Abstract
Opiate intoxication has been associated with life-threatening effects of sympathetic suppression and respiratory depression, but current literature is limited in describing its neurotoxic effects on the central nervous system. Here, we present the case of an otherwise high-functioning adolescent male who was found unresponsive after ingestion of approximately 3–4 fake oxycodone 10–325 mg pills laced with fentanyl. Magnetic resonance imaging showed evidence of diffuse T2 hyperintensities in the corpus callosum and bilateral frontal, parietal, and cerebellum indicative of diffuse white matter injury. In addition, there were distinct areas of restricted diffusion in the bilateral basal ganglia concerning for oxidative stress-mediated neuronal loss. His neurological exam improved with supportive treatment over the course of his hospitalization. Although limited literature has shown leukoencephalopathy to be associated with opioid overdose, we present a case of additional involvement of subcortical gray matter.
Keywords: Leukoencephalopathy, opioid overdose, white matter injury, adolescent
Introduction
Opioid use is a significant cause of morbidity and mortality. Among adolescents, the rate of opioid use is alarmingly high. In a recent survey of 67,000 high school students, 12.4% reported non-medical opioid use.1 Life-threatening effects of opioid toxicity, including sympathetic suppression and respiratory depression, have been well documented. However, current literature is limited in describing the neurotoxic effects of opiate intoxication on the central nervous system.2
Magnetic resonance imaging (MRI) is an important clinical tool to evaluate central nervous system pathology and has become an increasingly useful imaging modality in neurologic emergencies. The location of, as well as the features of, abnormalities seen on different MRI sequences can help determine underlying diagnosis. For example, diffusion weighted imaging sequences are an important tool for detecting ischemia.3 Here, we aim to present findings of opioid-induced neurotoxicity and outline a systematic walkthrough of the workup of opiate-induced central nervous system toxicity. We present these findings, as they will benefit clinicians to accelerate diagnosis, limit excess testing, and prevent unwarranted treatments in cases of opioid neurotoxicity, thereby reducing the morbidity and mortality of this growing epidemic.
Hospital course
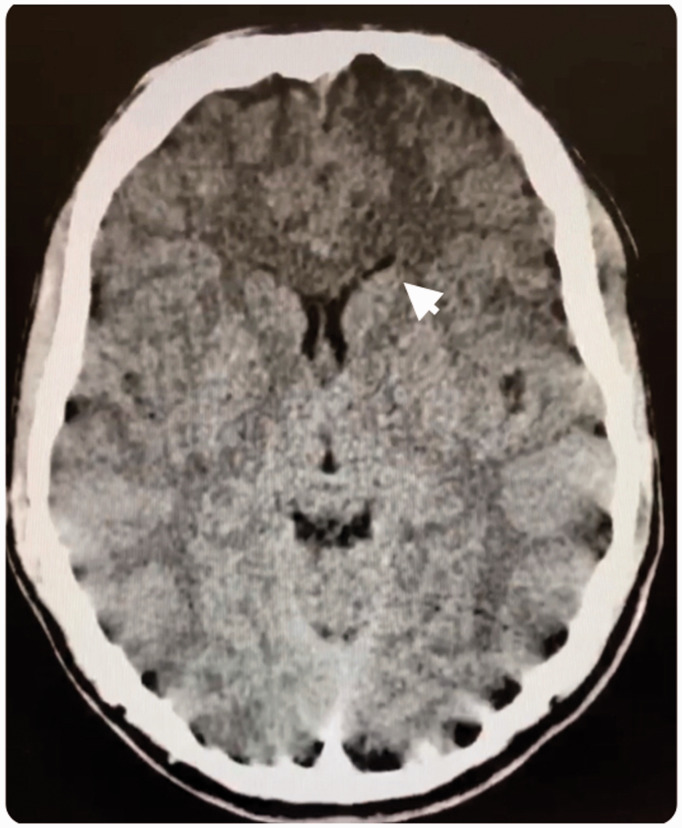
Our 16-year-old previously high-functioning male patient was found unresponsive by a friend approximately 12 h after ingestion of an unknown substance. He reportedly had no developmental abnormalities, past medical problems, or significant family history, and was not on any medications. According to the patient’s parents, he had no history of alcohol, tobacco, or other drug use. In the emergency department, he was lethargic and required intubation for respiratory protection. Head computed tomography scan with a display field of view of 44.24 cm × 23.2 cm and slice thickness of 3 mm showed hypodensity in bilateral caudate nuclei (see Figure 1). Basic labs were unremarkable except for an elevated creatine kinase of >3000. His urine drug screen was negative. The differential at this time was more suspicious of rhabdomyolysis from an inflammatory or infectious etiology. His respiratory status resolved gradually and creatinine kinase trended towards normal with supportive management. He was extubated the following morning. However, he remained minimally responsive to verbal and tactile stimuli and our Pediatric Neurology team was consulted.
Figure 1.
Computed tomography (CT) scan from a 16-year-old previously healthy male presenting with loss of consciousness after acute opiate intoxication. Arrow points to hypodensity in the caudate nucleus. CT parameters: display field of view 44.24 cm × 23.2 cm, slice thickness 3 mm.
Neurological exam revealed a drowsy and disoriented patient who only intermittently followed commands. No cranial nerve deficits were identified. Strength testing showed at minimum anti-gravity spontaneous movements in all extremities. Reflexes were diffusely increased with bilateral upgoing Babinski signs. Sensation, coordination, and gait could not be tested due to the patient’s inability to cooperate.
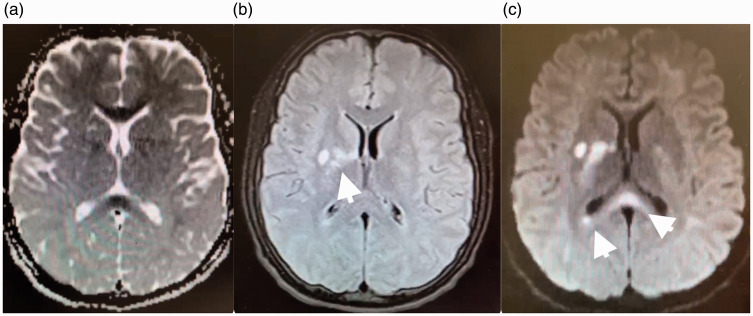
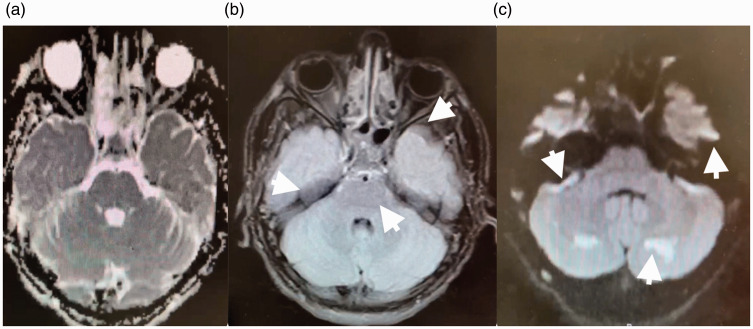
The patient’s MRI showed evidence of diffuse T2 hyperintensities in the corpus callosum and bilateral frontal, parietal and cerebellum indicative of diffuse white matter injury (see Figure 2 for scan acquisition parameters and images). In addition, there were distinct areas of restricted diffusion in the bilateral basal ganglia concerning for oxidative stress-mediated neuronal loss (see Figure 3 for scan acquisition parameters and images). A magnetic resonance angiogram of the head and neck was unremarkable. All studies were initially evaluated by the neuroradiology team, and then independently reviewed at a department-wide pediatric neurology conference. A wide differential was considered including toxic encephalopathy, vasculitis, autoimmune/post-infectious demyelinating disorders such as acute disseminated encephalomyelitis, congenital mitochondrial disorders, and paraneoplastic disorders.
Figure 2.
Magnetic resonance imaging (MRI) scan from a 16-year-old previously healthy male presenting with loss of consciousness after acute opiate intoxication. Apparent diffusion coefficient MRI (a) and corresponding diffusion-weighted MRI (b) show restricted diffusion (arrows) involving the corpus callosum and bilateral frontal, parietal, and cerebellar white matter. A T2 fluid attenuated inversion recovery MRI (c) shows multifocal hyperintensities (arrows). Findings are concerning for opiate-induced leukoencephalopathy. Diffusion-weighted imaging parameters: repetition time 6843 ms, echo time 114 ms, flip angle 90°, slice thickness 4mm. Apparent diffusion coefficient parameters: repetition time 6843 ms, echo time 114 ms, flip angle 90°, slice thickness 4mm. T2 fluid attenuated inversion recovery parameters: repetition time 8800 ms, echo time 82 ms, flip angle 150°, inversion time 2000 ms, slice thickness 4 mm.
Figure 3.
MRI scan from a 16-year-old previously healthy male presenting with loss of consciousness after acute opiate intoxication. Apparent diffusion coefficient (ADC) sequence (a) and corresponding diffusion-weighted (DWI) sequence (b) show restricted diffusion (arrow) in the basal ganglia. A T2 fluid attenuated inversion recovery (FLAIR) sequence (c) shows multifocal hyperintensities (arrows). Findings are concerning for opiate-induced oxidative stress and neuronal loss. DWI parameters: repetition time 6843 ms, echo time 114 ms, flip angle 90°, slice thickness 4mm. ADC parameters: repetition time 6843 ms, echo time 114 ms, flip angle 90°, slice thickness 4 mm. T2 FLAIR parameters: repetition time 8800 ms, echo time 82 ms, flip angle 150°, inversion time 2000 ms, slice thickness 4 mm.
In the process of considering lumbar puncture followed by empiric antibiotics versus steroids, the patient’s parents were able to obtain additional information in regard to the ingested substance. The pill appeared to be circular and light blue and the patient was under the impression that he had purchased oxycodone. The exact dose ingested was unknown but based on his history and collateral information from his friend, the patient likely consumed 3–4 fake oxycodone 10–325mg pills laced with variable amounts of fentanyl.
The patient continued to receive supportive treatment and a repeat neurological exam the following day showed an oriented talkative individual with intact higher-order cognitive processes. His muscular strength returned to baseline, though his hyperreflexia with bilateral upgoing Babinski signs was unchanged.
Discussion
Current scarcity of literature documenting the linear clinical and neuroimaging course of opioid-induced neurotoxicity often leads to delays in diagnosis, unwarranted testing, and potentially harmful treatments. Previous studies have shown that opioid abuse can lead to leukoencephalopathy.4,5 A recent case report outlined the presentation of a 16-year-old male with presumed heroin toxicity who presented with altered mental status and an elevated creatine kinase.5 His MRI findings showed symmetrical cerebral white matter changes in the frontal, parietal, occipital, and temporal lobes. Similarly, a case report of a 36-year-old male identified T2 hyperintensity in the subcortical white matter consistent with leukoencephalopathy following heroin abuse.6 In addition, a report of a three-year-old girl also showed increased T2 signal in the cerebellar hemispheres.7 Imaging of patients who used methadone and oxycodone also revealed diffuse hyperintensities on T2-weighted images.4,8 The uniqueness in the case is in the fact that there was not only diffuse radiographic involvement in the white matter, but also in the deep gray nuclei. These MRI findings may be suggestive of an alternative mechanism of injury in opioid neurotoxicity that has not yet been detailed. We hypothesize that high-potency opiates (like the fentanyl inadvertently consumed by our patient) can bind to G-protein coupled receptors in this region, causing excess dopamine release, thereby leading to oxidative stress and neuronal damage.9 Identifying the mechanism of injury for opioid-induced neurotoxicity may change prevention and management methods in order to improve acute and chronic recovery in these patients.
Opioid-induced neurotoxicity should be considered in the differential of previously healthy adolescents presenting with diffuse leukoencephalopathy with or without radiographic changes of the deep gray nuclei. Early diagnosis can prevent unsuccessful trials of potentially harmful treatments. Clinicians should be aware that routine urine toxicology tests are often not sensitive to fentanyl, norfentanyl or synthetic opiates. We encourage specialized testing for patients whose brain lesions match the morphology of those described in our report. In most cases, symptomatic management will be appropriate, though caution must be taken to preemptively prevent related life-threatening consequences such as edema, herniation, or respiratory/cardiovascular depression. Acute toxic leukoencephalopathy secondary to opioid use has been shown to be fatal in adolescents.10 As such, education regarding the neurologically devastating effects of opiate use so as to prevent their occurrence is paramount. Further research into the pathophysiology through which high-potency opiates cause neuronal toxicity can help target early therapies and shield adolescents from a lifetime of disability.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Andreea I Dinicu https://orcid.org/0000-0002-4390-5083
References
- 1.Palamar JJ, Shearston JA, Dawson EW, et al. Nonmedical opioid use and heroin use in a nationally representative sample of us high school seniors. Drug Alcohol Depend 2016; 158 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrot S, Poretti A, Tucker EW, et al. Acute brain injury following illicit drug abuse in adolescent and young adult patients: Spectrum of neuroimaging findings. Neuroradiol J 2017; 30: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozell JM, Li S. Recognition and appropriate use of magnetic resonance imaging for emergent neuroradiology. Semin Ultrasound CT MR 2017; 38: 424–438. [DOI] [PubMed] [Google Scholar]
- 4.Morales Odia Y, Jinka M, Ziai WC. Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit Care 2010; 13:93–97. [DOI] [PubMed] [Google Scholar]
- 5.Tamrazi B, Almast J. Your brain on drugs: Imaging of drug-related changes in the central nervous system. Radiographics 2012; 32: 701–719. [DOI] [PubMed] [Google Scholar]
- 6.Bega DS, McDaniel LM, Jhaveri MD, et al. Diffusion weighted imaging in heroin-associated spongiform leukoencephalopathy. Neurocrit Care 2009; 10: 352–354. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Mullen AJ, Hofstede D, et al. Malignant cerebellar edema in three-year-old girl following accidental opioid ingestion and fentanyl administration. Neuroradiol J 2019; 32: 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salgado RA, Jorens PG, Baar I, et al. Methadone-induced toxic leukoencephalopathy: MR imaging and MR proton spectroscopy findings. AJNR Am J Neuroradiol 2010; 31: 565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev 2008; 58: 192–208. [DOI] [PubMed] [Google Scholar]
- 10.Metkees M, Meesa IR, Srinivasan A. Methadone-induced acute toxic leukoencephalopathy. Pediatr Neurol 2015; 52: 256–257. [DOI] [PubMed] [Google Scholar]