Abstract
Allogeneic hematopoietic cell transplantation (HCT) is the most established form of cancer immunotherapy and has been successfully applied for the treatment and cure of otherwise lethal neoplastic blood disorders. Cancer immune surveillance is mediated to a large extent by alloreactive T- and natural killer (NK)-cells recognizing genetic differences between patient and donor. Profound insights into the biology of these effector cells has been obtained over recent years and used for the development of innovative strategies for intelligent donor selection, leading to improved graft-versus-leukemia effect without unmanageable graft-versus-host disease. The cellular composition of the stem cell source plays a major role in modulating these effects. This review summarizes the current state-of the-art of donor selection according to HLA, NK -alloreactivity and stem cell source.
Introduction
Selection of the best donor for allogeneic hematopoietic cell transplantation (HCT) is becoming an increasingly relevant issue, given the dramatic increase in donor options over the last years. Where human-leukocyte-antigen (HLA)-identical siblings were the sole donor source in the very early days, now over 30 million volunteer unrelated donors (VUD) and over 600.000 cord blood units (CBU) are registered worldwide, and a donor is available for most patients in need. Additionally, over recent years, HCT from HLA-haplotype (haplo) mismatched family donors has become a successful and widely used alternative. The complex mechanisms underlying graft-versus-leukemia (GvL) for the control of post-transplant relapse may vary considerably according to donor type, as they are modulated by both clinical and genetic factors of donor and host, as well as by the cellular composition of the stem cell graft. This review will summarize the current state of the art and outlook for donor selection according to HLA, to natural killer (NK) -cell alloreactivity and to stem cell source. These concepts were presented at the 3rd International Workshop on Biology, Prevention, and Treatment of Relapse after Stem Cell Transplantation held in Hamburg / Germany in November 2016 under the auspices of EBMT and ASBMT.
Donor selection according to HLA
Principles of HLA matching in HCT
The major histocompatibility complex (MHC) human chromosome 6p is the most polymorphic gene complex in eukaryotes, with 16,755 HLA alleles reported to date to the IMGT/HLA database (Release 3.28.0, 2017–04-13) 1. Ubiquitously and constitutively expressed HLA class I A, B, C-antigens, and cell-type-specific and inducible HLA class II DR, DQ, DP-antigens represent the major histocompatibility barrier to allogeneic tissue transplantation 2,3. HLA-alleles are inherited as haplotypes according to Mendelian rules and co-dominantly expressed, with a maximum of 12 different HLA-antigens encoded by the 6 HLA-loci on each chromosome. Except for cases of crossing-over due to genetic recombination, genotypically HLA-matched siblings share 12/12 HLA-alleles because they have inherited the same maternal and paternal copy of chromosome 6.Instead, siblings have a 50% likelihood of being HLA-haploidentical, i.e. to have inherited the same copy of chromosome 6 from one parent but not from the other. Parents are by definition HLA-haploidentical to their off-springs and vice versa, and a parental chromosome 6 can also be found in the extended family. This accounts for the availability of at least one HLA-haploidentical donor for most patients, with rising numbers of haplo HCT performed worldwide 4,5. An HLA-matched donor can also be identified in the international volunteer unrelated donor (VUD) or umbilical cord blood (UCB) registries 6, 7. Generally, these donors do not share the same ancestral haplotype but are matched by chance for at least the most relevant HLA alleles. The probability of finding a suitably HLA-matched VUD varies according to the ethnic group of the patient between 60 and 90% 8,9.
Mismatched HLA class I and class II-antigens expressed on patient antigen-presenting-cells (APC) are recognized by alloreactive donor T-cells after HCT. The precursor frequency of alloreactive T-cells is generally higher compared to conventional self-HLA restricted, peptide-antigen-specific T-cells, ranging from 1–10% 10. This is probably due to the cross-reactive nature of T-cell alloreactivity, whereby recognition of the same allogeneic HLA-molecule is mediated by conventional self-HLA-restricted T cells specific for different peptide-antigens (Figure 1). These peptide-antigens may or may not have been encountered previously, giving rise to alloreactive T-cells against mismatched HLA-antigens in the naïve and memory repertoire, respectively 11,12,10. This is in contrast to minor-histocompatibility-antigens (mHAgs), polymorphic peptides recognized in a conventional, self-HLA restricted manner by alloreactive T-cells which generally have not previously encountered the same mHAg and are therefore confined to the naïve repertoire 13,14,15 (Figure 1). These concepts need to be considered in the design of cellular immunotherapy protocols exploiting alloreactivity of specific T-cell subsets after HCT 16,17. Their differential pathophysiology also explains the weaker T-cell alloreactivity to mHAg compared to HLA-mismatches 17,18,19. Clinically, this translates into lower risks of clinically significant graft-versus-host-disease (GvHD) but also to less efficient beneficial GvL effects mediated by donor T-cells after gentoypically HLA-matched sibling HCT in which mHAgs are the sole targets of T-cell alloreactivity, compared to VUD, UCB or haploidentical HCT with varying degrees of additional HLA-mismatches.
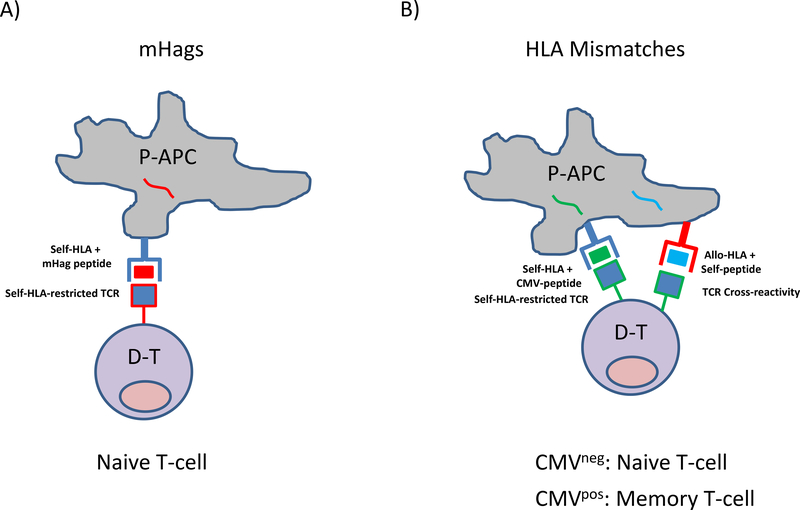
Figure 1. T-cell alloreactivity to mHAg or HLA-antigens mediating GvL and GvHD after HCT.
A) Self-HLA-restricted T-cell allorecognition of mHag peptides. Patient antigen-presenting-cells (P-APC) contain mHAg peptides (red) which are presented in the peptide-antigen binding groove of self-HLA-molecules (blue). HLA-matched donor T-cells (D-T) expressing a self-HLA-restricted T cell receptor (TCR) specific for the mHag peptide (red-lined blue square) recognize the mHAg-self-HLA complex on P-APC and mediate alloreactivity. Unless primed by previous events such as pregnancies or blood transfusions, the donor T-cells have not encountered the mHAg peptides before and are therefore in the naïve repertoire. B) Cross-reactive T-cell allorecognition of mismatched HLA-Antigens. Shown is a P-APC presenting, as an example, cytomegalovirus (CMV)-peptides (green) or self-peptides (blue) in the peptide-antigen-binding groove of self-HLA (blue) or allo-HLA molecules (red), respectively. D-T expressing a self-HLA-restricted TCR specific for the CMV-peptide (green-lined blue square) recognize the CMV-self-HLA complex on the P-APC, thereby mediating protective anti-viral immunity. In this example, the same TCR is also able to cross-recognize the allo-HLA presenting self-peptide due to molecular mimicry, thereby mediating alloreactivity. According to the donor’s CMV serostatus, the CMV-specific alloreactive T-cells will be predominant in the naive or in the memory compartment. CMV is shown here only as an example, self-HLA restricted T-cells specific for any foreign antigen can in principle display cross-reactive alloreactivity to mismatched HLA presenting self-peptides.
HLA mismatches and relapse according to different donor types
Based on the concepts outlined above, it is tempting to speculate that HLA-mismatches might be exploitable to reduce relapse by fostering GvL after HCT. Probably due to the clinically counterbalancing toxic effect of GvHD associated with the same HLA-mismatches, this concept is unfortunately not generally applicable (reviewed in 20). It is well established that the probability of overall survival after VUD HCT decreases significantly with every antigen- or allele-mismatch at HLA-A,-B,-C,-DRB1 (8/8 alleles), although the impact of HLA-disparity is lower in patients transplanted in advanced disease stage 21. The relevance of 8/8 HLA matching has been confirmed in numerous independent studies and is valid also in modern times 22–25. Nevertheless, a milestone study from the Japanese Registry showed that mismatches at HLA-C (but not at HLA-A,-B,-DRB1) are protective for relapse 26, an observation that might reflect the combined impact of T-cell and NK-cell alloreactivity, the latter being strongly influenced by missing HLA-C ligands as discussed in the following section. Importantly, VUD HCT is performed in over 80% of cases across mismatches at HLA-DPB1 which have been shown both experimentally and clinically to also be efficient GvL targets, with significantly lower relapse risks associated with HLA-DPB1 mismatches compared to matches 27. It has been proposed that genetically governed differential expression levels of certain HLA-C and DPB1 alleles modulates the risk of GvHD after VUD HSCT, whereby mismatched low expression alleles in the patient confer a lower GvHD-risk compared to high expression alleles 28, 29. Interestingly, these “GvHD permissive” mismatches were not associated with increased relapse risk, in line with the notion that lower levels of T-cell alloreactivity are needed for disease control than for the immune attack of healthy tissues 30, 31. Limited T-cell alloreactivity has also been proposed to be associated with matching for structural T-cell-epitopes (TCE) at HLA-DPB1, which in turn reflect the combined impact of amino acid polymorphism on T-cell alloreactivity, termed functional distance (FD) 23, 32–35. Permissive HLA-DPB1 mismatches between alleles of the same TCE group or with similar FD-scores were shown to provide a significant benefit for survival due to lower non-relapse-mortality and GvHD in the presence of a preserved GvL effect, compared to non-permissive HLA-DPB1 mismatches across different TCE groups or with distinct FD-scores 36. These proof-of-concept studies show that the identification of permissive mismatches in VUD HCT is feasible, and further insights into the role of presented peptides and/or the alloreactive T-cell repertoire might provide new avenues for their broader identification. This will be useful also in the context of UCB and haplo HCT where permissive mismatches are largely undefined to date 37, 38.
Overall, intelligent donor selection according to HLA is becoming increasingly accepted as a promising new strategy to harness T-cell alloreactivity after HCT, and is likely to become an instrumental tool complementary to cellular and pharmacological approaches that are being developed to this end.
Donor selection according to NK-cell alloreactivity
Relapse is the principal cause of death in acute myeloid leukemia (AML) patients after 100 days following HCT, making its reduction a paramount priority. A GVL effect in HCT has long been recognized 39), but harnessing its potential has been frustrated by gaps in knowledge about its exact mechanism. Recognition of the NK-cell as a major mediator of leukemic control has introduced a new optimism that donor selection based on NK-biology can be an effective intervention in capturing GVL alloreactivity, minimizing relapse, and increasing survival in allogeneic HCT for the treatment of AML.
NK-biology
First identified in 1975 40, the NK-cell discriminates self from non-self, targeting cells that specifically lack self-MHC determinants (“missing self”) 41. The basis of such discrimination resides in the NK-receptors that recognize MHC class I molecules, the Ly49 receptors in mice and the killer Ig-like receptors (KIR) in humans. The KIR genes demonstrate considerable inter-individual germline-encoded diversity, based on gene content 42,43, copy number 44, and polymorphism 45. Present in activating (KIR2DS1–4, KIR3DS1) and inhibitory (KIR2DL1–3, KIR3DL1–2) isoforms, KIR receptors interact with HLA class I ligands to “educate” the NK-cell and establish the degree of responsiveness. The inhibitory KIR2DL2/3, KIR2DL1, and KIR3DL1 interact with HLA-C1 (Ser77Asn80), HLA-C2 (Asn77Lys80), and HLA bearing the Bw4 epitope respectively.
While interaction between inhibitory KIR and its HLA class I ligand on the target cell leads to NK-cell inhibition, the same interaction in cis and trans titrate the response capacity of the NK-cell 46–49. NK-cells bearing an inhibitory KIR and cognate HLA ligand are “educated” for high response capacity. In contrast, NK-cells bearing an inhibitory KIR for which the individual lacks the HLA class I ligand are “uneducated” and display lower effector capacity. This results in educated NK-cells that are inhibited by self-HLA-bearing autologous cells but are highly effective at recognizing foreign or diseased cells that lack or have downregulated HLA. As a form of immune tolerance, uneducated NK-cells are hyporesponsive to autologous cells lacking the cognate ligand. Under inflammatory conditions, however, uneducated cells can be activated for effector function 50, 51.
With the exception of KIR2DS1, activating KIR have unknown ligands. HLA-C2 is the stimulatory ligand for KIR2DS1, except in the setting of HLA-C2 homozygosity, where the NK-response is suppressed 52, 53. Activating KIR typify KIR-B haplotypes, differing from the canonical KIR haplotype-A, which predominantly exhibits inhibitory KIR 43, 54.
Because expression of KIR receptors occurs largely stochastically, the behavior of the NK-cell at the single cell and population level is predictable based on genetics alone. This has facilitated several studies in allogeneic HCT to test how NK-genetics impacts HCT outcomes, outlining interventions that may finally capture the elusive GVL effect.
Missing self in HLA-mismatched HCT
Examination of educated NK activation according to “missing self” in HCT requires HLA mismatching across KIR ligands. Initial studies in haploidentical HCT demonstrated that “missing self” in a graft-versus-host vector is associated with lower relapse in AML, but not in acute lymphatic leukemia (ALL) patients 55, 56. This was followed by several studies in HLA-mismatched HCT 57–59, yielding inconsistent results. Nevertheless, the initial observation that “missing self” was associated with NK-activation and decreased AML relapse was the first confirmation that educated NK-cells play an important role in disease control in HCT.
NK-cell alloreactivity in HLA-matched HCT
Educated NK-cell activation requires HLA-mismatching, typically avoided in HCT due to the risk of GvHD. However, the prospect of NK-mediated relapse protection in HLA-matched HCT emerged when several retrospective studies observed decreased relapse and higher survival among patients who simply lack HLA ligands for donor inhibitory KIR. Among patients “missing ligand,” the greatest protection was among Bw6/Bw6 individuals 60. The highest relapse risk occurred for patients with all KIR ligands 60–62. Mediating the missing ligand protection is the uneducated NK-cell, whose hyporesponsiveness can be augmented in the setting of post-HCT inflammation 63.
Activating KIR: an argument for KIR-based donor selection
Numerous activating KIR populate the centromeric and telomeric portions of the KIR haplotype, collectively producing a diverse collection of KIR-B haplotypes 42. Telomeric KIR3DS1, the activating isoform to the inhibitory KIR3DL1, has been associated with lower transplant-related mortality (TRM) 64, 65. Furthermore, donors with haplotypes rich in centromeric activating KIR have been associated with lower relapse and higher survival 66, 67. Together, these early studies suggested that selecting donors with activating KIR can protect patients from relapse and TRM, increasing survival.
The HLA-C background of the individual shapes the activity of the KIR2DS1-bearing NK-cell, where homozygosity for the HLA-C2 ligand reduces NK-function 52, 53. Indeed, HLA-C2/C2 is a negative risk factor for AML relapse, neutralizing any KIR2DS1 benefit in HCT 68, 69. Thus, when selecting donors based on activating KIR, one must also consider the HLA background of the donor.
Donor selection based on KIR alleles
KIR polymorphism provides yet another exploitable possibility for increasing NK alloreactivity. HCT patients lacking Bw4 experience low relapse, presumed due to lack of inhibition of KIR3DL1+ NK-cells. Achieving a similar lack of inhibition, even in patients exhibiting the Bw4 epitope, can occur as a result of KIR3DL1 polymorphism, encoding allotypes with a range of specificities for ligand 48, 70, 71. Thus, NK-cells expressing alleles with poor avidity for Bw4 ligand signal less inhibition, resulting in higher activity against leukemic targets. Clinically translated, HLA-matched donor-recipients with low inhibition KIR3DL1-Bw4 allele combinations experience lower relapse and higher survival following HCT 72.
KIR/HLA-based donor selection feasible and realistic
Together, these studies offer increasing promise for capturing donor NK-cell alloreactivity and reducing AML relapse following HCT. From PCR-SSP and -SSOP 73, 74 to high-throughput sequencing 75, KIR typing technology is increasingly accessible. At the least, donor KIR typing is prognostic for patients with only one donor option; however, its greatest utility is for patients for whom more than one HLA-equivalent donor is available. 40% of patients exhibit all KIR ligands and are at high risk for relapse. The most relevant inhibitory KIR alleles and activating KIR are commonly found, making donor selection to avoid NK-inhibition and maximize NK activation a highly attainable goal.
Donor selection according to stem cell source
Cellular composition
The three commonly used cell sources for HCT are bone marrow (BM), GCSF-mobilized peripheral blood stem cells (PBSC) and UCB. The overall cellularity, as well as the cellular composition of these products differs, particularly with regards to CD34+ cell counts and T-cells 76–78, which may be expected to impact transplant outcomes, including relapse.
Relapse after HCT from BM vs PBSC
Holtick et al 79 performed a meta-analysis which included 1521 patients, transplanted between 1994 and 2009 in nine randomized control trials. In the cohort overall there was no significant difference in the incidence of disease relapse, although a trend in favor of reduced relapse with PBSC was reported (hazards ratio [HR]1.3; 95% confidence interval [CI] 0.98 to 1.72, P = 0.07). There was significant heterogeneity between trials with regards to this outcome, where a clear reduction in relapse was found for patients transplanted from related donors (HR 2.73; 95% CI 1.47 to 5.08, P = 0.001), but not for those transplanted from VUD (HR 1.07; 95% CI 0.78 to 1.47, P = 0.66). Only one of these studies 80 included patients receiving reduced intensity conditioning (RIC). To address this, two large retrospective registry-based analyses in the RIC-VUD setting have recently been performed. The Center for International Blood and Marrow Transplant Research (CIBMTR) 81 studied patients transplanted between the years 2000–2008, and found that relapse risk was higher with BM (relative risk [RR] 1.55, 95% CI 1.13–2.12, P = 0.006) in the setting of calcineurin inhibitor (CNI) and mycophenolate as GVHD prophylaxis (but not different when a CNI and methotrexate were given). Similar findings were reported by the European Society for Blood and Marrow Transplantation (EBMT) 82 in 602 patients with AML in complete remission transplanted between 2000 and 2007. On multivariate analysis, relapse incidence in the PBSC group was significantly reduced (HR, 0.61; P = 0.02). This group also studied this outcome in a similar population of HLA-identical siblings, where no difference in relapse incidence was found 83.
There is currently little comparative data available to directly address this question in the haplo setting, and none from prospective randomized studies. No difference in relapse was seen in several small retrospective studies 84, 85. However, a retrospective analysis which matched haplo-PBSC patients from several phase II studies on age and disease risk index 86 with haplo BM patients who had been transplanted on the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0603 87 study, found a significantly lower relapse incidence at 1, 2 and 3 years post-transplant in the PBSC patients 88.
In summary, PBSC is associated with a reduced risk of relapse in related, but not in VUD transplantation using myeloablative conditioning. In contrast, in certain RIC settings, relapse risk is reduced with PBSC in VUD transplantation, but the studies in related donor transplants are conflicting.
Relapse after HCT from UCB vs BM/PBSC
Shi-xia 89 performed a meta-analysis to address the comparative outcomes for pediatric recipients of BM vs UCB. They identified 1453 patients treated in seven comparative studies (not prospective or randomized). The relapse rate was reported in five studies, which showed a significantly lower rate in UCB recipients compared to BM recipients (OR 0.66, 95% CI (0.51, 0.86), p < 0.001). Fewer studies have reported this outcome in adult transplant recipients, where a difference in relapse risk does not seem to be found comparing UCB to BM 90, 91 or PBSC 90–93 grafts. A recent retrospective study 94 addressing this question only in adult recipients with myelodysplasia receiving RI regimens found a lower risk of relapse with PBSC grafts from a matched VUD (HR 0.57; 95% CI, 0.37 to 0.90; P = 0.02).
Recently it has been reported that there may be a relapse benefit when using UCB in the setting of minimal residual disease 95. Milano et al reported a retrospective analysis of 582 patients treated within a single institution with UCB or BM/PBSC from an VUD. They found an increased adjusted risk of relapse in the VUD (either stem cell source) compared to UCB (HR 1.95; 95% CI, 1.16 to 3.27; P=0.01). While relapse was significantly increased in recipients of BM/PBSC where MRD was present, this was not the case for recipients of UCB, where a non-significant increased HR of relapse was seen.
Single vs double UCB
Early retrospective studies suggested a reduced relapse risk in patients who received a double UCB graft compared to those receiving a single UCB unit 96, 97. This has not, however been confirmed in more recent retrospective studies 98, 99 or in a randomized study including 220 pediatric patients (BMT CTN 0501) 87. No prospective study in adult patients has been performed.
In summary, in pediatric recipients UCB is associated with a lower risk of relapse than BM. In adults, relapse risk with UCB vs PBSC is generally found to be similar. Studies regarding the impact on relapse of a single vs a double UCB unit are conflicting.
Conclusions
Relapse remains a major impediment to the clinical success of allo-HCT for the treatment of malignant blood disorders, in particular leukemias 100. In of the era of rapidly emerging targeted therapies for these diseases, improving the safety and efficacy of allo-HCT is more important than ever. Deep insights into the biology of cellular subsets involved in GvL and GvHD, including alloreactive T- and NK-cells, and cellular components of the different stem cell graft sources, have led to the development of innovative approaches.
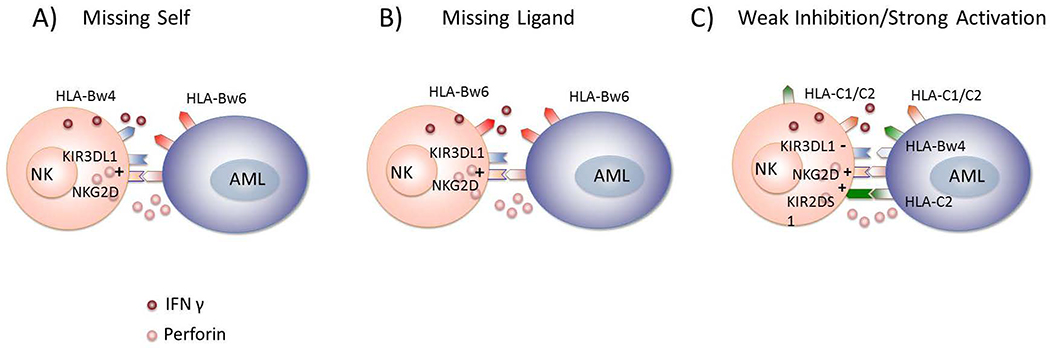
Figure 2. NK-cell alloreactivity mediating GvL after HCT.
A) NK-cell alloreactivity based on missing self-HLA. In HLA-mismatched HCT, educated donor NK cells stimulated by activating ligands, such as NKG2D ligand, are not inhibited from killing the target leukemia cell due to the lack of self-HLA ligand on the target cell. B) NK-cell alloreactivity based on missing ligand. In HLA-matched HCT, uneducated NK cells bearing KIR for which the patient lacks cognate HLA ligand can become activated under inflammatory conditions and can recognize and kill leukemic targets. C) NK-cell alloreactivity due to minimized inhibition and maximized activation. In HLA-matched HCT, educated donor NK-cells may experience less inhibition if the KIR-HLA interaction is characterized by low avidity. Heightened NK activity may occur if the donor NK cell expresses activating KIR.
Acknowledgements
This work was supported by grants from the Deutsche José Carreras Leukämie Stiftung (DJCLS R 15/02), the European Commission Transcan JTC2012 (Cancer12-045-HLALOSS), the Dr. Werner Jackstädt Stiftung, Germany and the Joseph Senker Stiftung, Germany to K.F.
Footnotes
Disclosure of Conflicts of Interest
The authors have no competing financial interests to declare.
References
- 1.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res 2015; 43(Database issue): D423–431. doi: 10.1093/nar/gku1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Rood JJ, van Leeuwen A, Persijn GG, Lansbergen Q, Goulmy E, Termijtelen A et al. Role of the HLA system in transplantation. HLA compatibility in clinical transplantation. Transplant Proc 1977; 9(1): 459–467. [PubMed] [Google Scholar]
- 3.Petersdorf EW. In celebration of Ruggero Ceppellini: HLA in transplantation. HLA 2017; 89(2): 71–76. doi: 10.1111/tan.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol 2016; 13(1): 10–24. doi: 10.1038/nrclinonc.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apperley J, Niederwieser D, Huang XJ, Nagler A, Fuchs E, Szer J et al. Haploidentical Hematopoietic Stem Cell Transplantation: A Global Overview Comparing Asia, the European Union, and the United States. Biol Blood Marrow Transplant 2016; 22(1): 23–26. doi: 10.1016/j.bbmt.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 6.Altaf SY, Apperley JF, Olavarria E. Matched unrelated donor transplants-State of the art in the 21st century. Semin Hematol 2016; 53(4): 221–229. doi: 10.1053/j.seminhematol.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Rocha V Umbilical cord blood cells from unrelated donor as an alternative source of hematopoietic stem cells for transplantation in children and adults. Semin Hematol 2016; 53(4): 237–245. doi: 10.1053/j.seminhematol.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 8.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 2014; 371(4): 339–348. doi: 10.1056/NEJMsa1311707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck K, Wadsworth K, Setterholm M, Maiers M, Confer D, Hartzman R et al. High-Resolution Match Rate of 7/8 and 9/10 or Better for the Be The Match Unrelated Donor Registry. Biol Blood Marrow Transplant 2016; 22(4): 759–763. doi: 10.1016/j.bbmt.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 10.Archbold JK, Macdonald WA, Burrows SR, Rossjohn J, McCluskey J. T-cell allorecognition: a case of mistaken identity or deja vu? Trends in immunology 2008; 29(5): 220–226. doi: 10.1016/j.it.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 11.Lakkis FG, Lechler RI. Origin and biology of the allogeneic response. Cold Spring Harb Perspect Med 2013; 3(8). doi: 10.1101/cshperspect.a014993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afzali B, Lechler RI, Hernandez-Fuentes MP. Allorecognition and the alloresponse: clinical implications. Tissue Antigens 2007; 69(6): 545–556. doi: 10.1111/j.1399-0039.2007.00834.x [DOI] [PubMed] [Google Scholar]
- 13.Falkenburg JH, Jedema I. Allo-reactive T cells for the treatment of hematological malignancies. Mol Oncol 2015; 9(10): 1894–1903. doi: 10.1016/j.molonc.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffioen M, van Bergen CA, Falkenburg JH. Autosomal Minor Histocompatibility Antigens: How Genetic Variants Create Diversity in Immune Targets. Front Immunol 2016; 7: 100. doi: 10.3389/fimmu.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spierings E Minor histocompatibility antigens: past, present, and future. Tissue Antigens 2014; 84(4): 374–360. doi: 10.1111/tan.12445 [DOI] [PubMed] [Google Scholar]
- 16.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest 2015; 125(7): 2677–2689. doi: 10.1172/JCI81229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Pira G, Di Cecca S, Montanari M, Moretta L, Manca F. Specific removal of alloreactive T-cells to prevent GvHD in hemopoietic stem cell transplantation: rationale, strategies and perspectives. Blood Rev 2016; 30(4): 297–307. doi: 10.1016/j.blre.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 18.Keever-Taylor CA, Passweg J, Kawanishi Y, Casper J, Flomenberg N, Baxter-Lowe LA. Association of donor-derived host-reactive cytolytic and helper T cells with outcome following alternative donor T cell-depleted bone marrow transplantation. Bone Marrow Transplant 1997; 19(10): 1001–1009. doi: 10.1038/sj.bmt.1700779 [DOI] [PubMed] [Google Scholar]
- 19.Irschick EU, Hladik F, Niederwieser D, Nussbaumer W, Holler E, Kaminski E et al. Studies on the mechanism of tolerance or graft-versus-host disease in allogeneic bone marrow recipients at the level of cytotoxic T-cell precursor frequencies. Blood 1992; 79(6): 1622–1628. [PubMed] [Google Scholar]
- 20.Fleischhauer K, Beelen DW. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin Hematol 2016; 53(2): 57–64. doi: 10.1053/j.seminhematol.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 2007; 110(13): 4576–4583. doi: 10.1182/blood-2007-06-097386 [DOI] [PubMed] [Google Scholar]
- 22.Furst D, Muller C, Vucinic V, Bunjes D, Herr W, Gramatzki M et al. High-resolution HLA matching in hematopoietic stem cell transplantation: a retrospective collaborative analysis. Blood 2013; 122(18): 3220–3229. doi: 10.1182/blood-2013-02-482547 [DOI] [PubMed] [Google Scholar]
- 23.Pidala J, Lee SJ, Ahn KW, Spellman S, Wang HL, Aljurf M et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood 2014; 124(16): 2596–2606. doi: 10.1182/blood-2014-05-576041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gratwohl A, Sureda A, Cornelissen J, Apperley J, Dreger P, Duarte R et al. Alloreactivity: the Janus-face of hematopoietic stem cell transplantation. Leukemia 2017. doi: 10.1038/leu.2017.79 [DOI] [PubMed] [Google Scholar]
- 25.Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 2016; 127(2): 260–267. doi: 10.1182/blood-2015-08-663823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morishima Y, Kashiwase K, Matsuo K, Azuma F, Morishima S, Onizuka M et al. Biological significance of HLA locus matching in unrelated donor bone marrow transplantation. Blood 2015; 125(7): 1189–1197. doi: 10.1182/blood-2014-10-604785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw BE, Gooley TA, Malkki M, Madrigal JA, Begovich AB, Horowitz MM et al. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood 2007; 110(13): 4560–4566. doi: 10.1182/blood-2007-06-095265 [DOI] [PubMed] [Google Scholar]
- 28.Petersdorf EW, Gooley TA, Malkki M, Bacigalupo AP, Cesbron A, Du Toit E et al. HLA-C expression levels define permissible mismatches in hematopoietic cell transplantation. Blood 2014; 124(26): 3996–4003. doi: 10.1182/blood-2014-09-599969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersdorf EW, Malkki M, O’HUigin C, Carrington M, Gooley T, Haagenson MD et al. High HLA-DP Expression and Graft-versus-Host Disease. N Engl J Med 2015; 373(7): 599–609. doi: 10.1056/NEJMoa1500140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9(9): 1144–1150. doi: 10.1038/nm915 [DOI] [PubMed] [Google Scholar]
- 31.van Bergen CA, van Luxemburg-Heijs SA, de Wreede LC, Eefting M, von dem Borne PA, van Balen P et al. Selective graft-versus-leukemia depends on magnitude and diversity of the alloreactive T cell response. J Clin Invest 2017; 127(2): 517–529. doi: 10.1172/JCI86175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zino E, Frumento G, Marktel S, Sormani MP, Ficara F, Di Terlizzi S et al. A T-cell epitope encoded by a subset of HLA-DPB1 alleles determines nonpermissive mismatches for hematologic stem cell transplantation. Blood 2004; 103(4): 1417–1424. doi: 10.1182/blood-2003-04-1279 [DOI] [PubMed] [Google Scholar]
- 33.Crocchiolo R, Zino E, Vago L, Oneto R, Bruno B, Pollichieni S et al. Nonpermissive HLA-DPB1 disparity is a significant independent risk factor for mortality after unrelated hematopoietic stem cell transplantation. Blood 2009; 114(7): 1437–1444. doi: 10.1182/blood-2009-01-200378 [DOI] [PubMed] [Google Scholar]
- 34.Fleischhauer K, Shaw BE, Gooley T, Malkki M, Bardy P, Bignon JD et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol 2012; 13(4): 366–374. doi: 10.1016/S1470-2045(12)70004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crivello P, Zito L, Sizzano F, Zino E, Maiers M, Mulder A et al. The impact of amino acid variability on alloreactivity defines a functional distance predictive of permissive HLA-DPB1 mismatches in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015; 21(2): 233–241. doi: 10.1016/j.bbmt.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 36.Crivello P, Heinold A, Rebmann V, Ottinger HD, Horn PA, Beelen DW et al. Functional distance between recipient and donor HLA-DPB1 determines nonpermissive mismatches in unrelated HCT. Blood 2016; 128(1): 120–129. doi: 10.1182/blood-2015-12-686238 [DOI] [PubMed] [Google Scholar]
- 37.McCurdy SR, Fuchs EJ. Selecting the best haploidentical donor. Semin Hematol 2016; 53(4): 246–251. doi: 10.1053/j.seminhematol.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 38.Barker JN, Kurtzberg J, Ballen K, Boo M, Brunstein C, Cutler C et al. Optimal Practices in Unrelated Donor Cord Blood Transplantation for Hematologic Malignancies. Biol Blood Marrow Transplant 2017. doi: 10.1016/j.bbmt.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75(3): 555–562. [PubMed] [Google Scholar]
- 40.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975; 5(2): 112–117. doi: 10.1002/eji.1830050208 [DOI] [PubMed] [Google Scholar]
- 41.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986; 319(6055): 675–678. doi: 10.1038/319675a0 [DOI] [PubMed] [Google Scholar]
- 42.Hsu KC, Liu XR, Selvakumar A, Mickelson E, O’Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol 2002; 169(9): 5118–5129. [DOI] [PubMed] [Google Scholar]
- 43.Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B et al. Human diversity in killer cell inhibitory receptor genes. Immunity 1997; 7(6): 753–763. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W, Johnson C, Jayaraman J, Simecek N, Noble J, Moffatt MF et al. Copy number variation leads to considerable diversity for B but not A haplotypes of the human KIR genes encoding NK cell receptors. Genome Res 2012; 22(10): 1845–1854. doi: 10.1101/gr.137976.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagtmann N, Rajagopalan S, Winter CC, Peruzzi M, Long EO. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity 1995; 3(6): 801–809. [DOI] [PubMed] [Google Scholar]
- 46.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006; 25(2): 331–342. doi: 10.1016/j.immuni.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol 2007; 179(9): 5977–5989. [DOI] [PubMed] [Google Scholar]
- 48.Boudreau JE, Mulrooney TJ, Le Luduec JB, Barker E, Hsu KC. KIR3DL1 and HLA-B Density and Binding Calibrate NK Education and Response to HIV. J Immunol 2016; 196(8): 3398–3410. doi: 10.4049/jimmunol.1502469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boudreau JE, Liu XR, Zhao Z, Zhang A, Shultz LD, Greiner DL et al. Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class I. Immunity 2016; 45(2): 280–291. doi: 10.1016/j.immuni.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 2005; 105(11): 4416–4423. doi: 10.1182/blood-2004-08-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005; 436(7051): 709–713. doi: 10.1038/nature03847 [DOI] [PubMed] [Google Scholar]
- 52.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol 2007; 179(2): 854–868. [DOI] [PubMed] [Google Scholar]
- 53.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood 2010; 115(6): 1166–1174. doi: 10.1182/blood-2009-09-245746 [DOI] [PubMed] [Google Scholar]
- 54.Dupont B, Hsu KC. Inhibitory killer Ig-like receptor genes and human leukocyte antigen class I ligands in haematopoietic stem cell transplantation. Curr Opin Immunol 2004; 16(5): 634–643. doi: 10.1016/j.coi.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 55.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 1999; 94(1): 333–339. [PubMed] [Google Scholar]
- 56.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295(5562): 2097–2100. doi: 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 57.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 2007; 110(1): 433–440. doi: 10.1182/blood-2006-07-038687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 2003; 102(3): 814–819. doi: 10.1182/blood-2003-01-0091 [DOI] [PubMed] [Google Scholar]
- 59.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant 2006; 12(8): 876–884. doi: 10.1016/j.bbmt.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 60.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 2005; 105(12): 4878–4884. doi: 10.1182/blood-2004-12-4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu KC, Gooley T, Malkki M, Pinto-Agnello C, Dupont B, Bignon JD et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant 2006; 12(8): 828–836. doi: 10.1016/j.bbmt.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 62.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood 2007; 109(11): 5058–5061. doi: 10.1182/blood-2007-01-065383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu J, Venstrom JM, Liu XR, Pring J, Hasan RS, O’Reilly RJ et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood 2009; 113(16): 3875–3884. doi: 10.1182/blood-2008-09-177055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Venstrom JM, Gooley TA, Spellman S, Pring J, Malkki M, Dupont B et al. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood 2010; 115(15): 3162–3165. doi: 10.1182/blood-2009-08-236943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mancusi A, Ruggeri L, Urbani E, Pierini A, Massei MS, Carotti A et al. Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces nonrelapse mortality. Blood 2015; 125(20): 3173–3182. doi: 10.1182/blood-2014-09-599993 [DOI] [PubMed] [Google Scholar]
- 66.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009; 113(3): 726–732. doi: 10.1182/blood-2008-07-171926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010; 116(14): 2411–2419. doi: 10.1182/blood-2010-05-283051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 2012; 367(9): 805–816. doi: 10.1056/NEJMoa1200503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Marsh SG et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol 2014; 192(10): 4592–4600. doi: 10.4049/jimmunol.1302517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parham P, Norman PJ, Abi-Rached L, Guethlein LA. Variable NK cell receptors exemplified by human KIR3DL1/S1. J Immunol 2011; 187(1): 11–19. doi: 10.4049/jimmunol.0902332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saunders PM, Pymm P, Pietra G, Hughes VA, Hitchen C, O’Connor GM et al. Killer cell immunoglobulin-like receptor 3DL1 polymorphism defines distinct hierarchies of HLA class I recognition. J Exp Med 2016; 213(5): 791–807. doi: 10.1084/jem.20152023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boudreau JE GF, Gooley TA, Stevenson PA, Le Luduec JB, Shaffer BC, Rajalingam R, Hou L, Hurley CK, Noreen H, Reed EF, Yu N, Vierra-Green C, Haagenson MD, Malkki M, Petersdorf EW, Spellman S, Hsu KC. KIR3DL1/HLA-B subtypes govern AML relapse after hematopoietic cell transplantation. Journal of Clinical Oncology 2017; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ordonez D, Moraru M, Gomez-Lozano N, Cisneros E, Vilches C. KIR typing by non-sequencing methods: polymerase-chain reaction with sequence-specific primers. Methods Mol Biol 2012; 882: 415–430. doi: 10.1007/978-1-61779-842-9_24 [DOI] [PubMed] [Google Scholar]
- 74.Boudreau JE, Le Luduec JB, Hsu KC. Development of a novel multiplex PCR assay to detect functional subtypes of KIR3DL1 alleles. PLoS One 2014; 9(6): e99543. doi: 10.1371/journal.pone.0099543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norman PJ, Hollenbach JA, Nemat-Gorgani N, Marin WM, Norberg SJ, Ashouri E et al. Defining KIR and HLA Class I Genotypes at Highest Resolution via High-Throughput Sequencing. Am J Hum Genet 2016; 99(2): 375–391. doi: 10.1016/j.ajhg.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pidala J, Anasetti C, Kharfan-Dabaja MA, Cutler C, Sheldon A, Djulbegovic B. Decision analysis of peripheral blood versus bone marrow hematopoietic stem cells for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009; 15(11): 1415–1421. doi: 10.1016/j.bbmt.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 77.Hassan HT, Stockschlader M, Schleimer B, Kruger W, Zander AR. Comparison of the content and subpopulations of CD3 and CD34 positive cells in bone marrow harvests and G-CSF-mobilized peripheral blood leukapheresis products from healthy adult donors. Transpl Immunol 1996; 4(4): 319–323. [DOI] [PubMed] [Google Scholar]
- 78.Chevallier P, Robillard N, Illiaquer M, Esbelin J, Mohty M, Bodin-Bressollette C et al. Characterization of various blood and graft sources: a prospective series. Transfusion 2013; 53(9): 2020–2026. doi: 10.1111/trf.12072 [DOI] [PubMed] [Google Scholar]
- 79.Holtick U, Albrecht M, Chemnitz JM, Theurich S, Shimabukuro-Vornhagen A, Skoetz N et al. Comparison of bone marrow versus peripheral blood allogeneic hematopoietic stem cell transplantation for hematological malignancies in adults - a systematic review and meta-analysis. Crit Rev Oncol Hematol 2015; 94(2): 179–188. doi: 10.1016/j.critrevonc.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 80.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012; 367(16): 1487–1496. doi: 10.1056/NEJMoa1203517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eapen M Unrelated donor transplantation: peripheral blood or bone marrow--does it matter? Best Pract Res Clin Haematol 2014; 27(3–4): 278–282. doi: 10.1016/j.beha.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 82.Nagler A, Labopin M, Shimoni A, Niederwieser D, Mufti GJ, Zander AR et al. Mobilized peripheral blood stem cells compared with bone marrow as the stem cell source for unrelated donor allogeneic transplantation with reduced-intensity conditioning in patients with acute myeloid leukemia in complete remission: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2012; 18(9): 1422–1429. doi: 10.1016/j.bbmt.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 83.Nagler A, Labopin M, Shimoni A, Mufti GJ, Cornelissen JJ, Blaise D et al. Mobilized peripheral blood stem cells compared with bone marrow from HLA-identical siblings for reduced-intensity conditioning transplantation in acute myeloid leukemia in complete remission: a retrospective analysis from the Acute Leukemia Working Party of EBMT. Eur J Haematol 2012; 89(3): 206–213. doi: 10.1111/j.1600-0609.2012.01811.x [DOI] [PubMed] [Google Scholar]
- 84.Bradstock K, Bilmon I, Kwan J, Blyth E, Micklethwaite K, Huang G et al. Influence of Stem Cell Source on Outcomes of Allogeneic Reduced-Intensity Conditioning Therapy Transplants Using Haploidentical Related Donors. Biol Blood Marrow Transplant 2015; 21(9): 1641–1645. doi: 10.1016/j.bbmt.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 85.Castagna L, Crocchiolo R, Furst S, Bramanti S, El Cheikh J, Sarina B et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant 2014; 20(5): 724–729. doi: 10.1016/j.bbmt.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 86.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014; 123(23): 3664–3671. doi: 10.1182/blood-2014-01-552984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wagner JE Jr.,, Eapen M, Carter S, Wang Y, Schultz KR, Wall DA et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med 2014; 371(18): 1685–1694. doi: 10.1056/NEJMoa1405584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Donnell PV, Eapen M, Horowitz MM, Logan BR, DiGilio A, Brunstein C et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: a matched-pair analysis. Bone Marrow Transplant 2016; 51(12): 1599–1601. doi: 10.1038/bmt.2016.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi-Xia X, Xian-Hua T, Xiang-Feng T. Unrelated umbilical cord blood transplantation and unrelated bone marrow transplantation in children with hematological disease: a meta-analysis. Pediatr Transplant 2009; 13(3): 278–284. doi: 10.1111/j.1399-3046.2008.01089.x [DOI] [PubMed] [Google Scholar]
- 90.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010; 11(7): 653–660. doi: 10.1016/S1470-2045(10)70127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marks DI, Woo KA, Zhong X, Appelbaum FR, Bachanova V, Barker JN et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica 2014; 99(2): 322–328. doi: 10.3324/haematol.2013.094193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood 2012; 119(23): 5591–5598. doi: 10.1182/blood-2011-12-400630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodrigues CA, Rocha V, Dreger P, Brunstein C, Sengeloev H, Finke J et al. Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica 2014; 99(2): 370–377. doi: 10.3324/haematol.2013.088997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robin M, Ruggeri A, Labopin M, Niederwieser D, Tabrizi R, Sanz G et al. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant 2015; 21(3): 489–495. doi: 10.1016/j.bbmt.2014.11.675 [DOI] [PubMed] [Google Scholar]
- 95.Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med 2016; 375(10): 944–953. doi: 10.1056/NEJMoa1602074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 2009; 114(19): 4293–4299. doi: 10.1182/blood-2009-05-220525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Labopin M, Ruggeri A, Gorin NC, Gluckman E, Blaise D, Mannone L et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica 2014; 99(3): 535–540. doi: 10.3324/haematol.2013.092254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia 2014; 28(4): 779–786. doi: 10.1038/leu.2013.259 [DOI] [PubMed] [Google Scholar]
- 99.Tsang KS, Leung AW, Lee V, Cheng FW, Shing MM, Pong HN et al. Indiscernible Benefit of Double-Unit Umbilical Cord Blood Transplantation in Children: A Single-Center Experience From Hong Kong. Cell Transplant 2016; 25(7): 1277–1286. doi: 10.3727/096368915X689631 [DOI] [PubMed] [Google Scholar]
- 100.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363(22): 2091–2101. doi: 10.1056/NEJMoa1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]