Abstract
Levofloxacin is a major antimicrobial agent that is used for the treatment of community-acquired lower respiratory tract infections (LRTIs). The present study was designed to investigate the pharmacokinetics (PK) and pharmacodynamics (PD) of levofloxacin in bronchial mucosa and lung tissue. A total of 32 patients undergoing pulmonary surgery were randomly assigned to one of four groups (8 subjects/group). All patients received a single dose of 500 mg levofloxacin orally prior to the operation. Blood, lung tissue and bronchial mucosa samples were collected prior to treatment and at 1.5, 4, 8, 12 and 24 h following treatment. The drug concentration was determined and PK and PD profiles were calculated using MATLAB software. The peak concentration of levofloxacin was 7.0±1.2 µg/g in lung tissues and 9.4±2.1 µg/g in bronchial mucosa. The corresponding area under the curve between 0 and 24 h (AUC0-24) was 85.7±8.5 and 137.3±19.4 µg h/g. The mean permeability of levofloxacin (ratio of concentration in tissue to that in plasma) was 2.4 in lung tissue and 4.4 in the bronchial mucosa. The PK profiles of levofloxacin in the plasma, lung and bronchial mucosa were described using an integrated one-compartment model. The probability of fAUC0-24/minimal inhibitory concentration (MIC) target attainment of levofloxacin against Streptococcus pneumoniae in the lung and bronchial mucosa was maintained at 100% when MIC ≤1 mg/l, while the cumulative fraction of fAUC0-24/MIC in the corresponding tissues was 94.4 and 98.1%, respectively. The present study demonstrated the high permeability of levofloxacin in the lung and bronchial mucosa of patients undergoing pulmonary surgery. In conclusion, treatment using 500 mg levofloxacin exhibits good clinical and microbiological efficacy for use in LRTIs that are caused by S. pneumoniae. This trial was registered retrospectively in the Chinese Clinical Trial Registry on January 13, 2020 (registration no. ChiCTR2000029096).
Keywords: levofloxacin, bronchial mucosa, lung tissue, pharmacokinetics, pharmacodynamics, pulmonary operation
Introduction
Levofloxacin is a third-generation fluoroquinolone antibiotic with wide-spectrum and potent in vitro antimicrobial activity against aerobic gram-negative and -positive microorganisms (1). Levofloxacin exhibits favorable pharmacokinetic (PK) and pharmacodynamic (PD) features, including high bactericidal activity, good absorption, high blood concentration, wide distribution, high tissue permeability and bioavailability (2-4). Levofloxacin is one of the major antimicrobial agents for the treatment of community-acquired lower respiratory tract infections (LRTIs) (5). The PK/PD parameters of levofloxacin, including the peak serum concentration (Cmax)/minimum inhibitory concentration (MIC) and area under the concentration-time curve from time 0 to 24 h (AUC0-24 h)/MIC, are closely associated with the clinical efficacy, bacterial eradication and prevention of the emergence of resistant bacteria in infectious diseases (6). Levofloxacin is used in clinical practice worldwide, particularly for the treatment of community-acquired pneumonia and acute exacerbation of chronic bronchitis (7-10).
Pulmonary operation is a clean-contaminated surgery (Altemeier Class II) and this procedure is likely to include contamination of bacteria colonizing the tracheal or bronchial mucosa, which is one of the major risk factors for peri-operative infection (11). Antimicrobial agents with good pathogen coverage and tissue penetration should be considered to prevent post-operative infections in clinical practice (12-20). Therefore, it is beneficial to perform studies in patients undergoing pulmonary operation who require antimicrobial agents to prevent potential infections due to external bacterial infection from an open airway and/or the colonizing microorganisms in the respiratory tract.
The present study was designed to examine the PK profiles and PK/PD of levofloxacin in bronchial mucosa and lung tissue. Patients undergoing pulmonary operation and those who required prophylactic antimicrobial therapy were included. All patients received levofloxacin prophylactically prior to the operation. The concentration of levofloxacin in the bronchial mucosa and lung tissues was determined and PK/PD analysis was performed using Monte Carlo simulation. The results provided information regarding the levofloxacin concentration in pulmonary tissues. An optimized dosing regimen allowing for the maximal bactericidal effect to be achieved in vivo was also recommended for patients with pulmonary disease.
Materials and methods
Study design
The present study was a randomized, single-center, open-label clinical trial. The protocol was reviewed and approved by the Ethics Committee of Huashan Hospital, Fudan University (Shanghai, China; approval no. 66; 2006). All patients signed an informed consent form prior to participation in the present study. The study was performed in compliance with the ethical principles outlined in the Declaration of Helsinki and all applicable regulatory requirements. The procedure of the present study is outlined in Table I. Since the study was designed as a pharmacokinetic study to explore the penetration of levofloxacin in tissue, it was not a comparative or controlled study. According to the Guidance for Industry on Population Pharmacokinetics released by the US Food and Drug Administration in July 2019, the data indicating the outcome of patients and the incidence of post-operative infections were not collected. The aim of the present study was to investigate the PK of a single drug, and therefore, no other drug was used for comparison.
Table I.
Flowchart of the study.
| Procedure | Screening | Experiment | ||||
|---|---|---|---|---|---|---|
| D-28 to D-2 | D-1 | D1 | D2 | D3 | D4 | |
| Informed consent | X | |||||
| Medical history | X | X | ||||
| Physical examination | X | X | X | |||
| Vital sign | X | X | X | X | ||
| Chest X-ray | X | |||||
| 12-lead ECG | X | X | X | |||
| Body weight | X | X | X | |||
| Inclusion/exclusion criteria | X | X | ||||
| Laboratory tests | ||||||
| Immunology | X | |||||
| Hematology, biochemistry, urinalysis | X | X | X | |||
| Endogenous creatinine clearance rate | X | X | X | |||
| Stay in hospital | X | X | X | X | X | |
| Drug administration | X | |||||
| Clinical observation | X | X | X | X | X | X |
| Pharmacokinetic evaluation | ||||||
| Blood sampling | X | X | ||||
| Tissue sampling | X | X | ||||
X means the procedure will be performed. ECG, electrocardiogram; D1, first day of the major experiment.
Participants
Recruitment of participants was performed in Shanghai. Patients who received a pulmonary operation at Huashan Hospital (Shanghai, China) due to pulmonary disease from June 2006 to June 2007 were enrolled according to the following inclusion criteria: i) age, at least 18 years; ii) requirement of pulmonary operation; and iii) voluntary agreement to participate and signing of informed consent form prior to the study procedure. Patients were excluded if they had severe pneumonia, moderate or severe renal impairment, or clinically significant abnormal liver function, which was defined as alanine aminotransferase or/and aspartate aminotransferase >3-fold the upper limit of the normal range (ULN) or total bilirubin >2-fold ULN. Creatinine clearance (CLcr) was calculated according to the Cockcroft-Gault formula (21):
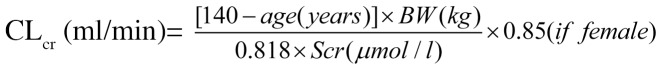 |
Where BW is the body weight and Scr indicates serum creatinine. Patients were excluded if they had a history of hypersensitivity to fluoroquinolones or other drugs, or photosensitivity, a history of epilepsy or other disorders of the central nervous system, documented QT prolongation or severe cardiac insufficiency. Pregnant or lactating females and patients that were treated with levofloxacin or other fluoroquinolones within 2 weeks prior to screening were also excluded from the present study.
Study drug
Levofloxacin 500 mg tablets (lot no. 0506G03) were provided by Daiichi Sankyo Co., Ltd.
Sample collection
The eligible patients were assigned randomly to one of four groups (8 subjects/group) according to the time of sampling. All patients received a single dose of 500 mg levofloxacin orally prior to pulmonary operation. Blood samples were collected prior to treatment, and at 1.5, 4, 8, 12 or 24 h following treatment. The samples of lung tissue and bronchial mucosa were collected at 4, 8, 12 or 24 h following treatment. Detailed sampling time points for the collection of blood or tissue samples are presented in Table II. The present study did not have a negative control, as the aim of the study was to observe PK of levofloxacin in the tissue and blood of patients.
Table II.
Time-points for collecting blood or tissue samples from patients undergoing pulmonary operation.
| Group | Number of subjects | Sampling time-point | |
|---|---|---|---|
| Blood | Lung tissue and bronchial mucosa | ||
| 1 | 8 | Pre-dose, 1.5 h; 4 h post-dose | 4 h post-dose |
| 2 | 8 | Pre-dose, 1.5 h; 8 h post-dose | 8 h post-dose |
| 3 | 8 | Pre-dose, 1.5 h; 12 h post-dose | 12 h post-dose |
| 4 | 8 | Pre-dose, 1.5 h; 24 h post-dose | 24 h post-dose |
All enrolled patients received a pulmonary operation due to lung cancer (n=23) or other pulmonary diseases, including old tuberculoma (n=3), pulmonary granuloma (n=3), right lung angioma, chronic cavity in right lower lobe, and bronchiectasis in left lung (one each). The patients with peripheral lung cancer received a lobectomy, and the patients with central type lung cancer were sampled using a unilateral pneumonectomy. A sample of lung tissue ~1 cm2 was collected from the external side of the excised lung lobe and rinsed twice with normal saline. The moisture on the surface was dried using a clean gauze. Bronchial rings were excised from the residual end of lung cancer specimen in patients with peripheral lung cancer, while adequate bronchial mucosa was collected from the patients with central type lung cancer to avoid tumor tissue. The bronchial mucosa samples were rinsed using the same procedures used for lung tissue samples. The samples of lung tissue and bronchial mucosa were sectioned into pieces, and a fixed volume of 50 mmol/l KH2PO4 buffer was added. Homogenate was extracted using an ultrasonic homogenizer, and tissues were centrifuged to harvest the supernatant. Blood samples were heparinized and centrifuged at 4°C, 1,500 x g for 10 min to separate plasma. All samples were stored in a refrigerator at -40°C and in dark conditions until subsequent analysis.
Levofloxacin assay and method validation
High-performance liquid chromatography was used to determine the concentration of levofloxacin in blood and tissue samples. The method used has been reported in a previous study (22). The analytical system consisted of a high-performance liquid chromatographer Waters model 2690 equipped with a fluorescent detector (model 474; Waters Corp.), which measured at wavelengths of 296 mm (excitation) and 504 nm (emission). The stationary phase was a TSK-gel ODS-80™ C18 column (4.6x150 mm; 5 µm; Tosoh Corporation). The mobile phase consisted of 50 mmol/l KH2PO4 (pH 2.0)-tetrahydrofuran-1 mol/l acetonitrile (92/7/1; v/v/v). The analysis was performed at a flow rate of 1.0 ml/min, a column temperature of 35°C and an injection volume of 10 µl. Compound DL-8493 was used as an internal control, which was provided by Daiichi Sankyo Co., Ltd. The lower limit of quantitation was 0.0100 µg/ml and the linear range was 0.01-5 µg/ml. The recovery of levofloxacin was 99.6±1.6%, 101.3±2.2% and 100.8±1.3% from plasma, lung tissue and bronchial mucosa, respectively. For the plasma and buffer samples, the intra-day relative standard deviation was ≤4.1 and ≤1.8%, while the inter-day variability was ≤2.5 and ≤4.2%, respectively. The corresponding accuracy was 96.1-101.9% and 96.2-103.1%.
PK evaluation
The PK parameters of levofloxacin in plasma, lung and bronchial mucosa were obtained using non-compartment analysis. The parameters included Cmax, peak time (Tmax), AUC0-24, area under the time-concentration curve from time zero to infinity (AUC0-∞), half life (T1/2), mean residence time until 24 h (MRT0-24), total apparent clearance (CLt/F), apparent volume of distribution (Vd/F), the ratio of Cmax in tissue vs. plasma (RCmax), the ratio of levofloxacin AUC0-24 in tissue vs. plasma (RAUC_0-24) and the ratio of levofloxacin AUC0-∞ in tissue vs. plasma (RAUC_0-∞), where the latter three parameters reflect the permeability of levofloxacin in lung or bronchial mucosa. Tmax is the time when levofloxacin concentration reaches Cmax. The area under the concentration-time curve AUC0-24 was calculated using the trapezoidal method: ∑ni=1Ci+Ci+1 (ti+1-ti)/2, where Ci and ti indicate the concentration and time, respectively. The number of time-points (n) was 5 for plasma and 4 for lung tissue and bronchial mucosa. T1/2 is calculated as 0.693/λ, where λ is the terminal elimination rate. MRT0-24 was obtained as AUMC0-24/AUC0-24, where AUMC0-24 is the integration of C x t vs. time from 0 to 24 h. The CLt/F of levofloxacin is calculated as Dose/AUC0-∞, while the Vd/F is obtained as (CLt/F)/λ. Since there was no single time-concentration curve from 0 to 24 h from the same subject, the non-parametric bootstrap method was used to obtain the above parameters (23). Specifically, a new replication of the dataset (bootstrap sample) at each time-point was obtained using eight random draws of individual data (with replacement) from the original dataset. The non-compartment analysis was performed using average values of each new dataset and this process was repeated 200 times with different random draws. All the calculations were performed using Matlab software (version 7.0.1; Mathworks Inc.).
A pharmacokinetic model was developed to analyze the time profiles of levofloxacin in plasma, lung tissue and bronchial mucosa simultaneously (Fig. 1). The time profiles of the levofloxacin concentration in plasma and tissues were described using a one-compartment model. The elimination of levofloxacin from the central, lung and bronchial compartment, as well as the drug transport from the central to lung or bronchial compartment, were all consistent with first-order kinetics. The differential equations are as follows:
Figure 1.
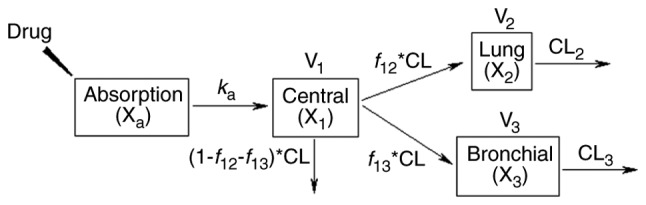
Pharmacokinetic model of levofloxacin in plasma, lung tissue and bronchial mucosa. Xa, X1, X2 and X3 denote the amount of levofloxacin in absorption, central, lung and bronchial compartment, respectively. ka represents the absorption rate of levofloxacin. CL is the clearance of levofloxacin. V1, V2, and V3 indicate the distribution volume in the central, lung, and bronchial compartment. f12 and f13 indicate the fraction of levofloxacin clearance from central to the lung and bronchial compartment, respectively. CL2 and CL3 are the clearance of levofloxacin from the lung and bronchial compartment, respectively.
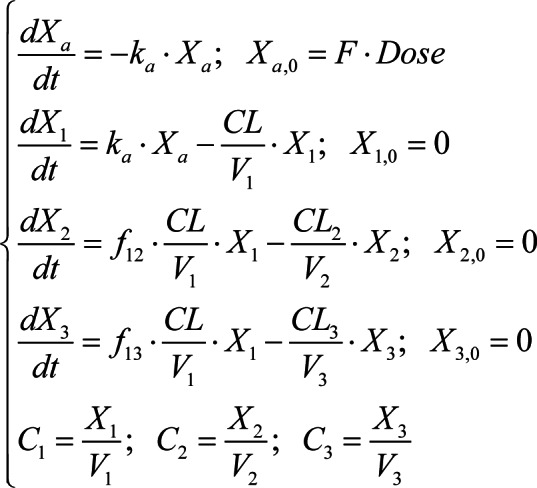 |
Where Xa, X1, X2 and X3 are the amount of levofloxacin in absorption, central, lung and bronchial compartment, respectively, and Xa,0, X1,0, X2,0 and X3,0 are the corresponding initial values. ka and F represent the absorption rate and bioavailability of levofloxacin. CL and V1 are the clearance of levofloxacin and distribution volume in the central compartment. f12 and f13 indicate the fraction of levofloxacin clearance from the lung and bronchial compartment, respectively. CL2 and CL3 are the clearance of levofloxacin from central to the lung and bronchial compartment. V2 is the weight of the lung, which was fixed as 1.2 kg according to previous literature (1/50 of the assumed body weight of 60 kg) (24-26). V3 is the weight of the bronchial mucosa, which was fixed as a value of 1, since the data were not identifiable. C1, C2 and C3 indicate the levofloxacin concentration in the plasma (mg/l), lung (mg/kg) and bronchial mucosa (mg/kg), respectively.
The PK model was developed in three steps: i) The mean C1 data were fit to obtain CL, volume of distribution in central department (V1/F) and ka; ii) the mean C2 data were fit to obtain f12 and CL2; iii) the mean C3 data were fit to obtain f13 and CL3. Non-compartment analysis was used to provide initial estimates of the parameters. Model fittings were performed by non-linear regression analysis using a maximum likelihood algorithm in Matlab software. The ordinary differential equation functions were used to solve differential equations. Goodness of fit was evaluated by the objective function (mean residual fraction ratio) and by visual inspection of diagnostic plots. Non-parametric bootstrap analysis was performed in order to obtain more accurate parameter estimates. This process was similar to that in the non-compartment analysis. The stability of the final model was evaluated by inspection of the distribution of parameter estimates from the new datasets and comparing these with values obtained from the fit of the original dataset.
Statistical analysis
The demographic and baseline characteristics of patients were summarized and compared between groups. Values are expressed as n or the mean ± standard deviation. Continuous data were assessed using analysis of variance and the least-significant difference test. The categorical data, including sex, history of smoking and concomitant medications, were compared using Fisher's exact test. All statistical analyses were performed using SPSS software (version 19.0; IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
PK/PD analysis
Levofloxacin is a concentration-dependent quinolone antibiotic. The major PK/PD parameters are AUC/MIC and Cmax/MIC (26-28). The MIC data of levofloxacin were obtained from a previous study (29). The fAUC0-24/MIC90 and fCmax/MIC90 of levofloxacin were calculated using the PK parameters obtained from a non-compartment analysis, where f is the free fraction of levofloxacin (0.7) (30). PK/PD analysis of levofloxacin was performed using Monte Carlo simulation. The simulated data of AUC0-24 and Cmax were obtained based on a logarithmic normal distribution. The simulated MIC values were generated based on a discrete distribution according to specified probability at each MIC level. The PK/PD targets of levofloxacin (fAUC0-24/MIC ≥30; fCmax/MIC ≥5) were used to predict the bacteriological efficacy of the drug against Streptococcus pneumonia (4,31-34). The probability of target attainment (PTA) of levofloxacin was calculated as the percentage of PK/PD parameter reaching the target at each specified MIC level, while the cumulative fraction of response (CFR) of levofloxacin was obtained as the percentage of PK/PD parameters attaining the target values (35). The simulation was performed in 5,000 patients using Matlab software.
Results
Baseline characteristics of patients
A total of 32 patients were enrolled in the present study. The underlying diseases of the patients included lung cancer (n=23), old tuberculosis (n=3), lung inflammatory granuloma (n=3), right lung angioma (n=1), chronic cavitation in the right lower lobe (n=1) and bronchiectasis in the left lower lobe (n=1). A total of 20 (62.5%) male and 12 (37.5%) female patients were enrolled, with an average age of 56±12 (range 23-80) years and an average CLcr of 92.2±20.9 ml/min. The baseline characteristics of sex, age, body weight, CLcr and alanine aminotransferase were well-balanced among the 4 patient groups (P>0.05; Table III). No significant difference between history of smoking or use of concomitant drugs was present among the 4 groups.
Table III.
Baseline characteristics of patients undergoing pulmonary operation (8 patients/group).
| Group | Sex (male/female) | Age (years) | Body weight (kg) | CLcr(ml/min) | ALT (U/l) | Smoking (yes/no) | Concomitant drugs (yes/no) |
|---|---|---|---|---|---|---|---|
| 1 | 7/1 | 48±13 | 65±10 | 105±17 | 23.9±13.8 | 6/2 | 0/8 |
| 2 | 6/2 | 61±10 | 64±7 | 80±14 | 18.8±7.21 | 4/4 | 0/8 |
| 3 | 3/5 | 58±13 | 65±10 | 94±23 | 22.3±8.43 | 2/6 | 1/7 |
| 4 | 4/4 | 58±12 | 61±12 | 90±23 | 21.6±14.8 | 2/6 | 3/5 |
| Total | 20/12 | 56.0±12.0 | 63.8±9.5 | 92.2±20.9 | 21.6±11.1 | 14/18 | 4/28 |
Values are expressed as n or the mean ± standard deviation. CLcr, creatinine clearance calculated using Cockcroft-Gault formula; ALT, alanine aminotransferase.
Permeability of levofloxacin in lung tissue and bronchial mucosa
The mean concentration-time curves of levofloxacin in lung tissue, bronchial mucosa and plasma of the patients are presented in Fig. 1. Table IV presents the concentration ratio of levofloxacin in lung tissue and bronchial mucosa at each time-point. The RCmax in lung tissue and bronchial mucosa was 1.7±1.0 and 2.2±1.1 at 4 h post-dose. These ratios increased to 3.8±2.0 and 9.0±8.0 at 24 h, respectively. Statistical analysis demonstrated that the concentration ratio of levofloxacin at 24 h was significantly higher compared with that at other sampling times in lung tissue and bronchial mucosa (Table IV). The results also indicated that the mean concentration ratio of levofloxacin in bronchial mucosa was significantly higher compared with that in lung tissue at 24 h post-dose.
Table IV.
Concentration ratio of levofloxacin in patients undergoing pulmonary operation after oral administration of a single 500-mg tablet (n=8 per group).
| Group | Actual sampling time (h) | Concentration ratioa | |
|---|---|---|---|
| Lung/plasma | Bronchial mucosa/plasma | ||
| 1 | 4.5±0.2 | 1.7±1.0b | 2.2±1.1b |
| 2 | 7.3±1.3 | 1.8±0.9b | 2.1±0.8b |
| 3 | 11.6±0.8 | 2.3±1.5c | 4.2±2.5a,c |
| 4 | 23.6±0.9 | 3.8±2.0 | 9.0±8.0 |
| Overall | 2.4±1.6 | 4.4±5.0 | |
aRatio of levofloxacin concentration in tissue samples to that in plasma at the same time-point. Values are expressed as n or the mean ± standard deviation.
bP<0.01 and
cP<0.05 compared with the corresponding ratio in group 4.
PK of levofloxacin
Non-compartment parameters of levofloxacin in plasma, lung tissue and bronchial mucosa are presented in Table V. For the majority of parameters, the values were ranked as plasma < lung tissue < bronchial mucosa. The AUC0-24 of levofloxacin in the plasma, lung tissue and bronchial mucosa was 65.2±4.0, 85.7±8.5 and 137.3±19.4 mg h/l, respectively. RAUC_0-24 was 1.3±0.2 in the lung and 2.1±0.3 in the bronchial mucosa. The levofloxacin concentration reached Cmax in plasma at 1.5 h, while the concentration in lung tissue and bronchial mucosa reached Cmax at 4 h post-dose. The Cmax in lung tissue and bronchial mucosa was increased by 32 and 77% compared with that in plasma. The T1/2 of levofloxacin was increased by 46% in lung tissue and 254% in bronchial mucosa compared with that in plasma. The MRT0-24 of levofloxacin exhibited a similar pattern of change to that of T1/2.
Table V.
Non-compartment parameters of levofloxacin following single oral administration of 500 mg tablet in patients undergoing pulmonary operation.
| Parameter | Plasma | Lung tissue | Bronchial mucosa |
|---|---|---|---|
| Cmax (mg/l or mg/kg) | 5.4±0.7 | 7.0±1.2 | 9.4±2.1 |
| Tmax (h) | 1.7±0.9 | 5.9±1.4 | 6.4±2.8 |
| AUC0-24 (mg h/l or mg h/kg) | 65.2±4.0 | 85.7±8.5 | 137.3±19.4 |
| AUC0-∞ (mg h/l or mg h/kg) | 69.4±4.1 | 106.6±8.1 | 273.7±129.7 |
| T1/2 (h) | 6.1±0.7 | 8.9±1.5 | 21.6±14.2 |
| MRT0-24 (h) | 8.3±0.3 | 9.9±0.4 | 11.2±0.7 |
| CLt/F (l/h) | 7.2± 0.4 | ||
| Vd/F (l) | 63.7±8.1 | ||
| RCmax (l/kg) | 1.3±0.3 | 1.8±0.5 | |
| RAUC_0-24 (l/kg) | 1.3±0.2 | 2.1±0.3 | |
| RAUC_0-∞ (l/kg) | 1.5±0.1 | 4.0±1.9 |
Values are expressed as n or the mean ± standard deviation. The results were obtained using a bootstrap method (n=200). The units of Cmax and AUC in plasma were mg/l and mg h/l. As for lung tissue and bronchial mucosa, the units of Cmax and AUC were mg/kg and mg h/kg, respectively. AUC0-24, area under the concentration-time curve between 0 and 24 h; Cmax, peak serum concentration; Tmax, peak time; T1/2, half life; MRT, mean residence time; CLt/F, total apparent clearance; Vd/F, apparent volume of distribution; RCmax, the ratio of Cmax in tissue vs. plasma.
The time profiles of levofloxacin in plasma, lung tissue and bronchial mucosa were well-described by the PK model (Fig. 2). The absorption rate of levofloxacin was 5.6 1/h, while the CLt/F was 7.8 l/h (Table VI). Bootstrapping for PK model showed that f12 and f13 were 0.128 and 0.074, which indicate that the percentage sof the levofloxacin dose distributed to the lung and bronchial compartment was 12.8 and 7.4%, respectively (Table VI). The parameter values obtained from the bootstrap dataset had almost the same order of magnitude as those from the original dataset. The coefficient of variation of parameters in tissue (f12, CL2, f13 and CL3) obtained from the bootstrap dataset was relatively high (Table VI). This is consistent with the high standard deviation of the levofloxacin concentration in lung tissue and bronchial mucosa (Fig. 2).
Figure 2.
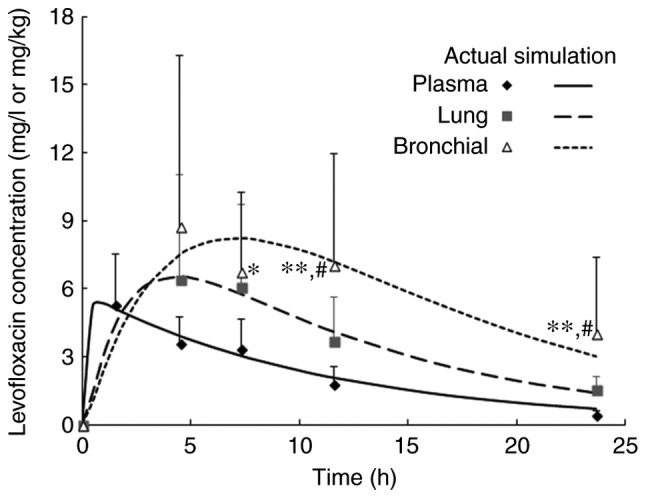
Time profiles of levofloxacin in plasma (µg/ml) or tissue samples (µg/g) after single oral administration of a 500-mg tablet in patients undergoing pulmonary operation. Values are expressed as the mean ± standard deviation (n=32 at 1.5 h or 8 at the other time-points) and predictions were made by a pharmacokinetic model. *P<0.05, **P<0.01 compared to plasma (least-significant difference test); #P<0.05 compared to lung tissue (least-significant difference test).
Table VI.
Pharmacokinetic parameters of levofloxacin in patients undergoing pulmonary operation.
| Parameter (Unit) | Original dataset | Bootstrap dataset | |
|---|---|---|---|
| Mean ± SDa | CV (%) | ||
| ka (l/h) | 5.6 | 6.2±2.0 | 32.6 |
| CL (l/h) | 7.8 | 8.2±0.8 | 9.8 |
| V1/F (l) | 86.4 | 85.6±11.9 | 13.9 |
| f12 | 0.128 | 0.2±0.1 | 77.7 |
| CL2 (l/h) | 0.6 | 0.8±0.6 | 72.3 |
| f13 | 0.074 | 0.1±0.08 | 69.3 |
| CL3 (l/h) | 0.2 | 0.3±0.3 | 75.9 |
aValues are expressed as the mean ± SD of individual estimates (n=200). SD, standard deviation; CV, coefficient of variation; f12, the fraction of levofloxacin clearance from central to the lung compartment; f13, the fraction of levofloxacin clearance from central to the bronchial compartment; ka, absorption rate; CL, clearance rate from plasma; CL2, the clearance of levofloxacin from the lung compartment; CL3, the clearance of levofloxacin from the bronchial compartment; V1/F, volume of distribution in central compartment.
Therapeutic implication of levofloxacin PK/PD
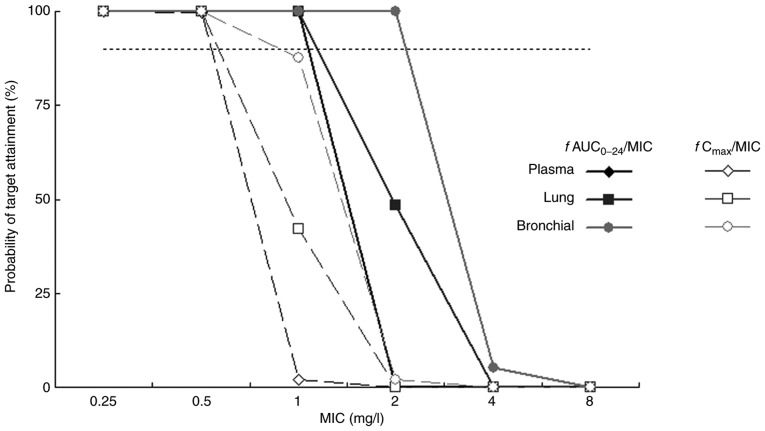
The PK/PD parameters of levofloxacin against common pathogens of community-acquired LRTIs are presented in Table VII. The fAUC0-24/MIC and fCmax/MIC of levofloxacin in lung tissue and bronchial mucosa were higher compared with those in plasma. The fAUC0-24/MIC was 45.6-182.5, 60.0-239.8 and 96.1-384.5 in plasma, lung tissue and bronchial mucosa, respectively, against gram-positive bacteria. The fCmax/MIC of levofloxacin was 3.8-15.1, 4.9-19.7 and 6.6-26.3 in the corresponding tissue. The PTA of PK/PD parameters of levofloxacin against S. pneumoniae are indicated in Fig. 3. The PTA of levofloxacin fAUC0-24/MIC was maintained at 100% in plasma and lung tissue when MIC ≤1 mg/l. The PTA of fAUC0-24/MIC was >90% in bronchial mucosa when the MIC was ≤2 mg/l. The CFR of levofloxacin for S. pneumoniae was 90.6, 94.4 and 98.1% in plasma, lung tissue and bronchial mucosa, respectively, when fAUC0-24/MIC=30. The CFR of levofloxacin fCmax/MIC was 44.7, 64.8 and 85.4% in the corresponding tissues.
Table VII.
Pharmacokinetic/pharmacodynamic parameters of levofloxacin in patients undergoing pulmonary operation following single oral administration of a 500-mg levofloxacin tablet.
| Bacteria (no. of strains) | MIC90 (mg/l) | fAUC0-24/MIC90 | fCmax/MIC90 | ||||
|---|---|---|---|---|---|---|---|
| Plasma | Lung tissue | Bronchial mucosa | Plasma | Lung tissue | Bronchial mucosa | ||
| MSSA (21) | 0.25 | 182.5 | 239.8 | 384.5 | 15.1 | 19.7 | 26.3 |
| S. pneumoniae (28) | 1 | 45.6 | 60.0 | 96.1 | 3.8 | 4.9 | 6.6 |
| H. influenzae (45) | 0.5 | 91.3 | 119.9 | 192.3 | 7.6 | 9.8 | 13.2 |
| K. pneumoniae (87) | 1 | 45.6 | 60.0 | 96.1 | 3.8 | 4.9 | 6.6 |
| P. aeruginosa (18) | 16 | 2.9 | 3.7 | 6.0 | 0.2 | 0.3 | 0.4 |
| Acinetobacter spp. (22) | 0.25 | 182.5 | 239.8 | 384.5 | 15.1 | 19.7 | 26.3 |
MIC90 was obtained from a previous study (29). f, unbound fraction of levofloxacin (0.7); MSSA, methicillin-susceptible S. aureus; MIC, minimum inhibitory concentration; AUC0-24, area under the concentration-time curve between 0 and 24 h; Cmax, peak serum concentration.
Figure 3.
PTA of levofloxacin fAUC0-24/MIC (target=30) and fCmax/MIC (target=5) against S. pneumoniae in patients undergoing pulmonary operation following single oral administration of a 500-mg tablet. The horizontal line indicates the PTA value of 90% and f is the unbound fraction of levofloxacin (0.7). MIC, minimal inhibitory concentration; AUC0-24, area under the concentration-time curve between 0 and 24 h; Cmax, peak serum concentration; PTA, probability target attainment.
Discussion
The results of the present study indicated that levofloxacin is able to rapidly penetrate into the lung tissue and bronchial mucosa. Following a single oral dose of 500 mg levofloxacin, the average concentration of levofloxacin in plasma, lung tissue and bronchial mucosa was 3.6 µg/ml, 6.4 and 8.7 µg/g at 4 h post-dose, and this was reduced to 0.5 µg/ml, 1.6 and 4.1 µg/g at 24 h. Compared with the plasma concentration, the levofloxacin concentration was higher in lung tissue and bronchial mucosa at 4-24 h post-dose. The average concentration ratio of the levofloxacin penetration into lung tissue was 2.4 (0.7-7.8) and the maximal concentration ratio value was observed at 24 h post-dose. The permeability of levofloxacin in lung tissue increased with time, demonstrating the high permeability of levofloxacin in lung tissue. Other studies have reported that the mean tissue permeability of levofloxacin was 4.0 within 24 h and 5.1 at 12 h following a single oral dose of 500 mg levofloxacin in patients receiving pulmonary biopsy or pneumonectomy (36). These data are inconsistent with the results of the present study and may be due to the patients in the aforementioned studies being from different ethnic groups and exhibiting high inter-subject variability due to the smaller patient sample size. In the present study, a total of 8 Chinese patients were enrolled in each group.
Pulmonary lobectomy is an effective treatment for intrapulmonary and bronchial diseases (37). However, this procedure to remove the source of contamination in bronchi may cause infection (38), and is a critical occasion for the prophylactic use of antimicrobial agents. Following oral administration of a single dose of 500 mg levofloxacin, the average concentration ratio of levofloxacin was 4.4 in bronchial mucosa within 24 h, which was significantly higher compared with that in lung tissue (2.4). Compared with water-soluble drugs, lipid-soluble antimicrobial agents, including fluoroquinolones, are more readily absorbed through the cell membrane and exhibit increased tissue permeability (39). Antimicrobial agents have been extensively studied in sputum (40). However, sputum is non-homogeneous sample and may be easily diluted by saliva (41). Therefore, bronchial mucosa is more reliable than sputum in the evaluation of antimicrobial agent permeability in lung tissues (42); however, it is more difficult to collect samples of bronchial mucosa and lung tissue than sputum. The present study provided direct evidence for the tissue permeability of levofloxacin.
Zhang et al (2) indicated that the Cmax/MIC90 ratio was 3-57 in epithelial lining fluid (ELF), and 1-6 in sputum following a single oral dose of 500 mg levofloxacin at the fasting state in patients with LRTI undergoing bronchoalveolar lavage (2). The AUC0-24 h/MIC90 of levofloxacin reached 35-138 and 9-38, respectively. Compared with the plasma concentration at the same time-point, the concentration ratio of levofloxacin in ELF was high (~1.04 at 24 h after dosing), but the concentration ratio in sputum was low (0.09) (2). The results of the present study demonstrated that the permeability of levofloxacin is more prominent in lung tissue and bronchial mucosa following an oral dose of 500 mg, and is higher compared with that in ELF and sputum.
The Vd of levofloxacin decreased significantly by 47.8% in patients undergoing pulmonary operation (1.0 vs. 1.9 l/kg), T1/2 decreased by 34.8% (6.1 vs. 9.4 h) and Cmax decreased by 23.1% (5.4 vs. 7.0 mg/l), while the AUC0-24 and AUC0-∞ of levofloxacin increased by 35.3% (65.2 mg h/l vs. 48.2 mg h/l) and 26.7% (69.4 mg h/l vs. 54.8 mg h/l), respectively, compared with that in healthy subjects (43). These results indicated that a decreased distribution volume of levofloxacin may be the reason for the higher drug exposure in patients undergoing pulmonary operation.
During the development of the compartment model, the levofloxacin concentration data in plasma and lung tissue was fit using a two-compartment model, where the peripheral compartment represents lung tissue. The results indicated that the inter-compartment clearance of levofloxacin was close to zero. Subsequently, a model was developed where drug elimination from the peripheral compartment was introduced. Although the model fittings improved, the simulated concentration of levofloxacin in plasma was higher than the actual values at all time-points. A three-compartment model was also used to analyze the PK data of levofloxacin, where the two peripheral compartments represented lung tissue and bronchial mucosa and the simulation results were not satisfactory. The final PK model was obtained by simplification of this model and by introducing an elimination pathway from peripheral compartments (Fig. 1). For instance, the elimination of levofloxacin from the lung compartment represented the process of drug efflux from lung tissue to blood. The structure of the final PK model was similar to that of the PK model of moxifloxacin in patients with bronchopneumonia (44). The CL of levofloxacin was close to the non-compartment parameter CLt/F and the distribution volume in the central compartment V1/F was similar to Vd/F. The results of most PK parameters derived from the bootstrap method were close to the results calculated from original dataset, and this was supported by the standard deviation for PK parameters. This suggested that the estimation of the results of the final PK model were reliable.
The PK/PD parameters of levofloxacin determined in the present study were similar to the literature reports following the adjustment by dose and drug unbound fraction. For S. pneumoniae, the Cmax/MIC90 and AUC/MIC90 of levofloxacin in plasma were similar to those in patients with bronchitis or obstructive pulmonary disease (32). The results of the present study were also similar to a PK study of levofloxacin in elderly adults receiving diagnostic bronchoscopy (45). The Cmax/MIC90 and AUC/MIC90 of levofloxacin in lung tissue against P. aeruginosa and K. pneumoniae exhibited the same orders of magnitude to the parameter values in patients undergoing off-pump coronary artery bypass grafting (46).
The results indicated that the fAUC0-24/MIC90 of levofloxacin was only 3.7 in lung tissue against P. aeruginosa. This result indicated that levofloxacin cannot be recommended as a first-line therapy of LRTIs where P. aeruginosa is isolated or is suspected to be the causative pathogen. This result is similar to a PK/PD report of levofloxacin in acutely hospitalized elderly patients (27). A previous study demonstrated that a PK/PD model predicted that 500 mg levofloxacin was not effective for treating multidrug- and drug-resistant tuberculosis (47-49). By contrast, the fAUC0-24/MIC of levofloxacin reached 60 in lung tissue against S. pneumoniae. The Monte Carlo simulation revealed that the PTA of levofloxacin fAUC0-24/MIC in lung tissue and bronchial mucosa was maintained at 100% when MIC ≤1 mg/l and the CFR of fAUC0-24/MIC was also >90% in these two tissues. These results are supported by a population PK (PPK) study of levofloxacin (26), which indicated an AUC0-24 of 66.19±1.30 mg h/l and the predicted CFR for a target AUC0-24/MIC ratio of 30 was 83.12% for S. aureus and 92.63% for S. pneumoniae. Due to the MIC90 of levofloxacin against S. pneumoniae being 1 mg/l, it may be suggested that a levofloxacin 500 mg dosing regimen has good clinical and microbiological efficacy in the treatment of pulmonary infections that are caused by S. pneumoniae.
In the present study, it was not possible to develop a PPK model of levofloxacin due to the small number of lung tissue and bronchial mucosa samples. It was impossible to estimate inter-subject variation due to there only being one data-point for each tissue sample type that was collected in one subject. Therefore, a compartment model was developed to analyze the PK data of levofloxacin in plasma, lung tissue and bronchial mucosa simultaneously. This provides more details about drug ADME compared with general non-compartment PK parameters. Treatment-emergent adverse events were not monitored, but the record of data did not indicate any significant or outstanding adverse events in the present study. The body mass index was also not calculated as patient height was not recorded. The standard error is particularly high for levofloxacin concentration in bronchial samples as compared with that in the other types of samples. This may be due to fewer number of samples at the time point of sampling and inter-individual variation. The bootstrap simulation in this study may offset this limitation to some extent.
To the best of our knowledge, the present study was the first to characterize the penetration of levofloxacin in bronchial mucosa. An integrated model was developed describing disposition and elimination of levofloxacin in plasma, lung tissue and bronchial mucosa, and PK/PD indices of levofloxacin in patients' bronchial mucosa were provided. The results indicated that levofloxacin is able to distribute to bronchi and lung tissues to reach an effective antimicrobial concentration after a single oral dose of 500 mg. The permeability of levofloxacin was demonstrated to be higher in bronchial mucosa compared with that in lung tissue. The PK/PD profiles of levofloxacin in lung tissues support the favorable efficacy of levofloxacin 500 mg regimen for managing the community-acquired LRTIs that are caused by S. pneumoniae.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the Ministry of Science and Technology of China (grant nos. 2012ZX09303004-001 and 2017ZX09304005) and the Natural Science Foundation of China (grant no. 81202582). Japan Daiichi Pharmaceutical Co. also sponsored this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GC, JZ and ZC conceived and designed the experiments, and wrote and modified the manuscript. YoZ and LP performed surgery and collected tissue samples. XX, YC and JY performed the PK/PD analysis. YiZ, JZ and YS critically interpreted the data and reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All study procedures in this study were approved by the Ethics Committee of Huashan Hospital, Fudan University (Shanghai, China). All patients provided written informed consent form prior to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torres A, Liapikou A. Levofloxacin for the treatment of respiratory tract infections. Expert Opin Pharmacother. 2012;13:1203–1212. doi: 10.1517/14656566.2012.688952. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Xie X, Zhou X, Chen YQ, Yu JC, Cao GY, Wu XJ, Shi YG, Zhang YY. Permeability and concentration of levofloxacin in epithelial lining fluid in patients with lower respiratory tract infections. J Clin Pharmacol. 2010;50:922–928. doi: 10.1177/0091270009355160. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T, Kaji Y, Miyawaki M. Penetration of single-dose levofloxacin into intestinal tissue. J Gastroen Hepatol Res. 2013;2:399–402. [Google Scholar]
- 4.Hutschala D, Skhirtladze K, Zuckermann A, Wisser W, Jaksch P, Mayer-Helm BX, Burgmann H, Wolner E, Müller M, Tschernko EM. In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob Agents Chemother. 2005;49:5107–5111. doi: 10.1128/AAC.49.12.5107-5111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman RF, Rotschafer JC, Tan JS. Antimicrobial treatment of lower respiratory tract infections in the hospital setting. Am J Med. 2005;118 (Suppl)(29S-38S) doi: 10.1016/j.amjmed.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: A critical review. Int J Antimicrob Agents. 2002;19:261–268. doi: 10.1016/s0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 7.Friedman H, Song X, Crespi S, Navaratnam P. Comparative analysis of length of stay, total costs, and treatment success between intravenous moxifloxacin 400 mg and levofloxacin 750 mg among hospitalized patients with community-acquired pneumonia. Value Health. 2009;12:1135–1143. doi: 10.1111/j.1524-4733.2009.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Frei CR, Jaso TC, Mortensen EM, Restrepo MI, Raut MK, Oramasionwu CU, Ruiz AD, Makos BR, Ruiz JL, Attridge RT, et al. Medical resource utilization among community-acquired pneumonia patients initially treated with levofloxacin 750 mg daily versus ceftriaxone 1000 mg plus azithromycin 500 mg daily: A US-based study. Curr Med Res Opin. 2009;25:859–868. doi: 10.1185/03007990902779749. [DOI] [PubMed] [Google Scholar]
- 9.Schein J, Janagap-Benson C, Grant R, Sikirica V, Doshi D, Olson W. A comparison of levofloxacin and moxifloxacin use in hospitalized community-acquired pneumonia (CAP) patients in the US: Focus on length of stay. Curr Med Res Opin. 2008;24:895–906. doi: 10.1185/030079908X273408. [DOI] [PubMed] [Google Scholar]
- 10.Lynch-JP III, File-TM Jr, Zhanel GG. Levofloxacin for the treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther. 2006;4:725–742. doi: 10.1586/eri.10.35. [DOI] [PubMed] [Google Scholar]
- 11.DiPiro JT. Short-term prophylaxis in clean-contaminated surgery. J Chemother. 1999;11:551–555. doi: 10.1179/joc.1999.11.6.551. [DOI] [PubMed] [Google Scholar]
- 12.Radu DM, Jauréguy F, Seguin A, Foulon C, Destable MD, Azorin J, Martinod E. Postoperative pneumonia after major pulmonary resections: An unsolved problem in thoracic surgery. Ann Thorac Surg. 2007;84:1669–1673. doi: 10.1016/j.athoracsur.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 13.Schussler O, Alifano M, Dermine H, Strano S, Casetta A, Sepulveda S, Chafik A, Coignard S, Rabbat A, Regnard JF. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med. 2006;173:1161–1169. doi: 10.1164/rccm.200510-1556OC. [DOI] [PubMed] [Google Scholar]
- 14.Belda J, Cavalcanti M, Ferrer M, Serra M, Puig de la Bellacasa J, Canalis E, Torres A. Bronchial colonization and postoperative respiratory infections in patients undergoing lung cancer surgery. Chest. 2005;128:1571–1579. doi: 10.1378/chest.128.3.1571. [DOI] [PubMed] [Google Scholar]
- 15.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical infection prevention project. Am J Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Sinnett D, Carpenter R, Preece PE, Royle GT. Antibiotic prophylaxis for post-operative wound infection in clean elective breast surgery. Eur J Surg Oncol. 2000;26:363–366. doi: 10.1053/ejso.1999.0899. [DOI] [PubMed] [Google Scholar]
- 17.Bratzler DW, Houck PM, Richards C, Steele L, Dellinger EP, Fry DE, Wright C, Ma A, Carr K, Red L. Use of antimicrobial prophylaxis for major surgery: Baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140:174–182. doi: 10.1001/archsurg.140.2.174. [DOI] [PubMed] [Google Scholar]
- 18.Malone DL, Genuit T, Tracy JK, Gannon C, Napolitano LM. Surgical site infections: Reanalysis of risk factors. J Surg Res. 2002;103:89–95. doi: 10.1006/jsre.2001.6343. [DOI] [PubMed] [Google Scholar]
- 19.Von Baum H, Böttcher S, Hoffmann H, Sonntag HG. Tissue penetration of a single dose of levofloxacin intravenously for antibiotic prophylaxis in lung surgery. J Antimicrob Chemother. 2001;47:729–730. doi: 10.1093/oxfordjournals.jac.a002697. [DOI] [PubMed] [Google Scholar]
- 20.Swoboda S, Oberdorfer K, Klee F, Hoppe-Tichy T, von Baum H, Geiss HK. Tissue and serum concentrations of levofloxacin 500 mg administered intravenously or orally for antibiotic prophylaxis in biliary surgery. J Antimicrob Chemother. 2003;51:459–462. doi: 10.1093/jac/dgk056. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Xie X, Zhang J, Yu JC, Shi YG, Zhang YY. HPLC assay of levofloxacin concentration in plasma, lung tissue, and body fluids. Chin J Clin Pharmacol Ther. 2008;17:158–162. doi: 10.1128/AAC.49.12.5107-5111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Cao Y, Zhou J, Liu X. Mechanism-based pharmacokinetic-pharmacodynamic modeling of bidirectional effect of danshensu on plasma homocysteine in rats. Pharm Res. 2009;26:1863–1673. doi: 10.1007/s11095-009-9899-x. [DOI] [PubMed] [Google Scholar]
- 24.Askenazi SS, Perlman M. Pulmonary hypoplasia: Lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Child. 1979;54:614–618. doi: 10.1136/adc.54.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi XL, Bo AH, Xia L. Determination of cerebral and lung weight of fetus. Chin J Birth Health Heredity. 1993;65(65) doi: 10.1002/uog.16017. [DOI] [PubMed] [Google Scholar]
- 26.Jaruratanasirikul S, Jaspattananon A, Wongpoowarak W, Nawakitrangsan M, Thengyai S, Samaeng M. Population pharmacokinetics and pharmacodynamics modeling of oral levofloxacin. J Med Assoc Thai. 2018;99:886–892. [PubMed] [Google Scholar]
- 27.Cojutti PG, Ramos-Martin V, Schiavon I, Rossi P, Baraldo M, Hope W, Pea F. Population pharmacokinetics and pharmacodynamics of levofloxacin in acutely hospitalized older patients with various degrees of renal function. Antimicrob Agents Chemothe. 2017;61(e02134-16) doi: 10.1128/AAC.02134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaglione F, Mouton JW, Mattina R, Fraschini F. Pharmacodynamics of levofloxacin and ciprofloxacin in a murine pneumonia model: Peak concentration/MIC versus area under the curve/MIC ratios. Antimicrob Agents Chemother. 2003;47:2749–2755. doi: 10.1128/aac.47.9.2749-2755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YY, Huang HH, Ren ZY. Clinical evaluation of oral levofloxacin 500 mg once-daily dosage for treatment of lower respiratory tract infections and urinary tract infections: A prospective multicenter study in China. J Infect Chemother. 2009;15:301–311. doi: 10.1007/s10156-009-0713-9. [DOI] [PubMed] [Google Scholar]
- 30.Cao G, Zhang J, Wu X, Yu J, Chen Y, Ye X, Zhu D, Zhang Y, Guo B, Shi Y. Pharmacokinetics and pharmacodynamics of levofloxacin injection in healthy Chinese volunteers and dosing regimen optimization. J Clin Pharm Ther. 2013;38:394–400. doi: 10.1111/jcpt.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodvold KA, Danziger LH, Gotfried MH. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and Azithromycin in healthy adults. Antimicrob Agents Chemother. 2003;47:2450–2457. doi: 10.1128/aac.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conte JE Jr, Golden JA, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int J Antimicrob Agents. 2007;30:422–427. doi: 10.1016/j.ijantimicag.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxac in: A new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Yu JC, Shi YG, Zhou L, Ye XY, Zhu DM, Zhang YY. Study of pharmacokinetics/pharmacodynamics of levofloxacin. Zhonghua Yi Xue Za Zhi. 2005;85:1926–1932. (In Chinese) [PubMed] [Google Scholar]
- 35.Wu XJ, Zhang J, Guo BN, Zhang YY, Yu JC, Cao GY, Chen YC, Zhu DM, Ye XY, Wu JF, et al. Pharmacokinetics and pharmacodynamics of multiple-dose intravenous nemonoxacin in healthy Chinese volunteers. Antimicrob Agents Chemother. 2015;59:1446–1454. doi: 10.1128/AAC.04039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee LJ, Sha X, Gotfried MH, Howard JR, Dix RK, Fish DN. Penetration of levofloxacin into lung tissue after oral administration to subjects undergoing lung biopsy or lobectomy. Pharmacotherapy. 1998;18:35–41. [PubMed] [Google Scholar]
- 37.Wang CQ, Wang W, Jin MH, Huang Q, Guan Q. Pulmonary resections in surgical treatment of lung tuberculosis. J Clin Pulmonary Med. 2009;14:906–908. doi: 10.1159/000485382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, Mao YB, Liu CT. Risk factors for surgical site infections in patients undergoing pneumonectomy. Chin J Nosocomiol. 2013;23(5198-5199-5202) [Google Scholar]
- 39.Pea F. Intracellular pharmacokinetics of antibacterials and their clinical implications. Clin Pharmacokinet. 2018;57:177–189. doi: 10.1007/s40262-017-0572-y. [DOI] [PubMed] [Google Scholar]
- 40.Moriarty TF, McElnay JC, Elborn JS, Tunney MM. Sputum antibiotic concentrations: Implications for treatment of cystic fibrosis lung infection. Pediatr Pulmonol. 2007;42:1008–1017. doi: 10.1002/ppul.20671. [DOI] [PubMed] [Google Scholar]
- 41.Kelly MM, Efthimiadis A, Hargreave FE. Induced sputum: Selection method. Methods Mol Med. 2001;56:77–91. doi: 10.1385/1-59259-151-5:77. [DOI] [PubMed] [Google Scholar]
- 42.Cazzola M, Blasi F, Terzano C, Matera MG, Marsico SA. Delivering antibacterials to the lungs: Considerations for optimizing outcomes. Am J Respir Med. 2002;1:261–272. doi: 10.1007/BF03256617. [DOI] [PubMed] [Google Scholar]
- 43.Yu JC, Zhang J, Cao GY. Single-dose and multiple-dose pharmacokinetics of levofloxacin in Chinese healthy subjects. J Third Mil Med Univ. 2013;35:2516–2520. [Google Scholar]
- 44.Simon N, Sampol E, Albanese J, Martin C, Arvis P, Urien S, Lacarelle B, Bruguerolle B. Population pharmacokinetics of moxifloxacin in plasma and bronchial secretions in patients with severe bronchopneumonia. Clin Pharmacol Ther. 2003;74:353–363. doi: 10.1016/S0009-9236(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 45.Capitano B, Mattoes HM, Shore E, O'Brien A, Braman S, Sutherland C, Nicolau DP. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest. 2004;125:965–973. doi: 10.1378/chest.125.3.965. [DOI] [PubMed] [Google Scholar]
- 46.Hutschala D, Kinstner C, Skhirtladze K, Mayer-Helm BX, Zeitlinger M, Wisser W, Müller M, Tschernko E. The impact of perioperative atelectasis on antibiotic penetration into lung tissue: An in vivo microdialysis study. Intensive Care Med. 2008;34:1827–1834. doi: 10.1007/s00134-008-1122-8. [DOI] [PubMed] [Google Scholar]
- 47.Van't Boveneind-Vrubleuskaya N, Seuruk T, van Hateren K, van der Laan T, Kosterink JGW, van der Werf TS, van Soolingen D, van den Hof S, Skrahina A, Alffenaar JC. Pharmacokinetics of levofloxacin in multidrug- and extensively drug-resistant tuberculosis patients. Antimicrob Agents Chemother. 2017;61(pii: e00343-17) doi: 10.1128/AAC.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Shaer MH, Alghamdi WA, Alsultan A, An G, Ahmed S, Alkabab Y, Banu S, Barbakadze K, Houpt E, Kipiani M, et al. Fluoroquinolones in drug-resistant tuberculosis: Culture conversion and pharmacokinetic/pharmacodynamic target attainment to guide dose selection. Antimicrob Agents Chemother. 2019;63(pii: e00279-19) doi: 10.1128/AAC.00279-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. U.S. Department of Health and Human Services. FDA guidance for industry: Population Pharmacokinetics, July 2019. https://www.fda.gov/media/128793/download. Accessed October 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.