Abstract
Background
Although physical activity has been associated with better cognitive function and reduced dementia risk, its association with cognitive decline in normal aging remains uncertain.
Objective
To determine whether physical activity in youth and older age are associated with age-related cognitive change.
Methods
Over a period of 27 years, 2,027 community-dwelling adults (mean age 73.5; 60% women) of the Rancho Bernardo Study of Healthy Aging completed up to seven cognitive assessments, including tests of global cognitive function, executive function, verbal fluency, and episodic memory. At each visit, participants reported concurrent physical activity. At baseline (1988–1992), participants additionally reported physical activity as a teenager and at age 30. For each age period, participants were classified as regularly active (3+ times/week) or inactive.
Results
Associations between concurrent physical activity and better cognitive function were stronger with advancing age on all tests, even after accounting for education, health, and lifestyle factors, as well as survival differences (ps < 0.05). Baseline physical activity did not predict rates of cognitive decline (ps > 0.40). Individuals who were physically active at age 30 and older age maintained the highest global cognitive function with advancing age (p = 0.002).
Conclusion
Regular physical activity is associated with better cognitive function with advancing age. Physical activity in young adulthood may contribute to cognitive reserve, which together with physical activity in later years, may act to preserve cognitive function with age.
Keywords: Aging, cognitive decline, cognitive reserve, exercise, longitudinal study, physical activity
INTRODUCTION
Regular exercise may support neurobiological functions that help to maintain cognitive abilities and stave off cognitive impairment in later life. Elucidating how physical activity influences cognitive aging is essential for developing strategies to preserve independence and quality of life into older age. There is compelling evidence that physical activity is beneficial for brain function, and is associated with better cognitive performance and lower dementia risk [1, 2]. However, it is less clear if physical activity attenuates cognitive decline in normal aging. Furthermore, it remains uncertain whether maintaining physical activity into older age is necessary to slow cognitive aging, or whether an active youth confers sufficient resilience against cognitive impairment in later life, when individuals may be unable or less likely to engage in regular exercise [3].
Prior studies have reported that physical activity is associated with lower risk for dementia and cognitive impairment [4, 5]. Physical activity comprises both light daily activities such as housework, walking, or gardening, and more vigorous exercise such as running or dancing [6]. While daily activities and exercise may elicit distinct cerebrovascular and neurobiological effects, positive associations with cognitive function have been reported for a range of activities. Both aerobic [7, 8] and strength [9, 10] training improve executive function, memory, and verbal fluency in older individuals. Although some longitudinal investigations have found that better fitness or greater physical activity is associated with slower decline in global cognitive function, memory, verbal fluency, attention, and processing speed [11–15], a meta-analysis [1] recommended further high-quality investigation to resolve incongruencies regarding the reliability of these findings. Evaluating associations between physical activity and cognitive function in large samples of aging adults followed for extended periods, while assessing potential confounding factors, will help to clarify the role of physical activity in preserving cognitive function into late life.
There is evidence that early-life physical activity supports late-life cognitive function. For example, exercise at younger ages has been linked to faster processing speed in older men [16] and lower risk of cognitive impairment in older women [17], whereas midlife exercise predicts better memory and slower memory decline [18]. We previously examined associations between physical activity at multiple age periods throughout life and cognitive function at a single time point in older age [19]. Consistent with prior findings, we observed positive interactive effects of teenage and current physical activity on executive function among older adults, such that current physical activity was associated with better cognitive performance primarily in individuals who were also active as teenagers. These associations were significant regardless of activity intensity, supporting the notion that even light activities may support healthy cognitive aging. However, longitudinal investigation of cognitive aging relative to physical activity over the lifespan is limited, and it is unclear whether physical activity throughout life modifies rates of cognitive decline with age.
This study builds upon our prior cross-sectional investigation, by examining associations between physical activity and trajectories of cognitive performance over a maximum 27-year follow-up in a large cohort of community-dwelling older adults. We examined both prospective associations of physical activity as a teenager, at age 30 and in older age, with subsequent cognitive change, and longitudinal associations of concurrent physical activity with cognitive change. We hypothesized that physical activity in older age would be associated with better performance and attenuated cognitive decline, and that physical activity at earlier ages would augment the benefits of older age physical activity on healthy cognitive aging.
METHODS
Participants
Participants were southern California residents who were members of the Rancho Bernardo Study (RBS) of Healthy Aging, a longitudinal observational study of community-dwelling older adults [20]. Cognitive function was first assessed at a 1988–1992 research clinic visit (baseline), with repeated assessments occurring approximately every four years, with the final assessment in 2014–2016.
A total of 2,212 RBS participants who attended the 1988–1992 visit were eligible for study inclusion. Of these, 185 were excluded due to missing cognitive data and three were excluded due to missing physical activity information, leaving 2,027 participants in our analytic sample. Study procedures were approved by the University of California, San Diego Human Research Protections Program Board, and all participants provided informed written consent prior to each visit.
Cognitive assessment
A cognitive test battery was administered by a trained interviewer at each research visit. The Mini-Mental State Exam (MMSE) [21], Trails Making Test Part B (Trails B) of the Halstead-Reitan Battery [22], and category fluency tests [23] were administered at all seven visits; the Buschke-Fuld Selective Reminding test [24] was administered at five visits. The MMSE evaluated orientation, attention, language, and memory to yield a measure of global cognition with a maximum score of 30. Trails B required participants to connect alternating letters and numbers in sequence, with a maximum completion time of 300 s, to provide a measure of psychomotor tracking and executive function. The category fluency test assessed verbal semantic fluency by having participants name as many unique animals as possible in 60 s. The Buschke-Fuld Selective Reminding test measured verbal episodic memory, requiring participants to recall as many words as possible from a list of ten words read by the examiner. Participants were reminded of any forgotten words and asked to recall all ten words again; this process was repeated six times. The number of correctly recalled words over all six trials (total recall) was analyzed.
Physical activity assessment
Participant health and lifestyle information was collected at each clinic visit through questionnaires. At the 1988–1992 assessment (baseline), participants reported how many times per week they engaged in light, moderate and strenuous exercise, as a teenager, at age 30, and currently. Examples were provided for each exercise level (e.g., light: yoga, archery, fishing; moderate: tennis, fast walking, skiing; strenuous: running, hockey, football). Participants were classified as physically active if they engaged in any level of physical activity at least three times per week. At all subsequent assessments, participants were simply asked whether they currently exercised at least three times per week.
Health and lifestyle assessment
At each visit, participants reported how many 12 oz bottles or cans of beer, 5 oz glasses of wine, or drinks containing 1.25 oz of hard liquor they consumed during an average week. The total number of alcoholic beverages consumed weekly was summed. Participants reported whether they never, previously, or currently smoked. Education level was acquired at enrollment (<7 years, junior high school, some high school, completed high school, some college, completed college, graduate school) and converted to years of education. Height and weight were measured in the clinic with participants wearing light clothing and no shoes, and body mass index (BMI; kg/m2) was calculated. Participants reported whether they had ever been diagnosed with various common medical conditions (yes/no), and a comorbidity index summed the number of positive responses for the following: angina, heart attack, congestive heart failure, stroke, diabetes, high blood pressure, pulmonary disease, asthma, thyroid disease, and arthritis. Positive responses were carried forward to subsequent visits in analyses.
Statistical analysis
Demographic, behavioral, and cognitive characteristics at the initial cognitive assessment were compared between currently active and inactive participants, using independent sample t-tests for continuous variables, and chi-squared tests for categorical variables.
Association of physical activity with cognitive function by age
Mixed-effects regression models investigated effects of physical activity on cognitive performance by age. Models included intercept and age as random effects, which allow individual subject baseline levels (intercept) and slopes to vary randomly about the mean trajectory described by the fixed effect terms. Both linear and quadratic age terms were included, as well as fixed effects of sex (0 = women, 1 = men), education (years), and time-varying concurrent (concurrent with the cognitive assessment; henceforth referred to as older age) physical activity (0 = inactive, 1 = active). Retest effects were included for the Trails B and MMSE (coded as 0 for the first assessment, and 1 for all subsequent assessments), as we previously found significant practice effects for these tests [25, 26]. An age by physical activity interaction term was included to assess whether associations between older age physical activity and cognitive function differ by age. Because there were no interactions between sex and older age physical activity or among sex, age, and physical activity (ps>0.10), these interactions were excluded from final models. Models were repeated with additional adjustment for birth decade, comorbidity index (updated to include newly reported comorbidities at each visit), and time-varying measures of BMI, alcohol consumption (average drinks per week), and smoking (never/former/current).
Models were repeated excluding participants demonstrating cognitive impairment, defined as an MMSE score greater than two standard deviations below the expected mean (based on age, sex, and education adjusted norms [27]) at baseline or final assessment.
To estimate the magnitude of effects of physical activity on cognitive aging, we computed predicted cognitive test scores from mixed effects models for active and inactive individuals at each age. For each test, the age at which an active individual would score equivalently to an inactive 70-, 80-, or 90-year-old was computed, and the estimated age difference (active - inactive) was calculated.
To evaluate whether differences in survival by physical activity influenced results, a longitudinal joint shared random effects model was constructed for each test, adjusting for age and sex.
To assess whether physical activity at earlier ages modified the association between older age physical activity and cognitive function, analyses were repeated including terms for physical activity at a younger age (teenage or age 30, in separate analyses), older age physical activity, the three-way interaction among younger age physical activity, older age physical activity and age, and all two-way interactions among these three terms. To test for multicollinearity between physical activity at younger and older ages, variance inflation factors were computed between models with one or both physical activity measures. No evidence of multicollinearity was found; variance inflation factors ranged from 1.01 to 1.21.
Associations of baseline physical activity with cognitive function over time
In our models using age as the temporal measure, associations between time-varying physical activity and cognitive function may reflect within-subject differences in rates of cognitive decline or between-subject differences in cognitive performance by age. Therefore, to directly examine associations between physical activity and subsequent cognitive change, mixed effects regression models were performed including baseline age, time as a random factor, and physical activity at the initial cognitive assessment as the exposure. In addition to an age by time interaction, we also included an interaction between physical activity and time to assess differences in rates of decline by activity status, and an interaction between physical activity by age to assess cross-sectional age effects on the association between activity and cognitive function. To examine whether associations between baseline physical activity and cognitive decline differed by age, we included a three-way interaction term for physical activity by time by age.
Exact p-values for two-sided tests are shown; p <0.05 was considered statistically significant. Analyses were conducted with SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics
Of the 2,027 participants, 15% attended six or seven cognitive assessments, 21% attended four to five, 33% attended two to three, and 31% attended one. Of those who attended more than one cognitive assessment, 10% participated for 20 or more years, 78% for 10–19 years, and 12% for fewer than 10 years. A total of 1,440 (71%) participants reported engaging in regular physical activity at the initial cognitive assessment, 82% as a teenager, and 63% at age 30. Between 69 and 80% of individuals who were active at the initial cognitive assessment reported being currently active at each subsequent assessment.
Characteristics for active and inactive participants at the initial cognitive assessment are presented in Table 1. Participants ranged in age from 44–99 years (mean ± SD = 73.5 ± 9.0; 91% were 60 or older and 60% women). Compared to inactive participants, those who were active were younger, more likely to be male, participated in more visits and for a longer follow-up, were less likely to be current smokers, and reported fewer medical conditions (ps< 0.001). Active participants were also less likely to demonstrate cognitive impairment, attained more years of education, and had lower BMI (ps<0.01). Alcohol consumption did not differ by physical activity status (p = 0.12).
Table 1.
Characteristics of participants by physical activity status at the initial cognitive assessment
| Inactive N = 587 | Active N = 1440 | Group effect | |
|---|---|---|---|
| Age (y) | 75.3 ± 9.4 | 72.8 ± 8.8 | t = 5.42, p < 0.001 |
| Sex (% women) | 68 | 57 | χ2 = 18.76, p < 0.001 |
| Education (y) | 14.1 ± 2.2 | 14.5 ± 2.3 | t = 3.01, p = 0.003 |
| Total number of visits | 2.5 ± 1.8 | 3.1 ± 1.9 | t = 7.08, p < 0.001 |
| Follow-up time (y) | 5.9 ± 6.9 | 8.4 ± 7.3 | t = 7.27, p < 0.001 |
| Active as teenager (%) | 79 | 84 | χ2 = 26.76, p < 0.001 |
| Active at age 30 (%) | 54 | 67 | χ2 = 8.35, p = 0.004 |
| Alcohol (drinks/week) | 5.2 ± 7.3 | 5.7 ± 7.2 | t = 1.58, p = 0.12 |
| Smoking (% former/current) | 44 / 12 | 51 / 7 | χ2 = 16.95, p < 0.001 |
| BMI (kg/m2)* | 25.5 ± 4.3 | 25.0 ± 3.5 | F = 8.55, p = 0.004 |
| # Medical conditions | 1.3 ± 1.0 | 1.1 ± 1.0 | t = 3.58, p < 0.001 |
| Cognitively Impaired at baseline (%) | 18 | 14 | χ2 = 6.45, p = 0.01 |
Adjusted for sex.
Physical activity and cognitive function by age
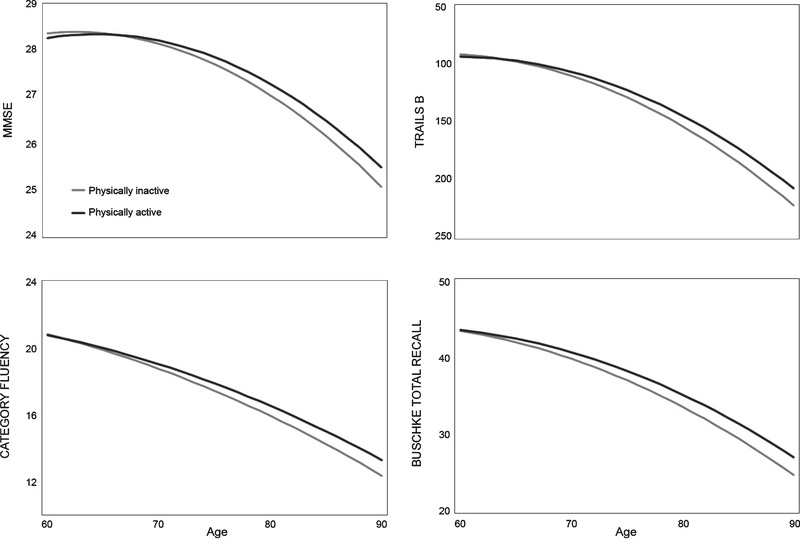
Base models of cognitive function by concurrent physical activity are presented in Table 2. There was an age-dependent association between physical activity and performance on all cognitive tests (MMSE, p = 0.003; Trails B, p<0.001; category fluency, p = 0.01; total recall, p = 0.02); physical activity was associated with better performance at older ages (Fig. 1). The effect size and significance of these associations were essentially unchanged (data not shown) after adjustment for birth decade, comorbidities, BMI, alcohol consumption, and smoking. Across tests, the association between physical activity and cognitive function was the equivalent of one to two years for a 70-year-old, two years for an 80-year-old, and two to 2.5 years for a 90-year-old. For example, models predicted that an inactive 80-year-old would score the equivalent of an active 82-year-old. Akaike information criterion (AIC) for models with and without physical activity are presented in Supplementary Table 4 for this and all mixed effects models.
Table 2.
Intercept and slope parameters (b-estimates) for each cognitive test from minimally adjusted linear mixed effects models of the longitudinal association between concurrent physical activity and cognitive function
| MMSE | Trails B | Category fluency | Total recall | |
|---|---|---|---|---|
| Intercept | 26.24*** | 145.55*** | 17.40*** | 39.55*** |
| Age | 1.06*** | −18.45*** | −0.94** | 0.29 |
| Age2 | −0.43*** | 12.27*** | −0.37*** | −1.29*** |
| Retest | 0.38*** | −3.93** | – | – |
| Sex | −0.42*** | −4.89* | 0.42* | −5.13*** |
| Education | 0.10*** | −2.97*** | 0.31*** | 0.51*** |
| Current physical activity | −0.28 | 7.78 | −0.36 | −0.61 |
| Current physical activity × age | 0.17** | −5.59*** | 0.32* | 0.72* |
p < 0.05
p < 0.01
p < 0.001.
Fig. 1.
Cognitive trajectories in older age by concurrent physical activity. Modeled trajectories for scores on each cognitive test as a function of time-varying physical activity status by age. Because 91% of participants were 60 years or older at baseline, the x-axis begins at age 60. The y-axis for Trails B is inverted because lower scores indicate better performance.
Overall, 524 (26%) participants met criteria for cognitive impairment at baseline or final assessment. Exclusion of these individuals did not alter the physical activity by age association for Trails B (base and full: ps < 0.001); but attenuated associations for category fluency and total recall (Supplementary Table 1). When individuals demonstrating cognitive impairment at baseline only were excluded, the physical activity by age interactions remained significant for all tests (ps<0.05).
Effects of survival on associations between physical activity and cognitive function by age
A total of 1,615 (80%) participants died over the course of cognitive follow-up (between 1988–2016). To determine whether differences in longevity influenced associations between physical activity and cognitive aging, analyses were repeated using joint models incorporating survival time. Concurrent physical activity was associated with lower risk of mortality during follow-up (hazard ratio = 0.79 [95% CI 0.70–0.89]; p<0.001). Age-dependent associations of physical activity and cognitive function remained significant and beta estimates were essentially unchanged after accounting for differences in survival time (age by activity interactions: MMSE, p = 0.001; Trails B, p<0.001; category fluency, p = 0.02; total recall, p = 0.01).
Physical activity at younger ages and cognitive function by age
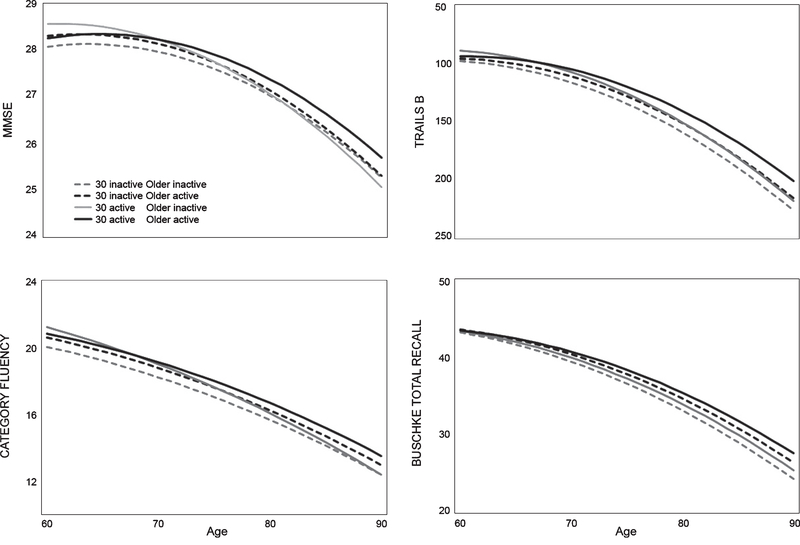
Teenage physical activity did not interact with the age-dependent association between older age physical activity and cognitive function for any test (Supplementary Table 2A). In contrast, there was a three-way interaction among age 30 physical activity, older age physical activity, and age for the MMSE (base: p = 0.002; full: p = 0.003); individuals active at both ages maintained the highest cognitive function over time (Fig. 2; Supplementary Table 2B). There was a trend for this interaction for category fluency (base: p = 0.08; full: p = 0.07). Multicollinearity between younger and older age physical activity was low (variance inflation factors = 1.01–1.21).
Fig. 2.
Cognitive trajectories in older age by age 30 and concurrent physical activity. Modeled trajectories for scores on each cognitive test as a function of time-varying physical activity status by older age (black/gray lines) and physical activity at age 30 (solid/dotted lines. The y-axis for Trails B is inverted because lower scores indicate better performance.
Baseline physical activity and cognitive function by time
To examine whether physical activity at baseline predicted cognitive change within individuals, mixed models used baseline rather than time-varying physical activity, and examined cognitive function over time adjusting for baseline age (Supplementary Table 3). There were no significant interactions between baseline physical activity and time for any test, or among physical activity, time, and age, indicating that baseline physical activity did not affect rate of decline over time, regardless of age at baseline.
DISCUSSION
In this cohort of older adults followed for up to 27 years, concurrent physical activity showed significant beneficial age-dependent associations with global cognitive function, executive function, memory, and verbal fluency. Building upon our prior cross-sectional study [19] that found the strongest associations with concurrent cognitive function for physical activity in older than younger ages, the present longitudinal investigation reports that associations of concurrent physical activity and cognitive function become more apparent with advancing age. These associations did not differ between men and women, were not affected by differences in survival, and were independent of several potential health and lifestyle confounders. Physical activity in early adulthood, but not during teenage years, augmented the positive association between cognitive function and physical activity in later life. While we did not find evidence that physical activity predicted rates of cognitive decline, our results suggest that physical activity throughout adulthood and into older age is important for supporting healthy cognitive aging.
Prior studies examining cognitive decline relative to physical activity have reported mixed findings. While some reported slower decline in global cognitive function, episodic memory, executive function, and verbal fluency [11–15], others found no link between physical activity and decline in executive function or verbal fluency [14, 15]. Discrepancies may stem from the challenge of distinguishing cross-sectional effects of age from longitudinal cognitive decline. A recent analysis of the Whitehall II cohort aimed to dissociate effects of physical activity on cross-sectional age differences in cognitive function from those on longitudinal change [28]. They observed that time-varying physical activity was associated with cognitive function with age, but not with cognitive decline over their 15-year follow-up of participants aged 45–69 at baseline. Our results, in an older cohort followed for a longer period of time, are consistent with their report. Together, the finding of an age-dependent association between physical activity and cognitive function implies that current physical activity is particularly important for cognitive health in the oldest old, consistent with lower rates of cognitive impairment for active adults 85 years of age or older [29].
The authors of the Whitehall study postulated that links between concurrent physical activity and cognitive function were driven by reverse causation, supported by their finding that declining physical activity began nine years prior to dementia diagnosis. In contrast, Buchman and colleagues [12] report that reduced physical activity predicted cognitive decline, but cognitive decline did not predict subsequent decline in physical activity. They also found that prior change in cognitive performance was not associated with physical activity, arguing against a reverse effect of cognitive impairment reducing activity. Although we did not have information on dementia diagnosis in our cohort, excluding individuals with cognitive impairment at first or last assessment did not change the age-dependent association between physical activity and executive function. Because the majority (80%) of our participants died over the course of study follow-up, failing physical or cognitive health in final years could have reduced physical activity levels during cognitive assessments close to time of death. However, we found that for all tests the age-dependent association of cognitive function with physical activity persisted after adjusting for comorbidities and after accounting for activity-related differences in survival time. This reduces, but does not eliminate, the likelihood that observed associations are due to reverse causation or declining health among inactive individuals.
The literature regarding effects of physical activity earlier in life on cognitive aging is sparse. Prior studies have reported better information processing speed in older men who were active as young adults [16], reduced risk of cognitive impairment for women who were active as teenagers [17], and slower memory decline during middle age for individuals who were physically active at age 36 [18]. However, none of these studies examined cognitive decline in older age relative to physical activity at younger ages. We add to these findings by reporting that physical activity during early life, independent of current activity status, did not reliably predict decline at older ages in any cognitive domain examined. Our finding that cognitive function is more tightly coupled to physical activity at older than younger ages is consistent with reports that physical activity in predominantly middle-aged participants did not predict cognitive decline, whereas physical activity in older age was associated with better cognitive function [28].
We observed that individuals who were active at both age 30 and in older age maintained better global cognitive function with advancing age, than individuals inactive at either age. Physical, social, or cognitive engagement early in life may elicit beneficial brain changes promoting “cognitive reserve” that may protect against cognitive deficits in later years [30]. Considering evidence that other lifestyle factors during youth and midlife, such as higher education or occupational status [31, 32], are cognitively protective, it is possible that the window of plasticity for establishing cognitive reserve through lifestyle choices extends beyond youth into adulthood. Alternatively, individuals who are active during adulthood may maintain a more active lifestyle throughout middle and older age, contributing to better overall cerebrovascular health or improved neural function compared to adults who are occasionally active.
Although reverse causality cannot be ruled out, there are several plausible biological pathways by which physical activity may act to preserve cognitive function during aging. Aerobic exercise has been found to alter neurotransmission [33] and to increase angiogenesis [34], trophic factors [35], synaptic plasticity [36], neurogenesis and long-term potentiation [37]. These studies point to improved cerebrovascular function, cultivation of an active pool of new hippocampal neurons, or enhanced synaptic plasticity, as prominent players underlying exercise-induced cognitive benefits. Notably, associations between physical activity and cognitive aging in the present study were robust to adjustment for various lifestyle factors and an overall health index. Thus, associations between physical activity and cognitive function may stem, at least partly, from positive neurobiological adaptations rather than from a generally healthy lifestyle or attenuation of comorbidities.
Limitations of this study include a homogeneous population of relatively well-educated, white, middle-class, southern California residents. While this limits generalizability to other populations, it minimizes confounding attributable to socioeconomic status, education, and access to healthcare. This study used self-reported physical activity; although this is a common method of assessing physical activity in cohort studies, it is subject to greater error than objective measurement. Furthermore, retrospective reporting of physical activity at younger ages may be less reliable due to recall bias, particularly in aging populations. Because physical activity intensity was not assessed after the initial cognitive evaluation, we were unable to dissociate effects of light activity versus strenuous exercise on rates of cognitive decline. We were also unable to differentiate effects of daily activities from those of exercise, given the phrasing of our physical activity questionnaire. Notable strengths include a large, well-characterized cohort, with one of the longest follow-up periods of any longitudinal study on physical activity and cognitive aging [38]. Furthermore, concurrent and retrospective assessment of physical activity at younger ages allowed us to examine associations of physical activity at different ages across the adult lifespan with later life cognitive change. Health histories obtained at each visit permitted accounting for current comorbidities. Although the proportion of participants engaging in regular physical activity was high (71%) in this relatively healthy, community-dwelling cohort, we were nonetheless able to identify meaningful associations with cognitive function.
In summary, this study identified a positive association between physical activity and better cognitive function that strengthened with older age, and found that physical activity earlier in adulthood augments this beneficial association. As the aging population continues to grow, there is mounting urgency to identify therapies to slow or delay aging-related cognitive decline. Efforts to encourage an active lifestyle in later years are a promising therapeutic avenue for maintaining cognitive health into older age, which may ultimately preserve independence and reduce clinical and caregiver burdens.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA021187); the National Institute of Aging (AG028507, AG00 7181); the National Institute of Diabetes and Digestive and Kidney Diseases (DK031801). Some study data were collected and managed using REDCap electronic data capture tools hosted at University of California San Diego and supported by a grant from the National Institutes of Health (UL1TR001442).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0491r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190491.
REFERENCES
- [1].Blondell SJ, Hammersley-Mather R, Veerman JL (2014) Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health 14, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chan JS, Yan JH, Payne VG (2013) The impact of obesity and exercise on cognitive aging. Front Aging Neurosci 5, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caspersen CJ, Pereira MA, Curran KM (2000) Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc 32, 1601–1609. [DOI] [PubMed] [Google Scholar]
- [4].Jedrziewski MK, Ewbank DC, Wang H, Trojanowski JQ (2010) Exercise and cognition: Results from the National Long Term Care Survey. Alzheimers Dement 6, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 58, 498–504. [DOI] [PubMed] [Google Scholar]
- [6].Caspersen CJ, Powell KE, Christenson GM (1985) Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep 100, 126–131. [PMC free article] [PubMed] [Google Scholar]
- [7].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, Liao L, Welsh-Bohmer KA, Browndyke JN, Kraus WE, Doraiswamy PM, Burke JR, Sherwood A (2019) Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 92, e212–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anderson-Hanley C, Nimon JP, Westen SC (2010) Cognitive health benefits of strengthening exercise for community-dwelling older adults. J Clin Exp Neuropsychol 32, 996–1001. [DOI] [PubMed] [Google Scholar]
- [10].Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT (2007) The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 39, 1401–1407. [DOI] [PubMed] [Google Scholar]
- [11].Barnes DE, Yaffe K, Satariano WA, Tager IB (2003) A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 51, 459–465. [DOI] [PubMed] [Google Scholar]
- [12].Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA (2012) Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78, 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hamer M, Muniz Terrera G, Demakakos P (2018) Physical activity and trajectories in cognitive function: English Longitudinal Study of Ageing. J Epidemiol Community Health 72, 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F (2004) Physical activity, including walking, and cognitive function in older women. JAMA 292, 1454–1461. [DOI] [PubMed] [Google Scholar]
- [15].Willey JZ, Gardener H, Caunca MR, Moon YP, Dong C, Cheung YK, Sacco RL, Elkind MS, Wright CB (2016) Leisure-time physical activity associates with cognitive decline: The Northern Manhattan Study. Neurology 86, 1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dik M, Deeg DJ, Visser M, Jonker C (2003) Early life physical activity and cognition at old age. J Clin Exp Neuropsychol 25, 643–653. [DOI] [PubMed] [Google Scholar]
- [17].Middleton LE, Barnes DE, Lui LY, Yaffe K (2010) Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc 58, 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Richards M, Hardy R, Wadsworth ME (2003) Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med 56, 785–792. [DOI] [PubMed] [Google Scholar]
- [19].Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Richard EL, Barrett-Connor E, McEvoy LK (2019) Lifetime physical activity and late-life cognitive function: The Rancho Bernardo study. Age Ageing 48, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barrett-Connor E (2013) The Rancho Bernardo Study: 40 years studying why women have less heart disease than men and how diabetes modifies women’s usual cardiac protection. Glob Heart 8. doi: 10.1016/j.gheart.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Folstein MF, Folstein SE, McHugh PR (1975) “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [22].Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8, 271–276. [Google Scholar]
- [23].Borkowski JG, Benton AL, Spreen O (1967) Word fluency and brain damage. Neuropsychologia 5, 135–140. [Google Scholar]
- [24].Buschke H, Fuld PA (1974) Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24, 1019–1025. [DOI] [PubMed] [Google Scholar]
- [25].Frank R, Wiederholt WC, Kritz-Silverstein DK, Salmon DP, Barrett-Connor E (1996) Effects of sequential neuropsychological testing of an elderly community-based sample. Neuroepidemiology 15, 257–268. [DOI] [PubMed] [Google Scholar]
- [26].Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Barrett-Connor E, McEvoy LK (2017) Effects of sex and education on cognitive change over a 27-year period in older adults: The Rancho Bernardo Study. Am J Geriatr Psychiatry 25, 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, Atri A (2011) A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sabia S, Dugravot A, Dartigues JF, Abell J, Elbaz A, Kivimaki M, Singh-Manoux A (2017) Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ 357, j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sumic A, Michael YL, Carlson NE, Howieson DB, Kaye JA (2007) Physical activity and the risk of dementia in oldest old. J Aging Health 19, 242–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scarmeas N, Stern Y (2004) Cognitive reserve: Implications for diagnosis and prevention of Alzheimer’s disease. Curr Neurol Neurosci Rep 4, 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW (1995) Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging 10, 578–589. [DOI] [PubMed] [Google Scholar]
- [32].White L, Katzman R, Losonczy K, Salive M, Wallace R, Berkman L, Taylor J, Fillenbaum G, Havlik R (1994) Association of education with incidence of cognitive impairment in three established populations for epidemiologic studies of the elderly. J Clin Epidemiol 47, 363–374. [DOI] [PubMed] [Google Scholar]
- [33].Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA (1989) Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand 136, 473–481. [DOI] [PubMed] [Google Scholar]
- [34].Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT (1992) Exercise and the brain: Angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab 12, 110–119. [DOI] [PubMed] [Google Scholar]
- [35].Neeper SA, Gomez-Pinilla F, Choi J, Cotman C (1995) Exercise and brain neurotrophins. Nature 373, 109. [DOI] [PubMed] [Google Scholar]
- [36].Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124, 71–79. [DOI] [PubMed] [Google Scholar]
- [37].van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C (2011) Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med 269, 107–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.