183971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delley C. L., Liu L., Sarhan M. F., Abate A. R. (2018). Combined aptamer and transcriptome sequencing of single cells. Sci. Rep. 8:2919. 10.1038/s41598-018-21153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C. A. (2012). Emerging and re-emerging viruses: a global challenge illustrated by Chikungunya virus outbreaks. World J. Virol. 1 11–22. 10.5501/wjv.v1.i1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon V., Li X. (2015). Single cell genome sequencing for viral-host interactions. J.Comput. Sci. Syst. Biol. 8 160–165. 10.4172/jcsb.1000183 [DOI] [Google Scholar]
- Diamond D. L., Syder A. J., Jacobs J. M., Sorensen C. M., Walters K. A., Sean C. P., et al. (2010). Temporal proteome and lipidome profiles reveal hepatitis c virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 6:e1000719. 10.1371/journal.ppat.1000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayman N., Karin O., Mayo A., Danon T., Shapira L., Rafael D., et al. (2017). Dynamic proteomics of herpes simplex virus infection. mBio 8:e01612-17. 10.1128/mbio.01612-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayman N., Patel P., Vistain L., Tay S. (2019). HSV-1 single-cell analysis reveals the activation of anti-viral and developmental programs in distinct sub-populations. eLife 2019:e46339. 10.7554/eLife.46339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld A. J., Halfmann P. J., Wendler J. P., Kyle J. E., Burnum-Johnson K. E., Peralta Z., et al. (2017). Multi-platform ’Omics analysis of human ebola virus disease pathogenesis. Cell Host Microbe 22 817–829.e8. 10.1016/j.chom.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bacha T., Struchiner C. J., Cordeiro M. T., Almeida F. C. L., Jr., Marques E. T., Da Poian A. T. (2016). 1H Nuclear magnetic resonance metabolomics of plasma unveils liver dysfunction in dengue patients. J. Virol. 90 7429–7443. 10.1128/JVI.00187-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etalo D. W., Díez-Simón C., de Vos R. C. H., Hall R. D. (2018). Laser ablation electrospray ionization-mass spectrometry imaging (LAESI-MS) for spatially resolved plant metabolomics. Methods Mol. Biol. 1778 253–267. 10.1007/978-1-4939-7819-9_18 [DOI] [PubMed] [Google Scholar]
- Fu X., Guo X., Wu S., Lin Q., Liu L., Liang H., et al. (2019). Non-Targeted UHPLC-Q-TOF/MS-based metabolomics reveals a metabolic shift from glucose to glutamine in cpb cells during ISKNV infection cycle. Metabolitis 9:174. 10.3390/metabo9090174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Wang Z., Li L., Dong S., Li Z., Jiang Z., et al. (2016). Novel chemical ligands to ebola virus and marburg virus nucleoproteins identified by combining affinity mass spectrometry and metabolomics approaches. Sci. Rep. 6:29680. 10.1038/srep29680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale T. V., Horton T. M., Grant D. S., Garry R. F. (2017). Metabolomics analyses identify platelet activating factors and heme breakdown products as Lassa fever biomarkers. PLoS Negl. Trop. Dis. 11:e0005943. 10.1371/journal.pntd.0005943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier B. K., Sogin E., Michellod D., Janda M., Kompauer M., Spengler B., et al. (2019). Spatial metabolomics of in situ, host-microbe interactions. bioRxiv [Preprint]. 10.1101/555045 [DOI] [PubMed] [Google Scholar]
- Gholipour Y., Balsells R. E., Nonami H. (2012). In situ pressure probe sampling and UV-MALDI MSfor profiling metabolites in living single cells. Mass Spectrom. 1:A0003. 10.5702/massspectrometry.A0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore I. S., Sven Heiles S., Pieterse C. L. (2019). Metabolic imaging at the single-cell scale: recent advances in mass spectrometry imaging. Annu. Rev. Anal. Chem. 12 201–224. [DOI] [PubMed] [Google Scholar]
- Godoy M. M. G., Lopes E. P. A., Silva R. O., Hallwass F., Koury L. C. A., Moura I. M. (2010). Hepatitis C virus infection diagnosis using metabonomics. J. Viral. Hepat. 17 854–858. 10.1111/j.1365-2893.2009.01252.x [DOI] [PubMed] [Google Scholar]
- Gong W., Jia J., Zhang B., Mi S., Zhang L., Xie X., et al. (2017). Serum metabolomic profiling of piglets infected with virulent classical swine fever virus. Front. Microbiol. 8:731. 10.3389/fmicb.2017.00731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Zhao Y., Cai S., Fu S., Yang C., Zhang S., et al. (2014). Single cell analysis with probe ESI-mass spectrometry: detection of metabolites at cellular and subcellular levels. Anal. Chem. 86 3809–3816. 10.1021/ac500882e [DOI] [PubMed] [Google Scholar]
- Grace B., Sheryl M. C., Boon P. L., Mah L. N., Sam F. Y. L. (2010). Metabolomics approach for investigation of effects of dengue virus infection using the EA. hy926 cell line. J. Proteome Res. 9 6523–6534. 10.1021/pr100727m [DOI] [PubMed] [Google Scholar]
- Gross A., Schoendube J., Zimmermann S., Steeb M., Zengerle R., Koltay P. (2015). Technologies for single-cell isolation. Int. J. Mol. Sci. 16 16897–16919. 10.3390/ijms160816897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Schöndube J., Niekrawitz S., Streule W., Riegger L., Zengerle R., et al. (2013). Single-cell printer. J. Lab. Autom. 18 504–518. 10.1177/2211068213497204 [DOI] [PubMed] [Google Scholar]
- Guido A., Gualdonia K. A., Mayera A. M. K., Katharina K., Alexander P., Philip K., et al. (2018). Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc. Natl. Acad. Sci. U.S.A. 115 E7158–E7165. 10.1073/pnas.1800525115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Li S., Caglar M. U., Mao Z., Liu W., Woodman A., et al. (2017). Single-cell virology: on-chip investigation of viral infection dynamics. Cell Rep. 21 1692–1704. 10.1016/j.celrep.2017.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K. T., SchÄfer R., Paul A., Gerber A., Ziemer G., Wendel H. P. (2006). A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific dna aptamers. Stem Cells 24 2220–2231. 10.1634/stemcells.2006-0015 [DOI] [PubMed] [Google Scholar]
- Hansen R. L., Lee Y. J. (2018). High spatial resolution mass spectrometry imaging: toward single cell metabolomics in plant tissues. Chem. Rec. 18 65–77. 10.1002/tcr.201700027 [DOI] [PubMed] [Google Scholar]
- Hansen R. L., Dueñas M. E., Looft T., Lee Y. J. (2018). Nanoparticle microarray for high-throughput microbiome metabolomics using matrix-assisted laser desorption ionization mass spectrometry. Anal. Bioanal. Chem. 411 147–156. 10.1007/s00216-018-1436-5 [DOI] [PubMed] [Google Scholar]
- Hegedus A., Kavanagh W. M., Khan M. B., Dias Zeidler J., Da Poian A. T., El-Bacha, et al. (2017). Evidence for altered glutamine metabolism in human immunodeficiency virus type 1 infected primary human cd4+ t cells. AIDS Res. Hum. Retroviruses 33 1236–1247. 10.1089/aid.2017.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewer R., Vorster J., Steffens F. E., Meyer D. (2006). Applying biofluid 1H NMR-based metabonomic techniques to distinguish between HIV-1 positive/AIDS patients on antiretroviral treatment and HIV-1 negative. J. Pharm. Biomed. Anal. 41 1442–1446. [DOI] [PubMed] [Google Scholar]
- Hiyama E., Ali A., Amer S., Harada T., Shimamoto K., Furushima R., et al. (2015). Direct lipido-metabolomics of single floating cells for analysis of circulating tumor cells by live single cell mass spectrometry. Anal. Sci. 31 1215–1217. 10.2116/analsci.31.1215 [DOI] [PubMed] [Google Scholar]
- Hofmann S., Krajewski M., Scherer C., Scholz V., Mordhorst V., Truschow P., et al. (2018). Complex lipid metabolic remodeling is required for efficient hepatitis C virus replication. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863 1041–1056. 10.1016/j.bbalip.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Hollenbaugh J. A., Montero C., Schinazi R. F., Munger J., Kim B. (2016). Metabolic profiling during HIV-1 and HIV-2 infection of primary human monocyte-derived macrophages. Virology 491 106–114. 10.1016/j.virol.2016.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbaugh J. A., Munger J., Kim B. (2011). Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC-MS/MS analysis. Virology 415 153–159. 10.1016/j.virol.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Zhang W., Xin H., Deng G. (2016). Single cell isolation and analysis. Front. Cell Dev. Biol. 4:116 10.3389/fcell.2016.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-Q., You J.-Q., Yuan B.-F., Feng Y.-Q. (2012). Sample preparation and direct electrospray ionization on a tip column for rapid mass spectrometry analysis of complex samples. Analyst 137:4593. 10.1039/c2an35856e [DOI] [PubMed] [Google Scholar]
- Jakó T., Szabó E., Tábi T., Zachar G., Csillag A., Szökõ É. (2014). Chiral analysis of amino acid neurotransmitters and neuromodulators in mouse brain by CE-LIF. Electrophoresis 35 2870–2876. 10.1002/elps.201400224 [DOI] [PubMed] [Google Scholar]
- Janssens S., Schotsaert M., Manganaro L., Dejosez M., Simon V., García-Sastre A., et al. (2018). FACS-mediated isolation of neuronal cell populations from virus-infected human embryonic stem cell-derived cerebral organoid cultures. Curr. Protoc. Stem Cell Biol. 48:e65. 10.1002/cpsc.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B., Xia B., Gao Y., Ma F., Ding L., Zhou Y. (2016). Generating electrospray ionization on ballpoint tips. Anal. Chem. 88 5072–5079. 10.1021/acs.analchem.5b03990 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Li Y., Zhou L., Zhang D. (2020). Comparative metabolomics unveils molecular changes and metabolic networks of syringin against hepatitis B mice by untargeted mass spectrometry. RSC Advances 10 461–473. 10.1039/c9ra06332c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua D. C., Xin H., Eun J. K., Soojin P., Young T. L., Micheal O., et al. (2016). Metabolic pathways of lung inflammation revealed by high-resolution metabolomics (HRM) of H1N1 influenza virus infection in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge M. T., Wu Y., Tayyari F., Hattori A., Glushka J., Ito T., et al. (2019). Continuous in vivo metabolism by NMR. Front. Mol. Biosci. 30:26. 10.3389/fmolb.2019.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacherovsky N., Cardle I. I., Cheng E. L., Yu J. L., Baldwin M. L., Salipante S. J., et al. (2019). Traceless aptamer-mediated isolation of CD8+ T cells for chimeric antigen receptor T-cell therapy. Nat. Biomed. Eng. 3 783–795. 10.1038/s41551-019-0411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama S., Harada K., Fukusaki E., Kobayashi A. (2006). Single cell-based analysis of toreniapetal pigments by a combination of ArFexcimer laser micro sampling and nano-high performance liquid chromatography (HPLC)-mass spectrometry. J. Biosci. Bioeng. 102 575–578. [DOI] [PubMed] [Google Scholar]
- Karla D. P., Jia L., Goodfellow I., Kolawole A. O., Arche J. R., Maddox R. J., et al. (2018). Central carbon metabolism is an intrinsic factor for optimal replication of a norovirus. bioRxiv [Preprint]. 10.1101/434019 434019 [DOI] [Google Scholar]
- Kawashima H., Oguchi M., Ioi H., Amaha M., Yamanaka G., Kashiwagi Y., et al. (2006). Primary biomarkers in cerebral spinal fluid obtained from patients with influenza-associated encephalopathy analyzed by metabolomics. Int. J. Neurosci. 116 927–936. 10.1080/00207450600550519 [DOI] [PubMed] [Google Scholar]
- Khalil J. Y. B., Langlois T., Andreani J., Sorraing J.-M., Raoult D., Camoin L., et al. (2017). Flow cytometry sorting to separate viable giant viruses from amoeba co-culture supernatants. Front. Cell. Infect. Microbiol. 6:202. 10.3389/fcimb.2016.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr A., Hegazy M. A., Kammouna A. K., Shehata M. A. (2016). Phospholipidomic identification of potential serum biomarkers in dengue fever, hepatitis B and hepatitis C using liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatography B Analyt. Technol. Biomed. Life Sci. 1009–1010 44–54. 10.1016/j.jchromb.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Khedra A., Hegazyb M., Kamala A., Shehatab M. A. (2014). Profiling of esterified fatty acids as biomarkers in the blood of dengue fever patients using a microliter-scale extraction followed by gas chromatography and mass spectrometry. J. Sep. Sci. 38 316–324. 10.1002/jssc.201400749 [DOI] [PubMed] [Google Scholar]
- Kollmer F., Paul W., Krehl M., Niehuis E. (2012). Ultra high spatial resolution SIMS with cluster ions—approaching the physical limits. Surf. Interface Anal. 45 312–314. 10.1002/sia.5093 [DOI] [Google Scholar]
- Konstantinos K. (2017). Metabolic Programming in Murine Cytomegalovirus Infected Macrophages. Ph.D. thesis, University of Edinburgh, Edinburgh. [Google Scholar]
- Korte A. R., Stopka S. A., Morris N., Razunguzwa T., Vertes A. (2016). Large-scale metabolite analysis of standards and human serum by laser desorption ionization mass spectrometry from silicon nanopost arrays. Anal. Chem. 88 8989–8996. 10.1021/acs.analchem.6b01186 [DOI] [PubMed] [Google Scholar]
- Kylea J. E., Burnum-Johnsona K. E., Wendlera J. P., Eisfeldb A. J., Halfmannb P. J., Watanabec T., et al. (2018). Plasma lipidome reveals critical illness and recovery from human Ebola virus disease. Proc. Natl. Acad. Sci. U.S.A. 116 3919–3928. 10.1073/pnas.1815356116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté J. M., Swan B. K., Poulos B., Luo H., Koren S., Hallam S. J., et al. (2015). Single-cell genomics-based analysis of virus–host interactions in marine surface bacterioplankton. ISME J. 9 2386–2399. 10.1038/ismej.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapainis T., Rubakhin S. S., Sweedler J. V. (2009). Capillary electrophoresis with electrospray ionizationmass spectrometric detection for single-cell metabolomics. Anal. Chem. 81 5858–5864. 10.1021/ac900936g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen K. (2011). Virus-host interactions. Cell 146 183–185. 10.1016/j.cell.2011.07.002 [DOI] [Google Scholar]
- Li H., Li H., Wang J., Guo L., Fan H., Zheng H., et al. (2019). The altered gut virome community in rhesus monkeys is correlated with the gut bacterial microbiome and associated metabolites. Virol J. 16:105. 10.1186/s12985-019-1211-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Chen P., Fan Y., Wang X., Xu K., Li L., et al. (2016). Multicolor fluorescence detection-based microfluidic device for single-cell metabolomics: simultaneous quantitation of multiple small molecules in primary liver cells. Anal. Chem. 88 8610–8616. 10.1021/acs.analchem.6b01775 [DOI] [PubMed] [Google Scholar]
- Lin S., Liu N., Yang Z., Song W., Wang P., Chen H., et al. (2010). GC/MS-based metabolomics reveals fatty acid biosynthesis and cholesterol metabolism in cell lines infected with influenza A virus. Talanta 83 262–268. 10.1016/j.talanta.2010.09.019 [DOI] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6:16. 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-X., Aerts J. T., Rubakhin S. S., Zhang X.-X., Sweedler J. V. (2014). Analysis of endogenous nucleotides by single cell capillary electrophoresis-mass spectrometry. Analyst 139 5835–5842. 10.1039/c4an01133c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Yin Y., Gong Y., Qiu X., Sun Y., Tan L., et al. (2019). In vitro and in vivo metabolomic profiling after infection with virulent newcastle disease virus. Viruses 11:962. 10.3390/v11100962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard-Banek C., Reddy S., Moody S. A., Nemes P. (2016). Label-free quantification of proteins in single embryonic cells with neural fate in the cleavage-stage frog (Xenopuslaevis) embryo using capillary electrophoresis electrospray ionization high-resolution mass spectrometry (CE-ESI-HRMS). Mol. Cell. Proteomics 15 2756–2768. 10.1074/mcp.m115.057760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungile S., Francois S., Tjaart P. J. K., Debra M. (2014). Mid-ATR-FTIR spectroscopic profiling of HIV/AIDS sera for novel systems diagnostics in global health. J. Integr. Biol. 18 513–523. 10.1089/omi.2013.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. G., Pranab K. M., Richard J. J., Mauricio R., Robert E. B., Masoumeh S., et al. (2013). Metabolomics reveals differential levels of oral metabolites in hiv-infected patients: toward novel diagnostic targets. J. Integr. Biol. 17 5–15. 10.1089/omi.2011.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Huang X., Yu K., Qu H., Chang-xiao L., Cheng Y. (2008). Metabonomic analysis of hepatitis B virus-induced liver failure: identification of potential diagnostic biomarkers by fuzzy support vector machine. J. Zhejiang Univ. Sci. B 9 474–481. 10.1631/jzus.B0820044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K. A., Stöckl J., Zlabinger G. J., Gualdoni G. A. (2019). Hijacking the supplies: metabolism as a novel facet of virus-host interaction. Front. Immunol. 10:1533. 10.3389/fimmu.2019.01533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C. F. O. R., de Oliveira D. N., Lima E., de O., Guerreiro T. M., Esteves C. Z., et al. (2016). A lipidomics approach in the characterization of zika-infected mosquito cells: potential targets for breaking the transmission cycle. PLoS One 11:e0164377. 10.1371/journal.pone.0164377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C. F. O. R., Delafiori J., Dabaja M. Z., Diogo N. O., Tatiane M. G., Tatiana E. C., et al. (2018). The role of lipids in the inception, maintenance and complications of dengue virus infection. Sci. Rep. 8:11826. 10.1038/s41598-018-30385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C. F. O. R., Delafiori J., de Oliveira D. N., Guerreiro T. M., Esteves C. Z., Lima E. D. O., et al. (2017). Serum metabolic alterations upon zikainfection. Front. Microbiol. 8:1954. 10.3389/fmicb.2017.01954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S., Muller W., Weichel W., Radbruch A. (1990). High gradient magnetic cell separation with MACS. Cytometry 11 231–238. 10.1002/cyto.990110203 [DOI] [PubMed] [Google Scholar]
- Minakshi P., Ghosh M., Kumar R., Patki H. S., Saini H. M., Ranjan K., et al. (2019a). “Single-cell metabolomics: technology and applications,” in Single-Cell Omics, eds Barh D., Azevedo V. (Cambridge, MA: Academic Press; ), 319–353. 10.1016/b978-0-12-814919-5.00015-4 [DOI] [Google Scholar]
- Minakshi P., Kumar R., Ghosh M., Saini H. M., Ranjan K., Brar B., et al. (2019b). “Single-Cell proteomics: technology and applications,” in Single-Cell Omics, eds Barh D., Azevedo V. (Cambridge, MA: Academic Press; ), 283–318. 10.1016/b978-0-12-814919-5.00014-2 [DOI] [Google Scholar]
- Munger J., Bennett B. D., Parikh A., Feng X. J., McArdle J., Rabitz H. A., et al. (2008). Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26 1179–1186. 10.1038/nbt.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi S. U., Rewari B. B., Bhavesh N. S., Jameel S. (2013). Nuclear magnetic resonance based profiling of biofluids reveals metabolic dysregulation in HIV-infected persons and those on anti-retroviral therapy. PLoS One 8:e64298. 10.1371/journal.pone.0064298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi S. U., Taneja S., Bhavesh N. S., Shastri J., Aggarwal R., Jameel S. (2011). Metabonomic analysis of hepatitis E patients shows deregulated metabolic cycles and abnormalities in amino acid metabolism. J.Viral. Hepat. 18 591–602. 10.1111/j.1365-2893.2011.01488.x [DOI] [PubMed] [Google Scholar]
- Nemes P., Rubakhin S., Aerts J., Sweedler J. (2013). Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Protoc. 8 783–799. 10.1038/nprot.2013.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurul S., Hasnah O., Tang T. H., Abdel-Hamid Z. A. H. (2017). Metabolomics approach for multibiomarkers determination to investigate dengue virus infection in human patients. Acta Biochim. Pol. 64 215–219. 10.18388/abp.2015_1224 [DOI] [PubMed] [Google Scholar]
- Onjiko R. M., Moody S. A., Nemes P. (2015). Single-cell mass spectrometry reveals small moleculesthat affect cell fates in the 16-cell embryo. Proc. Natl. Acad. Sci. U.S.A. 112 6545–6550. 10.1073/pnas.1423682112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia G., Astarita G. (eds). (2020). “Ion mobility-mass spectrometry”, in Methods in Molecular Biology (New York, NY: Springer; ), XI, 312. [DOI] [PubMed] [Google Scholar]
- Passalacqua K. D., Lu J., Goodfellow I., Kolawole A. O., Arche J. R., Maddox R. J., et al. (2018). Central carbon metabolism is an intrinsic factor for optimal replication of a norovirus. bioRxiv [Preprint]. 10.1101/434019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar N., Khan M. H., Peurla M., Chang H.-C., Hänninen P. E., Rosenholm J. M. (2017). Intracellular trafficking of fluorescent nanodiamonds and regulation of their cellular toxicity. ACS Omega 2 2689–2693. 10.1021/acsomega.7b00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu F., Chiang S., Zhang W., Ouyang Z. (2018). Direct sampling mass spectrometry for clinical analysis. Analyst 144 1034–1051. 10.1039/c8an01722k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Philip M. C., Yang N., Sweedler J. V. (2017). Single cell neurometabolomics. ACS Chem. Neurosci. 9 40–50. 10.1021/acschemneuro.7b00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter J. B., Wahl A. S., Freund S., Genezel Y. (2010). Metabolic effects of influenza virus infection in cultured animal cells: intra- and extracellular metabolite profiling. BMC Syst. Biol. 4:61. 10.1186/1752-0509-4-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser S., Sheyn U., Frada M. J., Pilzer D., Rotkopf R., Vardi A. (2019). Unmasking cellular response of a bloom-forming alga to viral infection by resolving expression profiles at a single-cell level. PLoS Pathog. 15:e1007708. 10.1371/journal.ppat.1007708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B., Elshina E., Kowalsky J. R., teVelthuis A. J. W., Bloom J. D. (2019). Single-cell virus sequencing of influenza infections that trigger innate immunity. J. Virol. 93:e00500-19. 10.1128/jvi.00500-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer G., Shahaf N., Ziv C., Dong Y., Meoded R. A., Helfrich E. J. N., et al. (2019). In plaque-mass spectrometry imaging of a bloom-forming alga during viral infection reveals a metabolic shift towards odd-chain fatty acid lipids. Nat. Microbiol. 4 527–538. 10.1038/s41564-018-0336-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman J. C. (2020). Virus-host Metabolic Interactions: Using Metabolomics to Probe Oxidative Stress, Inflammation and Systemic Immunity. Ph.D. thesis, University of Leiden, Chicago, IL. [Google Scholar]
- Schoeman J. C., Hou J., Harms A. C., Vreeken R. J., Berger R., Hankemeier T., et al. (2016). Metabolic characterization of the natural progression of chronic hepatitis B. Genome Med. 8:64. 10.1186/s13073-016-0318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoendube J., Wright D., Zengerle R., Koltay P. (2015). Single-cell printing based on impedance detection. Biomicrofluidics 9:014117 10.1063/1.4907896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalabaeva V., Lovato L., La Rocca R., Messina G. C., Dipalo M., Miele E., et al. (2017). Time resolved and label free monitoring of extracellular metabolites by surface enhanced Raman spectroscopy. PLoS One 12:e0175581. 10.1371/journal.pone.0175581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B., Vertes A. (2009). In situ metabolic profiling of single cells by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 81 8265–8271. 10.1021/ac901525g [DOI] [PubMed] [Google Scholar]
- Shrinet J., Shastri J., Gaind R. (2016). Serum metabolomics analysis of patients with chikungunya and dengue mono/co-infections reveals distinct metabolite signatures in the three disease conditions. Sci. Rep. 6:36833. 10.1038/srep36833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. M. (2019). An efficient protocol for single-cell cloning human pluripotent stem cells. Front. Cell Dev. Biol. 7:11. 10.3389/fcell.2019.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Wright K. L., Ashton L. (2016). Raman spectroscopy: an evolving technique for live cell studies. Analyst 141 3590–3600. 10.1039/c6an00152a [DOI] [PubMed] [Google Scholar]
- Stopka S. A., Samarah L., Shaw J. B., Liyu A. V., Velièkoviæ D., Agtuca B. J., et al. (2019). Ambient metabolic profiling and imaging of biological samples with Ultrahigh molecular resolution using laser ablation electrospray ionization 21 tesla FTICR Mass spectrometry. Anal. Chem. 91 5028–5035. 10.1021/acs.analchem.8b05084 [DOI] [PubMed] [Google Scholar]
- Strzelecka P. M., Ranzoni A. M., Cvejic A. (2018). Dissecting human disease with single-cell omics: application in model systems and in the clinic. Dis. Model. Mech. 11:dmm036525. 10.1242/dmm.036525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Song L., Feng S., Li L., Yu H., Wang Q., et al. (2018). Fatty acid metabolism is associated with disease severity after h7n9 infection. EBioMedicine 33 218–229. 10.1016/j.ebiom.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatos A. (2011). Single cell metabolomics comes of age: new developments in mass spectrometry profiling and imaging. Anal. Chem. 83 5037–5044. 10.1021/ac2003592 [DOI] [PubMed] [Google Scholar]
- Tejedor M. L., Mizuno H., Tsuyama N., Harada T., Masujima T. (2009). Direct single-cell molecular analysis of plant tissues by video mass spectrometry. Anal. Sci. 25 1053–1055. [DOI] [PubMed] [Google Scholar]
- Thai M., Nicholas A. G., Braas D., Nehil M., Komisopoulou E., Kurdistani S. K. (2014). Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 19 694–670. 10.1016/j.cmet.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker S. K., Chapa T., Garcia G., Jr., Gong D., Schmid E. W., Arumugaswami V., et al. (2019). Differential metabolic reprogramming by zika virus promotes cell death in human versus mosquito cells. Cell Metab. 29 1206–1216.e4. 10.1016/j.cmet.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Zhang K., Min J., Chen C., Cao Y., Ding C., et al. (2019). Metabolomic analysis of influenza a virus A/WSN/1933 (H1N1) infected A549 cells during first cycle of viral replication. Viruses 11:1007. 10.3390/v11111007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyama N., Mizuno H., Tokunaga E., Masujima T. (2008). Live single-cell molecular analysis by video-mass spectrometry. Anal. Sci. 24 559–561. 10.2116/analsci.24.559 [DOI] [PubMed] [Google Scholar]
- Vanderpoorten O., Peter Q., Challa P. K., Keyser U. F., Baumberg J., Kaminski C. F., et al. (2019). Scalable integration of nano-, and microfluidics with hybrid two-photon lithography. Microsyst. Nanoeng. 5:40. 10.1038/s41378-019-0080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastag L., Koyuncu E., Grady S. L., Shenk T. E., Rabinowitz J. D. (2011). Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 7:e1002124. 10.1371/journal.ppat.1002124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voge N. V., Perera R., Mahapatra S., Gresh L., Balmaseda A., Loroño-Pino M. A., et al. (2016). Metabolomics-based discovery of small molecule biomarkers in serum associated with dengue virus infections and disease outcomes. PLoS Negl. Trop. Dis. 10:e0004449. 10.1371/journal.pntd.0004449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. N., Stolee J. A., Vertes A. (2012). Nanophotonic ionization for ultratrace and single-cell analysis by mass spectrometry. Anal.Chem. 84 7756–7762. 10.1021/ac301238k [DOI] [PubMed] [Google Scholar]
- Wang H., Chen L., Sun L. (2017). Digital microfluidics: a promising technique for biochemical applications. Front. Mech. Eng. 12:510–525. 10.1007/s11465-017-0460-z [DOI] [Google Scholar]
- Wang S., Zhang Q., Hui H., Agrawal K., Karris M. A. Y. (2019). An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. bioRxiv [Preprint]. 10.1101/678763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Liang H., Cao H., Zhang B., Li J., Wang W., et al. (2019). Efficient ligand discovery from natural herbs by integrating virtual screen, affinity mass spectrometry and targeted metabolomics. Analyst 144 2881–2890. 10.1039/c8an02482k [DOI] [PubMed] [Google Scholar]
- Warrick J. W., Timm A., Swick A., Yin J. (2016). Tools for single-cell kinetic analysis of virus-host interactions. PLoS One 11:e0145081. 10.1371/journal.pone.0145081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- William R., Wikoff, Siuzdak G., Howard S., Fox J. (2008). Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. Clin. Invest. 118 2661–2669. 10.1172/JCI34138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Brito I. L., Alm E. J., Blainey P. C. (2016). Virtual microfluidics for digital quantification and single-cell sequencing. Nat. Methods 13 759–762. 10.1038/nmeth.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Zhang J., Yuan D., Li W. (2016). Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 38 238–249. 10.1002/elps.201600386 [DOI] [PubMed] [Google Scholar]
- Yuan S., Chu H., Chan J. F. W., Ye Z. W., Wen L., Yan B., et al. (2019). SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 10:120. 10.1038/s41467-018-08015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini F., Robinson M. L., Croote D., Sahoo M. K., Sanz A. M., Ortiz-Lasso E., et al. (2018). Virus-inclusive single-cell RNA sequencing reveals the molecular signature of progression to severe dengue. Proc. Natl. Acad. Sci. U.S.A. 115 E12363–E12369. 10.1073/pnas.1813819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Chou C. K., Xia X., Hung M. C., Qin L. (2014). Block-Cell-Printing for live single-cell printing. Proc. Natl. Acad. Sci. U.S.A. 111 2948–2953. 10.1073/pnas.1313661111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Sevinsky C. J., Davis B. M., Vertes A. (2018). Single-cell mass spectrometry of subpopulations selected by fluorescence microscopy. Anal. Chem. 90 4626–4634. 10.1021/acs.analchem.7b05126 [DOI] [PubMed] [Google Scholar]