Abstract
Background
Suspected cholesteatoma recurrence is commonly investigated with magnetic resonance imaging (MRI) of the temporal bone. Non-echo planar diffusion-weighted imaging (non-EP DWI) has become the sequence of choice.
Purpose
To assess the agreement between an MRI protocol incorporating both non-EP DWI and contrast-enhanced sequences, and a shortened protocol without contrast-enhanced sequences in the assessment of suspected cholesteatoma recurrence.
Materials and methods
One hundred consecutive MRIs, consisting of T2-weighted, non-EP DWI and pre- and post-contrast T1-weighted sequences, were reviewed by two radiologists at a tertiary referral centre. Agreement between the two protocols was assessment by means of a weighted Cohen kappa coefficient.
Results
We found a near perfect agreement between the two protocols (kappa coefficient with linear weighting 0.98; 95% confidence interval 0.95–1.00). There were two cases in which the two protocols were discordant. In both cases, the lesion measured <3 mm and images were degraded by artefact at the bone–air interface. The shortened protocol without post-contrast sequences yielded a 32% reduction in acquisition time.
Conclusion
When non-EP DWI is available, contrast-enhanced sequences can be omitted in the vast majority of cases without compromising diagnostic accuracy. Contrast-enhanced sequences may provide additional value in equivocal cases with small (<3 mm) lesions or in cases where images are degraded by artefact.
Keywords: Recurrent cholesteatoma, diffusion-weighted imaging, MRI, temporal bone
Introduction
Cholesteatoma is an aggressive temporal bone mass composed of keratinizing stratified squamous epithelium. Congenital cholesteatomas account for a small minority of cases (2%) and can occur anywhere in the temporal bone, including the middle ear, mastoid air cells and petrous apex.1 Acquired cholesteatomas account for the vast majority of cases and occur almost exclusively in the middle ear. Cholesteatoma can recur after a seemingly complete surgical resection and may be complicated by recurrent infection, otorrhea, sensorineural and conductive hearing loss, facial nerve paralysis, vertigo, tinnitus, otalgia, headache or meningitis. The frequency of recurrence ranges from 5 to 15% but can be as high as 61%, particularly following canal wall up techniques.2
Traditionally, suspected recurrence was investigated by second-look surgery, high-resolution computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) of the temporal bone. In the last decade, non-echo planar diffusion-weighted imaging (non-EP DWI) has become the gold standard. However, despite excellent diagnostic accuracy, it still displays occasional false-negative and false-positive findings.3–7
We hypothesized that non-EP DWI could obviate the need for delayed post-contrast sequences. To test this hypothesis, we compared our standard protocol, consisting of T1, T2, non-EP DWI and delayed post-contrast sequences, with a shortened protocol without delayed post-contrast imaging. To the authors’ knowledge, this is the largest series evaluating MRI techniques in the assessment of recurrent cholesteatoma, and is the only study to directly compare two protocols, one with both non-EP DWI and post-contrast sequences and the other with non-EP DWI but without post-contrast imaging.
Materials and methods
Study design
A retrospective study was performed of 100 consecutive MRIs acquired for the investigation of cholesteatoma recurrence. All imaging was performed in a tertiary referral centre over a four-year period. Studies were identified by searching the radiology information system for all MRI temporal bones and reviewing the indication and clinical details. One study was excluded as post-contrast imaging had not been performed. Ethical approval was granted by the chairman of our institution’s Ethics and Medical Research Committee. All patients underwent imaging as per our institution’s standard protocol as detailed below. Images were read in consensus by a fellowship-trained neuroradiologist with 10 years’ experience and a senior radiology resident.
MRI imaging protocol
Imaging was performed on a General Electric Signa HD×1.5T MRI scanner. The initial non-contrast scan protocol was as follows: axial T2 (TR 2920 ms; flip angle 90°; TE 120 ms; slice thickness 1.7 mm; NEX 4; ETL 16; FOV 22×22 cm2; matrix 288×192), axial T1 (TR 400 ms; TE 10 ms; flip angle 73°; slice thickness 1.7 mm; NEX 4; FOV 22×22 cm2; matrix 256×192), coronal T2 (TR 2200 ms; flip angle 90°; TE 120 ms; slice thickness 1.7 mm; NEX 4; ETL 17; FOV 18×18 cm2; matrix 288×192), coronal T1 (TR 400 ms; TE 10 ms; flip angle 73°; slice thickness 1.7 mm; NEX 4; FOV 18×18 cm2; matrix 256×192), axial T2 DWI (TR 4000 ms; TE 85 ms; flip angle 90°; slice thickness 3 mm; NEX 2; ETL 16; FOV 20×20 cm2; matrix 128×128). This was then followed by delayed post-contrast imaging performed 45–60 min after intravenous administration of a gadolinium-based contrast agent (GBCA): coronal T1 (TR 400 ms; TE 10 ms; flip angle 73°; slice thickness 1.7 mm; NEX 4; FOV 18×18 cm2; matrix 288×192) and axial T1 (TR 400 ms; TE 10 ms; flip angle 73°; slice thickness 1.7 mm; NEX 4; FOV 18×18 cm2; matrix 256×192).
Imaging assessment
We defined two groups of sequences that we called the ‘short protocol’ and the ‘long protocol’. The short protocol was defined as all sequences except for the post-contrast images. The long protocol (our standard imaging protocol) was defined as the short protocol plus the delayed post-contrast images. The short and long protocols were reviewed independently with a minimum time interval of four weeks. Assessment of the short protocol was performed first. In order to minimize bias, the studies were presented in a random order and the readers were blinded to the patients’ identity and clinical details. The study was considered positive for cholesteatoma recurrence if a lesion was moderately hyperintense relative to grey matter on T2-weighted sequences and markedly hyperintense on diffusion-weighted images with a b value of 1000 s/mm2.7,8 After a minimum interval of four weeks, the long protocol was reviewed. The studies were again presented in a random order and the readers blinded to the patients’ identity and clinical details. The above criteria were used to diagnose cholesteatoma recurrence with the added criterion of lack of post-contrast enhancement in the lesion. If the DWI and the post-contrast imaging were discordant (i.e. if a lesion demonstrated diffusion restriction but also demonstrated delayed post-contrast enhancement), the study was recorded as equivocal.
Statistical analysis
The primary endpoint was agreement between the short and long protocols as assessed by a kappa coefficient with linear weighting and presented as point and 95% confidence interval (CI) estimates. This coefficient was interpreted as follows: 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.80, almost perfect agreement.9 Sensitivity, specificity, positive and negative predictive values and accuracy for the short protocol using the long protocol as the gold standard were also calculated. Statistical analysis was performed using Stata Statistical Software: Release 12 (StataCorp LP, College Station, TX, USA).
Results
The mean age in our population was 43.7 years (range 14–76 years). There were 49 females and 51 males (p = 0.842): 57 cases (57%) were considered positive for cholesteatoma recurrence on both the long and short protocols (Table 1); 26 were on the right side and 31 on the left (p = 0.51). The foci of recurrence had a mean size of 7 mm (range 2–27 mm). There was no difference in age between the patients with and without a recurrence, with mean ages of 43.5 years and 43.9 years, respectively (p = 0.899). The mean acquisition time was 25 min for the short protocol and 37 min for the long protocol.
Table 1.
Number of MRI studies considered positive, equivocal and negative using the long and short protocols.
|
Long protocol |
||||
|---|---|---|---|---|
| Short protocol | Positive | Equivocal | Negative | |
| Positive | 57 | 2 | 0 | 59 |
| Equivocal | 0 | 0 | 0 | 0 |
| Negative | 0 | 0 | 41 | 41 |
| 57 | 2 | 41 | 100 | |
Assessment of this study’s primary endpoint, agreement between the short and long protocols, reveals a kappa coefficient with linear weighting of 0.98 (CI: 0.95–1.00), indicating a near perfect agreement. In 98/100 cases, the short and long protocols agreed on lesion characterization. There were two cases in which the short and long protocols were discordant. In both of these cases, the lesion was mildly hyperintense on the DWI sequences and was deemed to represent a positive cholesteatoma recurrence on the short protocol. However, the lesions also demonstrated equivocal delayed post-contrast enhancement and so were considered equivocal on the long protocol. In both cases the lesions measured <3 mm and were difficult to accurately interpret on both the short and long protocols due to their small size and surrounding susceptibility artifact at the bone-air interface.
If the long protocol is considered our gold standard, the sensitivity and specificity of the short protocol would be 100% (95% CI: 93.7–100) and 95.3% (95% CI: 84.2–99.4), respectively. The accuracy of the short protocol compared to the long protocol would be 98% (95% CI: 94–100), the positive predictive value 96.6% (95% CI: 88.3–99.6) and the negative predictive value 100% (95% CI: 91.4–100).
Discussion
The early and accurate detection of cholesteatoma is critical due to the aggressive nature of the lesion and its myriad potential complications. Historically, the diagnosis was made on clinical, otoscopic and auditory findings. In cases of recurrent disease, second-look surgery was considered the gold standard. Nowadays, modern imaging techniques have reduced the need for invasive second-look surgery with its associated risks and operative costs.
High-resolution CT provides exquisite anatomic detail and accurately depicts osseous erosion but is unable to differentiate between the various types of soft tissue that may be present in the post-operative ear.10 MRI is more expensive and time-consuming but has the added advantage of superior soft tissue characterization, allowing the reader to distinguish between fluid, granulation tissue and cholesteatoma. (Figures 1 to 3).
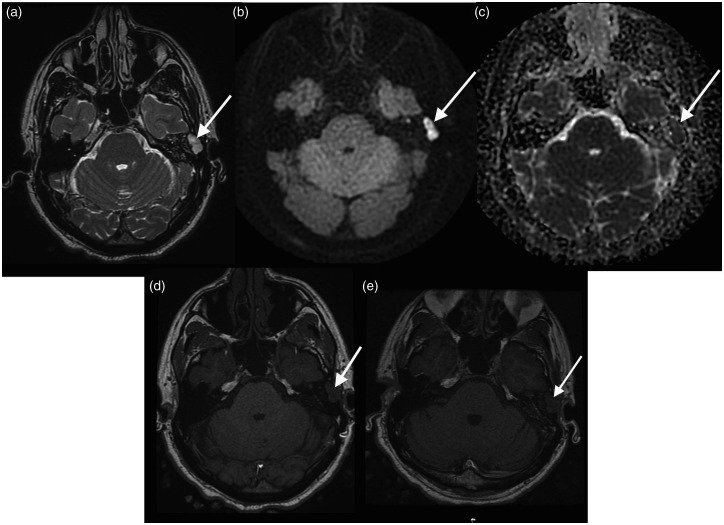
Figure 1.
Cholesteatoma recurrence. (a) T2 weighted image demonstrating abnormal high T2 signal in the region of the left mastoid cavity (arrow); (b) PROPELLER DWI showing high signal in the corresponding region; (c) apparent diffusion coefficient (ADC) map showing corresponding low signal; (d) T1 pre-contrast showing no intrinsic T1 hyperintensity; and (e) T1 delayed post-contrast image showing no enhancement in this lesion consistent with cholesteatoma recurrence.
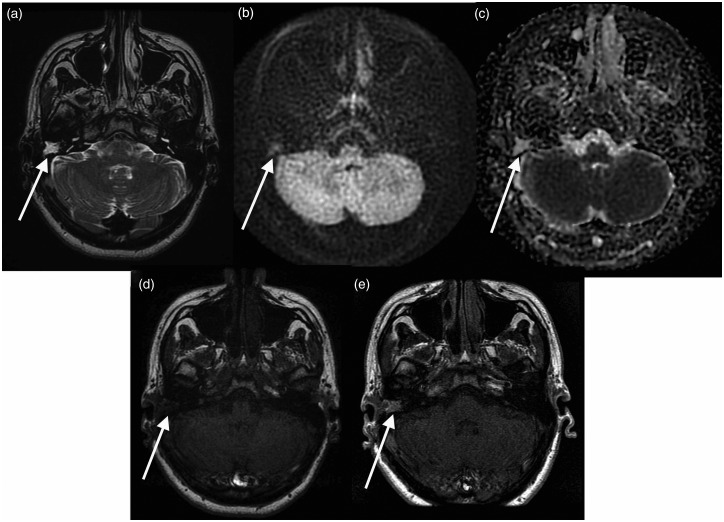
Figure 2.
Granulation tissue. (a) T2 weighted image demonstrating abnormal high T2 signal in the region of the right mastoid cavity (arrow); (b) PROPELLER DWI showing signal isointense to brain in the corresponding region; (c) apparent diffusion coefficient (ADC) map showing corresponding intermediate signal; (d) T1 pre-contrast; and (e) T1 delayed post-contrast showing enhancement in this lesion consistent with granulation tissue.
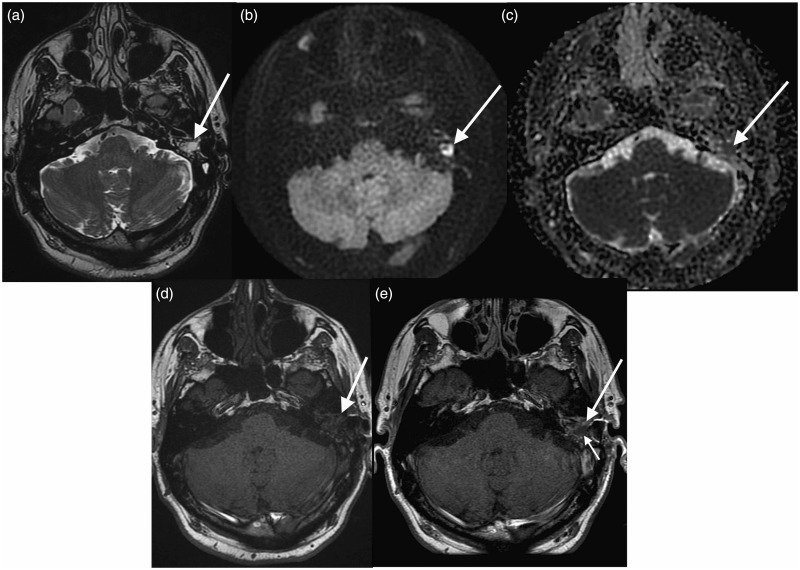
Figure 3.
Combined cholesteatoma and granulation tissue. (a) T2 weighted image demonstrating abnormal high T2 signal in the region of the left mastoid cavity (arrow); (b) PROPELLER DWI showing high signal in the corresponding region; (c) apparent diffusion coefficient (ADC) map showing corresponding low signal; (d) T1 pre-contrast; and (e) T1 delayed post-contrast image showing no enhancement in the lesion demonstrating diffusion restriction (short arrow) but enhancement of the surrounding soft tissue (long arrow) consistent with cholesteatoma recurrence with surrounding granulation tissue.
Diffusion-weighted imaging has become more popular due to its higher accuracy and shorter acquisition times relative to contrast-enhanced MRI.11–14 Traditional EP DWI, first described in the assessment of cholesteatoma in a case report in 2002, suffers from considerable susceptibility artefact at the bone–air interface.15 Non-EP DWI is a newer technique which is less prone to such artefact and has become the sequence of choice over the last 10 years. Several studies have shown that non-EP DWI is more accurate than EP DWI and contrast-enhanced MRI.4,8,14,16–18 Despite this, non-EP DWI has its pitfalls and occasionally gives rise to false-positive and false-negative results.
Our study demonstrates a near perfect agreement between two protocols: one consisting of T2, non-EP DWI and pre- and post-contrast T1-weighted sequences, and the other consisting of the same sequences but without the post-contrast T1 weighted sequences. These results indicate that the post-contrast sequences rarely alter the interpretation of the study and provide little additional value in the vast majority of cases. Indeed, of 100 patients included in the study, the two protocols were discordant in two cases. In both cases, the lesions were 2–3 mm in size and were degraded by artefact at the bone–air interface. Unfortunately, surgical specimens were not available which may have provided additional insights into the value, if any, of post-contrast imaging in these cases. Nonetheless, our findings are in agreement with previous studies which have also found that non-EP DWI may be limited in cases of small lesions in the 2–3 mm size range.19,20
The two major drawbacks of performing additional contrast-enhanced sequences are the longer acquisition time and the need for intravenous contrast. Post-contrast imaging is typically acquired 45–60 min after contrast injection and required a mean acquisition times of 12 min, giving a mean total scan time of 37 min for this study’s long protocol. If the post-contrast images were omitted, a mean saving of 32% total scan time (25 v. 37 min) and up to 74% reduction in the time the patient spends in the department (25 v. 97 min) could be expected. By omitting the contrast-enhanced sequences, capacity and efficiency in the MRI department could be improved without a drop-off in diagnostic performance.
The risks associated with GBCA are well described and include anaphylactoid reactions and nephrogenic systemic fibrosis in the setting of impaired renal function.21 Although the risk of an adverse reaction is low, occurring in approximately one in 10,000–40,000 injections, care must be taken to administer GBCAs only when necessary.22 Gadolinium deposition in brain tissue has been detected radiologically and pathologically, including within the dentate nucleus, pons, globus pallidus and thalamus in patients who have undergone multiple MRIs using GBCAs.23,24 The clinical significance of this deposition is uncertain but has prompted the European Medicine Agency’s Pharmacovigilance Risk Assessment Committee recommending the suspension of marketing authorization for four of the linear GBCAs.
We acknowledge three limitations of our study. Firstly, we did not use histology as the gold standard for cholesteatoma detection. However, several previous studies have investigated the radiological–pathological correlation for both non-EP DWI and contrast-enhanced MRI.25 Rather than adding to this body of literature, we sought to demonstrate agreement between two protocols, highlighting any discordant cases in which contrast-enhanced sequences may provide additional value. Secondly, although all studies were read by two radiologists, they were read in consensus rather than isolation. This was because any interobserver variation could have been attributed to the difference in experience between the two readers and therefore interobserver variation related to the imaging protocols could not have been determined with confidence. Lastly, our study was designed only to evaluate for the presence or absence of recurrent cholesteatoma and did not consider alternative diagnoses such as inflammation, fluid collections and granulation tissue. However, our findings suggest that even in such cases, the delayed post-contrast imaging does not alter the reader’s interpretation. Larger prospective studies with pathological correlation would be useful to validate these findings.
Conclusion
In summary, this study demonstrates almost perfect agreement between an MRI protocol incorporating both non-EP DWI and contrast-enhanced sequences, and a shortened protocol without contrast-enhanced sequences in the assessment of suspected cholesteatoma recurrence. These findings suggest that contrast-enhanced sequences could be omitted in the vast majority of cases, yielding a considerable saving in terms of time and expense, without compromising diagnostic accuracy. Contrast-enhanced sequences may provide additional value in equivocal cases with small (<3 mm) lesions or in cases where images are degraded by artefact.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical statement
This manuscript complies with ethical standards and ethical approval was granted by the Chairperson of our institution’s Ethics Committee.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Stephen Liddy https://orcid.org/0000-0001-8726-6255
References
- 1.Baráth K, Huber AM, Stämpfli P, et al. Neuroradiology of cholesteatomas. Am J Neuroradiol 2011; 32: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerckhoffs KGP, Kommer MBJ, Van Strien THL, et al. The disease recurrence rate after the canal wall up or canal wall down technique in adults. Laryngoscope 2016; 126: 980–987. [DOI] [PubMed] [Google Scholar]
- 3.Lingam RK, Nash R, Majithia A, et al. Non-echoplanar diffusion weighted imaging in the detection of post-operative middle ear cholesteatoma: navigating beyond the pitfalls to find the pearl. Insights Imaging 2016; 7: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateos-Fernández M, Mas-Estellés F, de Paula-Vernetta C, et al. The role of diffusion-weighted magnetic resonance imaging in cholesteatoma diagnosis and follow-up: study with the diffusion PROPELLER technique. Acta Otorrinolaringol 2012; 63: 438–442. [DOI] [PubMed] [Google Scholar]
- 5.von Kalle T, Amrhein P, Koitschev A. Non-echoplanar diffusion-weighted MRI in children and adolescents with cholesteatoma: reliability and pitfalls in comparison to middle ear surgery. Pediatr Radiol 2015; 45: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 6.Nash R, Wong PY, Kalan A, et al. Comparing diffusion weighted MRI in the detection of post-operative middle ear cholesteatoma in children and adults. Int J Pediat. Otorhinolaryngol 2015; 79: 2281–2285. [DOI] [PubMed] [Google Scholar]
- 7.Khemani S, Singh A, Lingam RK, et al. Imaging of postoperative middle ear cholesteatoma. Clin Radiol 2011; 66: 760–767. [DOI] [PubMed] [Google Scholar]
- 8.Venail F, Bonafe A, Poirrier V, et al. Comparison of Echo-planar diffusion-weighted imaging and delayed postcontrast T1-weighted MR imaging for the detection of residual cholesteatoma. Am J Neuroradiol 2008; 29: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viera AJ, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med 2005; 37: 360–363. [PubMed] [Google Scholar]
- 10.Rogha M, Hashemi SM, Mokhtarinejad F, et al. Comparison of preoperative temporal bone CT with intraoperative findings in patients with cholesteatoma. Iran J Otorhinolaryngol 2014; 26: 7–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Henninger B, Kremser C. Diffusion weighted imaging for the detection and evaluation of cholesteatoma. World J Radiol 2017; 9: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz KM, Lane JI, Bolster BD, et al. The utility of diffusion-weighted imaging for cholesteatoma evaluation. Am J Neuroradiol 2011; 32: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubrulle F, Souillard R, Chechin D, et al. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology 2006; 238: 604–610. [DOI] [PubMed] [Google Scholar]
- 14.De Foer B, Vercruysse JP, Spaepen M, et al. Diffusion-weighted magnetic resonance imaging of the temporal bone. Neuroradiology 2010; 52: 785–807. [DOI] [PubMed] [Google Scholar]
- 15.Maheshwari S, Mukherji SK. Diffusion-weighted imaging for differentiating recurrent cholesteatoma from granulation tissue after mastoidectomy: Case report. Am J Neuroradiol 2002; 23: 847–849. [PMC free article] [PubMed] [Google Scholar]
- 16.Pennanéach A, Garetier M, Ollivier M, et al. Diagnostic accuracy of diffusion-weighted MR imaging versus delayed gadolinium enhanced T1-weighted imaging in middle ear recurrent cholesteatoma: A retrospective study of 39 patients. J Neuroradiol 2016; 43: 148–154. [DOI] [PubMed] [Google Scholar]
- 17.Vercruysse JP, De Foer B, Pouillon M, et al. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: A surgical verified study of 100 patients. Eur Radiol 2006; 16: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 18.Dremmen MHG, Hofman PAM, Hof JR, et al. The diagnostic accuracy of non-echo-planar diffusion-weighted imaging in the detection of residual and/or recurrent cholesteatoma of the temporal bone. Am J Neuroradiol 2012; 33: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrales CE, Blevins NH. Imaging for evaluation of cholesteatoma: Current concepts and future directions. Curr Opin Otolaryngol Head Neck Surg 2013; 21: 461–467. [DOI] [PubMed] [Google Scholar]
- 20.De Foer B, Vercruysse JP, Bernaerts A, et al. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol 2008; 29: 513–517. [DOI] [PubMed] [Google Scholar]
- 21.Tsai LL, Grant AK, Mortele KJ, et al. A practical guide to MR imaging safety: What radiologists need to know. Radiographics 2015; 35: 1722–1737. [DOI] [PubMed] [Google Scholar]
- 22.Beckett KR, Moriarity AK, Langer JM. Safe use of contrast media: What the radiologist needs to know. Radiographics. 2015; 35: 1738–1750. [DOI] [PubMed] [Google Scholar]
- 23.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadoliniumbased contrast material. Radiology 2014; 270: 834–841. [DOI] [PubMed] [Google Scholar]
- 24.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275: 772–782. [DOI] [PubMed] [Google Scholar]
- 25.Muzaffar J, Metcalfe C, Colley S, et al. Diffusion-weighted magnetic resonance imaging for residual and recurrent cholesteatoma: a systematic review and meta-analysis. Clin Otolaryngol 2017; 42: 536–543. [DOI] [PubMed] [Google Scholar]