Abstract
Objectives
We aimed to evaluate the antifertility activity and vaginal irritation effects of tideglusib in vivo using rabbit models and to evaluate the cytotoxical effects of tideglusib to sperm, vaginal cells and vaginal bacteria (L. acidophilus) in vitro.
Study design
We treated female rabbits with vaginal tideglusib 1 mM, nonoxynol-9 (N-9) or vehicle control (Poloxamer 407). In experiment 1, we sacrificed females (n = 6 each) after 10 days of daily administration and assessed vaginal histological changes using Eckstein irritation score. In experiment 2, females (n = 9 each) received estradiol benzoate to induce ovulation 24 h prior to vaginal treatment followed by introduction of a fertile male. These females underwent necropsy at the 21st day to assess pregnancy status. In experiment 3, we used an HTM-TOX IVOS sperm motility analyzer and scanning electron microscopy (SEM) to evaluate the effect of tideglusib on human sperm samples. In experiment 4, we evaluated the effect of tideglusib on lactobacillus and vaginal cell growth in vitro.
Results
The total irritation score of tideglusib vs. N-9 was 3.4 ± 2.07 vs. 7.8 ± 3.82, p <.05. The pregnancy rate of tideglusib, N-9 and control group was 11.1%, 0% and 88.9%, respectively. Tideglusib exhibited a dose-dependent spermostatic/spermicidal activity, and the minimum effective concentrations of tideglusib and N-9 were 8.724 ± 3.047 μM and 219.75 ± 41.78 μM, respectively. SEM and transmission electron microscopy revealed acrosomal membrane impairments caused by tideglusib. Tideglusib was much less toxic to vaginal cells and L. acidophilus than N-9 in vitro.
Conclusions
Evaluation using rabbit models indicated that tideglusib is a prospective spermicidal contraceptive with low vaginal irritation effects.
Implications
Tideglusib or tideglusib analogues may be a contraceptive with perspective to replace N-9. It is possible for a spermicide to balance spermicidal activity and vaginal/cervical irritation effects very well.
Keywords: Tideglusib, Sperm, Contraceptive, Nonoxynol-9, Spermicide
1. Introduction
Safe contraception is an unmet need, resulting in health burdens. Almost 10% of fertile women in China have unintended pregnancy [1], and 51% of pregnancies annually in the United States [2] are unintended. In China, between 2005 and 2012, heterosexual transmission of human immunodeficiency virus (HIV) among infected women surged from 25.8% to 87.4%, with the majority of reported cases in the 20–39 age groups [3]. A woman's choice of contraception can affect her risks for both unintended pregnancy and sexually transmitted infections (STIs), especially HIV transmission during sexual contact with her infected partners. Unfortunately, most contraceptives do not protect women from HIV infection or other sexually transmitted diseases (STDs).
Spermicides, designed to prevent fertilization by killing or inactivating sperm through chemical toxic (most commonly nonoxynol-9, N-9) to sperm and a formulation, have been used alone or with barrier products [4], [5], for example, condoms to prevent HIV infection [6]. However, the high incidence of unwanted pregnancy (Pearl index for ideal use: 18) [7] associated with spermicidal method is one of its disadvantages. Although N-9 had protective effects against STDs [8] and HIV [9], recent clinical trials showed that spermicide actually increased urinary tract infections among spermicide-exposed women [10], and frequent use of N-9 even increased the risks of rectal transmission of HIV and other STIs through genital lesions (mucosal inflammation) [11], [12]. Thus, WHO considered spermicide a category 4 method (contraindicated) for women at high risk of HIV infection.
However, spermicides have important advantages. At present, young, urban women in China (north or southwest) prefer user-controlled methods [13]. Spermicides as readily available, over-the counter, woman-controlled contraceptive methods, highly acceptable to most women [14], may meet the need. Because spermicide use is not influenced by acceptability [15], spermicide may be considered as a route of administration for contraception for women, especially suitable for perimenopausal [16] and breastfeeding women [17].
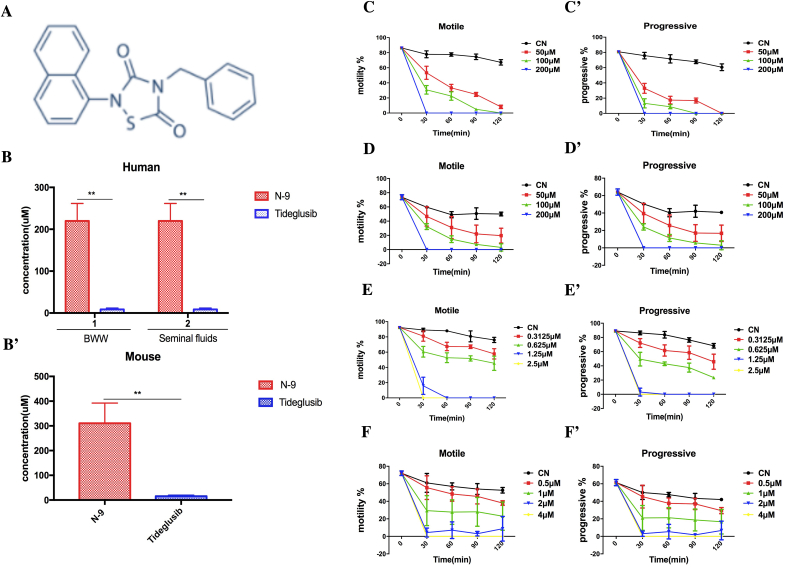
The major challenges are to improve protective effects of spermicides and maintain the balance between contraceptive efficacy and disturbance to the vaginal environments [18]. Therefore, only spermicides with higher efficacy and fewer adverse effects than N-9 would be novel alternatives. We identified tideglusib (Fig. 2A, Cas 865854-05-3), a selective and irreversible non-ATP competitive inhibitor of glycogen synthase kinase 3β (GSK-3β) [19], as a promising compound by screening > 7000 compounds. The objectives are to evaluate the antifertility activity and vaginal irritation effects of tideglusib in vivo and the cytotoxical effects to sperm, vaginal cells and vaginal bacteria (L. acidophilus) in vitro.
Fig. 2.
Novel spermostatic/spermicidal activity of tideglusib in vitro.
Spermostatic/spermicidal effects of (C, C′, D and D′) N-9 and (E, E′, F, and F′) tideglusib on human and mouse sperm in vitro. (A) Structure of tideglusib. (B) Measured MECs of N-9 and tideglusib in human and (B′) mouse spermatozoa within 20 s were compared. (B) Effect of human seminal fluids on spermostatic/spermicidal activity of tideglusib and N-9. **p <.01. (C, E) Time- and dose-dependent effects of compounds on human sperm motility, (C′, E′) progressive motility, (D, F) mouse sperm motility and (D′, F′) progressive motility. Motility (%): percentage of motile sperm (A + B + C%); progressive motility (%): percentage of forward spermatozoa (A + B%); CN: blank control. The data were presented as mean ± SD.
2. Materials and methods
2.1. Reagents and cell lines
The bioactive compound library screened and tideglusib was purchased from Selleck Co., Ltd., (Houston, TX, USA). N-9 was obtained from Xi'an Ruilin Biotechnology Co., Ltd.2, (Shanxi, China). A Live/Dead sperm viability kit (L-7011) was purchased from Invitrogen, Life Technologies Ltd., (Paisley, UK). The cell counting kit-8 (CCK-8) was from Dojindo, Co. Ltd., Japan. Defined keratinocyte serum-free medium (GibcoTM10744–019) was from Thermo Fisher Scientific Inc., Waltham, MA, USA.
The human vaginal epithelial Vk2/E6E7 cell line (ATCC CRL-2616) was a gift from Professor Wenliang Zhou at Sun Yat-sen University, Guangzhou, China. The cells were cultured in keratinocyte serum-free medium supplemented with epidermal growth factor (0.01 ng/mL) and penicillin (100 U/mL) in the incubator at 37 °C with 5% CO2.
2.2. Tideglusib-loaded vaginal gel
Add poloxamer 407 to distilled water with uniform stirring and store at 4°C until the polymer dissolved completely to prepare 20% poloxamer 407 (w/v) gels. Mix the prepared gel and tideglusib stock solution in DMSO with constant stirring to obtain a colorless transparent gel at 1 mM (approx. 100 × minimum effective concentration [MEC]), as the highest solubility of tideglusib in the present gel was approximately 100 × MEC (1 mM).
2.3. Antifertility and vaginal irritation effects of tideglusib gels
The animal use and welfare committee of SIPPR approved all experiments. We used breeding rabbits with proven fertility and divided the females into three groups receiving treatments as follows: vehicle only (Poloxamer 407, 20%), tideglusib gel at 1 mM (approx. 100 × MEC) and N-9 gel at 40 mg/mL. We inserted a catheter-equipped 5-mL disposable syringe 8–9 cm into the orificium of rabbits to administer 2.5-mL gel intravaginally.
2.3.1. Experiment 1
After daily administration of the vaginal gels for 10 days, we euthanized the female rabbits (n = 6/group) and removed the vaginas and uteri. We longitudinally incised each rabbit vagina, observed the morphological changes in the tissue, and then fixed the tissues in PBS containing 10% formaldehyde at 25°C for 4 h, dehydrated in graded ethanol solutions and embedded in paraffin. We stained paraffin sections with hematoxylin and eosin (H&E) followed by observation under a light microscope (Nikon 80i, Japan) and assessments of the Eckstein irritation score [20].
2.3.2. Experiment 2
We hypodermically injected 0.1 mg/kg estradiol benzoate to induce ovulation (females, n = 9/group) 24 h prior to the vaginal treatment. Ten minutes after the gel administration, we allowed females to monogamously mate with fertile males. On day 21, we euthanized the females and conducted exploratory laparotomies to observe ovulation points, implantation sites and fetus and then calculated pregnancy rates.
2.4. Spermostatic/spermicidal effects of tideglusib on human sperm in vitro
The Institutional Ethics Committee of Shanghai Institute of Planned Parenthood Research (SIPPR, Shanghai, China) approved this study, and all participants provided the informed consent. According to established guidelines (WHO manual 5th edition, 2010), we analyzed human semen [21] using a HTM-TOX IVOS sperm motility analyzer (Hamilton Thorne Research, Beverly, MA, USA) to select samples with > 15 × 106 mL sperm counts and > 70% motility with normal sperm morphology for subsequent assays. We collected the “swim-up” spermatozoa (WHO manual 5th edition, 5.4.2 procedure) [21] and diluted in pre-equilibrated Biggers–Whitten–Whittingham (BWW) medium to 10–20 × 106 cells/mL. To prepare mouse sperm, we euthanized male C57BL/6 mice and squeezed the cauda of the epididymis with tweezers to release the sperm [22]. We cultured the sperm in 1 mL prewarmed BWW medium (37 °C) for 15 min and diluted the removed suspension of uppermost sperm to 4–10 × 106 cells/mL.
2.4.1. Experiment 3
In this study, we defined the MEC of a compound as the lowest concentration that irreversibly immobilizes sperms within 20 s. Using a modified Sander–Cramer assay, we mixed the motile spermatozoa (5-10 × 106/mL) with BWW medium (v/v,1/1) containing diluted tideglusib for 20 s to determine the MEC. Then, we washed the immotile sperms twice and resuspended in fresh BWW medium to observe the revival at 37°C for 60 min.
To prepare SEM samples, we spread sperm on slides, fixed with 2.5% glutaraldehyde for 2 h, washed in PBS, fixed with 1% osmium tetroxide and then dehydrated using a graded acetone series. After critical point drying, we coated the samples with gold and observed under a field emission SEM (FEI Quanta FEG 250, Thermo Fisher Scientific Inc.). To prepare transmission electron microscopy (TEM) samples, we fixed human sperm precipitates, then washed, dehydrated and embedded them in EP812 for 48 h. We stained the sliced ultrathin sections and examined by TEM (Philips CM 120, Netherlands). We also used hypo-osmotic swelling (HOS) test (WHO Manual 2010, 2.6.3 procedure) [21] and flow cytometry (BD LSR II, BD Biosciences, San Jose, CA, USA) with a Live/Dead sperm viability kit to evaluate sperm viability and membrane integrity.
2.5. Cytotoxicity of tideglusib in vitro
2.5.1. Experiment 4
We cultured L. acidophilus (ATCC 4356) in De Man, Rogosa and Sharpe agar (MRS) medium (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 36 h and diluted to 1.0 × 106 CFU/mL and then incubated the diluted suspensions with N-9 or tideglusib in MRS medium. We then diluted the mixture and spread on the MRS agar plates before incubation under anaerobic condition at 37°C for 48 h.
We co-incubated Vk2/E6E7 cells (5 × 103 cells/well) with nurture medium containing compounds for 24 h and quantified the cell proliferation using a CCK-8 kit and a microplate reader (BioTek Instruments Inc., VT, USA) [23]. Then, we calculated according to the formula: (%)=[(AC − Ab)−(AS − Ab)]/(AC − Ab)× 100, where AS, AC and Ab are the average OD of the experimental, control and blank wells, respectively.
2.6. Statistical analysis
Statistical analysis was performed with the Graphpad prism software using one-way ANOVA (HOS, cytotoxic effects) and nonpaired Student's t tests (MEC) as appropriate. The analysis of ovulation points, histopathologic scores of rabbit vagina (one-way ANOVA analysis) and implantation sites, and survived fetus (Kruskal–Wallis test) was performed with SPSS software. Quantitative data were expressed as mean ± SD. p <.05 was considered statistically significant.
3. Results
3.1. Pathological changes in rabbit vagina
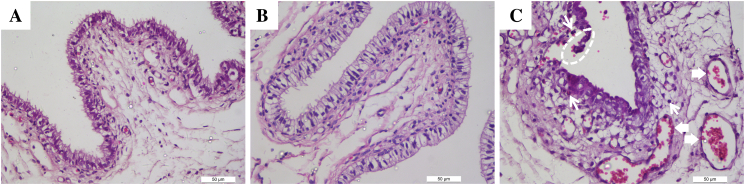
Consecutive intravaginal exposure of rabbits to tideglusib for 10 days did not result in significant microscopic abnormalities of vagina tissues. The light microscopy examination revealed intact vaginal epithelium, lack of leukocyte influx and slight vascular congestion in the representative vaginal sections of rabbits receiving gel alone (Fig. 1A) or gel with tideglusib (Fig. 1B). However, ulceration of the epithelial cell layers, vascular congestion, submucosal edema and increased leukocyte infiltration (Fig. 1C) were prominent in N-9 group (a positive control). Accordingly, the total pathological score of tideglusib group (3.4 ± 2.07) was lower than N-9 (7.8 ± 3.82) (p <.05) but not significantly different from negative control (1.4 ± 0.82), as shown in Table 1.
Fig. 1.
Light microscopic changes in rabbit vagina.
H&E staining of histological sections of rabbit vaginal mucosa after being consecutively exposed to gels for 10 days. Representative light micrographs of sections of rabbit vaginal tissue (n = 6) after a 10-day intravaginal administration of gel (A) alone, (B) gel with tideglusib or (C) gel with N-9. Note the intactness of vaginal epithelium and only a small number of vascular congestion in control and tideglusib-treated rabbit vaginal mucosa. The dashed circles indicate the ulceration of the epithelial cell layers. The thick arrows indicate vascular congestion, and thin arrows indicate increased leukocyte infiltration in the N-9 gel group (original magnification × 400). The detailed pathological scores were shown in Table 1.
Table 1.
Histopathologic scores of rabbit vagina
| Groups | Vaginal mucosal involvement score |
Total scores | |||
|---|---|---|---|---|---|
| Epithelial injury | Leukocyte infiltration | Vessel congestion | Interstitial edema | ||
| Control | 0 ± 0 | 0.8 ± 0.27 | 0.1 ± 0.22 | 0.5 ± 0.5 | 1.4 ± 0.82# |
| Tideglusib group | 1 ± 1.06 | 1.1 ± 0.65 | 1 ± 0.61 | 0.3 ± 0.27 | 3.4 ± 2.07# |
| N-9 group | 2 ± 1.58 | 1.6 ± 1.56 | 1.8 ± 0.84 | 2.4 ± 1.34 | 7.8 ± 3.82⁎ |
0 = no obvious change, 1 (minimal), 2 (mild), 3 (moderate) to 4 (marked irritation).
The data are presented as mean ± SD of three independent experiments; n = 6 rabbits in each group.
p <.05 vs. blank control.
p <.05 vs. N-9 group.
3.2. Contraceptive efficacy of vaginal tideglusib in rabbits
No significant difference regarding ovulation rates was observed among the three groups except the implantation rates and pregnancy rates (Table 2). In contrast, both active treatments significantly reduced implantation sites (tideglusib 0.3 ± 1, N-9 0) compared to control (3.1 ± 2.4) and prevented pregnancy [tideglusib 1/9 (11.1%), N-9 0/9 (0%), control females 8/9 (88.9%)] (p <.01). Although intravaginal administration of tideglusib in rabbits effectively reduced pregnancy rate, N-9 appears better in contraceptive efficacy in their present formulations.
Table 2.
Comparison of the contraceptive activity of tideglusib gel and N-9 gel in rabbits (n = 9)
| Groups | Number of animals | Ovulation points | Implantation sites | Pregnancy | Survived fetus | Pregnancy rates (%) |
|---|---|---|---|---|---|---|
| Control | 9 | 5.3 ± 2.5 | 3.1 ± 2.4 | 8 | 28 | 88.9 |
| Tideglusib group | 9 | 4.1 ± 1.9 | 0.3 ± 1.0⁎⁎ | 1 | 3 | 11.1 |
| N-9 group | 9 | 4.8 ± 2.3 | 0.0 ± 0.0⁎⁎ | 0 | 0 | 0 |
The data shown are the mean from three independent experiments and are presented as mean ± SD of three determinations.
p <.01.
3.3. Novel spermostatic/spermicidal effects of tideglusib on human sperm
Both tideglusib and N-9 exhibited immobilizing activity in a dose-dependent manner (Fig. 2C–F′). The mean MECs of tideglusib (Fig. 2B–B′) were 8.724 ± 3.047 μM (n = 4, human) and 15.50 ± 4.107 μM (n = 5, mouse), which were lower than those of N-9 at 219.75 ± 41.78 μM (n = 4, human) and 310.80 ± 81.44 μM (n = 5, mouse). Tideglusib was more effective than N-9 in vitro. No revival of human sperm motility was observed in sperm revival tests when tideglusib was washed out.
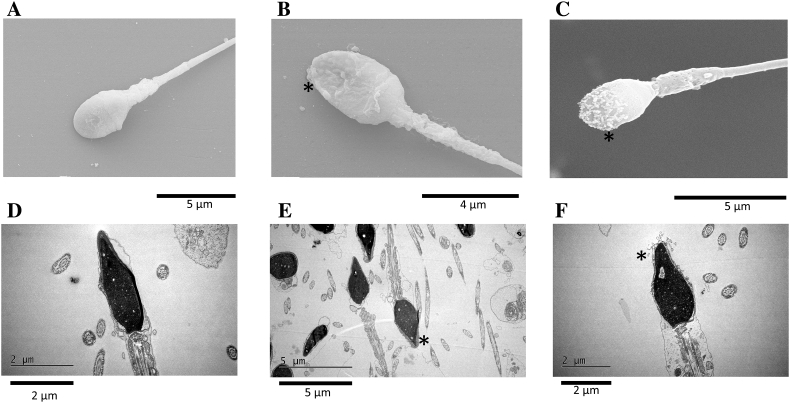
The combination of the SEM and TEM analysis revealed considerable damage to the acrosomal and sperm membrane of middle piece after tideglusib treatments (Fig. 3). Intact sperms (Fig. 3A, D) had a smooth membrane surrounding the oval nucleus, whereas the damaged sperms exhibited dissolution, distortion and detachment of the acrosomal cap membrane (Fig. 3E, F) and blebbing of the acrosomal membrane (Fig. 3B, C) as well. Expansion and separation of the acrosomal cap membrane from the nucleus, vesiculation of the acrosomal membrane and disintegration of the plasma membrane after treatments were also revealed by TEM (Fig. 3E, F).
Fig. 3.
Ultrastructural impairments of human sperm.
(A–C) Typical damages to human sperm membrane caused by tideglusib were observed using SEM and (D–F) TEM. (A, D) Intact human spermatozoa and (B, C, E, F) membrane damage after treatment with 10 μM tideglusib for 20 s. * indicates typical membrane impairment.
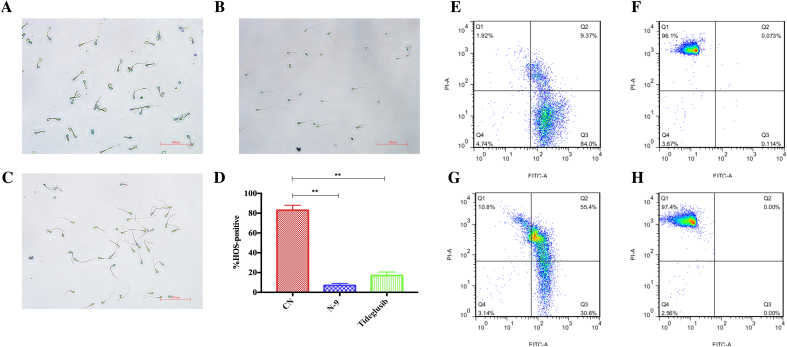
N-9 and tideglusib treatments for 20 s at their MECs decreased HOS responsiveness of human sperm from 83% to 7% and 17%, respectively (Fig. 4A–D), which suggested the overall loss of sperm membrane integrity after tideglusib treatment (**p <.01). Treatments with different concentration of tideglusib resulted in different sperm mortality (1.25 μM 60 min 10.8%, 10 μM 20 s 97.4%; Fig. 4G, H). Because dying moribund sperms were double-stained [24], the subpopulation between the quadrant regions of SYBR-14 +/propidium iodide (PI)− and SYBR-14 −/PI + featured dying sperms after treatment.
Fig. 4.
Impairment of sperm membrane integrity by tideglusib.
(A–D) Evaluation of sperm membrane integrity using HOS assays and (E–H) SYBR-14/PI staining. HOS assays of (A) intact (blank control), (B) N-9 treatment (at 1 × MEC, approx. 260 μM for 20 s) and (C) tideglusib treatment (10 μM for 20 s) on human sperm. (D) Percentage of HOS-positive sperms after treatment with N-9 or tideglusib at MECs for 20 s or blank control. Data are expressed as percentage (%) of total sperm counts. CN: blank control; **p <.01. (E–H) SYBR-14/PI staining and flow cytometry analysis. (E) Blank control. (F) N-9 treatment (260 μM) for 20 s. (G) Tideglusib treatment (1.25 μM) for 60 min. (H) Tideglusib treatment (10 μM) for 20 s.
3.4. Cytotoxicity of tideglusib to L. acidophilus and vaginal cells
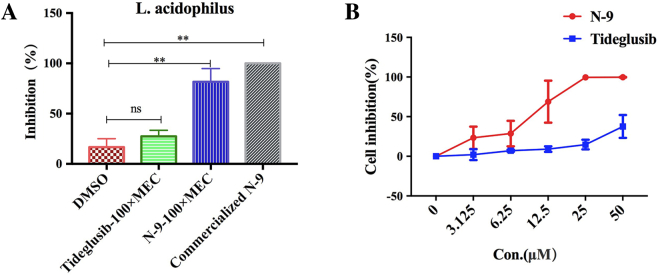
The inhibition (%) on L. acidophilus by DMSO, 100 × MEC of tideglusib, 100 × MEC of N-9 and commercialized N-9 was 17%, 27%, 82% and 100%, respectively. Tideglusib was less toxic than N-9 to L. acidophilus (p <.01) (Fig. 5A.) As assessed in vivo, tideglusib was much less toxic to vaginal cells than N-9 (Fig. 5B) in vitro.
Fig. 5.
Cytotoxicity of tideglusib to vaginal cells and L. acidophilus.
Cytotoxic effects of tideglusib and N-9 on Lactobacillus acidophilus (A, n = 3) and vaginal cells (B, n = 3). (A) The inhibition (%) on L. acidophilus colonies after treatment with DMSO (17%), 100 × MEC of tideglusib (27%), 100 × MEC of N-9 (82%), commercialized N-9 (100%). (B) Inhibition (%) of the compounds on VK2/E6E7 cell proliferation after incubation for 24 h. **p <.01, ns: p >.05.
4. Discussion
The in vivo assays in rabbits suggested that tideglusib had antifertility activity through decreasing implantation sites, survived fetus and pregnancy rate (Table 2). However, the tideglusib gels at 100 × MEC did not completely protect the females from pregnancy. There were limitations in the present research that may account for the incomplete contraceptive efficacy. In the present study, the solubility of tideglusib was limited, and 100 × MEC was the highest dose at which tideglusib could be completed dissolved in the basal formulation. A proper preparation of tideglusib may help to make preparations of higher doses to achieve complete contraceptive efficacy.
As there is a correlation between humans and rabbits in the irritation potential of vaginal gels [25], the vaginal irritation tests and histopathological scores (Table 1) support the potential use of tideglusib in humans in future. The vaginal cytotoxicity tests using Vk2/E6E7 cells (Fig. 5B) also support the prospects of tideglusib as a contraceptive agent compared with N-9. However, there are still limitations in the present assays. The present irritation tests and histopathological scores could not reflect the irritations of higher doses that may acquire complete contraception.
In vitro tests showed that tideglusib was a better spermicide than N-9 compared their MECs. The spermicidal potency of tideglusib was higher or comparable to that of many reported nondetergent spermicides such as DSE-37 [26], [27], c-butyrolactone derivatives [28] and N, N′-dithiobisphthalimide [29]. Compared with N-9, tideglusib did not significantly affect the growth of L. acidophilus even at 100 × MEC (Fig. 5A). These data suggested that tideglusib might be an alternative to N-9 considering its less disturbing effects to vaginal environments.
The highly “selective” toxicity of tideglusib to sperms would facilitate the discovery of novel contraceptive agents and male contraceptive targets. The novel structure of heterocyclic thiadiazolidinones provides a template to produce and test active derivatives of tideglusib, with the aim of identifying novel and druggable candidates. Identification and characterization of molecular “targets” of tideglusib on the sperm membrane would also facilitate the discovery of male contraceptive targets.
To the best of our knowledge, the rapid in vitro spermostatic activity of tideglusib identified in this study is a novel finding. Tideglusib is the first non-ATP competitive, irreversible inhibitor of GSK-3β with a novel structure derived from a small heterocyclic thiadiazolidinone [30]. Our data suggest that the rapid spermicidal activity of tideglusib on sperms may be derived from its detergent-like activity (partition coefficient [ClogP 5.21]) rather than its GSK-3β inhibitory activity, although the GSK-3β inhibitory activity is essential for most all of its known applications [31]. We observed that ATP-competitive GSK-3β inhibitors did not have spermostatic effects (data not shown). According to previous hypotheses, inhibition of Wnt/GSK-3β signaling would increase sperm motility [32], [33], which is contrary to the observed spermostatic activity of tideglusib. GSK-3α, rather than GSK-3β, is the major GSK-3 subtype in mouse sperm [34], and the targeted disruption of GSK-3α in males affects sperm motility and causes male infertility [33]. Therefore, whether tideglusib inhibits sperm motility by inhibiting GSK-3α is worth further investigation.
Tideglusib or its derivatives, as an alternative to N-9, may be prepared in variant contraceptive preparations or products such as condoms for further assessment. The final success of a spermicide with high activity and low irritation may encourage the repeated use by women without the risks of increased susceptibility to HIV infection and other STDs, especially for those in whom hormonal products may be contraindicated.
Acknowledgment
This work was supported by the Science and Technology Commission of Shanghai Municipality (grant number 15431903000), National Natural Science Foundation of China (grant number 81671508), the National Key Research and Development Program of China (grant number 2016YFC1000905) and Science and Technology Climbing Fund of SIPPR (grant numbers PD2017-11 and PD2017-8).
Contributor Information
Weihua Li, Email: iamliweihua@foxmail.com.
Heguo Yu, Email: yuheguo@hotmail.com.
Hua Diao, Email: diaohua@sippr.org.cn.
References
- 1.Mao Y., Tang M., Chen Y. Disease burden of unintended pregnancy in China. Value Health. 2014;17:A751. doi: 10.1016/j.jval.2014.08.201. [DOI] [PubMed] [Google Scholar]
- 2.Committee Opinion No. 654: reproductive life planning to reduce unintended pregnancyObstet Gynecol. 2016;127:e66–e69. doi: 10.1097/AOG.0000000000001314. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X.Y., Huang T., Feng Y.B. Characteristics of the HIV/AIDS epidemic in women aged 15–49 years from 2005 to 2012 in China. Biomed Environ Sci. 2015;28:701–708. doi: 10.3967/bes2015.100. [DOI] [PubMed] [Google Scholar]
- 4.Craig S., Hepburn S. The effectiveness of barrier methods of contraception with and without spermicide. Contraception. 1982;26:347–359. doi: 10.1016/0010-7824(82)90102-0. [DOI] [PubMed] [Google Scholar]
- 5.Faundes A., Elias C., Coggins C. Spermicides and barrier contraception. Curr Opin Obstet Gynecol. 1994;6:552–558. [PubMed] [Google Scholar]
- 6.Merino G., Murrieta S., Sandoval C. HIV infections / preventative medicine: condoms / spermicide agents. Adv Contracept Deliv Syst. 1994;10:387–389. [PubMed] [Google Scholar]
- 7.Wiegratz I., Thaler C.J. Hormonal contraception — what kind, when, and for whom? Dtsch Arztebl Int. 2011;108(28–29):495–505. doi: 10.3238/arztebl.2011.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly J.P., Reynolds R.B., Stagno S. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob Agents Chemother. 1985;27:760–762. doi: 10.1128/aac.27.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malkovsky M., Newell A., Dalgleish A.G. Inactivation of HIV by nonoxynol-9. Lancet. 1988;1:645. doi: 10.1016/s0140-6736(88)91440-7. [DOI] [PubMed] [Google Scholar]
- 10.Fihn S.D., Boyko E.J., Normand E.H. Association between use of spermicide-coated condoms and Escherichia coli urinary tract infection in young women. Am J Epidemiol. 1996;144:512–520. doi: 10.1093/oxfordjournals.aje.a008958. [DOI] [PubMed] [Google Scholar]
- 11.Phillips D.M., Sudol K.M., Taylor C.L. Lubricants containing N-9 may enhance rectal transmission of HIV and other STIs. Contraception. 2004;70:107–110. doi: 10.1016/j.contraception.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson D., Tholandi M., Ramjee G. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomised controlled trials including more than 5000 women. Lancet Infect Dis. 2002;2:613–617. doi: 10.1016/s1473-3099(02)00396-1. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X., Guo C., Pang L. Provider-controlled or user-dependent contraceptive methods: levels and pattern among married women of reproductive age in China, 1988–2006. Eur J Obstet Gynecol Reprod Biol. 2017;211:68–73. doi: 10.1016/j.ejogrb.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Raymond E., Alvarado G., Ledesma L. Acceptability of two spermicides in five countries. Contraception. 1999;60:45–50. doi: 10.1016/s0010-7824(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 15.Raymond E.G., Chen P.L., Condon S. Acceptability of five nonoxynol-9 spermicides. Contraception. 2005;71:438–442. doi: 10.1016/j.contraception.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serfaty D. Contraception during perimenopause: the spermicides option. J Gynecol Obstet Hum Reprod. 2017;46:211–218. doi: 10.1016/j.jogoh.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Serfaty D. Contraception in breastfeeding women: place for spermicides. J Gynecol Obstet Biol Reprod (Paris) 2015;44:18–27. doi: 10.1016/j.jgyn.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Grimes D.A., Lopez L.M., Raymond E.G. Spermicide used alone for contraception. Cochrane Database Syst Rev. 2013;5(12) doi: 10.1002/14651858.CD005218.pub4. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez J.M., Fuertes A., Orozco L. Evidence for irreversible inhibition of glycogen synthase kinase-3beta by tideglusib. J Biol Chem. 2012;287:893–904. doi: 10.1074/jbc.M111.306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein P., Jackson M.C., Millman N. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . 5th ed. World Health Organization [c]; Geneva: 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 22.Zhao L.L., Ru Y.F., Liu M. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan L., Yuan P., Yuan H. miR-542-3p inhibits colorectal cancer cell proliferation, migration and invasion by targeting OTUB1. Am J Cancer Res. 2017;7:159–172. [PMC free article] [PubMed] [Google Scholar]
- 24.Garner D.L., Johnson L.A. Viability assessment of mammalian sperm using Sybr-14 and propidium iodide. Biol Reprod. 1995;53:276–284. doi: 10.1095/biolreprod53.2.276. [DOI] [PubMed] [Google Scholar]
- 25.D'Cruz O.J., Waurzyniak B., Uckun F.M. Mucosal toxicity studies of a gel formulation of native pokeweed antiviral protein. Toxicol Pathol. 2004;32:212–221. doi: 10.1080/01926230490274362. [DOI] [PubMed] [Google Scholar]
- 26.Jain R.K., Maikhuri J.P., Kiran Kumar S.T. Novel disulphide esters of carbothioic acid as potent, non-detergent spermicides with low toxicity to Lactobacillus and HeLa cells in vitro. Hum Reprod. 2007;22:708–716. doi: 10.1093/humrep/del448. [DOI] [PubMed] [Google Scholar]
- 27.Jain R.K., Jain A., Kumar R. Functional attenuation of human sperm by novel, non-surfactant spermicides: precise targeting of membrane physiology without affecting structure. Hum Reprod. 2010;25:1165–1176. doi: 10.1093/humrep/deq036. [DOI] [PubMed] [Google Scholar]
- 28.Pandey R.R., Srivastava A., Pachauri S.D. Design and synthesis of gamma-butyrolactone derivatives as potential spermicidal agents. Bioorg Med Chem Lett. 2014;24:3903–3906. doi: 10.1016/j.bmcl.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 29.Florez M., Diaz E.S., Brito I. N,N'-Dithiobisphthalimide, a disulfide aromatic compound, is a potent spermicide agent in humans. Syst Biol Reprod Med. 2011;57:309–317. doi: 10.3109/19396368.2011.613977. [DOI] [PubMed] [Google Scholar]
- 30.Martinez A., Alonso M., Castro A. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J Med Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 31.Neves V.C., Babb R., Chandrasekaran D. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci Rep. 2017;7:39654. doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch S., Acebron S.P., Herbst J. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell. 2015;163:1225–1236. doi: 10.1016/j.cell.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharjee R., Goswami S., Dudiki T. Targeted disruption of glycogen synthase kinase 3A (GSK3A) in mice affects sperm motility resulting in male infertility. Biol Reprod. 2015;92:65. doi: 10.1095/biolreprod.114.124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid A.T., Anderson A.L., Roman S.D. Glycogen synthase kinase 3 regulates acrosomal exocytosis in mouse spermatozoa via dynamin phosphorylation. FASEB J. 2015;29:2872–2882. doi: 10.1096/fj.14-265553. [DOI] [PubMed] [Google Scholar]