Abstract
Objective
This systematic review aims to evaluate the benefits of oral continuous combined hormonal contraceptives (CHCs) in managing dysmenorrhea by comparing randomized controlled trials (RCTs) evaluating the efficacy of continuous vs. cyclic CHC use for the following outcomes: (a) reducing dysmenorrhea duration and frequency, (b) severity, (c) recurrence and (d) interference with daily activity.
Study design
Cochrane, PUBMED and Popline databases were searched from 1934 to 2018 for all relevant studies evaluating CHC for treatment of dysmenorrhea. A study was selected if it (a) compared continuous regimen vs. cyclic regimen of oral CHC, (b) measured dysmenorrhea as a primary or secondary outcome, (c) was an RCT and (d) was published in English. Due to differences in CHC used and outcome measurement, a systematic analysis of individual study results and a limited meta-analysis were conducted.
Results
Of 780 studies that were screened by title and abstract, 8 were included in the final analysis; 6 evaluated cyclic vs. continuous CHC, and 2 evaluated cyclic vs. extended/flexible CHC use. Quality of evidence was low for all outcome measures. Overall, compared to cyclic use, flexible/extended CHC resulted in 4 fewer days of dysmenorrhea. Studies revealed conflicting results for interference with daily activity, pain severity and pain recurrence. Side effects were few in both comparison groups.
Conclusions
Continuous or extended/flexible CHC use may reduce dysmenorrhea duration compared to cyclic regimen; however, more rigorous research is needed.
Implications
This systematic review shows that continuous CHC use may reduce dysmenorrhea duration compared to cyclic regimen, although the quality of evidence is low. Future double-blinded RCTs with more rigorous study design, consistent outcome measures and comprehensive outcome reporting are needed.
Keywords: Dysmenorrhea, Oral contraceptives, Hormonal contraceptives, Treatment
1. Introduction
Dysmenorrhea is defined as cyclic crampy lower abdominal or pelvic pain that occurs just before and/or during menstruation, affecting approximately 50%–90% of reproductive age women worldwide [1], [2], [3], [4]. Primary dysmenorrhea does not have discernable macroscopic pathology, while secondary dysmenorrhea results from diseases such as endometriosis, adenomyosis or uterine fibroids [5]. Dysmenorrhea pain scores are moderate in 37%–47% and severe in 17%–18% of women [6], [7], and dysmenorrhea is associated with decreased quality of life, depression and anxiety [5], [8], [9], [10].
In recent studies, women with dysmenorrhea demonstrate functional and structural changes in the areas of the brain responsible for pain processing, like women with noncyclic chronic pelvic pain due to endometriosis [11], [12]. Initial longitudinal studies suggest that central changes associated with chronic pain may be reversible once the nociceptive input is removed. Researchers speculate that untreated dysmenorrhea may increase the risk of developing chronic pelvic pain and associated comorbidities [5] and that early screening and treatment of dysmenorrhea may prevent women from developing chronic pelvic pain.
Cyclic combined hormonal contraceptives (CHCs) are commonly used as second-line therapy for dysmenorrhea, following first-line therapy of nonsteroidal anti-inflammatory drugs [13], [14], [15]. CHCs may be prescribed as continuous, extended or flexible regimens. Cyclic use consists of 21 days of active hormone tablets followed by a 7-day hormone-free interval during which the patient experiences withdrawal bleeding. Continuous regimens skip the hormone-free interval to eliminate menstruation. Extended regimens lengthen the interval of active hormone to greater than 21 days, resulting in decreased and delayed menstruation. Flexible regimens allow women to initiate a hormone-free interval at their discretion, usually in response to unscheduled or “breakthrough” bleeding.
CHCs have been shown in randomized controlled trials (RCTs) to be similarly effective when used in cyclic, continuous, extended or flexible regimens for contraception [16]. A Cochrane systematic review of 10 studies confirmed that cyclic CHCs also significantly improve dysmenorrhea [15]. Continuous/extended regimen contraceptives are part of American College of Obstetricians and Gynecologists dysmenorrhea management guidelines [17]. However, their efficacy in the treatment of dysmenorrhea has not been studied in a systematic fashion.
Our research goal is to describe the existing evidence gap and systematically review all relevant RCTs evaluating the efficacy of continuous/flexible vs. cyclic CHC for the management of dysmenorrhea.
2. Materials and methods
2.1. Search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [18]. The research question was defined a priori. Three databases — Popline, Cochrane and PUBMED — were queried. We searched Popline using keywords dysmenorrhea and contraception, Cochrane for reviews comparing continuous vs. cyclic contraception and dysmenorrhea, and the PUBMED computerized database from 1934 to 2018 (last searched on September 23,2018) using the following search strategy of Keywords: (oral contracept* OR hormonal contracept* OR combined contracept*) AND (menstrual pain[tw] OR pelvic pain[tw] OR dysmenorrhea OR dysmenorrhea) AND (flexible OR extended OR cyclic OR cyclical OR continuous).
2.2. Selection criteria
Articles were included in this review if they were RCTs that (a) compared continuous or extended vs. cyclic CHC and (b) measured dysmenorrhea as a primary or secondary outcome. We excluded studies not published in English. The population of interest was any reproductive age woman desiring contraception. The following dysmenorrhea outcomes were reviewed: (a) duration, (b) frequency, (c) severity, (d) recurrence, (e) days when dysmenorrhea interfered with activity and (f) side effects and adverse effects.
2.3. Study selection, data synthesis and quality of evidence assessment
The primary reviewer (T.D.) evaluated titles and abstracts identified from the literature search of Cochrane, PUBMED and Popline to determine papers requiring full-text review as per the inclusion and exclusion criteria. Two authors (T.D. and C.O.) independently evaluated the included studies for risk of bias and quality of evidence according to the GRADE Handbook [19] using Review Manager 5 (RevMan 2014) [20] and GRADEpro [21], with reviewer G.L. serving as adjudicator. Factors that were considered when evaluating risk of bias included random sequence generation, allocation concealment, blinding, incomplete outcome reporting and selective reporting. Evidence quality for each outcome was rated as high, moderate, low or very low based on assessment of the studies' risk of bias, inconsistency, indirectness, imprecision and publication bias. Due to the differences in type of contraceptive used and method of outcome measurements in each study, only limited meta-analysis was performed, and the outcomes of studies not included in the meta-analysis are reported in narrative form.
3. Results
A total of 794 articles were extracted from Cochrane, PUBMED and Popline search. After removal of duplicates, 780 studies were screened for eligibility (Fig. 1). Of the 15 articles that were assessed for eligibility by full text, 7 were excluded for the following reasons: not a randomized control trial [22], [23], [24], [25], [26], control group was using placebo [27] and continuous intervention was using progestin only [28]. From the eight studies included in descriptive analysis, two had similar methodology and were evaluated in a meta-analysis [35], [36]. All eight studies in the final analysis were RCTs published between 2002 and 2017. Cyclic regimen consisted of 21 days of active hormone with a 7-day hormone-free interval unless otherwise defined.
Fig. 1.
Flowchart of study selection.
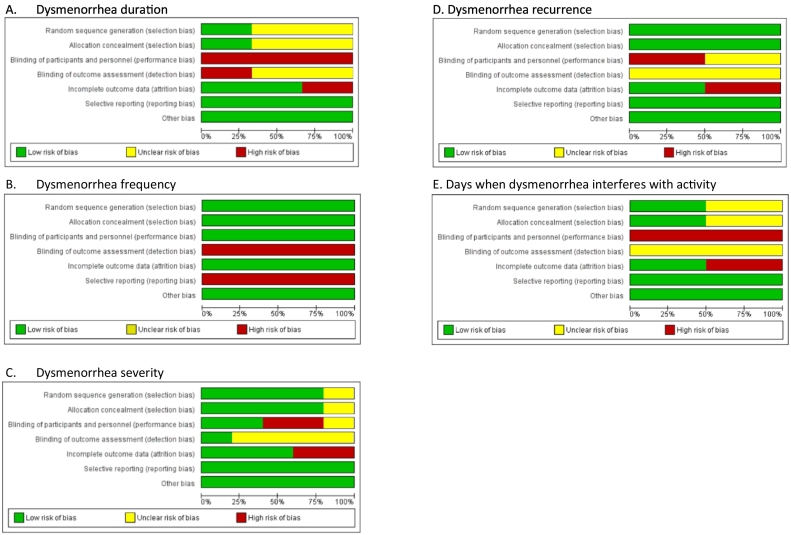
Characteristics of studies are summarized in Table 1. Study duration ranged from 6 to 24 months. Study locations spanned seven countries. Five types of progestins were used across studies. Risk of bias for each outcome is represented in Fig. 2. Overall, all studies had serious risk of bias. Two studies had unknown random sequence generation [29], [36]. Four studies had unknown allocation concealment [29], [31], [33], [36]. Seven studies had unknown blinding or were open label [29], [31], [32], [33], [34], [35], [36]. Incomplete outcome accounting occurred in two studies [33], [36]. Selective reporting occurred in one study [32]. Summary of evidence evaluating quality of evidence and effect of intervention for each outcome is presented in Table 2. Quality of evidence was (a) low for duration, (b) very low for frequency, (c) very low for severity, (d) low for recurrence rate and (e) low for days with dysmenorrhea interfering with daily activity.
Table 1.
Characteristics of studies
| Study | Location | Design | Study duration | Medical intervention | Type of OCP | Mean age (S.D.) | Number of patients | Outcomes and time points measured |
|---|---|---|---|---|---|---|---|---|
| Kwiecien 2002 | USA | RCT | 6 months | CYC: 21/7 days CON: 168 days | 20 mcg EE/0.1 mg levonorgestrel | CYC: 26.5 (4.5) CON: 29.3 (6.7) |
CYC: 16 CON: 16 |
Number of days with dysmenorrhea measured over 6 months. |
| Legro 2008 | USA | RCT | 6 months | CYC: 21/7 days CON: 168 days | 20 mcg EE/1 mg norethindrone | CYC: 27.5 (4.7) CON: 26.9 (5.6) |
CYC: 31 CON: 31 |
MMDQ (cyclical perimenstrual symptoms including dysmenorrhea) measured at baseline and again at 6 months. |
| Seracchioli 2010 | Italy | RCT | 24 months | CYC: 21/7 days CON: 730 days | 20 mcg EE/0.075 mg gestodene | CYC: 30.2 (2.4) CON: 29.6 (2.7) |
CYC: 92 CON: 95 |
Dysmenorrhea recurrence rate defined as VAS > 4 out of 10-point scale and change in severity of dysmenorrhea (10-point VAS scale) after endometrioma excision, measured at 6, 12, 18 and 24 months. |
| Machado 2010 | Brazil | RCT | 6 months | CYC: 21/7 days CON: 168 days | 30 mcg EE/3 mg drospirenone | CYC: 27.7 (5.1) CON: 27.9 (4.4) |
CYC: 39 CON: 39 |
Change in percentage of women experiencing dysmenorrhea recorded by patient diary at 1 and 6 months. |
| Muzii 2011 | Italy | RCT | 6 months | CYC: 21/7 days CON: 168 days | 20 mcg EE/0.15 mg desogestrel | CYC: 30.3 (2.9) CON: 30.6 (3.1) |
CYC: 28 CON: 29 |
Dysmenorrhea recurrence rate defined as VAS > 4 out of 10-point scale and change in severity of dysmenorrhea (10-point VAS scale) after endometrioma excision, measured at 3, 6, 12 and 24 months. |
| Dmitrovic 2012 | Croatia | RCT | 6 months | CYC: 21/7 days CON: 168 days | 20 mcg EE/0.075 mg gestodene | CYC: 21.1 (4.3) CON: 20.7 (3.9) |
CYC: 19 CON: 19 |
Reduction in dysmenorrhea as measured by 100-mm VAS scale and dysmenorrhea severity as assessed by MMDQ at 1, 3 and 6 months. |
| Strowitzki 2012 | UK and Germany | RCT | 140 days | CYC: 24/4 days FLEX: up to 140 days | 20 mcg EE/3 mg drospirenone | CYC: 25.3 (5.0) FLEX: 25.6 (5.1) |
CYC: 108 FLEX: 115 |
Numbers of days with dysmenorrhea and days in which dysmenorrhea interfered with daily activities as measured by patient diary for 140 days. |
| Momoeda 2017 | Japan | RCT | 364 days | CYC: 24/4 days FLEX: up to 364 days | 20 mcg EE/3 mg drospirenone | CYC: 30.4 (6.6) FLEX: 28.9 (6.0) |
CYC: 107 FLEX: 105 |
Numbers of days with dysmenorrhea and days in which dysmenorrhea interfered with daily activities as measured by patient diary for 364 days. Pain severity reduction was measured by a 100-mm VAS scale over 364 days. |
OCP, oral contraceptive pill; EE, ethinyl estradiol; CYC, cyclic regimen; CON, continuous regimen; FLEX, flexible regimen.
Fig. 2.
Risk of bias.
Table 2.
Summary of evidence
| Certainty assessment |
No. of patients |
Effect |
Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Continuous oral combined contraception | Cyclic oral combined contraception | Relative (95% CI) | Absolute (95% CI) | |
| Dysmenorrhea duration (follow-up: median 140 days; assessed with: daily journal) | |||||||||||
| 3 | Randomized trials | Seriousa | Not serious | Seriousb | Not serious | None | 236 | 231 | - | MD 4.0 days lower (5.7 lower to 2.3 lower)k | ⨁⨁◯◯ Low |
| Dysmenorrhea frequency (follow-up: mean 168 days; assessed with: daily journal) | |||||||||||
| 1 | Randomized trials | Seriousc | Not serious | Seriousd | Seriouse | None | Continuous and cyclic regimen each included 39 women. Frequency was measured as the percentage of women with dysmenorrhea after the 1st and 6th pill pack. Frequency declined from 59% to 28.2% (p <.02) in the continuous regimen group and from 44.4% to 27.8% in the cyclic regimen (noted as not statistically significant but p value not reported). Based on the available evidence, we are uncertain whether there is any difference in dysmenorrhea frequency among patients taking either cyclic or continuous CHC. | ⨁◯◯◯ Very low | |||
| Dysmenorrhea severity (follow-up: range 1 months to 6 months; assessed with: 10-point VAS scale, 100-mm VAS scale, MMDQ) | |||||||||||
| 5 | Randomized trials | Seriousf | Not serious | Very seriousg | Not serious | None | There were 277 women in the cyclic and 279 continuous group. Studies differed in measurement of pain severity, study duration and progestins used. Dmitrovic 2012 and Legro 2008 both measured severity using the MMDQ. Dmitrovic 2012 reported no difference in MMDQ pain score, mean difference 4.5 (95% CI − 22.2 to 13.2, p =.6). Legro 2008 reported a mean difference of 8.4 (95% CI 2.0–14.7, p =.01) at 6 months, favoring continuous CHC. Seracchioli 2010 reported median difference of 2 on 10-point VAS scores at 6 months (p <.0005), favoring continuous CHC. Muzii 2011 also evaluated pain severity on 10-point VAS but found no significant difference (no numerical data were provided). Momoeda 2017 and Dmitrovic 2012 evaluated pain severity using 100-mm VAS. Momoeda 2017 reported that there was no significant difference in pain reduction over 6 months (no numerical data were provided). Dmitrovic 2012 reported a mean difference in favor of continuous CHC at 1 month of − 27.3 (95% CI − 40.5 to − 14.2, p <.001) and at 3 months of − 17.8 (95% CI − 33.4 to − 2.1, p =.03); however, the authors noted no difference in dysmenorrhea severity at 6 months, − 16.0 (95% CI − 32.2 to 0.1, p =.05). Based on the available evidence, we are uncertain whether there is any difference in dysmenorrhea severity among patients taking either cyclic or continuous CHC. | ⨁◯◯◯ Very low | |||
| Dysmenorrhea recurrence (follow-up: range 6 months to 48 months; assessed with: 10-point VAS) | |||||||||||
| 2 | Randomized trials | Serioush | Not serious | Seriousi | Not serious | None | There were a total of 120 women in the cyclic regimen and 124 in the continuous regimen. Recurrence rates were defined as pain severity VAS > 4 during treatment. Seracchioli 2010 showed that, after 24 months of treatment, recurrence rates were < 5% in the continuous regimen compared to 25%–30% in the cyclic regimen (p <.005). Muzii 2011 showed that, after 12 months of treatment, recurrence rates were 17% in the continuous regimen compared to 32% in the continuous regimen (p =.54). Based on the available evidence, we are uncertain if there is any difference in dysmenorrhea recurrence among patients taking either cyclic or continuous CHC. | ⨁⨁◯◯ Low | |||
| Numbers of days when dysmenorrhea interfered with daily activity (follow-up: range 140 days to 168 days; assessed with: daily journal) | |||||||||||
| 2 | Randomized trials | Seriousj | Not serious | Not serious | Seriouse | None | There were a total of 215 women in the cyclic regimen and 220 in the flexible regimen. Strowitzki 2012 reported a mean difference of 2.2 fewer days (95% CI − 4.2 to − 0.1) in favor of flexible regimen, and Momoeda 2017 reported 2.0 days fewer (95% CI − 7.5 to 3.5), not statistically significant. Standard deviations not reported by studies; therefore, we were unable to compile meta-analysis. Based on the available evidence, we are uncertain if there is any difference in number of days with dysmenorrhea that interfered with daily activities among patients taking either cyclic or flexible CHC. | ⨁⨁◯◯ Low | |||
| Side effects | |||||||||||
| 5 | Randomized trials | - | - | - | - | - | All studies reported side effect profiles except for Seracchioli 2010 and Muzii 2011. Types of side effects assessed varied among studies. Kwiecien 2002 reported decrease of bloating in the continuous group, with a mean difference of 10.4 days less (p =.04). Machado 2010 found that there was a significant decrease of headache (p <.02), nausea (p <.02), appetite (p <.05) and acne (p <.05) in the continuous compared to cyclic group. However, Momoeda 2017, Strowitzki 2012 and Kwiecien 2002 found no difference in headaches, nausea or vomiting, and Legro 2008 also reported no difference in nausea and vomiting. Dmitrovic 2012 found greater weight gain in the continuous group (mean difference 2.3 kg, 95% CI 0.8–3.8, p =.003) and decrease in systolic blood pressure (p <.05); however, no differences were reported by Legro 2008, Machado 2010 or Strowitzki 2012. Legro 2008, Dmitrovic 2012 and Strowitzki 2012 found no difference in triglycerides, LDL and total cholesterol. While Legro 2008 found an increase in serum HDL-C in the cyclic group, (mean difference 5.0, 95% CI 0.7–9.3, p =.02), neither Dmitrovic 2012 and Strowitzki 2012 found a difference. | - | |||
MD, mean difference; RR, risk ratio.
Lack of blinding in all studies. Risk of incomplete accounting in Momoeda 2017 due to higher discontinuation rates in the cyclic group (22%) compared to continuous group (7%). Unclear allocation concealment in Kwiecien 2002 and Momoeda 2017.
Kwiecien 2002 examined women seeking birth control regardless of dysmenorrhea status, whereas Momoeda 2017 and Strowitzki 2012 only included women who reported dysmenorrhea prior to enrollment. Study locations were varied including Japan, Europe and the United States. Two different progestins were assessed.
This study was open label, and more participants in the continuous group (15.4%) discontinued due to adverse effects than in the cyclic group (7.7%).
This study population included any woman seeking birth control regardless of dysmenorrhea status in Brazil.
Sample size does not meet optimal information size criteria.
Lack of blinding in Momoeda 2017 and Seracchioli 2010. Incomplete accounting of patients and outcome events in Momoeda 2017 due to variance in discontinuation rates (23 women in continuous group vs. 7 women in cyclic group). In Muzii 2011, there were a difference in discontinuation rate between continuous vs. cyclic group (41% vs. 14%) and imbalanced crossover (high crossover from continuous to cyclic group but none from cyclic to continuous group).
Differences in study populations: Seracchioli 2010 and Muzii 2011 studied women using contraception after excision of endometriomas; Legro 2008 studied women seeking birth control regardless of dysmenorrhea status; Momoeda 2017 and Dmitrovic 2012 studied women who reported dysmenorrhea prior to enrollment. Study locations were varied including Japan, Europe and the United States. Outcome measurement scales varied: 10-point VAS was used by Seracchioli 2010 and Muzii 2011, 100-mm VAS was used by Momoeda 2017 and Dmitrovic 2012, and MMDQ was used by Legro 2008 and Dmitrovic 2012. Four different progestins were assessed in the studies.
Unclear allocation concealment and lack of blinding in Seracchioli 2010. Incomplete accounting of patients and outcome events in Muzii 2011.
Both trials studied Italian women who had dysmenorrhea likely from endometriosis and who underwent surgical excision of endometriomas prior to starting combined hormonal contraception.
Lack of blinding in both studies. Incomplete outcome data, unclear lack of allocation concealment and randomization of patients in Momoeda 2017.
Kwiecien 2002 reported mean difference of 11.4 less days (p <.01) between continuous and cyclic regimen. This study could not be included in the meta-analysis due to lack of reporting of standard deviation and usage of different type of progestin.
Kwiecien et al. [29] studied 32 women who were evenly randomized to receive either 168 days of cyclic or continuous regimen of 20 mcg ethinyl estradiol/0.1 mg levonorgestrel. One of the study's secondary outcome was number of days with dysmenorrhea. Patients taking continuous CHC had fewer days with dysmenorrhea than those taking cyclic CHC (1.9 vs. 13.3 days, p <.01).
Legro et al. [30] studied 62 women, evenly randomized to receive 20 mcg ethinyl estradiol/1 mg norethindrone in either a cyclic or continuous regimen for 168 days. Dysmenorrhea was a secondary outcome and was assessed using the Moos-Menstrual Distress Questionnaire (MMDQ) administered at baseline and once per menstrual cycle thereafter. The change in dysmenorrhea severity during treatment compared to baseline was greater (p =.010) in the continuous group (− 5.8) vs. the cyclic group (2.6).
Seracchioli et al. [31] studied women with secondary dysmenorrhea from endometriosis who had undergone laparoscopic excision of symptomatic ovarian endometrioma. After surgery, they were randomly assigned to one of three groups: (a) no additional treatment (104 allocated and 87 completed the trial), (b) cyclic oral CHC (103 allocated and 92 completed the trial) and (c) received continuous oral CHC (104 allocated and 95 completed the trial). Participants used 0.020 mg ethinyl estradiol/0.075 mg gestodene for 24 months. The primary outcome, dysmenorrhea recurrence [defined as 10-point Visual Analogue Scale (VAS) score ≥ 4], decreased in the continuous CHC group compared to cyclic CHC and placebo groups (p <.005).
Machado et al. [32] studied 78 women evenly randomized to a cyclic regimen or continuous regimen using 30 mcg ethinyl estradiol/3 mg drosperinone for 168 days. Twenty-nine women in each group (74%) successfully completed the trial. Frequency of dysmenorrhea was a secondary outcome. There was a decrease in dysmenorrhea frequency from 59% at 1 month to 29% at 6 months among the women taking continuous regimen (p <.02). No statistical difference was found for the cyclic group (44% at 1 months to 28% at 6 months).
Muzii et al. [33] studied participants who were previously diagnosed with endometriomas larger than 3 cm, had moderate to severe dysmenorrhea or chronic pelvic pain (≥ 4 on a 10-point VAS scale) and had not used estroprogestins in the last 6 months. All women underwent laparoscopic excision of ovarian endometriomas. Twenty-eight women postoperatively received 20 mcg ethinyl estradiol/0.15 mg desogestrel in a cyclic regimen, and 29 women received continuous active hormone for 168 days. One of the primary outcomes of the study was pain recurrence defined as dysmenorrhea or chronic pain that was graded ≥ 4 on the 10-point VAS scale. There was no significant difference in pain recurrence rate between the continuous and the cyclic groups (17% vs. 32%, p =.54). The discontinuation rate was significantly increased in the continuous group (41% vs. 14% in the cyclic regimen, p =.03) and was mainly attributed to breakthrough bleeding. Six women who discontinued the continuous regimen were crossed over to the cyclic regimen, and the other six discontinued all hormonal treatments. The four patients who did not complete the cyclic regimen were observed without further treatment.
Dmitrovic et al. [34] studied 38 women with history of primary dysmenorrhea (onset < 3 years after menarche) and 3 months of moderate to severe primary dysmenorrhea prior to enrollment, and used 0.075 mg gestodene and 20 mcg ethinyl estradiol as either cyclic or continuous regimen for 168 days. Fourteen women in the cyclic group and 15 in the continuous group completed the study. Pain severity, the primary outcome, was measured using 100-mm VAS and the MMDQ over 6 months. There was no difference in MMDQ pain score, mean difference 4.5 [95% confidence interval (CI) − 22.2 to 13.2, p =.61]. There was a mean difference in favor of continuous CHC at 1 month, − 27.3 (95% CI − 40.5 to − 14.2, p <.001), and at 3 months, − 17.8 (95% CI − 33.4 to − 2.1, p =.03). However, there was no difference in dysmenorrhea severity at 6 months, − 16.0 (95% CI − 32.2 to 0.1, p =.05).
Strowitzki et al. [35] studied participants with moderate to severe primary dysmenorrhea in at least 4 of 6 preceding menses and used 20 mcg ethinyl estradiol/3 mg drosperinone. There were 108 women in the cyclic group (24 days active tablets/3 days hormone-free tablets) and 115 women in the extended/flexible group, where women could use the CHC for as long as they desired until they experienced 3 days of consecutive bleeding. At that point, participants started a 4-day hormone-free period before resuming active tablets. Of the 223 participants, 210 women completed the trial (110 in the extended regimen and 100 in the cyclic regimen). The primary outcome was the number of days with dysmenorrhea. The investigators found that women in the extended/flexible regimen spent fewer days with dysmenorrhea (− 4.2 days, 95% CI − 6.5 to − 2.0, p <.001) and with dysmenorrhea that interfered with daily activities (− 2.2 days, 95% CI − 4.2 to − 0.1) when compared to women on the cyclic regimen.
Momoeda et al. [36] studied women with dysmenorrhea baseline score of ≥ 3 points in 2 prior consecutive menses and used extended/flexible vs. cyclic CHC (20 mcg ethinyl estradiol/3 mg drospirenone). A total of 216 patients were evenly randomized into the 2 groups. Women in the flexible regimen used CHC ≥ 24 days and up to 120 days. After 3 consecutive days of bleeding, patients started a 4-day hormone-free interval prior to resuming CHC. Women in the cyclic regimen received 24 days of active tablets and 4 days of hormone-free tablets. Ninety-eight participants (91%) in the flexible/extended group and 84 participants (78%) in the cyclic group completed the 168-day study. Of the 98 women in the flexible/extended group, 59 continued with long-term follow-up for a total of 364 days. The primary outcome was defined as number of days with at least mild dysmenorrhea. Women using the flexible/extended regimen reported 3.4 fewer days of dysmenorrhea when compared to women using the cyclic regimen (95% CI − 6.5 to − 0.3, p =.030). There was no difference in the number of days with dysmenorrhea that interfered with daily activity (mean difference 2.0, 95% CI − 7.5 to 3.5). Both the flexible/extended regimen and the cyclic regimen were associated with a decrease in dysmenorrhea severity from baseline; however, no statistical difference was found. After 364 days of flexible/extended regimen, there was no statistical difference in the reduction in dysmenorrhea severity when compared to 168 days of treatment.
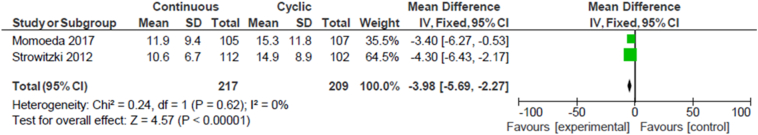
Meta-analysis was performed for two studies: Momoeda et al. and Strowitzki et al. [35], [36]. The analysis showed that women who used the flexible/extended regimen reported 3.98 fewer days of dysmenorrhea (95% CI − 5.69 to − 2.27) when compared to women using the cyclic regimen (Fig. 3). The other studies had different methods of outcome measurements, were using different progestin formulations of CHC or did not publish sufficient data to be compiled into a meta-analysis.
Fig. 3.
Dysmenorrhea duration.
Due to the very low quality of evidence, we are uncertain whether there is any difference in dysmenorrhea frequency and severity among patients taking either cyclic or continuous CHC. Due to the conflicting results from available evidence, we are uncertain if there is any difference in dysmenorrhea or in the number of days with dysmenorrhea that interfered with activities among patients taking either cyclic or continuous/flexible CHC.
The number of adverse events reported by studies was low and comparable between groups. Several studies measured various side effects. Kwiecien et al. [29] reported decrease of bloating in the continuous group, with a mean difference of 10.4 days less (p =.04). Machado et al. [32] found that there was a significant decrease of headache (p <.02), nausea (p <.020), appetite (p <.05) and acne (p <.05) in the continuous compared to the cyclic group. However, others found no significant difference in headaches, nausea or vomiting [27], [28], [33], [34]. Dmitrovic et al. [34] found greater weight gain in the continuous group (mean difference 2.3 kg, 95% CI 0.8–3.8, p =.003) and decrease in systolic blood pressure (p <.050); however, again, no differences were reported by others [30, 32, 36]. No significant difference was found in triglycerides, low-density lipoprotein (LDL) and total cholesterol [30], [34], [36]. While Legro et al. [30] found an increase in serum high-density lipoprotein cholesterol (HDL-C) in the cyclic group (mean difference 5.0, 95% CI 0.7–9.3, p =.02), neither Dmitrovic et al. [34] and Strowitzki et al. [36] reported a difference.
4. Discussion
CHCs are routinely used in a variety of regimens for contraception. Although both cyclic and continuous regimens are recommended for treatment of dysmenorrhea [37], there has not been a systematic review comparing the efficacy of the different regimens. Our research suggests that continuous and flexible CHC may result in 4 days less spent in pain when compared to cyclic CHC. We are uncertain whether continuous and flexible CHC decreases dysmenorrhea severity, frequency, recurrence and days when dysmenorrhea interferes with activity due to the very low quality of evidence or conflicting evidence. Side effects and adverse events were few, and there is not enough high-quality evidence about differences in side effects that can be attributed to cyclic vs. continuous or flexible CHC.
Although our findings are based on RCTs, there are several limitations. Nearly all trials used different formulations of CHC and measured outcomes using different scales, which limited our ability to perform a large meta-analysis. Our study examined CHC only and did not include the many formulations of progestin-only contraceptive. During our literature review, we did find some studies examining progestin-only contraceptives, and further research regarding their efficacy on dysmenorrhea would be valuable. Study populations were heterogeneous, with eight RCTs conducted in three different continents, which might account for conflicting results regarding dysmenorrhea severity. Only English studies were reviewed, further limiting generalizability. Quality of evidence was low for three outcomes (duration, severity and days when dysmenorrhea interfered with activity) and was very low for dysmenorrhea recurrence and frequency. Attrition bias was a concern in two studies. Majority of studies were either open label or had unclear blinding, and several had unclear allocation concealment, leading to an increase in risk of bias. More high-quality double-blind RCTs are needed to address these limitations. Lastly, the number of participants enrolled in each study was small. However, there were a total of 889 patients when all studies were combined, and the optimal information size criteria were met in all outcomes except for days when dysmenorrhea interfered with daily activity and dysmenorrhea frequency.
In conclusion, our findings suggest that continuous or flexible CHC may be more effective than cyclic CHC in decreasing dysmenorrhea duration without increasing side effects or adverse events. Given the recent evidence suggesting that untreated dysmenorrhea may increase the risk for developing chronic pelvic pain and long-standing psychiatric dysfunction [5], it is important that high-quality research is conducted to more confidently elucidate the effect of continuous CHC on dysmenorrhea.
Acknowledgments
We would like to thank Shalu Gillum, J.D., M.L.S., AHIP, for her assistance with query of the literature databases.
Footnotes
Disclosures: Dr. Chensi Ouyang, Dr. Georgine Lamvu, Dr. Jorge Carrillo and Dr. Jessica Feranec are employees of the Veterans Affairs Medical Center in Orlando, Florida. Dr. Georgine Lamvu serves as Chairman of the Board for the International Pelvic Pain Society. In 2017, she served on the Obstetrics and Gynecology Advisory Board for Daiichi Sankyo Inc. and as a consultant for Abbvie Pharmaceuticals and Uroshape Inc. Additionally, Dr. Lamvu has research funding from Pfizer, Grants for Learning and Change and the National Vulvodynia Association.
Funding: none.
References
- 1.Jamieson D.J., Steege J.F. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996;87(1):55–58. doi: 10.1016/0029-7844(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 2.Gebeyehu M.B., Mekuria A.B., Tefera Y.G. Prevalence, impact, and management practice of dysmenorrhea among University of Gondar students, northwestern Ethiopia: a cross-sectional study. Int J Reprod Med. 2017;2017 doi: 10.1155/2017/3208276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersch B., Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144(6):655–660. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- 4.Unsal A., Ayranci U., Tozun M. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115(2):138–145. doi: 10.3109/03009730903457218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacovides S., Avidon I., Baker F.C. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi: 10.1093/humupd/dmv039. [DOI] [PubMed] [Google Scholar]
- 6.Kural M., Noor N.N., Pandit D. Menstrual characteristics and prevalence of dysmenorrhea in college going girls. J Family Med Prim Care. 2015;4(3):426–431. doi: 10.4103/2249-4863.161345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hailemeskel S., Demissie A., Assefa N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in Ethiopia. Int J Womens Health. 2016;8:489–496. doi: 10.2147/IJWH.S112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A., Kiran D., Singh H. Prevalence and severity of dysmenorrhea: a problem related to menstruation, among first and second year female medical students. Indian J Physiol Pharmacol. 2008;52(4):389–397. [PubMed] [Google Scholar]
- 9.Gagua T., Tkeshelashvili B., Gagua D. Assessment of anxiety and depression in adolescents with primary dysmenorrhea: a case-control study. J Pediatr Adolesc Gynecol. 2013;26(6):350–354. doi: 10.1016/j.jpag.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Balik G., Ustuner I., Kagitci M. Is there a relationship between mood disorders and dysmenorrhea? J Pediatr Adolesc Gynecol. 2014;27(6):371–374. doi: 10.1016/j.jpag.2014.01.108. [DOI] [PubMed] [Google Scholar]
- 11.Vincent K., Warnaby C., Stagg C.J. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975. doi: 10.1016/j.pain.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Wu T.H., Tu C.H., Chao H.T. Dynamic changes of functional pain connectome in women with primary dysmenorrhea. Sci Rep. 2016;6 doi: 10.1038/srep24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osayande A.M., Suarna Diagnosis and initial management of dysmenorrhea. Am Fam Physician. 2014;89(5):341–346. [PubMed] [Google Scholar]
- 14.Lefebvre G., Pinsonneault O., Antao V. Primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can. 2005;27(12):1117–1146. doi: 10.1016/s1701-2163(16)30395-4. [DOI] [PubMed] [Google Scholar]
- 15.CL, Farquhar C., Roberts H. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD002120.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelman A., Micks E., Gallo M.F. Continuous or extended cycle vs cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD004695.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The American College of Obstetricians and Gynecologists Dysmenorrhea: painful periods. https://www.acog.org/Patients/FAQs/Dysmenorrhea-Painful-Periods Available:
- 18.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. 2013. fromguidelinedevelopment.org/handbook Available. [Google Scholar]
- 20.Review Manager (RevMan) [computer program] The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2014. Version 5.3. [Google Scholar]
- 21.GRADEpro GDT: GRADEpro guideline development tool [software] McMaster University, 2015 (developed by Evidence Prime, Inc.) gradepro.org Available from.
- 22.Caruso S., Iraci M., Cianci S. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 microg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Invest. 2016;39(8):923–931. doi: 10.1007/s40618-016-0460-6. [DOI] [PubMed] [Google Scholar]
- 23.Coffee A.L., Sulak P.J., Kuehl T.J. Long-term assessment of symptomatology and satisfaction of an extended oral contraceptive regimen. Contraception. 2007;75(6):444–449. doi: 10.1016/j.contraception.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Vercellini P., Frontino G., De Giorgi O. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80(3):560–563. doi: 10.1016/s0015-0282(03)00794-5. [DOI] [PubMed] [Google Scholar]
- 25.Seidman D.S., Yeshaya A., Ber A. A prospective follow-up of two 21/7 cycles followed by two extended regimen 84/7 cycles with contraceptive pills containing ethinyl estradiol and drospirenone. Isr Med Assoc J. 2010;12(7):400–405. [PubMed] [Google Scholar]
- 26.Vlahos N., Vlachos A., Triantafyllidou O. Continuous versus cyclic use of oral contraceptives after surgery for symptomatic endometriosis: a prospective cohort study. Fertil Steril. 2013;100(5):1337–1342. doi: 10.1016/j.fertnstert.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Harada T., Momoeda M., Terakawa N. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2011;95(6):1928–1931. doi: 10.1016/j.fertnstert.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 28.Razzi S., Luisi S., Ferretti C. Use of a progestogen only preparation containing desogestrel in the treatment of recurrent pelvic pain after conservative surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;135(2):188–190. doi: 10.1016/j.ejogrb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Kwiecien M., Edelman A., Nichols M.D. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67(1):9–13. doi: 10.1016/s0010-7824(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 30.Legro R.S., Pauli J.G., Kunselman A.R. Effects of continuous versus cyclical Oral contraception: a randomized controlled trial. J Clin Endocrinol Metabol. 2008;93(2):420–429. doi: 10.1210/jc.2007-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seracchioli R., Mabrouk M., Frasca C. Long-term oral contraceptive pills and postoperative pain management after laparoscopic excision of ovarian endometrioma: a randomized controlled trial. Fertil Steril. 2010;94(2):464–471. doi: 10.1016/j.fertnstert.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 32.Machado R.B., de Melo N.R., Maia H., Jr. Bleeding patterns and menstrual-related symptoms with the continuous use of a contraceptive combination of ethinylestradiol and drospirenone: a randomized study. Contraception. 2010;81(3):215–222. doi: 10.1016/j.contraception.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Muzii L., Maneschi F., Marana R. Oral estroprogestins after laparoscopic surgery to excise endometriomas: continuous or cyclic administration? Results of a multicenter randomized study. J Minim Invasive Gynecol. 2011;18(2):173–178. doi: 10.1016/j.jmig.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Dmitrovic R., Kunselman A.R., Legro R.S. Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol. 2012;119(6):1143–1150. doi: 10.1097/AOG.0b013e318257217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strowitzki T., Kirsch B., Elliesen J. Efficacy of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen in women with moderate-to-severe primary dysmenorrhoea: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):94–101. doi: 10.1136/jfprhc-2011-100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momoeda M., Kondo M., Elliesen J. Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study. Int J Womens Health. 2017;9:295–305. doi: 10.2147/IJWH.S134576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptivesObstet Gynecol. 2010;115(1):206–218. doi: 10.1097/AOG.0b013e3181cb50b5. [DOI] [PubMed] [Google Scholar]