Abstract
The NMDA-type ionotropic glutamate receptors play pivotal roles in many brain functions, but are also involved in numerous brain disorders. Seven NMDA receptor subunits exist (GluN1, GluN2A-D, and GluN3A-B) that assemble into a diverse array of tetrameric receptor subtypes with distinct functional properties and physiological roles. Most NMDA receptors are composed of two GluN1 and two GluN2 subunits, which can assemble into four diheteromeric receptors subtypes composed of GluN1 and one type of GluN2 subunit (e.g. GluN1/2A), and presumably also six triheteromeric receptor subtypes composed of GluN1 and two different GluN2 subunits (e.g. GluN1/2A/2B). Despite accumulating evidence that a large proportion of native NMDA receptors are triheteromers, little is known about their function and pharmacology due to the lack methods to faithfully express triheteromeric NMDA receptors in heterologous expression systems. The problem is that co-expression of GluN1 with two different GluN2 subunits generates two distinct diheteromeric receptor subtypes as well as one triheteromeric receptor subtype, thereby confounding studies on a homogenous population of triheteromeric NMDA receptors. Here, we will describe a method to selectively express recombinant triheteromeric GluN1/2A/2B receptors without interfering co-expression of diheteromeric GluN1/2A and GluN1/2B receptors. This method enables quantitative evaluation of functional and pharmacological properties of triheteromeric GluN1/2A/2B receptors, which are presumably the most abundant NMDA receptors in the adult cortex and hippocampus.
Keywords: Ionotropic glutamate receptor, ligand-gated ion channel, assembly, retention signals, coiled-coil, endoplasmic reticulum, trafficking, Xenopus oocytes
1. Introduction
NMDA receptors have been extensively studied for more than three decades as principle mediators of excitatory synaptic transmission. Molecular cloning in the early 1990’s identified cDNA for individual NMDA receptors subunits and ensuing studies in heterologous expression systems revealed pronounced variation in pharmacological and functional properties, depending on the subunit composition of the different NMDA receptor subtypes [1]. Initially, five NMDA receptor subunits were identified, GluN1 and four different GluN2 subunits (GluN2A-D), and it was quickly discovered that co-expression of GluN1 and GluN2 subunits is necessary for the formation of functional NMDA receptors [1]. By now, it is firmly established that two GluN1 and two GluN2 subunits assemble into functional receptors with an alternating subunit arrangement around a central ion channel pore (i.e. 1–2-1–2) [2–4] (Fig. 1a). Later, two GluN3 subunits (GluN3A-B) were identified that can also assemble with GluN1 and GluN2, but the stoichiometry of GluN3-containing receptors is still unknown [5]. Recombinant studies almost exclusively describe diheteromeric NMDA receptors that are assembled from GluN1 and only one type of GluN2 (i.e. GluN1/2A, GluN1/2B, GluN1/2C, and GluN1/2D) (Fig. 1b). However, a compelling body of biochemical and functional evidence, dating back to 1994, shows that a large proportion of native NMDA receptors are triheteromers assembled from two GluN1 and two different GluN2 subunits (Fig. 1b). For example, GluN1/2A/2B is the major subtype in hippocampus and cortex of the adult rodent brain [6–10], GluN1/2B/2D is found in cerebellar Golgi cells [11], hippocampal granule cells [12], substantia nigra [13], and the subthalamic nucleus [14], and GluN1/2A/2C is identified in adult cerebellar granule cells [15,16]. Despite the prevalence of triheteromeric NMDA receptors in vivo, the biophysical and pharmacological properties for most triheteromeric NMDA receptor subtypes are poorly understood, primarily due to our inability to study a homogenous population of triheteromeric receptors in heterologous expression systems without accompanying diheteromeric receptors [8,15,17–20].
Figure 1. Control of NMDA receptor composition using engineered peptide tags.
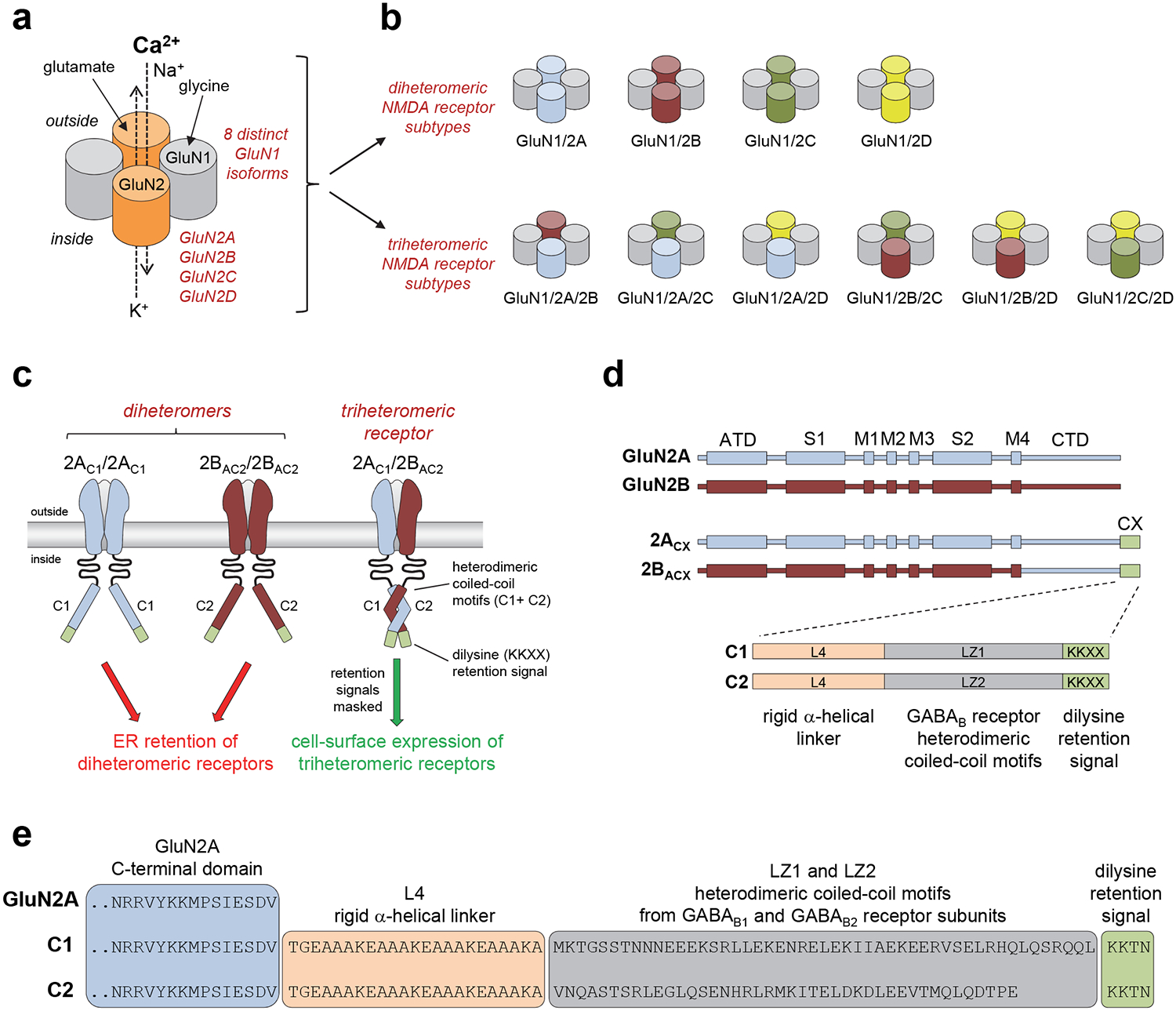
a) Schematic representation of the subunit arrangement in NMDA receptors composed of glycine-binding GluN1 and glutamate-binding GluN2 subunits. The GluN1 subunit exist in eight different isoforms (i.e. splice variants) and four different genes encoding GluN2 subunits have been identified (GluN2A-D). b) Schematic representation of possible subunit compositions in NMDA receptors assembled from GluN1 and GluN2 subunits. c) Co-expression of GluN1, GluN2A, and GluN2B subunits in heterologous systems normally generates three populations of functional NMDA receptors (i.e. GluN1/2A, GluN1/GluN2B, and GluN1/2A/2B). However, fusing the engineered C1 and C2 tags to the intracellular C-termini of GluN2 subunits prevents cell-surface expression of receptors that contain two C1-tagged or two C2-tagged GluN2 subunits. GluN1 is omitted for clarity. d) Linear representations of the polypeptide chains show the amino-terminal domain (ATD), S1 and S2 segments that form the agonist binding domain, and the transmembrane domain formed by M1, M2, M3, and M4 of GluN2A (blue), GluN2B (red), GluN2A with C1- or C2-tags fused to the intracellular C-terminus (2ACX), and chimeric GluN2B subunits with the C-terminal domain (CTD) replaced by that of C1- or C2-tagged GluN2A (2BACX). The C1 and C2 tags are composed of a rigid α-helical linker (L4), the coiled-coil motifs of GABAB1 (LZ1) and GABAB2 (LZ2) receptors, and a di-lysine endoplasmic reticulum (ER) retention/retrieval signal. e) Sequences for the C-termini of wildtype GluN2A and subunits with C1- or C2-tags fused to the C-terminus. Adapted with permission from Hansen et al. [18].
In order to enable functional studies on triheteromeric NMDA receptors, we developed a method to selectively express NMDA receptors composed of two GluN1 and two different GluN2 subunits at the cell surface in heterologous expression systems (e.g. HEK cells and Xenopus oocytes) [18]. This method was designed to prevent surface expression of diheteromeric NMDA receptors without affecting functional and pharmacological receptor properties. Inspired by previous studies that selectively expressed heterodimeric metabotropic glutamate receptors in heterologous systems [21], we adapted the trafficking control system of the G protein-coupled GABAB receptors to control the expression of triheteromeric NMDA receptors. Functional GABAB receptors are heterodimers composed of GABAB1 and GABAB2 subunits [22]. The intracellular C-terminal domain (CTD) of the GABAB1 subunit contains a retention/retrieval signal (RXR motif) that retains the subunit in the endoplasmic reticulum (ER), thereby preventing expression of homomeric GABAB1 at the cell surface [23]. The RXR retention signal is masked through a heterodimeric coiled-coil interaction between leucine zipper motifs (LZ1 and LZ2) in the CTDs of GABAB1 and GABAB2 subunits, resulting in trafficking of the heterodimer to the cell surface [23–25]. The intracellular CTD of the GABAB2 subunit is lacking ER retention/retrieval signals, but homomeric GABAB2 are non-functional [22]. Thus, this trafficking control system ensures that functional GABAB receptors expressed at the cell surface are composed of one GABAB1 and one GABAB2 subunit. We modified this GABAB trafficking control system to only allow surface expression of triheteromeric NMDA receptors containing GluN1 with one GluN2A, and one GluN2B subunit (i.e. GluN1/2A/2B) [18] (Fig. 1c).
We engineered two different peptide tags intended for fusion to the C-terminus of GluN2 subunits. The peptide tags are composed of the leucine zipper motifs from GABAB1 and GABAB2 (LZ1 and LZ2, respectively) immediately followed by C-terminal di-lysine (KKXX) ER retention/retrieval signals (Fig. 1d). The KKXX retention signals, which must be located at the distal C-termini, will localize the subunits in the ER [26] unless masked by coiled-coil formation between LZ1 and LZ2 [27]. In order to minimize steric effects that could prevent coiled-coil formation between LZ1 and LZ2, we included a peptide linker (L4) to generate the tags L4-LZ1-KKXX and L4-LZ2-KKXX (hereafter denoted C1 and C2, respectively) (Fig. 1d). The L4 peptide linker was designed to generate a rigid α-helix composed of four repeats of amino acids EAAAK [28]. Placement of the engineered C1 and C2 tags at the intracellular GluN2 C-termini should enable selective surface expression of NMDA receptors composed of two GluN1, one C1-tagged GluN2, and one C2-tagged GluN2 subunit (Fig. 1c). To remove differences between the CTDs of GluN2A and GluN2B subunits that could potentially prevent the interaction between C1 and C2 or affect ER retention (see Note 1), we replaced the CTD of GluN2B with that of GluN2A (Fig. 1d). The intracellular CTDs of C1- and C2-tagged GluN2A and GluN2B subunits (also denoted 2AC1, 2AC2, 2BAC1, and 2BAC2) therefore only differ by the leucine zipper motifs (LZ1 and LZ2) (Fig. 1e).
To assess whether co-expression of GluN1 with 2AC1 and 2BAC2 generates triheteromeric GluN1/2AC1/2BAC2 receptors and whether the method prevents expression of diheteromeric GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2 receptors, it is critical to carefully develop a set of control experiments (see Note 2). In addition, controls can be designed to determine if fusion of the C1 and C2 tags to the GluN2 subunits affects receptor function (see Note 3). According to the experimental design, the current responses activated by glutamate + glycine from cells co-expressing GluN1 with 2AC1 and 2BAC2 should, in theory, be mediated by triheteromeric GluN1/2AC1/2BAC2 receptors with masked retention signals. However, some diheteromeric GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2 receptors may also escape ER retention and could contribute to the measured current responses. To evaluate the contribution of escaped receptors (escape current) to the total current response, we generated 2AC1 and 2BAC2 subunits with mutations in the agonist binding pocket that abolish glutamate binding (R518K + T690I in 2AC1-RKTI and R519K + T691I in 2BAC2-RKTI) (see Note 4). Thus, the RKTI mutations effectively render the subunit non-functional. NMDA receptors containing one copy of a non-functional subunit are non-responsive to glutamate (i.e. in the presence of glycine), since agonist occupancy at all four subunits are required for NMDA receptor activation [29,30] (Fig. 2a). Thus, co-expression of GluN1 + 2AC1 + 2BAC2-RKTI produces only one population of functional receptors, namely escaped GluN1/2AC1/2AC1. Comparison of current responses from cells expressing GluN1 + 2AC1 + 2BAC2 with responses from cells co-expressing GluN1 + 2AC1 + 2BAC2-RKTI allows estimation of the fractional current mediated by escaped GluN1/2AC1/2AC1. Similarly, comparison to current responses from cells expressing GluN1 + 2AC1-RKTI + 2BAC2 allows estimation of the fractional current mediated by escaped GluN1/2BAC2/2BAC2 (Fig. 2b). The sum of the fractional currents from escaped GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2 will provide an estimate of the percent “escape” current in cells co-expressing GluN1 + 2AC1 + 2BAC2. Alternative control experiments can be designed to evaluate the efficiency by which the engineered peptide tags express a homogenous population of triheteromeric receptors. However, the RKTI controls have the distinct advantage that they would also detect if C1 and C2 tags interact between receptors, rather than within one receptor as intended (Fig. 2c). For example, GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2 could in theory interact via the C1 and C2 tags, thereby enabling surface expression. This scenario would manifest in current responses for cells co-expressing GluN1 + 2AC1 + 2BAC2-RKTI or GluN1 + 2AC1-RKTI + 2BAC2. Alternative control experiments could evaluate current responses from cells co-expressing GluN1 + 2AC1 or GluN1 + 2BAC2, which should not allow surface expression, but these controls would not assess whether inter-receptor interactions occur between C1 and C2 tags of GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2. This problem emphasizes the need to carefully design control experiments to assess the effectiveness of the method, especially when the experimental conditions are changed.
Figure 2. Evaluation of selective triheteromeric NMDA receptor expression.
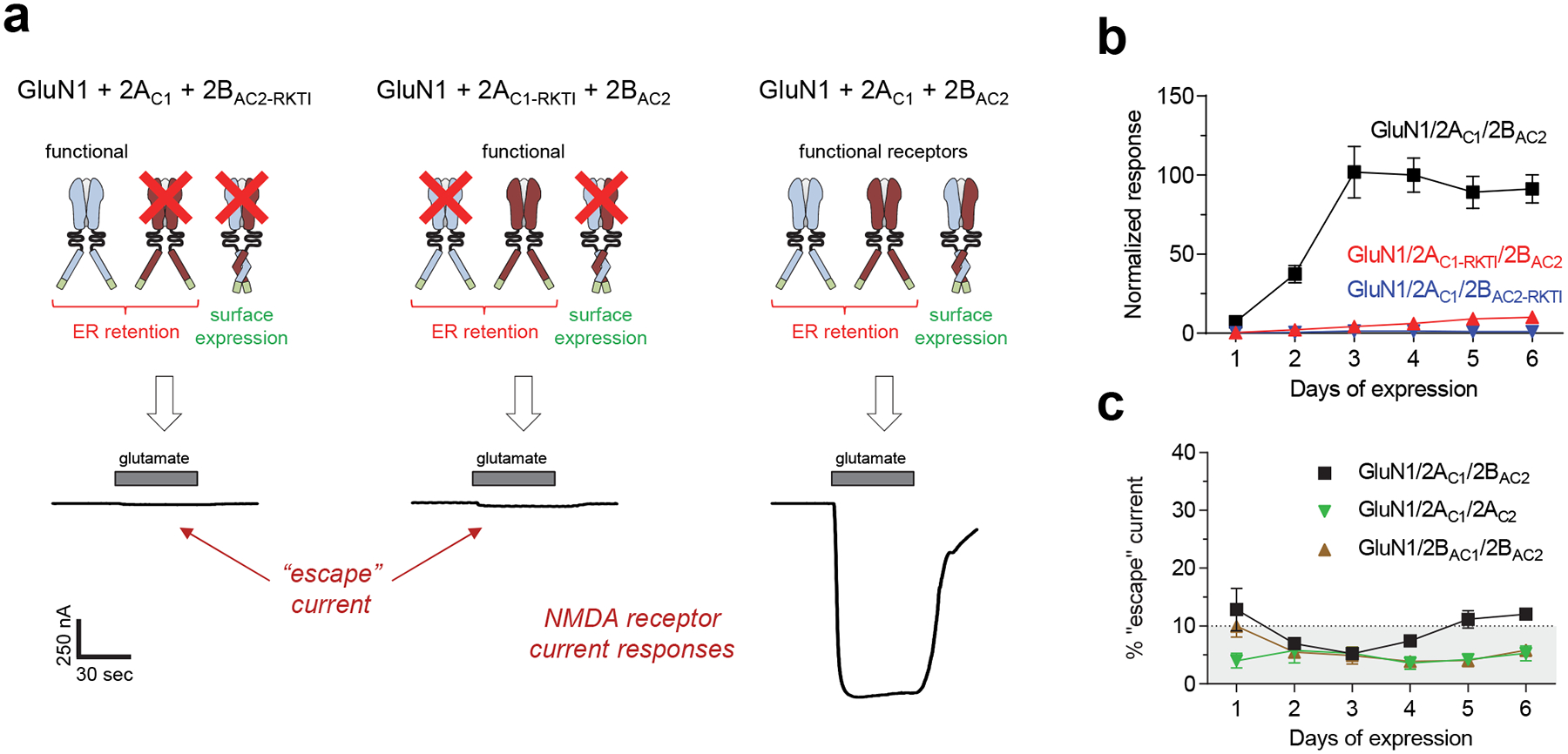
a) Representative two-electrode voltage-clamp recordings of responses from recombinant NMDA receptors expressed in Xenopus oocytes activated by 100 μM glutamate in the continuous presence of 50 μM glycine. GluN1 was co-expressed with either 2AC1 + mutated 2BAC2 (R519K + T691I in 2BAC2-RKTI) (left), mutated 2AC1 (R518K + T690I in 2AC1-RKTI) + 2BAC2 (middle), or 2AC1 + 2BAC2 (right). The RKTI mutations abolish binding of glutamate, thereby causing NMDA receptors with this subunit to be non-functional (indicated by red X). b) Co-expression of GluN1 with 2AC1 and 2BAC2 (GluN1/2AC1/2BAC2) produces robust current responses that increase in the days following cRNA injection (black), whereas current responses from diheteromeric GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2 receptors that may have escaped ER retention remain small (i.e. escape currents). Data are normalized to the averaged response from GluN1/2AC1/2BAC2 on day 4, and are mean ± SEM. Each data point is from 5 batches of oocytes with 6 oocytes for each batch (N = 30). c) The sum of the fractional currents assessed using the RKTI mutations (i.e. shown in panel b) provides an estimate of the percent “escape” current in oocytes co-expressing GluN1 with 2AC1 and 2BAC2 (GluN1/2AC1/2BAC2). The method can also be used for expression of GluN1/2AC1/2AC2 and GluN1/2BAC1/2BAC2 receptors as indicated by the percent “escape” current included in the graph, which are similarly assessed using RKTI mutations. Adapted with permission from Hansen et al. [18].
The method, which was described in 2014, has been applied in multiple studies and revealed surprising pharmacological and functional properties of triheteromeric GluN1/2A/2B receptors that are distinct from the properties of the respective diheteromeric GluN1/2A and GluN1/2B receptors [18,20,31–35]. In addition, the method is facilitating new opportunities to develop therapeutic agents that target triheteromeric NMDA receptors with one copy of a disease-causing mutation [32–37]. The method also paves the way for new experiments into the relationship between NMDA receptor structure and function by enabling unprecedented control of the subunit composition. Future work will show if the method can be optimized for the expression of triheteromeric NMDA receptors with other subunit compositions (e.g. GluN1/2B/2D or GluN1/2A/2C) (see Note 5).
2. Materials
The protocol described here use Xenopus laevis oocytes as heterologous expression system for triheteromeric GluN1/2A/2B receptors, and recordings of receptor function are performed using two-electrode voltage-clamp electrophysiology. DNA constructs for GluN1 as well as C1- and C2-tagged GluN2A and GluN2B subunits can be designed as described (Fig. 1).
2.1. Linearization of Plasmid DNA and In Vitro cRNA Transcription
Purified plasmid DNA (5–10 μg) and suitable restriction enzymes.
QIAquick PCR Purification Kit (Qiagen)
RNase-free 3 M sodium acetate, pH 5.2 (Sigma-Aldrich)
96–100 % ethanol and 70 % ethanol prepared using RNase-free water.
TE buffer (DNA/RNA storage buffer): 1 mM EDTA, 10 mM Tris·Cl, pH 8.0 prepared using RNase-free water.
mMESSAGE mMACHINE High Yield Capped RNA Transcription Kit (Ambion) with a suitable RNA polymerase (SP6, T3, or T7).
RNase-free 1.5 mL microcentrifuge tubes.
Refrigerated centrifuge for 1.5 mL microcentrifuge tubes.
2.2. Injection and Maintenance of Xenopus Oocytes
Stereomicroscope with at least 10-fold magnification and a working distance of at least 10 cm.
Microinjector and micromanipulator: Cytoplasmic injections in oocytes can be performed in volumes between ~2–70 nL using either an automated injection device (e.g. Nanoject III, Drummond Scientific) or using a manual injection device. Precise positioning of the microinjector requires a micromanipulator.
Micropipette puller (e.g. PC-10 Puller, Narishige).
Glass capillaries suitable for the injection device (Drummond Scientific or World Precision Instruments) and mineral oil (e.g. light oil for molecular biology, Sigma-Aldrich) for back filling the glass capillaries using a flexible needle (e.g. MicroFil, 34 ga., World Precision Instruments).
Incubator: This method requires incubation of the oocytes at 19 °C (see Note 6).
Injection chamber: Can be custom-made with different designs (see Note 7).
Pipettes for handling Xenopus oocytes: Can be prepared by breaking the tip of glass Pasteur pipettes (9”, Fischer Scientific) to create a tip with an internal diameter of 1.5–2.5 mm. It is important to fire polish the tip to prevent damage to the oocytes.
Modified Barthś solution (MBS) (in mM): 88 NaCl, 1.0 KCl, 2.4 NaHCO3, 0.41 CaCl2, 0.82 MgSO4, 0.33 Ca(NO3)2, 10 HEPES, pH 7.5 supplemented with 1 IU/mL penicillin, 1 μg/mL streptomycin (i.e. add 100 μL from Penicillin/Streptomycin solution (10,000 IU/ml and 10,000 μg/ml, respectively; Invitrogen)), and 50 μg/mL gentamycin (Fisher BioReagents).
Defolliculated Xenopus oocytes (see Note 8).
2.3. Electrophysiological Recordings from Xenopus Oocytes
3. Methods
3.1. Linearization of Plasmid DNA
To produce template DNA for cRNA synthesis, dilute 5–10 μg plasmid DNA in 200 μL of the suitable restriction enzyme buffer, add 20–40 units of restriction enzyme, and incubate 2–3 hours at 37 °C. Restriction enzyme that leaves a 5’ protruding overhang are preferred (see Note 11). Proceed to purify the linearized DNA or store the reaction at −20 °C after incubation.
The QIAquick PCR Purification Kit will be used for purification of template DNA: Following incubation at 37 °C, add 1 mL (5 volumes) Buffer PB to the reaction and mix. Transfer the DNA solution to a spin column and centrifuge at room temperature (RT) until the solution has passed the column. Apply half of the 1.2 ml DNA solution (i.e. 200 μL reaction + 1 mL Buffer PB) at a time, since the spin column can only hold 800 μL. Remove solution after it has passed the column. The DNA is now bound to the column.
Wash by adding 750 μL Buffer PE and centrifuge at RT until the solution has passed the column. Remove solution and centrifuge spin column for an additional 3 min at max speed to remove residual ethanol. Transfer spin column to a new, RNase-free 1.5 mL tube.
To elute DNA, add 100 μL Buffer EB (RNase-free) to the spin column and centrifuge at max speed for 1 min. The eluted DNA can be stored at −20 °C.
Add 1/10 volume (10 μL) 3 M sodium acetate, pH 5.2 (ice cold), mix, and then add 2.5 volume (250 μL) 96–100 % ethanol (ice cold). Mix well and incubate 10 min on dry ice (or 30 min to overnight at −80 °C).
Centrifuge at 20,000 x g for 30 min (4 °C) and carefully remove the supernatant without disturbing the barely visible pellet containing the DNA. Add 200 μL 70 % ethanol (ice cold). Do not resuspend the DNA. Centrifuge at 20,000 x g for 30 min (4 °C).
Carefully remove the supernatant. Dry the pellet at RT or low heat (e.g. 37 °C for 3–5 minutes). Do not over-dry, since this will make it difficult to resuspend the DNA.
Resuspend pellet in 5–10 μL TE buffer (see Note 12). Run 0.5 μL on an agarose gel to assess the concentration and the quality of the purified DNA. The plasmid DNA should be completely linearized and appear as a single band on the gel (see Note 13). The concentration of DNA can be determined using a spectrophotometer or estimated by comparing band intensity with those of bands in a DNA ladder with known concentration. Store the linearized DNA template at −20 °C.
3.2. In Vitro cRNA Transcription
Prepare the reaction for RNA transcription using 1–2 μg linearized DNA template according to the instructions included in the mMESSAGE mMACHINE High Yield Capped RNA Transcription Kit (Ambion). Incubate the reaction at 37 °C for 2 hours.
Add 1 μL TURBO DNase (included in the transcription kit) to the reaction, mix carefully, and incubate at 37 °C for 15 min. This step is required to digest the template DNA prior to cRNA precipitation.
Precipitate the cRNA by adding 30 μL RNase-free water (included in the transcription kit) and 30 μL LiCl precipitation solution (included in the transcription kit), vortex, and incubate at −20 °C for ≥30 min or overnight.
Transfer the frozen tubes to a refrigerated centrifuge and pellet the cRNA for 30 min at 20,000 x g (4 °C). Carefully remove the supernatant without disturbing the pellet, carefully add 200 μL 70 % ethanol (do not resuspend), and re-centrifuge for 30 min at 20,000 x g (4 °C). The ethanol can be added to the side of the tube in order not to disturb the pellet.
Carefully remove the ethanol, briefly dry the cRNA (e.g. at 37°C for a couple of minutes), and resuspend the cRNA in 20 μL TE buffer (see Note 12). Run 1 μL on an agarose gel to assess the quality of the RNA, which should appear as a single band on the gel (see Note 13). It is recommended that the cRNA concentration is determined using small-volume spectrophotometer (e.g. Nanodrop, Thermo Scientific). Keep RNA ice cold at all times and store at −80 °C.
3.3. Expression of triheteromeric NMDA receptors in Xenopus Oocytes
Prepare injection pipettes using a standard pipette puller. The tips of the injection pipettes are broken under the stereomicroscope to an outer diameter of about 10–20 μm. Use gloves when handling injection pipettes and protect injection pipettes from contamination by RNases.
Place oocytes in the injection chamber containing MBS solution. The oocytes can be manipulated using customized Pasteur pipettes.
Back-fill an injection pipette with mineral oil and mount it to the microinjector syringe according to the manufacturer’s instructions. The plunger will force mineral oil out of the tip of the injection pipette, resulting in a complete seal of oil between the tip and the plunger. Make sure there are no air bubbles in the injection pipette. The injection pipette can be moved and precisely positioned using a manual micromanipulator once it has been mounted to the microinjector syringe.
- Combine cRNA encoding GluN1 as well as C1- and C2-tagged GluN2A and GluN2B for injection. In order to evaluate escape currents from diheteromeric receptors, make the following combinations of cRNA:
- GluN1 + 2AC1 + 2BAC2 (i.e. to measure total current amplitudes)
- GluN1 + 2AC1 + 2BAC2-RKTI (i.e. escape currents from GluN1/2AC1/2AC1)
-
GluN1 + 2AC1-RKTI + 2BAC2 (i.e. escape currents from GluN1/2BAC2/2BAC2)In order to enable comparisons of current amplitudes, it is critical that the quantity of total injected cRNA and the molar ratios of the cRNAs are kept constant among the three combinations. Optimization of total injected cRNA and molar ratios are likely required to minimize escape currents for the specific constructs used and under the given experimental conditions (see Note 14).
Transfer cRNA to the injection pipette by placing a small volume, usually 2–3 μL, in the cap from a 1.5 mL RNase-free microcentrifuge tube (separate the cap from the tube using a scissor) under the stereomicroscope. Load the cRNA into the injection pipette using the microinjector. Watch the cRNA solution as it is drawn into the injection pipette to ensure the tip has not been clogged and that there are no air bubbles in the injection pipette.
Place the injection chamber containing oocytes under the stereomicroscope and inject cRNA by positioning the injection pipette over each oocyte and gently lowering the tip until it pierces the membrane. Some oocytes may leak after the injection pipette has been pulled back out of the membrane. To allow proper diffusion of the RNA inside the oocyte, it is advisable to wait approximately 5 seconds after the cRNA has been injected before retracting the injection pipette. Each oocyte can be injected with anywhere between 10 nL to 100 nL RNA solution depending on the desired level of expression, which usually increase as more RNA is injected. Use a new injection pipette for each type of cRNA to prevent cross-contamination.
Repeat steps 5–6 for each cRNA combination, ensuring that the volume of injected cRNA is constant for all injections.
Following cRNA injection, place the oocyte in a petri dish containing Barthś solution and incubate at 19 °C until the day of recording (see Note 14).
3.4. Electrophysiological Recordings of NMDA Receptor Function
- In order to evaluate expression of triheteromeric GluN1/2A/2B receptors as described in Figure 2, make the following solutions:
- Wash (i.e. extracellular recording solution with 50 μM glycine)
- 100 μM L-glutamate in wash.
Apply solutions to the perfusion system of the two-electrode voltage clamp setup and adjust flow rates.
Place the injected oocyte in the recording chamber of the two-electrode voltage clamp setup, penetrate the membrane with the two electrodes, and clamp the oocyte at the desired holding potential (i.e. membrane potential). Holding potentials between −80 to −40 mV are suitable for recordings of NMDA receptor-mediated currents, albeit the holding potential must be kept constant for all oocytes to enable comparison of current amplitudes.
Start the recording when the baseline current has stabilized and activate the NMDA receptor current response by applying glutamate, i.e. start in wash (solution 1), switch to glutamate (solution 2), and return to wash after a steady-state response is achieved. Typically, 30–60 second applications of glutamate is sufficient to reach a steady-state (i.e. equilibrium) response or to determine if no response can be observed (i.e. due to the lack of functional NMDA receptor expression). It is important though to ensure you reach equilibrium in order to accurately measure the response amplitude (see Note 15).
Determine the glutamate-activated current response (i.e. steady-state current in the presence of glutamate minus the baseline current) for an equal number of oocytes injected with cRNA from each of the three combinations. The number of oocytes should be sufficient to allow an estimate of the average current response for each cRNA combination. Typically this can be achieved using 6–8 oocytes per cRNA combination, depending on response variability. It is important that current responses are measured using the same experimental conditions for each cRNA combination.
Calculate the mean current response for each cRNA combination. The mean current response from oocytes expressing GluN1 + 2AC1 + 2BAC2 divided by the sum of the mean current responses from oocytes expressing GluN1 + 2AC1 + 2BAC2-RKTI and GluN1 + 2AC1-RKTI + 2BAC2 provides an estimate of the contribution of escaped diheteromeric receptors to the total current response (i.e. escape current). This method should consistently yield <10% escape currents (i.e. >90% of the total current response is mediated by triheteromeric GluN1/2A/2B). However, the method may underperform for some batches of oocytes (see Note 16) and if the cRNA quality is poor (i.e. due to degradation). It is therefore strongly advised that the estimation of escape current is performed on the day of all quantitative pharmacological experiments (e.g. determination of concentration-response relationships for triheteromeric GluN1/2A/2B receptors).
4. Notes
The idea behind replacing the GluN2B CTD with the GluN2A CTD in triheteromeric GluN1/2A/2B receptors was two-fold. First, the CTD replacement ensures that the structure of the two GluN2 subunit is identical, thereby resulting in a symmetrical placement of the C-termini with the coiled-coil motifs and the retention signals relative to the membrane and the GluN1 subunits. Thus, the CTD replacement would ideally optimize coiled-coil formation in triheteromeric GluN1/2AC1/2BAC2 and minimize biased coiled-coil formation in diheteromeric GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2. Second, variation may exit among GluN2 CTDs with respect to their trafficking properties, which could interfere with the method in subunits-specific manner.
Ideally, selective antagonists for GluN1/2AC1/2AC1 and GluN1/2BAC2/2BAC2 would enable measurements of the escape currents in oocytes co-expressing GluN1 with 2AC1 and 2BAC2, but such ligands with no effects on triheteromeric GluN1/2A/2B receptors are currently not available.
The functional properties of diheteromeric GluN1/2B receptors containing the GluN2A CTD are indistinguishable from those of wild type GluN1/2B [18,38,39]. In addition, fusion of the C1 and C2 tags at the C-terminus of the GluN2 subunits has no detectable effects on the pharmacological and functional properties of the receptors [18]. This can be shown by comparing function and pharmacology of wildtype GluN1/2A and GluN1/2B receptors with those of GluN1/2AC1/2AC2 and GluN1/2BAC1/2BAC2, respectively [18].
Diheteromeric GluN1/2A receptors with the R518K mutation in both GluN2A subunits (GluN1/2ARK/2ARK) are not activated by 100 μM glutamate (in the presence of glycine). However, small current responses are activated by 100 μM glutamate for triheteromeric GluN1/2A receptors with only one R518K mutation (GluN1/2A/2ARK) (data not shown). Thus, in order to completely abolish glutamate binding, the T690I mutation was introduced to the GluN2A subunit in addition to the R518K mutation [18]. Similarly, both the R519K and T691I mutations are required to completely abolish glutamate binding to the GluN2B subunit [18].
The method described here works well for expression of GluN1/2AC1/2AC2 and GluN1/2BAC1/2BAC2 receptors (Fig. 2c), which can be used in studies on the relationship between NMDA receptor structure and function by enabling expression of receptors with mutations in only one of the two GluN2 subunits [18]. In theory, the method could also be adapted and optimized for the expression of other triheteromeric NMDA receptors, such as GluN1/2A/2C or GluN1/2B/2D. However, expression of these subunit combinations are complicated by functional differences endowed by the different GluN2 subunits. The probability that the channel will be open when all the agonist binding sites are fully occupied by agonists (i.e. the open probability) is ~0.5 for diheteromeric GluN1/2A, ~0.1 for GluN1/2B, and <0.05 for GluN1/2C and GluN1/2D [1]. For example, escaped diheteromeric GluN1/2A will produce large current responses compared to escaped diheteromeric GluN1/2C, which may be problematic if the open probability of triheteromeric GluN1/2A/2C is closer to that of GluN1/2C.
The success of the method to express triheteromeric receptors is highly dependent on the incubation temperature during cRNA expression in the oocytes. At 16 °C, the expression of triheteromeric receptors small compared to escaped diheteromeric receptors. However, this problem was solved by raising the incubation temperature to 19 °C.
An injection chamber is required in order to prevent oocytes from moving during injection. Such chamber can be custom-made by carving a V-shaped groove in a silicone elastomer (e.g. Sylgard 184 silicone elastomer kit, World Precision Instruments), which has been applied to the bottom of a petridish. Alternatively, a chamber can be made by gluing a fine polypropylene mesh to the bottom of a dish.
Defolliculated healthy-looking stage V-VI oocytes are needed for cRNA injection and NMDA receptor expression. Oocytes can be purchased from several vendors (Rob Weymouth (Xenopus 1) or Ecocyte Bioscience), but can also be surgically removed from captive-bred Xenopus laevis and treated with collagenase in order to remove the surrounding follicular layer. Several publications provide detailed protocols on the maintenance and surgery of Xenopus laevis as well as treatment, isolation, and selection of oocytes [40–44].
Most electrophysiological recordings using oocytes are performed with a two-electrode voltage-clamp setup. The principles and design of the two-electrode voltage-clamp setup is described in detail elsewhere [41].
The concentrations of divalent cations and the pH of the extracellular recording solution can be varied to meet special requirements of the receptor of interest and the experimental design. During two-electrode voltage-clamp recordings, the oocytes are stable when divalent cations (e.g. Ca2+, Ba2+, or Mg2+) are included in the extracellular recording solution, but the oocytes are unstable in the complete absence of divalent cations. The central ion channel pore of NMDA receptors is blocked by extracellular Mg2+, whereas the channel is highly permeable to extracellular Ca2+. NMDA receptor-mediated Ca2+ influx into the cytoplasm of oocytes strongly activates calcium-activated chloride channels, which will impede electrophysiological recordings of NMDA receptor responses [45,46]. Thus, Ca2+ and Mg2+ should generally be avoided, leaving Ba2+ as the preferred divalent cation in the extracellular recording solution for studies on NMDA receptors expressed in Xenopus oocytes.
Several factors affect the expression of NMDA receptor subunits in Xenopus oocytes, but one important factor is the choice of DNA template. The open reading frame of the subunits can be inserted into a vector immediately following a RNA polymerase promoter (e.g. from SP6, T7, or T3 bacteriophage) for successful transcription and translation. Expression may be increased by using a vector specifically designed for expression in Xenopus oocytes, such as pGEMHE [47] or pXOOM [48]. The plasmid DNA must be linearized with a restriction enzyme that leaves a 5’ protruding overhang (or blunt end), since a 3’ protruding overhang can function as a primer for unintended RNA synthesis and generate antisense RNA that strongly interferes with translation. To increase the stability of the RNA, the site of linearization should be located following an artificial poly(A) tail or 200–500 base pairs from the stop codon.
Patience and a great deal of pipetting are often required when resuspending DNA or RNA pellets in storage buffers (e.g. TE buffer). In addition, resuspension can be further complicated if the DNA/RNA pellets have been “over-dried”. DNA/RNA that has not been properly resuspended may be absent from the agarose gel. If the pellets have been “over-dried” or are otherwise resisting resuspension, then repeated freeze/thawing of the sample can facilitate dissolution of DNA/RNA.
The quality of the linearized DNA template critically affects RNA transcription. It is important that the linearized DNA appear as a single band on the gel. The presence of higher order bands could indicate incomplete linearization of the DNA plasmid, which will produce higher order poorly-expressing RNA products during in vitro transcription (i.e. RNA polymerase will continue transcription on the DNA plasmid instead of producing several run-off transcripts). RNA that does not appear as a single band on the gel, but rather appear as a smear, is often caused by the presence of RNases during transcription and/or in the RNA solution. To prevent RNase contamination, it is important to always use cloves and preferentially filter tips when handling template DNA and RNA solutions.
It is advised that the molar ratios of cRNA for the different subunits, the total amount of cRNA injected, and the duration of expression are optimized to maximize the expression of triheteromeric receptors and to minimize the escape of diheteromeric receptors. In the previously published study, we injected a total of approximately 10 ng cRNA encoding GluN1, 2AC1, and 2BAC2 at a 1:6:6 ratio in a total volume of 50 nl, which consistently resulted in selective expression of triheteromeric receptors on days 2–4 after injection [18]. However, these experimental conditions are likely dependent on multiple factors (e.g. the plasmids used, the quality of the cRNA, or the preparation of oocytes) and will presumably vary among experimenters and laboratories.
Activity-dependent increases in NMDA receptor response amplitudes can be observed for Xenopus oocytes expressing GluN2A- or GluN2B-containing NMDA receptors (e.g. GluN1/2A and GluN1/2B) [49–51]. This “run-up” of responses can strongly interfere with the ability to perform quantitative pharmacological experiments, such as the determination of ligand concentration-response relationships. However, activity-dependent increases in response amplitude can be prevented by gentle injection with 20 nl of 50 mM BAPTA (in water and adjusted to pH 7.4 using KOH) approximately 10–30 min before the recordings are performed [49]. Injection with BAPTA will not interfere with the protocol described here [35]. However, long-term viability is low for oocytes injected with BAPTA and they are usually discarded following the subsequent recording.
Xenopus laevis oocytes have low-level expression of endogenous NMDA receptor subunits that can, under some circumstances, assemble with recombinant NMDA receptor subunits to produce functional receptors [52–54]. In most batches of oocytes, the expression of Xenopus NMDA receptor subunits is too low to interfere with the expression of recombinant NMDA receptors. However, there are occasional batches of oocytes with unusually high levels endogenous Xenopus NMDA receptor subunits, resulting in unexpected functional and pharmacological results. Such batches of oocytes are also expected to interfere with the protocol described here and should be avoided.
Acknowledgements
This work was supported by grants from National Institutes of Health (R01NS065371, P20GM103546, and R01NS097536).
References
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62 (3):405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X, Gouaux E (2014) NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 511 (7508):191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulbrich MH, Isacoff EY (2007) Subunit counting in membrane-bound proteins. Nat Methods 4 (4):319–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344 (6187):992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Otano I, Larsen RS, Wesseling JF (2016) Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat Rev Neurosci 17 (10):623–635 [DOI] [PubMed] [Google Scholar]
- 6.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ (2007) NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci 27 (31):8334–8343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauner C, Kohr G (2011) Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286 (9):7558–7566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tovar KR, McGinley MJ, Westbrook GL (2013) Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci 33 (21):9150–9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo JH, Wang YH, Yasuda RP, Dunah AW, Wolfe BB (1997) The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51 (1):79–86 [DOI] [PubMed] [Google Scholar]
- 10.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY (1994) Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368 (6467):144–147 [DOI] [PubMed] [Google Scholar]
- 11.Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG (2003) NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci 23 (12):4958–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pina-Crespo JC, Gibb AJ (2002) Subtypes of NMDA receptors in new-born rat hippocampal granule cells. J Physiol 541 (Pt 1):41–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones S, Gibb AJ (2005) Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J Physiol 569 (Pt 1):209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y, Traynelis SF (2015) NMDA Receptors Containing the GluN2D Subunit Control Neuronal Function in the Subthalamic Nucleus. J Neurosci 35 (48):15971–15983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chazot PL, Coleman SK, Cik M, Stephenson FA (1994) Molecular characterization of N-methyl-D-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem 269 (39):24403–24409 [PubMed] [Google Scholar]
- 16.Cathala L, Misra C, Cull-Candy S (2000) Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci 20 (16):5899–5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatton CJ, Paoletti P (2005) Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46 (2):261–274 [DOI] [PubMed] [Google Scholar]
- 18.Hansen KB, Ogden KK, Yuan H, Traynelis SF (2014) Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 81 (5):1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JAH, Wolfe BB, Grayson DR (1998) Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol 79 (2):555–566 [DOI] [PubMed] [Google Scholar]
- 20.Stroebel D, Carvalho S, Grand T, Zhu S, Paoletti P (2014) Controlling NMDA receptor subunit composition using ectopic retention signals. J Neurosci 34 (50):16630–16636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP (2004) Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol 11 (8):706–713 [DOI] [PubMed] [Google Scholar]
- 22.Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84 (3):835–867 [DOI] [PubMed] [Google Scholar]
- 23.Margeta-Mitrovic M, Jan YN, Jan LY (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27 (1):97–106 [DOI] [PubMed] [Google Scholar]
- 24.Kammerer RA, Frank S, Schulthess T, Landwehr R, Lustig A, Engel J (1999) Heterodimerization of a functional GABAB receptor is mediated by parallel coiled-coil alpha-helices. Biochemistry 38 (40):13263–13269 [DOI] [PubMed] [Google Scholar]
- 25.Burmakina S, Geng Y, Chen Y, Fan QR (2014) Heterodimeric coiled-coil interactions of human GABAB receptor. Proc Natl Acad Sci U S A 111 (19):6958–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerangue N, Malan MJ, Fried SR, Dazin PF, Jan YN, Jan LY, Schwappach B (2001) Analysis of endoplasmic reticulum trafficking signals by combinatorial screening in mammalian cells. Proc Natl Acad Sci U S A 98 (5):2431–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brock C, Boudier L, Maurel D, Blahos J, Pin JP (2005) Assembly-dependent surface targeting of the heterodimeric GABAB Receptor is controlled by COPI but not 14–3-3. Mol Biol Cell 16 (12):5572–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai R, Wriggers W, Nishikawa Y, Nagamune T, Fujisawa T (2004) Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins 57 (4):829–838 [DOI] [PubMed] [Google Scholar]
- 29.Benveniste M, Mayer ML (1991) Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys J 59 (3):560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clements JD, Westbrook GL (1991) Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 7 (4):605–613 [DOI] [PubMed] [Google Scholar]
- 31.Cheriyan J, Balsara RD, Hansen KB, Castellino FJ (2016) Pharmacology of triheteromeric N-Methyl-D-Aspartate Receptors. Neurosci Lett 617:240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi F, Mou T-C, Dorsett KN, Volkmann RA, Menniti FS, Sprang SR, Hansen KB (2016) Structural basis for negative allosteric modulation of GluN2A-containing NMDA receptors. Neuron In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serraz B, Grand T, Paoletti P (2016) Altered zinc sensitivity of NMDA receptors harboring clinically-relevant mutations. Neuropharmacology 109:196–204 [DOI] [PubMed] [Google Scholar]
- 34.Hackos DH, Lupardus PJ, Grand T, Chen Y, Wang TM, Reynen P, Gustafson A, Wallweber HJ, Volgraf M, Sellers BD, Schwarz JB, Paoletti P, Sheng M, Zhou Q, Hanson JE (2016) Positive Allosteric Modulators of GluN2A-Containing NMDARs with Distinct Modes of Action and Impacts on Circuit Function. Neuron 89 (5):983–999 [DOI] [PubMed] [Google Scholar]
- 35.Yi F, Mou TC, Dorsett KN, Volkmann RA, Menniti FS, Sprang SR, Hansen KB (2016) Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors. Neuron 91 (6):1316–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan H, Hansen KB, Zhang J, Pierson TM, Markello TC, Fajardo KV, Holloman CM, Golas G, Adams DR, Boerkoel CF, Gahl WA, Traynelis SF (2014) Functional analysis of a de novo GRIN2A missense mutation associated with early-onset epileptic encephalopathy. Nat Commun 5:3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khatri A, Burger PB, Swanger SA, Hansen KB, Zimmerman S, Karakas E, Liotta DC, Furukawa H, Snyder JP, Traynelis SF (2014) Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator. Mol Pharmacol 86 (5):548–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maki BA, Aman TK, Amico-Ruvio SA, Kussius CL, Popescu GK (2012) C-terminal domains of N-methyl-D-aspartic acid receptor modulate unitary channel conductance and gating. J Biol Chem 287 (43):36071–36080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Punnakkal P, Jendritza P, Kohr G (2012) Influence of the intracellular GluN2 C-terminal domain on NMDA receptor function. Neuropharmacology 62 (5–6):1985–1992 [DOI] [PubMed] [Google Scholar]
- 40.Bossi E, Fabbrini MS, Ceriotti A (2007) Exogenous protein expression in Xenopus oocytes: basic procedures. Methods Mol Biol 375:107–131 [DOI] [PubMed] [Google Scholar]
- 41.Stuhmer W (1998) Electrophysiologic recordings from Xenopus oocytes. Methods Enzymol 293:280–300 [DOI] [PubMed] [Google Scholar]
- 42.Goldin AL (1992) Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol 207:266–279 [DOI] [PubMed] [Google Scholar]
- 43.Goldin AL, Sumikawa K (1992) Preparation of RNA for injection into Xenopus oocytes. Methods Enzymol 207:279–297 [DOI] [PubMed] [Google Scholar]
- 44.Matten WT, Vande Woude GF (1995) Microinjection into Xenopus oocytes. Methods Enzymol 254:458–466 [DOI] [PubMed] [Google Scholar]
- 45.Leonard JP, Kelso SR (1990) Apparent desensitization of NMDA responses in Xenopus oocytes involves calcium-dependent chloride current. Neuron 4 (1):53–60 [DOI] [PubMed] [Google Scholar]
- 46.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature 354 (6348):31–37 [DOI] [PubMed] [Google Scholar]
- 47.Liman ER, Tytgat J, Hess P (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9 (5):861–871 [DOI] [PubMed] [Google Scholar]
- 48.Jespersen T, Grunnet M, Angelo K, Klaerke DA, Olesen SP (2002) Dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytes. Biotechniques 32 (3):536–538, 540 [DOI] [PubMed] [Google Scholar]
- 49.Williams K (1993) Ifenprodil Discriminates Subtypes of the N-Methyl-D-Aspartate Receptor - Selectivity and Mechanisms at Recombinant Heteromeric Receptors. Mol Pharmacol 44 (4):851–859 [PubMed] [Google Scholar]
- 50.Logan SM, Rivera FE, Leonard JP (1999) Protein kinase C modulation of recombinant NMDA receptor currents: roles for the C-terminal C1 exon and calcium ions. J Neurosci 19 (3):974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS (1997) Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. J Neurosci 17 (22):8676–8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terhag J, Cavara NA, Hollmann M (2010) Cave Canalem: how endogenous ion channels may interfere with heterologous expression in Xenopus oocytes. Methods 51 (1):66–74 [DOI] [PubMed] [Google Scholar]
- 53.Schmidt C, Hollmann M (2009) Molecular and functional characterization of Xenopus laevis N-methyl-d-aspartate receptors. Mol Cell Neurosci 42 (2):116–127 [DOI] [PubMed] [Google Scholar]
- 54.Schmidt C, Klein C, Hollmann M (2009) Xenopus laevis oocytes endogenously express all subunits of the ionotropic glutamate receptor family. J Mol Biol 390 (2):182–195 [DOI] [PubMed] [Google Scholar]


