Abstract
Objectives
To evaluate contraceptive effectiveness and safety of oral drospirenone 4 mg 24/4-day regimen in the United States.
Study design
We performed a prospective, single-arm, multicenter phase 3 trial in sexually active women for up to thirteen 28-day treatment cycles. Primary outcome was the Pearl index, calculated using confirmed on-drug pregnancies and evaluable cycles in nonbreastfeeding women aged ≤ 35 years. We assessed adverse events (AEs), including hyperkalemia and venous thromboembolism.
Results
Of 1006 women who received at least one dose of drospirenone, 352 women (35.0%) completed the trial and 654 (65.0%) women discontinued before trial end. Most participants (92.2%) were ≤ 35 years; one third had a body mass index (BMI) ≥ 30 kg/m2. Among nonbreastfeeding women aged ≤ 35 years, there were 17 pregnancies (Pearl index: 4.0; 95% confidence interval [CI], 2.3–6.4; n = 953), of which three were unconfirmed and two were from sites excluded from the main analysis for major breaches of Food and Drug Administration regulations. The Pearl index was 2.9 (95% CI: 1.5–5.1) for confirmed pregnancies among 915 nonbreastfeeding women aged ≤ 35 years from sites with no protocol violations. Nearly all (95.4%) treatment-emergent AEs were mild or moderate in intensity. No cases of venous thromboembolism were reported. The frequency of hyperkalemia was 0.5%. Women with baseline systolic/diastolic blood pressure ≥ 130/85 mmHg had a mean reduction from baseline in blood pressure at exit visit (− 8.5/− 4.9 mmHg; n = 119). No other clinically relevant changes were observed. Participant satisfaction was high.
Conclusion
Drospirenone 4 mg 24/4 regimen provides effective contraception with a good safety/tolerability profile in a broad group of women, including overweight or obese women.
Implications
This new progestin-only contraceptive, drospirenone 4 mg in a 24/4 regimen, provides a contraceptive option for the majority of women regardless of blood pressure or BMI.
Keywords: Progesterone/progestin-only pill, 24/4-day regimen, Efficacy, Safety, Scheduled bleeding
1. Introduction
There is a need for progestin-only contraceptive pills (POPs) because of the cardiovascular risk associated with estrogens in combined oral contraceptive (COC) pills [1,2]. Estrogens are contraindicated in 13%–29% of reproductive-age women due to migraines or cardiovascular risk factors (e.g., hypertension or smoking) [3,4]. Nevertheless, POPs taken continuously often cause breakthrough bleeding, which can result in discontinuation [5,6]. A broad range of contraceptive methods are required to support diverse users and individual choice. Approximately 1 in 10 women residing in the United States (U.S.) who are at risk of unintended pregnancy do not use highly effective hormonal contraceptive methods [7,8], and 45% of pregnancies were unintended in 2011 [9].
Drospirenone is a progestin extensively studied in combination with an estrogen as a COC. Drospirenone inhibits follicular development and ovulation by suppressing luteinizing hormone, increases cervical mucus viscosity and reduces ovarian androgenic hormone production [10]. Drospirenone may reduce blood pressure and excretion of potassium due to its antimineralocorticoid activity [[11], [12], [13]]. It has a terminal half-life of between 25 and 30 h [14].
Drospirenone 4 mg taken for 24 days followed by placebo for 4 days (24/4-day regimen) throughout two 28-day cycles demonstrated effective ovulation inhibition equivalent to daily desogestrel 75 mcg with no subjects ovulating at cycle 1 and one subject ovulating in each group at cycle 2 [10]. The 24/4-day regimen was chosen in order to induce scheduled bleeding and reduce unscheduled bleeding in contrast to other POPs with a continuous regimen. A European trial of 713 women demonstrated contraceptive efficacy of drospirenone 4 mg 24/4 dosing regimen with a Pearl index of 0.51 [95% confidence interval (CI), 0.11–1.49] over thirteen 28-day cycles [15]. Previous drospirenone trials were predominantly conducted with women < 30 years old, and few (< 5%) were obese [10,[15], [16], [17]]. The objectives of this phase 3 trial were to evaluate contraceptive efficacy, safety and tolerability of oral drospirenone 4 mg 24/4-day regimen in U.S. women, including both women > 35 years old and those with a body mass index (BMI) > 30 kg/m2.
2. Materials and methods
2.1. Trial design
We performed a prospective, open-label, single-arm, multicenter trial. Each center's Institutional Review Board approved the protocol. Women provided written informed consent prior to enrollment. Registration was at clincaltrials.gov: NCT02269241.
2.2. Participants
Sexually active, healthy, nonpregnant women aged ≥ 15 years seeking contraception were eligible for enrollment. Breastfeeding women at least 6 weeks postpartum were allowed to enroll for safety evaluations only. Nonmenopausal participants had regular menstrual cycles during the previous 6 months when not using hormonal contraception or three complete cycles after birth if not breastfeeding, and screening systolic and diastolic blood pressure (SBP/DBP) of ≤ 159/99 mmHg. We excluded women with polycystic ovary syndrome, known infertility, current or history of venous thromboembolism (VTE) and abnormal Papanicolaou smear, and those with known contraindications to drospirenone, including renal insufficiency, hepatic dysfunction, adrenal insufficiency, current or history of cerebral–vascular or coronary artery disease, valvular heart disease with thrombogenic complications, diabetes with vascular involvement, headaches with focal neurological symptoms, major surgery with prolonged immobilization, known or suspected breast carcinoma, known or suspected sex-steroid sensitive malignancies or undiagnosed abnormal genital bleeding. Use of a hormonal contraceptive implant or intrauterine device in place within 2 months prior to enrollment or injectable contraceptives within the previous 6 or 9 months, depending on type, also precluded enrollment. Participants could have previous experience using COCs.
2.3. Study drug intervention
Participation included up to thirteen 28-day treatment cycles. Up to 8 study visits were scheduled: screening (baseline); dispensation; and cycles 1, 3, 6, 9 and 13. A final follow-up visit was scheduled for 10 or 14 days after cycle 13 or after early discontinuation (EDV). Urine pregnancy tests were conducted at every study visit. Participants also performed home urine pregnancy tests at the start of each cycle. Investigators asked women with a positive urine pregnancy test to return to the study site for a confirming quantitative serum human chorionic gonadotropin test.
During each cycle, participants swallowed one active tablet containing drospirenone 4 mg for 24 consecutive days followed by 4 days of placebo. Switchers from other COCs took their first study drug dose the day following the last active tablet of their previous COC; all others started drospirenone on the first day of menses. Participants took missed tablets as soon as remembered if within 24 h or with the next scheduled dose if more than 24 h late. If a participant forgot more than three consecutive tablets, then they took two tablets immediately and left the remaining missed tablet(s) in the pack. In case of forgotten tablets, the investigator advised the participant to start the next pack on schedule so that each medication cycle had a length of 28 days.
2.4. Outcomes
2.4.1. Contraceptive efficacy
The primary outcome was the Pearl index calculated using confirmed on-drug pregnancies and evaluable cycles in nonbreastfeeding women aged ≤ 35 years (at enrollment). An exposure (medication) cycle was defined as 28 days starting with the administration of the first tablet from the blister containing 28 tablets and ending with the last day of intake. Evaluable cycles were defined as exposure cycles with sexual intercourse without backup contraceptive and any cycle in which a participant became pregnant. An exposure cycle was defined as nonevaluable if the participant did not become pregnant and had intercourse with additional contraception, or had no intercourse, or if the cycle had a missing e-diary answer about intercourse. Pregnancies were defined as “confirmed” if both urine test and a positive quantitative serum β-human chorionic gonadotropin (β-hCG) test were performed at a central laboratory and “unconfirmed” if the quantitative serum β-hCG test was not conducted or recorded. For confirmed pregnancies, we determined gestational age by either first trimester ultrasound or quantitative β-hCG when necessary.
Secondary outcomes comprised Pearl indexes from overall exposure or evaluable cycles in nonbreastfeeding women aged ≤ 35 years and > 35 years at time of enrollment based on all on-drug confirmed pregnancies and all pregnancies, including those nonconfirmed. We also report Pearl indexes for BMI subgroups (< 30 kg/m2 and ≥ 30 kg/m2).
2.4.2. Safety and tolerability
Participants were monitored for adverse events (AEs), clinical laboratory parameters (hematology, serum biochemistry and urinalysis), vital signs, physical examination and cervical cytology. Site staff contacted participants on day 10 of each cycle to collect information about any AEs that may have occurred. Investigators assessed routine laboratory parameters and vital signs at every site visit. Participants were also monitored at every site visit for hyperkalemia (defined as two serum potassium measurements higher than the reference range 3.5–5.3 mmol/L; women with one measurement above reference range were contacted immediately to retest) and VTE, including symptoms of or risk factors for deep vein thrombosis and pulmonary embolism. All cases of VTE and hyperkalemia were reported as serious AEs to the Safety Manager within 24 h and were considered important for the evaluation of the safety profile of drospirenone independent from the classification of seriousness, expectedness and intensity.
2.4.3. Bleeding patterns
Participants reported bleeding in an e-diary. Scheduled withdrawal bleeding comprised any bleeding or spotting that occurred during hormone-free intervals (cycle days 25–28) lasting up to 8 consecutive days. Unscheduled bleeding or spotting occurred at any other time, and prolonged bleeding lasted > 14 consecutive days.
2.4.4. Participant satisfaction
The acceptability of drospirenone was assessed at visits 3 and 6/EDV. Each participant was asked “Are you satisfied with this method?” (answer options: strongly agree, agree, undecided, disagree, strongly disagree). Women with previous COC experience were asked “How was your well-being during the intake of the study medication in comparison to the time when you took your former oral contraception?” (answer options: better, unchanged, worse).
2.4.5. Participant's e-diary
Participants were asked to complete an e-diary. Data collected from the e-diary were used in the primary endpoint analysis, as well as for documentation of vaginal bleeding pattern, AEs, concomitant contraceptives, intake (or forgotten intake) of a tablet from blister pack and confirmation of sexual activity for each cycle.
2.5. Sample size
The Pearl index was hypothesized to be less than 3.0, and we planned a study large enough that the upper confidence interval (CI) would not exceed 5.0 (95% CI). Our target was to yield at least 5000 evaluable cycles (with intercourse without backup contraception at least once per month) for the Pearl index calculation in nonbreastfeeding women ≤ 35 years old. A minimum of 75 women > 35 years old was also allocated to treatment to evaluate safety. Based on previous drospirenone trials, we assumed that 24.8% of women would be excluded due to using backup contraception or having no intercourse during a cycle and that the trial early discontinuation rate would be 45% [18].
2.6. Statistical methods
Safety was assessed using data from all women who received at least one dose of drospirenone 4 mg (the safety set). Contraceptive efficacy was assessed using data from all nonbreastfeeding women who received at least one dose of drospirenone 4 mg and were not pregnant when drospirenone was first taken (full-analysis set). Additional contraceptive efficacy analyses were conducted on a dataset that included two study sites that had major breaches of U.S. Food and Drug Administration (FDA) regulations and current Good Clinical Practice (cGCP) and trial protocol procedures. The primary analysis excluded these sites.
The Pearl index was calculated as [on-drug pregnancies/evaluable cycles] × 1300 [19]. An “on-drug pregnancy” comprised all conceptions that occurred from day 1 (initiation of study medication) through 7 days after final tablet (active or placebo) intake. The Pearl index was also stratified by age (≤ 35 years and > 35 years) and, in addition to the planned analyses, by subgroup based on BMI (< 30 kg/m2 and ≥ 30 kg/m2). Two-sided 95% CIs were calculated assuming that confirmed pregnancy events have a Poisson distribution.
We summarized all AEs, including treatment-emergent AEs (TEAEs) by number and percentage of women, and number of AEs by severity. All AEs were recorded using MedDRA primary system class and preferred term.
3. Results
3.1. Participant disposition and baseline data
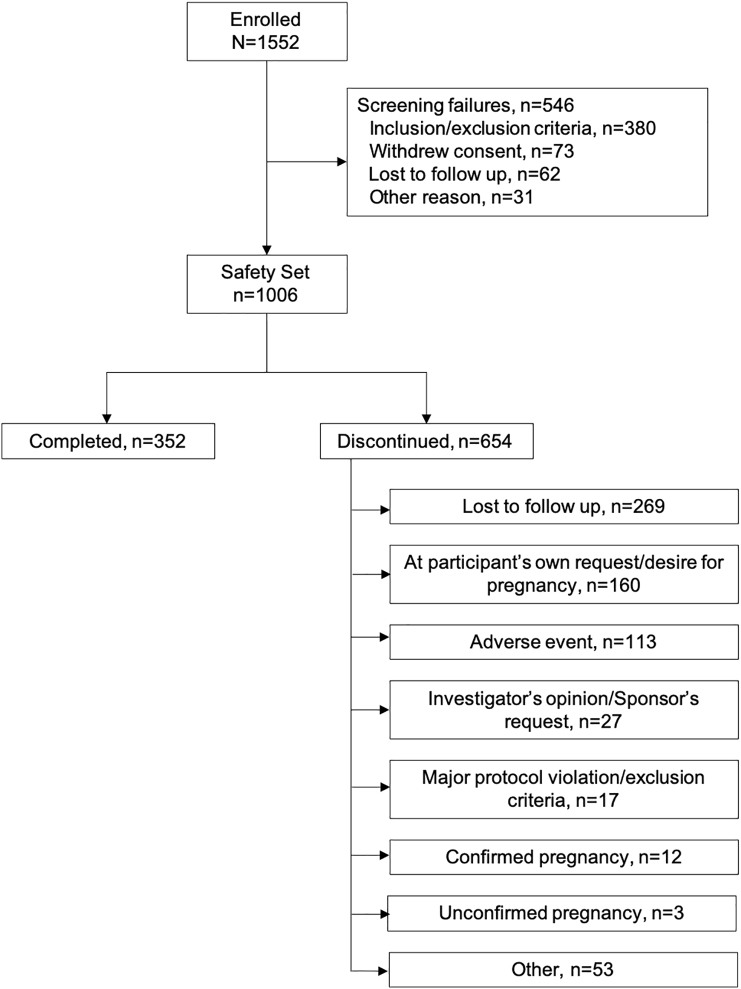
The study began with 43 sites in the U.S. We excluded two sites with 63 participants (not included below because the data reliability could not be assured) due to major breaches of FDA regulations, cGCP and trial protocol procedures. With the agreement of the relevant Institutional Review Boards, these sites were closed. The main analysis comprised 41 sites that enrolled 1552 women between October 2014 and October 2017. Of these, 546 women failed screening and were discontinued before taking drospirenone (Fig. 1). Of 1006 women who received at least one dose of drospirenone (safety set), 352 (35.0%) completed the trial and 654 (65.0%) discontinued before the trial end. The full-analysis set comprised 1004 women who were not pregnant at time of first dose. Two women already pregnant at the date of first dose of the study drug were excluded from the efficacy analysis. The modified full-analysis set excluded 11 breastfeeding women, comprising 993 women. The most common reasons for discontinuation were loss to follow-up (269 women, 26.7%) and withdrawal of consent (155 women, 15.4%). Regarding withdrawal of consent, 105 (10.4%) participants stated that the reasons were unrelated to drospirenone, 26 (2.6%) stated the reasons were related, and 24 (2.4%) were uncategorized. Median duration of exposure to drospirenone was 168 days (range: 1–411 days). Five hundred and six women (50.3%) were exposed to drospirenone for at least 168 days.
Fig. 1.
Flowchart of study participant disposition for a phase 3, multicenter, 13-cycle trial of a drospirenone 4 mg 24/4-day contraceptive regimen.
Table 1 presents participant baseline characteristics. Most participants (92.2%) were ≤ 35 years; one third had a BMI ≥ 30 kg/m2, and 18.1% had a BMI ≥ 35 kg/m2. At screening, 11.8% had SBP/DBP ≥ 130/85 mmHg. Investigators assessed one third of participants to have VTE risk factors (Table 1). Most women (79.2%) had prior hormonal contraceptive experience, with about one quarter of women switching at time of enrollment.
Table 1.
Baseline demographic and clinical characteristics (safety set) enrolled in phase 3, multicenter, 13-cycle trial of a drospirenone 4 mg 24/4-day contraceptive regimen
| Non-breastfeeding women |
Breastfeeding women |
Total |
|
|---|---|---|---|
| n = 995 | n = 11 | n = 1006 | |
| Age, years (Mean ± SD) | 27.5 ± 5.95 | 27.0 ± 4.96 | 27.5 ± 5.94 |
| ≤ 35 years, n (%) | 917 (92.2) | 11 (100.0) | 928 (92.2) |
| > 35 years, n (5) | 78 (7.8) | 0 | 78 (7.8) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 226 (22.7) | 3 (27.3) | 229 (22.8) |
| Not Hispanic or Latino | 769 (77.3) | 8 (72.7) | 777 (77.2) |
| Race, n (%) | |||
| Caucasian | 561 (56.4) | 10 (90.9) | 571 (56.8) |
| African–American | 357 (35.9) | 1 (9.1) | 358 (35.6) |
| Asian | 20 (2.0) | 0 | 20 (2.0) |
| American Indian or Alaska Native | 13 (1.3) | 0 | 13 (1.3) |
| Native Hawaiian or other Pacific | 5 (0.5) | 0 | 5 (0.5) |
| Islander | 39 (3.9) | 0 | 39 (3.9) |
| Other | |||
| Highest level of education | |||
| No high-school diploma | 35 (3.5) | 1 (9.1) | 36 (3.6) |
| High-school diploma or equivalent | 233 (23.4) | 2 (18.2) | 235 (23.4) |
| Some college education | 408 (41.0) | 4 (36.4) | 412 (41.0) |
| College degree or higher | 319 (32.1) | 4 (36.4) | 323 (32.1) |
| Bodyweight, kg | |||
| Mean ± SD | – | – | 76.7 ± 21.92 |
| Median (min, max) | – | – | 72 (39, 206) |
| BMI, n (%) | |||
| ≤ 25 kg/m2 | 387 (38.9) | 1 (9.1) | 388 (38.6) |
| > 25–<30 kg/m2 | 256 (25.7) | 8 (72.7) | 264 (26.2) |
| < 30 kg/m2 | 643 (64.6) | 9 (81.8) | 652 (64.8) |
| ≥ 30 kg/m2 | 352 (35.4) | 2 (18.2) | 354 (35.2) |
| ≥ 35 kg/m2 | 182 (18.3) | – | 182 (18.1) |
| ≥ 40 kg/m2 | 84 (8.4) | – | 84 (8.3) |
| Blood pressure (SBP/DBP) | |||
| < 130/85 mmHg, n (%) | 876 (88.0) | 11 (100.0) | 887 (88.2) |
| ≥ 130/85 mmHg, n (%) | 119 (12.0) | 0 | 119 (11.8) |
| Previous exposure to hormonal contraceptives, n (%) | |||
| Naïve user | – | – | 209 (20.8) |
| Non-switching previous user | |||
| ≥ 3 months | 463 (46.0) | ||
| < 3 months | 70 (7.0) | ||
| Switcher | 264 (26.2) | ||
| VTE risk factors, n (%) | |||
| Family history of thromboembolic illness | – | – | |
| Yes | 12 (1.2) | ||
| No | 993 (98.8) | ||
| Missing | 1 | ||
| Current smoker ≥ 35 years or non-smoker ≥ 40 years | |||
| Yes | – | – | 51 (5.1) |
| No | 955 (94.9) | ||
| BMI ≥ 30 kg/m2 | |||
| Yes | – | – | 353 (35.1) |
| No | 653 (64.9) | ||
| Number of VTE risk factorsa | |||
| No risk factors | – | – | 611 (60.8) |
| 1 risk factor | 367 (36.5) | ||
| 2 risk factors | 27 (2.7) | ||
| ≥ 3 risk factors | 0 |
Risk factors: family history of thromboembolic illness, current smoker ≥ 35 years or non-smoker ≥ 40 years or BMI ≥ 30 kg/m2. Missing data for 1 participant.
3.2. Contraceptive outcomes
In the modified full-analysis set, 993 women had 6566 exposure cycles, of which 6004 cycles were evaluable (Table S1). Tables 2 provides Pearl index details. Among nonbreastfeeding women, the study identified 15 pregnancies, 12 of which were confirmed. Of the three unconfirmed pregnancies, two of the women were lost to follow-up, and the remaining woman reported an elective termination of the pregnancy without making any return visit to the study site. One pregnancy in a breastfeeding woman was not included in Pearl index calculations. No woman aged > 35 years became pregnant during the treatment period. The Pearl indexes were similar when analyzed by BMI subgroup (Table 3). Two sites excluded from the main analysis reported two pregnancies among nonbreastfeeding women aged ≤ 35 years.
Table 2.
Pearl indexes among nonbreastfeeding women enrolled in a phase 3, multicenter, 13-cycle trial of a drospirenone 4 mg 24/4-day contraceptive regimen
|
Study sites with no protocol violations |
All sites |
|||
|---|---|---|---|---|
| Women ≤ 35 years (at time of enrollment) n = 915a |
Women > 35 years n = 78a |
All women n = 993a |
Women ≤ 35 years (at time of enrollment) from all sites n = 953b |
|
| Pearl index among women with confirmed on-drug pregnancies and evaluable cycles | ||||
| Women with a confirmed pregnancy,dn (%) | 12 (1.3) | 0 | 12 (1.2%) | 14 (1.5) |
| Evaluable cycles,e n | 5337 | 667 | 6004 | 5547 |
| Pearl index | 2.9 (1.5–5.1)c | 0 (NC–7.2) | 2.6 (1.3–4.5) | 3.3 (1.8–5.5) |
| Overall Pearl index among women with confirmed on-drug pregnancies and exposure cycles | ||||
| Women with a confirmed pregnancy,dn (%) | 12 (1.3) | 0 | 12 (1.2%) | 14 (1.5) |
| Exposure cycles,f n | 5835 | 731 | 6566 | 6073 |
| Pearl index (95% CI) | 2.7 (1.4–4.7) | 0 (NC–6.6) | 2.4 (1.2–4.2) | 3.0 (1.6–5.0) |
| Pearl index among women with either confirmed or unconfirmed pregnancies and evaluable cycles | ||||
| Women with a pregnancy,gn (%) | 15 (1.6) | 0 | 15 (1.5) | 17 (1.8) |
| Evaluable cycles,e n | 5337 | 667 | 6004 | 5547 |
| Pearl index (95% CI) | 3.7 (2.0–6.0) | 0 (NC–7.2) | 3.2 (1.8–5.4) | 4.0 (2.3–6.4) |
Modified full-analysis set included women from 41 sites with no major protocol or regulatory violations.
Includes women from 43 sites, including women who were enrolled at two study sites that were excluded from the main analysis set due to major breaches of FDA regulations, and ICH GCP and trial protocol procedure.
Primary endpoint.
Pregnancies were defined as “confirmed” if a positive quantitative serum human chorionic gonadotropin test was recorded and “unconfirmed” if this test was not conducted or recorded.
Evaluable cycles were defined as exposure cycles with sexual intercourse without backup contraceptive and any cycle in which a participant became pregnant.
An exposure cycle was defined as nonevaluable if the participant did not become pregnant and had intercourse with additional contraception, or had no intercourse, or if the cycle had a missing e-diary answer about intercourse.
Includes pregnancies reported by women that were not confirmed by a quantitative serum pregnancy test.
Table 3.
Pearl index by BMI among nonbreastfeeding women ≤ 35 years enrolled in a phase 3, multicenter, 13-cycle trial of a drospirenone 4 mg 24/4-day contraceptive regimen (n = 915; modified full-analysis set)
| BMI < 30 kg/m2 (n = 590) |
BMI ≥ 30 kg/m2 (n = 325) |
|
|---|---|---|
| Confirmed pregnancies | ||
| Women with a pregnancy, n (%) | 8 (1.4) | 4 (1.2) |
| Exposure cycles | 3520 | 1817 |
| Pearl index (95% CI) | 3.0 (1.3–5.8) | 2.9 (0.8–7.3) |
| Confirmed and unconfirmed pregnancies | ||
| Women with a pregnancy, n (%) | 11 (1.9) | 4 (1.2) |
| Exposure cycles | 3520 | 1817 |
| Pearl index (95% CI) | 4.1 (2.0–7.3) | 2.9 (0.8–7.3) |
3.3. Safety and tolerability
3.3.1. Adverse events, physical examinations and laboratory parameters
Six hundred fourteen women (61.0%) reported 1771 TEAEs (Table 4). Seventeen women (1.7%) reported 32 serious AEs; all but two of these resolved without sequelae. One woman experienced a ruptured intracranial aneurysm with neurological sequelae, and one woman with hyperkalemia had an unknown outcome. Most women (95.4%) had TEAEs that were classified as mild or moderate in intensity. One hundred thirteen women (11.2%) discontinued early from the trial due to TEAEs, of whom 100 (9.9%) had at least one possibly related TEAE, including 19 (1.9%) who discontinued due to metrorrhagia. No cases of VTE were reported. The frequency of hyperkalemia was low: five (0.5%) had asymptomatic hyperkalemia, four of which were reported as serious AEs possibly related to study drug; all were considered mild by the investigator, and none were hospitalized. One participant with hyperkalemia was lost to follow-up; all other cases of hyperkalemia resolved without sequelae. Six additional women had an AE of increased blood potassium (defined as one occurrence above the upper reference range).
Table 4.
Summary of adverse events for women enrolled in a phase 3, multicenter, 13-cycle trial of a drospirenone 4 mg 24/4-day contraceptive regimen (n = 1006; safety set)
| Women, n (%) | Events, n | |
|---|---|---|
| Women with at least 1 AE | 667 (66.3) | 2008 |
| Women with at least 1 TEAE | 614 (61.0) | 1771 |
| Women with at least 1 related TEAEa | 341 (33.9) | 640 |
| Women with at least 1 serious AE | 17 (1.7) | 32 |
| Women with at least 1 serious TEAE | 15 (1.5) | 24 |
| Women with at least 1 serious related TEAE | 3 (0.3) | 3 |
| Women with at least 1 TEAE leading to trial discontinuation | 113 (11.2) | 163 |
| Women with at least 1 related TEAE leading to trial discontinuation | 100 (9.9) | 123 |
| Deaths | 0 | 0 |
| Frequency of women with TEAEs ≥ 2.0% | ||
| Nasopharyngitis | 77 (7.7) | 87 |
| Headache | 64 (6.4) | 72 |
| Nausea | 63 (6.3) | 64 |
| Dysmenorrhea | 58 (5.8) | 62 |
| Metrorrhagia | 53 (5.3) | 54 |
| Breast pain | 51 (5.1) | 54 |
| Upper respiratory tract infection | 36 (3.6) | 38 |
| Acne | 35 (3.5) | 36 |
| Urinary tract infection | 34 (3.4) | 36 |
| Weight increase | 34 (3.4) | 34 |
| Breast tenderness | 33 (3.3) | 34 |
| Cervical dysplasia | 29 (2.9) | 39 |
| Abdominal pan | 26 (2.6) | 28 |
| Vulvovaginal mycotic infection | 24 (2.4) | 24 |
| Diarrhea | 23 (2.3) | 23 |
| Sinusitis | 22 (2.2) | 25 |
Related TEAEs were “possibly related,” “probably related” or “definitely related” as assessed by the investigator.
No clinically relevant changes occurred in other laboratory parameters, blood pressure, heart rate, body weight or gynecological examination. We evaluated blood pressure changes according to baseline blood pressure. Women who had SBP/DBP ≥ 130/85 mmHg at baseline were observed to have a mean reduction from baseline in blood pressure at visit 6/EDV (− 8.5/− 4.9 mmHg; n = 119). Women with baseline SBP/DBP < 130/85 mmHg had no mean change in blood pressure (0.7/0.6 mmHg; n = 887). The 113 women with the lowest baseline BP had a mean change of + 7.3/+3.5 mmHg.
3.3.2. Bleeding pattern changes
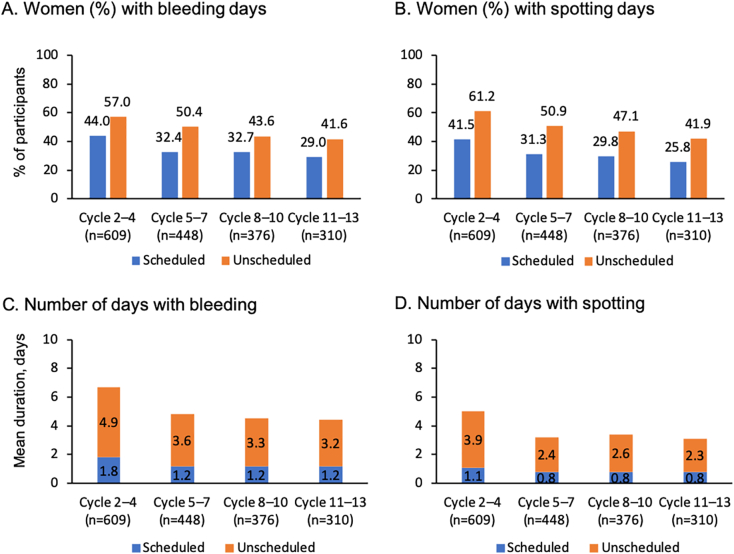
About one third of participants (169/523; 32.3%) reported scheduled withdrawal bleeding in the second cycle, and the frequency declined with continued use (Fig. 2). Unscheduled bleeding was recorded by 187/523 women (45.5%) in the second cycle, and this also declined with continued use; approximately one third (71/239) recorded unscheduled bleeding in cycle 13. Mean duration of all bleeding and/or spotting episodes decreased over time with a trend towards fewer recording prolonged bleeding and/or spotting (Table 5). Over time, a greater proportion reported amenorrhea.
Fig. 2.
Scheduled and unscheduled bleeding and spotting in a phase 3, multicenter, 13-cycle trial of drospirenone 4 mg 24/4-day contraceptive regimen (full-analysis set).
Table 5.
Bleeding and spotting pattern data for women enrolled in a phase 3, multicenter, 13-cycle trial of a drospirenone 4 mg 24/4-day contraceptive regimen
| Bleeding duration, days Median (min, max) |
Spotting duration, days Median (min, max) |
Prolonged bleeding/spotting > 9 days n/m (%) [95% CI] |
Prolonged bleeding/spotting > 14 days n/m (%) [95% CI] |
Women with no bleeding/spotting episodes m (%) |
|
|---|---|---|---|---|---|
| Cycles 2–4 N = 609 |
4.0 (0, 65) |
3.0 (0, 34) |
132/467 (28.3) [24.2–32.6] |
55/467 (11.8) [9.0–15.1] |
142 (23.3) |
| Cycles 5–7 N = 448 |
3.0 (0, 44) |
2.0 (0, 24) |
57/317 (18.0) [13.9–22.7] |
20/317 (6.3) [3.9–9.6] |
131 (29.2) |
| Cycles 8–10 N = 376 |
2.0 (0, 48) |
1.0 (0, 37) |
57/252 (22.6) 17.6–28.3) |
18/252 (7.1) [4.3–11.1] |
124 (33.0) |
| Cycles 11–13 N = 310 |
1.0 (0, 42) |
1.0 (0, 31) |
32/199 (16.1) [11.3–21.9] |
14/199 (7.0) [3.9–1.5] |
111 (35.8) |
Full-analysis set for combined scheduled and unscheduled bleeding data; N, number of women with data available; n, number of women with prolonged bleeding/spotting in respective cycle; m, number of women with data available in respective cycle; CI, Clopper–Pearson 95% confidence interval.
3.4. Participant satisfaction
Most women agreed or strongly agreed that they were satisfied with drospirenone at visit 3 (585/679; 86.2%) and visit 6/EDV (484/631; 76.7%). Of 349 who completed visit 6 and did not discontinue the trial, 90.8% agreed or strongly agreed that they were satisfied with drospirenone. Of 540 who attended both visits 3 and 6/EDV, 70.2% who were satisfied with drospirenone at visit 3 reported the same satisfaction at visit 6/EDV. Most women with past COC experience rated their well-being as “better” (visit 3, 30.9%; visit 6/EDV, 29.5%) or “unchanged” (visit 3, 51.0%; visit 6/EDV, 40.5%) compared with when they were taking a previous COC.
4. Discussion
This trial demonstrated that using drospirenone 4 mg 24/4-day regimen over 13 cycles shows good contraceptive efficacy in women with varied characteristics regarding weight, BMI, age and blood pressure, comparable or better than recently approved COCs [20]. Approximately 1000 nonbreastfeeding women ≤ 35 years old reported 12 confirmed and 3 unconfirmed pregnancies, with 2 additional pregnancies reported in women from excluded study sites. Drospirenone maintains contraceptive effectiveness even with 24-h delayed or missed-pill errors [16]. Among nonbreastfeeding women aged ≤ 35 years, the Pearl index was 2.9 using evaluable cycles (i.e., not including cycles with no intercourse or additional contraception) and confirmed pregnancies. We did not include data from the two excluded study sites in our main analysis because it was potentially inaccurate but nevertheless have reported the Pearl index for all study sites, including those with protocol violations.
The trial indicated that this regimen was safe. No VTEs were reported during the study, although the sample size may have been too low to observe rare events. A previous trial had demonstrated no effect of the drospirenone 4 mg 24/4-day regimen on hemostatic parameters [17]. Women with elevated blood pressure at baseline had a mean reduction in SBP/DBP at visit 6/EDV, which was expected due to drospirenone's antimineralocorticoid effects [21,22]. Similar reductions in blood pressure have been observed in other drospirenone trials [15,23]; however, our observed changes in blood pressure may have been due to measurements regression to the mean. We did not observe hypotension among participants. In this study, all cases of hyperkalemia were reported as serious AEs as prespecified in the study protocol because of drospirenone's antimineralocorticoid potency, which reduces the excretion of potassium [24]. More serious symptoms of hyperkalemia include slow heartbeat and weak pulse. Severe hyperkalemia can result in respiratory paralysis or cardiac arrest [24]; however, the incidence of hyperkalemia in our trial was low (0.5%), and all cases were asymptomatic.
Our estimates for the sample size were based on previous studies for drospirenone, and as such, we had assumed that the Pearl index would be similar; however, our trial had a higher Pearl index. Although our trial had more pregnancies than a previous European trial with a pregnancy rate of 0.4% and Pearl index of 0.5 [15], we demonstrated contraceptive efficacy similar to levels observed with COCs [20] and continuous POPs [6]. Pregnancy rates are often higher in U.S. contraception trials compared with European trials due to multiple unclear reasons [25]. The participants in our current study and those in the comparable European study of drospirenone with a 24/4-day regimen differed: approximately 35% of participants in our study had a BMI > 30 kg/m2 compared with 6% of participants in the European study [15]. Our study enrolled women of whom 36% had at least one VTE risk, whereas only 15% of the participants of the European study had VTE risks [15]. Lastly, participants in our study were younger, with 92% ≤ 35 years old compared with 80% in the European study [15]. As such, we believe that our study shows that drospirenone as a 24/4-day regimen provides an appropriate contraceptive option for a much broader group of women than the group for whom previous POPs were recommended.
Continuous POPs are associated with the limitation of more days of bleeding than COCs [6]. We have shown that the number of unscheduled bleeding days with drospirenone 24/4-day regimen decreased over time, the proportion of women who had no bleeding increased with each cycle, and few (1.9%) participants discontinued due to bleeding. In comparison, a double-blind study comparing daily desogestrel 75 mcg (n = 989) and levonorgestrel 30 mcg (n = 331) had much higher discontinuation rates due to abnormal bleeding (22.5%, desogestrel; 18%, levonorgestrel) [26]. There have been no recent studies of norethindrone, but a 1971 report described 9/154 (5.8%) women using daily norethindrone 0.35 mg discontinuing with the primary reason of irregular bleeding; the author noted that many more may have discontinued, with bleeding being a contributing factor [27].
This was a single-arm, noncomparator study; therefore, no direct comparisons can be made with other types of contraception. The study product, trial procedures (such as multiple site visits) or socioeconomic factors may have contributed to a high dropout rate. Overall, there was a high level of participant satisfaction. Although 86% agreed or strongly agreed that they were satisfied with drospirenone, some of the 269 women lost to follow-up may have disliked the study drug due to bleeding, a tolerability issue or even pregnancy. Nevertheless, the dropout rate was similar to a historical trial for norethindrone 0.35 mg, in which 65.6% of women discontinued the trial, 22.3% due to reasons considered to be related to the study drug [28]. A 12-month European POP trial had a discontinuation rate of 44.4% for women taking desogestrel and 39.0% for women taking levonorgestrel [26].
In summary, drospirenone 4 mg 24/4 regimen provides clinical contraceptive efficacy similar to historical efficacy of many currently marketed COC pills, with a good safety profile and favorable cycle control in a broad group of women, including those who are overweight or obese, have high blood pressure or are older than 35 years.
The following are the supplementary data related to this article.
Protocol deviations leading to exclusion of cycles from analyses in nonbreastfeeding women from a phase 3, multicenter, 13-cycle trial of drospirenone 4 mg 24/4-day contraceptive regimen
Acknowledgments
Study investigators were R. Ackerman, M.D., FACOG; E. Andruczyk, M.D., FACOG, MBA; K.T. Barnhard, M.D., MSCR; R. Beyer, M.D., M.A., FACOG, CPI; P.P. Bhiwandiwala (Bhiwandi), M.D., FACOG; S. Blank, M.D.; A.E. Burke, M.D., M.P.H.; S.E. Chavoustie, M.D., FACOG, CCRP; T. Copper, M.D., A.J. Donovan, M.D.; S.E. Eder, M.D., FACOG, FACS; C.D. Eubank, Jr., M.D., FACOG; R.A. Felman, M.D.; F. Fisk, M.D., FACOG; H. Green, M.D.; R. Hedrick, M.D.; J.N. Hernandez, M.D., FACOG; C. Huffman, M.D.; M.A. Jacobs, M.D.; TD Kimble, M.D.; S. Kim, M.D., R. Kroll, M.D., FACOG; S.N. Lederman, M.D.; G.C. Lefebvre, M.D.; A. London, M.D.; J.A. Mickelson, M.D., CPI; A.S. Murthy, M.D., M.P.H., FACOG; D.M. Radin, M.D.; R. Reagan, M.D.; S.W. Robison, M.D.; W.D. Summers, M.D.; S. Sussman, M.D.; M.A. Thomas, M.D.; P. Thompson, M.D.; M. Twede, M.D., FACOG; A.S. Waldbaum, M.D.; K.K. Waldrep, M.D.; D. Walland, M.D.; L.S. Wan, M.D.; C. Westhoff, M.D.; P. Winkle, M.D., FACP, FACOG, CPI; and D.B. Young, M.D.
Celia J Parkyn, Ph.D., provided medical writing and editorial support, funded by Exeltis, Madrid, Spain.
Footnotes
Funding: The clinical trial was fully funded by Exeltis, Madrid, Spain.
Declaration of interest: Thomas Kimble disclosed the following — Exeltis: other research support includes receipt of drugs, supplies, equipment or other in-kind support, principal investigator.
Anne Burke disclosed the following — Exeltis: other research support includes receipt of drugs, supplies, equipment or other in-kind support, principal investigator; Bayer: research support mediated through Johns Hopkins University.
Kurt Barnhart disclosed the following — AbbVie: consultant; Bayer: consultant; Exeltis: other research support includes receipt of drugs, supplies, equipment or other in-kind support, principal investigator.
Enrico Colli disclosed the following — Exeltis: employment.
David F. Archer disclosed the following — AbbVie: consultant/advisory board; other research support includes receipt of drugs, supplies, equipment or other in-kind support; Agile: consultant/advisory board, ownership interest includes stock, stock options, patent or other intellectual property. Bayer: other research support includes receipt of drugs, supplies, equipment or other in-kind support; Endoceutics: consultant/advisory board; other research support includes receipt of drugs, supplies, equipment or other in-kind support. Exeltis: consultant/advisory board; Mithra: consultant advisory board; Myovant: other research support includes receipt of drugs, supplies, equipment or other in-kind support; Obs-Eva: consultant; other research support includes receipt of drugs, supplies, equipment or other in-kind support; TherapeuticsMD: consultant/advisory board; other research support includes receipt of drugs, supplies, equipment or other in-kind support.
Carolyn L. Westhoff disclosed the following — AbbVie: consultant/advisory board; Agile: consultant/advisory board; Mithra: consultant; Bayer and Merck: DSMB member; Exeltis and Sebela: other research support includes receipt of drugs, supplies, equipment or other in-kind support.
References
- 1.Roach R.E., Helmerhorst F.M., Lijfering W.M., Stijnen T., Algra A., Dekkers O.M. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;8 doi: 10.1002/14651858.CD011054.pub2. CD011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stegeman B.H., de Bastos M., Rosendaal F.R. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;f5298:347. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Judge C.P., Zhao X., Sileanu F.E., Mor M.K., Borrero S. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1–234.e9. doi: 10.1016/j.ajog.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shortridge E., Miller K. Contraindications to oral contraceptive use among women in the United States, 1999-2001. Contraception. 2007;75:355–360. doi: 10.1016/j.contraception.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Regidor P.A. The clinical relevance of progestogens in hormonal contraception: present status and future developments. Oncotarget. 2018;9:34628–34638. doi: 10.18632/oncotarget.26015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimes D.A., Lopez L.M., O’Brien P.A., Raymond E.G. Progestin-only pills for contraception. Cochrane Database Syst Rev. 2013;11 doi: 10.1002/14651858.CD007541.pub3. CD007541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones J., Mosher W., Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since. Natl Health Stat Report. 1995;2012:1–25. [PubMed] [Google Scholar]
- 8.Kavanaugh M.L., Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97:14–21. doi: 10.1016/j.contraception.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finer L.B., Zolna M.R. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duijkers I.J., Heger-Mahn D., Drouin D., Skouby S. A randomised study comparing the effect on ovarian activity of a progestogen-only pill (POP) containing desogestrel and a new POP containing drospirenone in a 24/4 regimen. Eur J Contracept Reprod Health Care. 2015;20:419–427. doi: 10.3109/13625187.2015.1044082. [DOI] [PubMed] [Google Scholar]
- 11.Foidart J.M. Added benefits of drospirenone for compliance. Climacteric. 2005;8(Suppl. 3):28–34. doi: 10.1080/13697130500330309. [DOI] [PubMed] [Google Scholar]
- 12.Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8(Suppl. 3):4–12. doi: 10.1080/13697130500330382. [DOI] [PubMed] [Google Scholar]
- 13.Bird S.T., Pepe S.R., Etminan M., Liu X., Brophy J.M., Delaney J.A. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi: 10.1186/1472-6904-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blode H., Kowal K., Roth K., Reif S. Pharmacokinetics of drospirenone and ethinylestradiol in Caucasian and Japanese women. Eur J Contracept Reprod Health Care. 2012;17:284–297. doi: 10.3109/13625187.2012.677076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer D.F., Ahrendt H.-J., Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439–444. doi: 10.1016/j.contraception.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Duijkers I.J.M., Heger-Mahn D., Drouin D., Colli E., Skouby S. Maintenance of ovulation inhibition with a new progestogen-only pill containing drospirenone after scheduled 24-h delays in pill intake. Contraception. 2016;93:303–309. doi: 10.1016/j.contraception.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Regidor P.A., Colli E., Schindler A.E. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24 + 4 cycle with desogestrel 75 mug per day. Gynecol Endocrinol. 2016;32:749–751. doi: 10.3109/09513590.2016.1161743. [DOI] [PubMed] [Google Scholar]
- 18.Palacios S., Colli E., Regidor P.A. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019 doi: 10.1111/aogs.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearl R. Factors in human fertility and their statistical evaluation. The Lancet. 1933;222:607–611. [Google Scholar]
- 20.Gerlinger C., Trussell J., Mellinger U. Different Pearl indices in studies of hormonal contraceptives in the United States: impact of study population. Contraception. 2014;90:142–146. doi: 10.1016/j.contraception.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oelkers W. Drospirenone, a progestogen with antimineralocorticoid properties: a short review. Mol Cell Endocrinol. 2004;217:255–261. doi: 10.1016/j.mce.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Sica D.A. Drospirenone: an antihypertensive in waiting. Hypertension. 2006;48:205–206. doi: 10.1161/01.HYP.0000232180.72752.f8. [DOI] [PubMed] [Google Scholar]
- 23.White W.B., Pitt B., Preston R.A., Hanes V. Antihypertensive effects of drospirenone with 17beta-estradiol, a novel hormone treatment in postmenopausal women with stage 1 hypertension. Circulation. 2005;112:1979–1984. doi: 10.1161/CIRCULATIONAHA.104.501502. [DOI] [PubMed] [Google Scholar]
- 24.Mount D.B. In: Chapter 49: Fluid and electrolyte disturbances. Principles of internal medicine 19th edition. Jameson J.L., Kasper D., Hauser S., Longo D., Fauci A., Harrison J.L., editors. Mcgraw-Hill Education; United States: 2015. [Google Scholar]
- 25.Trussell J., Portman D. The creeping Pearl: why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills? Contraception. 2013;88:604–610. doi: 10.1016/j.contraception.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A double-blind study comparing the contraceptive efficacy, acceptability and safety of two progestogen-only pills containing desogestrel 75 micrograms/day or levonorgestrel 30 micrograms/day Collaborative study group on the desogestrel-containing progestogen-only pill. Eur J Contracept Reprod Health Care. 1998;3:169–178. doi: 10.3109/13625189809167250. [DOI] [PubMed] [Google Scholar]
- 27.Board J.A. Continuous norethindrone, 0.35 mg., as an oral contraceptive agent. Am J Obstet Gynecol. 1971;109:531–535. doi: 10.1016/0002-9378(71)90624-7. [DOI] [PubMed] [Google Scholar]
- 28.Micronor 0.35 mg tablets (Ortho) 01/02/1973 approval [contraception]: medical officers review: FDA summary basis of approval, medical original review NDA 15-954. Accessed July 23, 2019. Available from URL: www.foiservices.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol deviations leading to exclusion of cycles from analyses in nonbreastfeeding women from a phase 3, multicenter, 13-cycle trial of drospirenone 4 mg 24/4-day contraceptive regimen