Abstract
Background
Preoperative imaging of salivary gland tumors is important for predicting and differentiating benign from malignant tumors, and for aiding management planning. We aimed to investigate the accuracy of combined quantitative diffusion-weighted magnetic resonance imaging (MRI) and routine contrast-enhanced MRI in the evaluation of salivary gland tumors and the differentiation of benign from malignant tumors.
Results
This study included 51 patients with a total of 16 benign and 35 malignant lesions that were detected by histopathological analysis. There was a statistically significant difference between the apparent diffusion coefficient values (ADC) of malignant and benign lesions (0.69 ± 0.22 × 10−3 mm2/s and 1.39 ± 0.52 × 10−3 mm2/s respectively). The optimal cut-off ADC value was 1.08 with 75% specificity and 97% sensitivity. The routine contrast-enhanced MRI had predicted benign and malignant tumors with 65% sensitivity and 44% specificity. The sensitivity and specificity were greatly increased when quantitative diffusion-weighted MRI was combined with routine contrast-enhanced MRI: 100%, and 88% respectively. A receiver operating curve was generated. The area under curve was 0.88 (p < 0.001, 95% CI: 0.76–0.99).
Conclusion
Combined quantitative diffusion-weighted MRI with ADC measurements and routine contrast-enhanced magnetic resonance imaging are helpful tools for the evaluation of salivary gland tumors and help differentiate benign from malignant lesions.
Keywords: Diffusion-weighted MRI, apparent diffusion coefficient, contrast-enhanced MRI, salivary gland tumors
Background
Salivary gland tumors (SGTs) represent 2–4% of head and neck neoplasms.1 These may be broadly categorized into benign tumors, tumor-like lesions and malignant neoplasms.2
The general rule of SGTs states that the smaller the salivary gland, the greater the possibility the tumor is malignant.3 Within the parotid glands, approximately 20% of tumors are malignant. The incidence of malignancy increases in the submandibular, sublingual and other minor salivary glands, and represents approximately 50%, and 85% respectively.4
Common clinical indications for salivary gland imaging are pain and swelling. Preoperative imaging of the salivary gland masses is paramount to identification and assessment. The ability of imaging to differentiate SGTs from the masses of adjacent cervical spaces, especially the parapharyngeal, masticator, and submental spaces as well as mandibular lesions is of significant value.5 Preoperative imaging aids management planning as the surgical approaches differ significantly between benign to malignant tumors, for example, superficial parotidectomy with facial nerve sparing versus radical parotidectomy with or without adjuvant chemoradiotherapy.6 The imaging modalities for salivary gland imaging vary widely but generally include plain radiography, sialography (conventional fluoroscopic, computed tomography (CT), and magnetic resonance imaging (MRI)), ultrasonography, CT, MRI, radionuclide scintigraphy, and positron emission tomography (PET)/CT.4 Ultrasound is the modality of choice but has limitations in assessments of the deep lobe of the parotid gland, deep extension of SGTs, and infiltration of adjacent structures, for example, the mandible. CT is very helpful in the evaluation of internal calcifications and bony invasions, yet it lacks the soft tissue contrast that MRI provides.7 CT also carries the risk of ionizing radiation. The use of iodinated contrast material with CTs is another limiting factor that is contraindicated in specific cases, for example, iodine allergy or renal impairment. MRI is the imaging modality of choice for SGTs as it facilitates accurate delineation of tumor extension, characterization of internal tumor composition, detection of perineural invasion, and differentiation between benign and malignant tumors.3
Diffusion-weighted MRI has a pertinent role in the evaluation of head and neck cancer. It aids locoregional staging by detection of metastatic cervical lymph nodes. Diffusion-weighted MRI can differentiate recurrent tumors from post-therapeutic changes.8 Furthermore, advanced diffusion imaging modules such as diffusion tensor imaging, intravoxel incoherent motion, and diffusion kurtosis imaging can provide more imaging features to facilitate differentiation of benign from malignant tumors.9 These imaging techniques have the advantages of being noninvasive and not dependent on radiation exposure or contrast-medium administration.9
The purpose of this study was to determine the accuracy of a combined approach using routine contrast-enhanced MRI and quantitative diffusion-weighted MRI in the differentiation of SGTs in the head and neck regions into benign and malignant groups.
Methods
In our retrospective analytical observational study, we identified 51 patients with SGTs in our hospital database, which included radiological and pathological records of patients treated in our tertiary care cancer center from January 1 2012 to December 31 2017. The study protocol was approved by the Institutional Review Board (IRB) of National Cancer Institute, Cairo University.
Technique
Routine contrast-enhanced MRI
All patients underwent routine contrast-enhanced MRI (Achieva 1.5 Tesla superconducting MR imager). A standard head and neck circularly polarized coil was used for MRI while the patients lay in a supine position. A routine MRI sequence was as follows: axial T1-weighted spin-echo (SE) sequence (time to repeat/time to echo: 529/15 ms); axial and coronal T2-weighted turbo SE (time to repeat (TR)/time to echo (TE): 4005/100 ms); axial 5-mm slice thickness STIR (short tau inversion recovery) sequence (TR/TE: 8085/20 ms). The contrast-enhanced series included a T1-weighted fat-saturated post-gadolinium administration sequence in the axial, coronal, and sagittal planes. We used gadolinium-DTPA at a dose of 0.1 mmol/kg. Two radiologists with 15- and 12-years’ experience in neuroradiology evaluated the lesions regarding number (solitary versus multiple) and location (parotid, submandibular, sublingual, other minor salivary glands, and unilateral versus bilateral). The tumor margins were also evaluated and classified into the categories of well-defined, lobulated, and ill-defined. The signal of these lesions was assessed in T1, T2, and STIR images and classified accordingly into low, intermediate-isointense, heterogenous with signal void, heterogenous signal, and high signal groups. A post-contrast series was used to evaluate the enhancement pattern of these lesions, which were further classified into non-enhancing, faint homogenously enhancing, ring-enhancing, intense homogenously enhancing and heterogeneously enhancing patterns. The invasion of surrounding structures, for example, the parapharyngeal space, mandible, external carotid artery, Retromandibular vein, and masticator muscles; perineural invasion; and intracranial extension were evaluated. Regional lymphadenopathy, for example, submental (level 1a), submandibular (level 1b), intra-parotid, and the pre- and postauricular lymph node groups were also evaluated.
Diffusion-weighted MRI
Axial diffusion-weighted MRI was performed by using a single-shot echo planar SE sequence with the following parameters: 1600/107; diffusion gradient encoding in three (x, y, z) orthogonal directions; b values of 0 and 800 s/mm2; field of view 220 mm; matrix size 128 × 128; section thickness 4mm; section gap 0 mm; and one signal acquired. For each b value, x-, y-, and z single-direction diffusion-weighted MRIs and a baseline image (b0 s/mm2) were acquired; a combined ([x–y–z]/3) diffusion-weighted MRI was calculated and performed automatically by the magnetic resonance instrument.
All diffusion-weighted MRI data were transferred to a computer for determination of signal intensity and ADC measurement. Each image used for the creation of the ADC maps was obtained with one signal acquired. The two radiologists used b800 diffusion-weighted MRIs to characterize signal intensity of the masses; they then subjectively correlated the signal intensity on the b800 images with the signal on the ADC maps to exclude T2 shine-through phenomenon. The lesions with a hyperintense signal on b800 and a dark signal on the ADC maps were considered to be actual restricted diffusivity, whereas the lesions that appeared hyperintense on both b800 and ADC maps were considered to be T2 shine-through. The two radiologists determined the most suitable solid enhancing components of the masses, and measured ADC values manually by placing regions of interest (ROI) on the ADC map. They placed at least three ROIs of at least 1 cm diameter within the solid enhancing components of the tumors and calculated the mean ADC values. Thus, cystic-, necrotic-, and hemorrhagic tumor areas were avoided. Whenever possible ROIs were also placed in apparently normal gland tissue or the contralateral gland as controls to obtain relative ADC values.
Histopathologic classification
All patients underwent either surgical resection of the tumor or imaging-guided biopsy. Histopathological evaluation is the gold standard in diagnosis, hence, the results were obtained and compared with ADC values.
Statistical analysis
Data were coded and entered using SPSS (Statistical Package for the Social Sciences, version 22; SPSS Inc., Chicago, IL, USA). Our data were checked for normality of data distribution with the Kolmogorov–Smirnov test. The data was summarized using mean, standard deviation, median, minimum, and maximum in quantitative data as well as frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables such as benign and malignant tumors were carried out using the nonparametric Kruskal–Wallis and Mann–Whitney tests.10 For comparing categorical data such as the signal characteristics, the chi-square (χ2) test was performed. However, when the expected frequency was less than 5, the exact test was used instead.11 ROC curves were constructed with area under curve (AUC) analysis performed to detect the best ADC cut-off value for detection of malignancy. P-value was considered statistically significant if <0.05 with a confidence interval (CI) of 95%. Standard diagnostic indices including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic efficacy were calculated as described by Galen.12
Results
Demographic data
This study included 51 patients (24 male and 27 female). Patients ages ranged from 2–70 years with a mean of 36.2 ± 22.68 year (mean ± SD). The benign group included 16 patients (10 female and 6 male), their ages ranged from 2–63 years with a mean age of 34. The malignant group included 35 patients (17 female and 18 male) whose ages ranged from 2–70 years with a mean age of 32.
Locations of the lesions
Approximately 90% of the cases were found within the parotid gland, while the remaining 10% were found within the submandibular (Figure 1) and minor salivary glands (Figure 2).
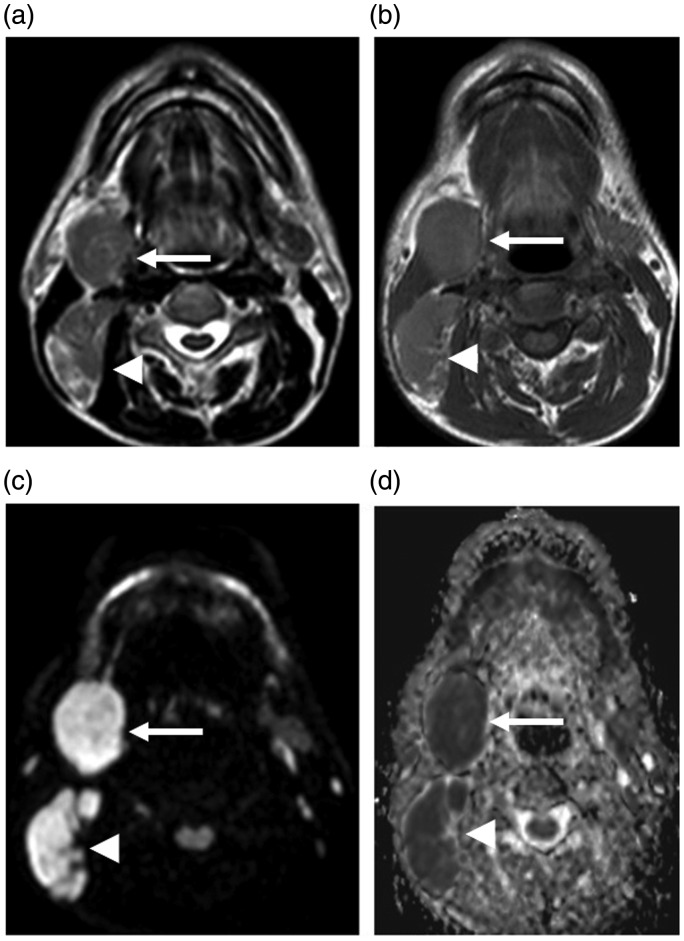
Figure 1.
Fifty-six year-old male with a history of right cervical mass. A well-defined right submandibular mass lesion with intermediate signal intensity on T1WI (b) and T2WI (a) (arrows). Enlarged right cervical lymph nodes are also observed (arrowheads). They show a bright signal on diffusion-weighted MRI (c) and have an ADC value (d) of 0.5 × 10−3 mm2/s. Pathological assessment shows classical Hodgkin lymphoma with mixed cellularity.
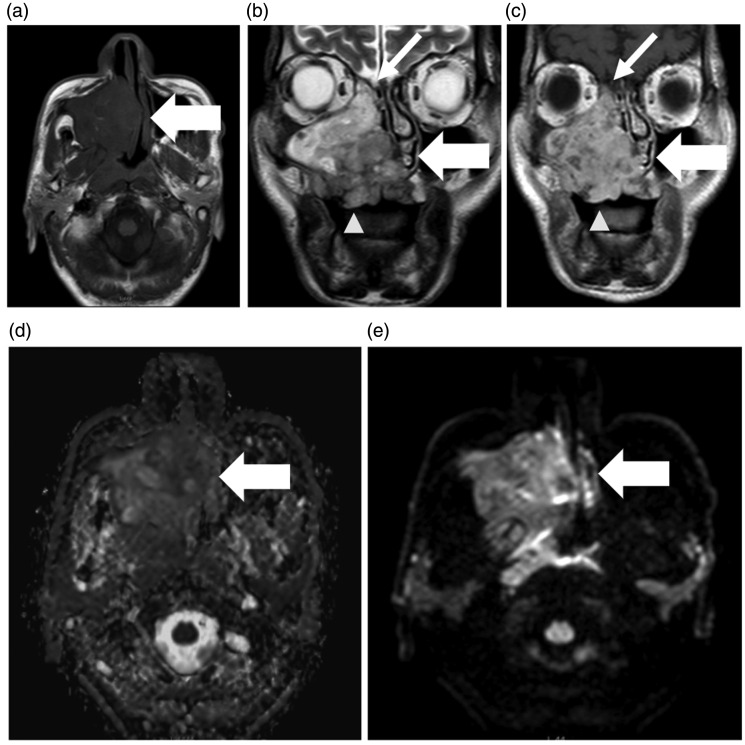
Figure 2.
Seventy year-old male with swelling over the right maxillary region with nasal obstruction on the right side. MRI shows an ill-defined, irregular-shaped soft tissue mass lesion involving the hard palate and right alveolar margin (wide arrows). The mass infiltrates most of the right maxillary sinus and extends superiorly to the ethmoid sinus (narrow white arrows (b) and (c)). It also infiltrates the right nasal cavity, the nasal septum, and the medial pterygoid plate with an invasion of the inferior orbital canal as well as the hard palate (arrowheads (b, c)). The mass has low signal on T1WI (a) and high SI on T2WI (b) with internal cystic areas of higher T2 signal within, and heterogenous enhancement on post-contrast series (c). Diffusion-weighted MRI findings: bright signal in diffusion-weighted MRI (d) with an estimated ADC (e) of 1.03 × 10−3mm 2/s. The pathological assessment revealed adenoid cystic carcinoma.
Location of the lesions within the parotid gland
Nearly half of the lesions (49% of the lesions) were found in the superficial lobe, and 12% of lesions were found within the deep lobe. The remaining (39%) lesions were found to involve both the superficial and deep lobes (Figure 3). Forty-six percent of the lesions were in the right parotid gland, 45% of the lesions were found in the left parotid gland. Bilateral involvement of the parotid glands was found in the remaining 9% of lesions.
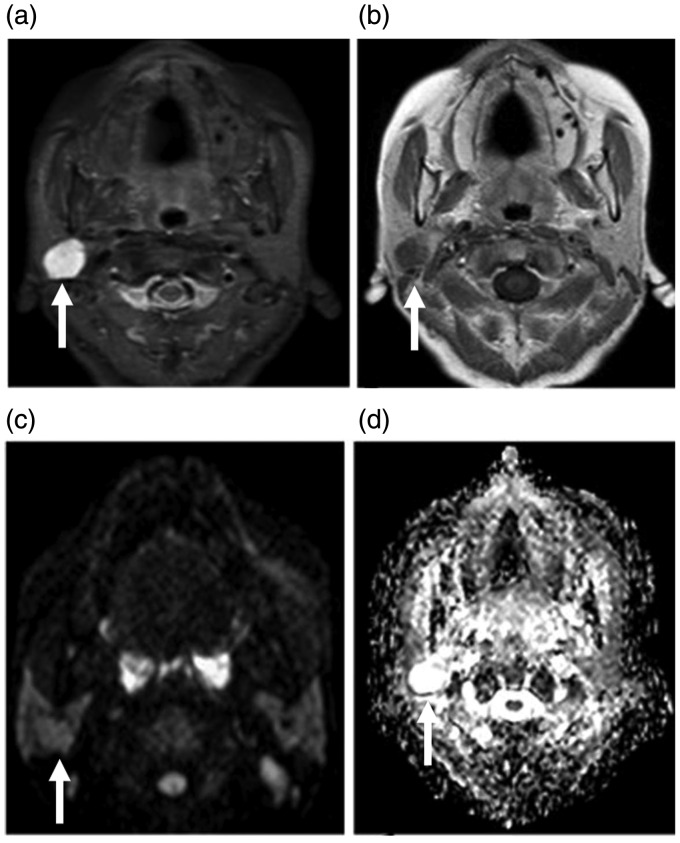
Figure 3.
Sixty-three year-old female presenting with nasal discharge, with a history of nasopharyngeal carcinoma. A well-defined mass lesion is seen at the junction between the superficial and deep lobes of the right parotid gland (arrows). The mass shows hypointense T1WI (not shown), and hyperintense T2WI signal (a) with faint enhancement on post-contrast series (b). This mass has an isointense signal on diffusion-weighted MRI (c) and a bright signal on the ADC map (d) with an ADC value of 2.1 × 10−3 mm2/s. Pathological diagnosis indicated pleomorphic adenoma.
Pathological analysis
We divided the patients into two groups according to the pathological results: benign and malignant. The benign group included 16 patients, the malignant, 35 patients. The pathological diagnoses were diverse, as listed in tables 1 and 2. Pleomorphic adenoma was the most common benign lesion in our study, which accounted for approximately 31%. The most common malignant tumor was adenoid cystic carcinoma (AdCC), representing 20% of all malignant tumors.
Table 1.
Represents all detected benign pathological conditions in our cohort.
| Benign | Pathological lesion | Frequency | Percentage |
|---|---|---|---|
| Pleomorphic adenoma | 5 | 10% | |
| Hemangioma | 2 | 4% | |
| Inflammatory intra-parotid lymph node | 2 | 4% | |
| Sialosis | 2 | 4% | |
| BLEL or atypical myoepithelial lesion | 2 | 4% | |
| Hemangioendothelioma | 1 | 2% | |
| Chronic nonspecific inflammation | 1 | 2% | |
| Warthin’s tumor | 1 | 2% |
Note: BLEL = benign lympho-epithelial lesion.
Table 2.
Represents all detected malignant tumors.
| Malignant | Pathological lesion | Frequency | Percentage |
|---|---|---|---|
| Adenoid cystic carcinoma | 7 | 14% | |
| Lymphoma | 6 | 12% | |
| Leukemia | 6 | 12% | |
| Metastatic IPLN | 6 | 12% | |
| Primitive neuroectodermal tumor | 2 | 4% | |
| Rhabdomyosarcoma | 2 | 4% | |
| Mixed salivary gland carcinoma | 2 | 4% | |
| Squamous cell carcinoma | 2 | 4% | |
| Mucoepidermoid carcinoma | 1 | 2% | |
| Atypical teratoid rhabdoid tumor | 1 | 2% |
Note: IPLN = intra-parotid lymph node.
Routine contrast-enhanced MRI
Based on a signal intensity on the T1 weighted image (T1WI), the T2WI and the enhancement pattern described in the section titled Routine contrast-enhanced MRI, the lesions were distributed as presented in Table 3.
Table 3.
Evaluation of variable imaging features and signal characteristics in benign and malignant lesions.
| Benign |
Malignant |
P-value | |||
|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | ||
| T1 | |||||
| Isointense | 9 | 56% | 16 | 46% | 0.065 |
| Heterogenous signal intensity | 2 | 13% | 3 | 9% | |
| Heterogenous with signal void | 2 | 13% | 0 | 0% | |
| Low signal intensity | 3 | 19% | 16 | 46% | |
| T2 | |||||
| High signal intensity | 8 | 50% | 16 | 46% | 0.332 |
| Heterogenous signal intensity | 5 | 31% | 9 | 26% | |
| Intermediate signal intensity | 2 | 13% | 10 | 29% | |
| Low signal intensity | 1 | 6% | 0 | 0% | |
| Enhancement | |||||
| Homogenous | 1 | 6 % | 18 | 51% | <0.001 |
| Heterogenous | 6 | 38% | 15 | 43% | |
| Heterogenous ring | 3 | 19% | 0 | 0% | |
| No uptake | 0 | 0% | 1 | 3% | |
| Faint | 3 | 19% | 1 | 3% | |
| Intense | 3 | 19% | 0 | 0% | |
| Relationship to surrounding structures | |||||
| Not invading | 10 | 67% | 17 | 52% | 0.028 |
| Invading surrounding | 1 | 7% | 10 | 30% | |
| Invading parapharyngeal space | 1 | 7% | 5 | 15% | |
| Indenting overlying muscles and skin | 3 | 20% | 0 | 0% | |
| Associated with another intracranial mass | 0 | 0% | 1 | 3% | |
| Lymph node | |||||
| Not significant | 3 | 19% | 10 | 30% | 0.545 |
| Bilateral cervical lymph node or positive lymph node | 6 | 38% | 14 | 42% | |
| Bilateral nonspecific | 7 | 44% | 9 | 27% | |
Accuracy of routine contrast-enhanced MRI
After evaluation of the lesions with the routine contrast-enhanced MRI, we categorized the patients into benign- or malignant-looking, based on suspicious imaging criteria for malignancy. These criteria included ill-defined margins, a hypointense signal on T2WIs, heterogenous enhancement patterns, and infiltration of the surrounding structures (e.g., mandible, vascular, and perineural invasion). The results revealed 32 malignant- and 19 benign-looking lesions. These results correlated with the pathological data and demonstrated 23 true positive, 7 true negative, 9 false positive, and 12 false negative results for malignancy. Thus, the sensitivity and specificity were 66% (95% CI: 46–80%) and 44% (95% CI: 20–70%) respectively.
Diffusion-weighted MRI signal intensity
We found a significant difference between the benign and malignant groups, as 91% of the malignant tumors exhibited a restricted diffusion pattern, while 67% of the benign tumors showed a facilitated diffusion pattern or T2 shine-through phenomenon.
ADC values
The calculated ADC values ranged from 0.30–2.4 × 10−3 mm2/s. The calculated ADC value of the lesions was found to be statistically significant when differentiating between benign and malignant lesions. The mean ADC value for benign lesions was 1.39 ± 0.52 × 10−3 mm2/s, while the mean ADC value for malignant lesions was 0.69 ± 0.22 × 10−3 mm2/s (p-value < 0.001). Thus, the mean ADC value of benign was significantly higher than that of malignant lesions.
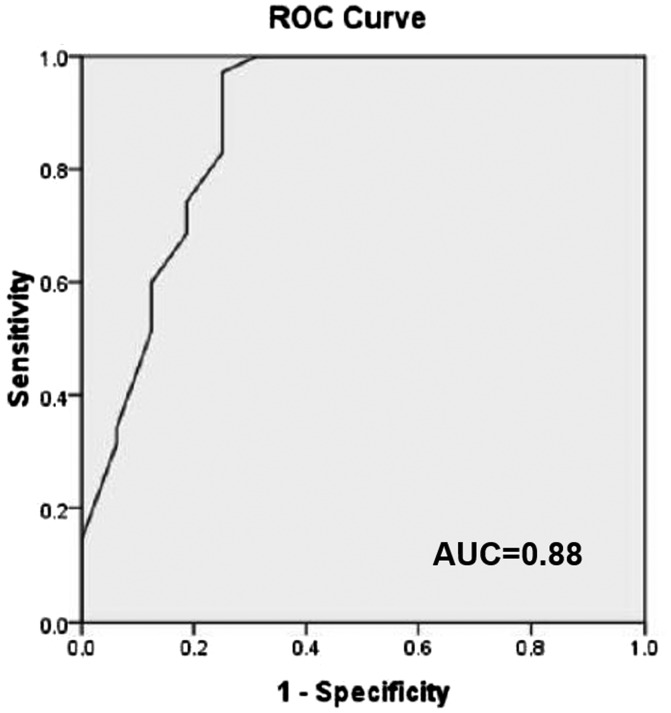
The ADC value of 1.08 × 10−3 mm2/s was suggested as the optimal cut-off between benign and malignant entities, with 97% sensitivity and 75% specificity. The AUC was 0.88 (p < 0.001, 95% CI 0.76–0.99) (Figure 4).
Figure 4.
ROC analysis using various ADC values of the tumors to differentiate benign from malignant salivary gland tumors. AUC was 0.88 and found to be statistically significant p < 0.001 (95% CI: 0.76–0.99). ROC = receiver operating curve; ADC = apparent diffusion coefficient; AUC = area under curve.
Accuracy of interpretation of routine contrast-enhanced MRI and quantitative diffusion-weighted MRI together
We combined the routine contrast-enhanced MRI findings with the quantitative diffusion-weighted MRI results. This resulted in 37 malignant- and 14 benign-looking lesions. Correlation with the pathological findings revealed 35 true positive, 2 false positive, and 14 true negative cases. Based on these results, the sensitivity and specificity were 100% (95% CI: 90–100%) and 88% (95% CI: 62–98%) respectively, with 95% (95% CI: 81–99%) PPV, and 100% (95% CI: 77–100%) NPV.
Discussion
Histopathological subtypes of the salivary gland neoplasms are diverse and very variable. These represent the most complex groups of head and neck tumors encountered by clinicians.13
Our results demonstrated that combined routine contrast-enhanced MRI and quantitative diffusion-weighted MRI is of significant value in differentiating between benign and malignant SGTs. The ADC values of malignant lesions were significantly lower than those of benign lesions, except for cases of Warthin’s tumor. There was still an overlap between the mean ADC value of Warthin’s tumors and the mean ADC value of malignant salivary tumors. Warthin’s tumors have propensity of lymphoid tissue which may be attributed to low ADC values as seen in lymphoma.14
In this study, pleomorphic adenomas demonstrated high ADC values compared with malignant and Warthin’s tumors. This was attributed to the high propensity of pleomorphic adenomas to contain chondroid and myxoid matrices that contribute to the facilitated diffusivity.15
Mucoepidermoid carcinomas (MECs) demonstrated slightly high ADC values 1.02 × 10−3 mm2/s. Generally, MECs are classified into low, intermediate, or high grades.16 High-grade MECs usually have low ADC values reflective of restricted diffusivity, while low- to intermediate-grade MECs have higher ADC values with relatively increased diffusivity. The presence of micro- and macro-cystic changes within low-grade MECs make the cells less densely packed with resultant facilitated diffusivity.9
Previous studies showed that AdCCs have ADC values higher than other malignancies.17–19 In our cohort, AdCCs had lower ADC values (0.94 × 10−3 mm2/s); this might be explained as most of our cases were high-grade tumors with significant heterogeneously enhancing solid components.18
Our results were like Eida et al.’s19 results, where they concluded that most malignant tumors have low ADC values whereas benign tumors tend to have high ADC values (≥1.8 × 10−3 mm2/s). They also observed that primary salivary gland malignant lymphomas were associated with extremely low ADC values (less than 0.6 × 10−3 mm2/s) owing to the homogenous growth patterns of lymphoma cells.19
Balçık et al.’s20 study included 40 patients with 41 parotid gland masses. The mean ADC values of benign and malignant salivary tumors were 1.74 ± 0.58 × 10−3 mm2/s and 1.13 ± 0.13 × 10−3 mm2/s, respectively. This agrees with our study results. Malignant lesions were fewer in number and, thus, were not compared with each other. Balçık et al.20 confirmed that the ADC values of pleomorphic adenomas were significantly higher than the ADC values of malignant salivary- and Warthin’s tumors. Balçık et al.20 concluded there was no absolute optimal cut-off, preferring to separate the cut-off between dual groups of pleomorphic adenomas, Warthin’s tumors, and malignant lesions. In our study, due to the paucity of Warthin’s tumors, no dual comparison between the Warthin’s tumor group and the malignant lesions group was done. Low ADC values in Warthin's tumor might be attributed to the intense lymphoid accumulation in the stroma and proliferation of the epithelial component leading to a decrease in the extracellular extravascular space and therefore a decrease in ADC, even less than in malignant lesions.20 Abdel Razek et al. investigated the utility of dynamic susceptibility MRI perfusion to further differentiate Warthin’s from malignant tumors as Warthin’s tumors require a less aggressive surgical approach.21
Celebi et al.22 undertook a study of 75 patients with a total of 81 salivary gland masses. They found 49 benign lesions and the remaining 32 were diagnosed as malignant. They concluded that the mean ADC value of benign tumors (1.72 × 10−3 mm2/s) was significantly higher than that of malignant tumors (1.05 × 10−3 mm2/s). The cut-off proposed for ADC values to distinguish malignant from benign SGTs was 1.17 × 10−3 mm2/s with a sensitivity, specificity, PPV, and NPV of 63%, 72%, 79%, and 79%, respectively. They also commended the combination of diffusion-weighted- and routine MRI sequences for better diagnostic value. Thus, differentiation of lesions would be very helpful with respect to a surgical approach.22
The current study had a few limitations. The study design was a single-center retrospective study that included a small sample size in each pathological category to get statistical significance. Therefore, a multicentric prospective study with a larger cohort would emphasize and consolidate these results. We found significant overlap between parotid benign lympho-epithelial and malignant lesions as well as both have high ADC values and overlap with AdCC. Parotid benign lympho-epithelial lesions have diffuse, well-organized lymphoid tissue and lymphocytic interstitial infiltrate.
Conclusion
The combined interpretation of contrast-enhanced MRI and quantitative diffusion-weighted MRI provides an effective diagnostic tool for characterization of SGTs and can discriminate between benign and malignant masses.
Author contributions
Formulation of idea and data collection: DH; writing, editing, and revising the manuscript: AN; revising the integrity of the manuscript and professional language revision: AY, EM; final revision of the manuscript: REA.
Availability of data and material
Available.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
This study was approved by the research committee of National Cancer Institute, Cairo University.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ORCID iD
Ayman Nada https://orcid.org/0000-0002-9296-9227
References
- 1.El-Naggar AK, Chan JKC, Takata T, et al. The fourth edition of the head and neck World Health Organization blue book: editors’ perspectives. Hum Pathol 2017; 66: 10–12. [DOI] [PubMed] [Google Scholar]
- 2.Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol 2009; 3: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler AT, Bhatt AA. Review of the major and minor salivary glands, Part 2: neoplasms and tumor-like lesions. J Clin Imaging Sci 2018; 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sentani K, Ogawa I, Ozasa K, et al. Characteristics of 5015 salivary gland neoplasms registered in the Hiroshima Tumor Tissue Registry over a period of 39 years. J Clin Med 2019; 8: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastogi R, Bhargava S, Mallarajapatna GJ, et al. Pictorial essay: salivary gland imaging. Indian J Radiol Imaging 2012; 22: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thielker J, Grosheva M, Ihrler S, et al. Contemporary management of benign and malignant parotid tumors. Front Surg 2018; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicero G, D’angelo T, Racchiusa S, et al. Cross-sectional imaging of parotid gland nodules: a brief practical guide. J Clin Imaging Sci 2018; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel Razek AAK, Mukherji SK. State-of-the-art imaging of salivary gland tumors. Neuroimaging Clin N Am 2018; 28: 303–317. [DOI] [PubMed] [Google Scholar]
- 9.Abdel Razek AAK, Elkhamary SM, Nada N. Correlation of apparent diffusion coefficient with histopathological parameters of salivary gland cancer. Int J Oral Maxillofac Surg 2019; 48: 995–1000. [DOI] [PubMed] [Google Scholar]
- 10.Chan YH. Biostatistics102: quantitative data – parametric and non-parametric tests. Singapore Med J 2003; 44: 391–396. [PubMed] [Google Scholar]
- 11.Chan YH. Biostatistics 103: qualitative data – tests of independence. Singapore Med J 2003; 44: 498–503. [PubMed] [Google Scholar]
- 12.Galen RS. Predictive values and efficiency of laboratory testing. Pediatr Clin North Am 1980; 27: 861–869. [DOI] [PubMed] [Google Scholar]
- 13.Salama AA, El-Barbary AH, Mlees MA, et al. Value of apparent diffusion coefficient and magnetic resonance spectroscopy in the identification of various pathological subtypes of parotid gland tumors. Egypt J Radiol Nucl Med 2015; 46: 45–52. [Google Scholar]
- 14.Rastogi R, Bhargava S, Mallarajapatna GJ, et al. Pictorial essay: salivary gland imaging. Mini-Symp Head Neck 2013; 22: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nada A, Youssef A, El Basmy A, et al. Diffusion-weighted imaging of the parotid gland: can the apparent diffusion coefficient discriminate between normal and abnormal parotid gland tissues? Erciyes Med J 2017; 39: 125–130 [Google Scholar]
- 16.Sato K, Akiba J, Nakamura K, et al. Mucoepidermoid carcinoma of the sublingual gland harboring a translocation of the MAML2 gene: a case report. Oncol Lett 2017; 14: 2970–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Bhalla AS, Sharma R, et al. Can diffusion-weighted imaging aid in differentiating benign from malignant sinonasal masses?: A useful adjunct. Pol J Radiol 2017; 82: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng N, Li R, Liu W, et al. The diagnostic value of combining conventional, diffusion-weighted imaging and dynamic contrast-enhanced MRI for salivary gland tumors. Br J Radiol 2018; 91: 20170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eida S, Sumi M, Sakihama N. Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJR Neuroradiol 2007; 28: 116–121. [PMC free article] [PubMed] [Google Scholar]
- 20.Balçık Ç, Akan H, İncesu L. Evaluating of parotid gland tumors according to diffusion-weighted MRI. Eur J Gen Med 2014; 11: 77–84. [Google Scholar]
- 21.Abdel Razek AA, Samir S, Ashmalla GA. Characterization of parotid tumors with dynamic susceptibility contrast perfusion-weighted magnetic resonance imaging and diffusion-weighted MR Imaging. J Comput Assist Tomogr 2017; 41: 131–136. [DOI] [PubMed] [Google Scholar]
- 22.Celebi I, Mahmutoglu AS, Ucgul A, et al. Quantitative diffusion-weighted magnetic resonance imaging in the evaluation of parotid gland masses: a study with histopathological correlation. Clin Imaging 2013; 37: 232–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available.