Abstract
Background & Aims
Although coronavirus disease 2019 (COVID-19) is characterized by fever and respiratory symptoms, some patients have no or mild symptoms. Severe acute respiratory syndrome-coronavirus (SARS-CoV-2) has been detected in feces of patients. We investigated gastrointestinal symptoms and shedding of virus into feces of patients with asymptomatic or mild COVID-19.
Methods
We collected data from 46 patients (median age, 26 y; 46% men) with asymptomatic or mild COVID-19 (without fever and pneumonia) and prolonged respiratory shedding of SARS-CoV-2, quarantined from April 4, 2020, through April 24, 2020, in Korea. Respiratory specimens included upper respiratory specimens (nasopharyngeal and oropharyngeal swabs) and lower respiratory specimens (sputum), and were collected twice per week. The median interval between COVID-19 diagnosis to the start of fecal sample collection was 37 days (range, 29–41 d); 213 stool specimens were collected from 46 patients. We used real-time reverse-transcription polymerase chain reaction to detect SARS-CoV-2 in the respiratory and fecal specimens.
Results
Gastrointestinal manifestations were observed in 16 of the 46 patients (35%); diarrhea was the most common (15%), followed by abdominal pain (11%), dyspepsia (11%), and nausea (2%). Virus RNA was detected in feces from 2 patients without gastrointestinal symptoms (4%). Mean cycle threshold values from the time of quarantine to the time of fecal collection tended to be lower in patients with virus detected in fecal samples than in patients without virus in fecal samples (29.91 vs 33.67 in the first week, 29.47 vs 35.71 in the fifth week, respectively). Shedding of virus into feces persisted until day 50 after diagnosis; fecal samples began to test negative before or at approximately the time that respiratory specimens also began to test negative.
Conclusions
In an analysis of fecal and respiratory specimens from patients with COVID-19 in quarantine in Korea, we found that the gastrointestinal tract could be a route of transmission of SARS-CoV-2 even in patients with asymptomatic or mild disease, with no gastrointestinal symptoms. The viral load of the respiratory specimens appears be related to shedding of the virus into feces in this group of patients.
Keywords: SARS-CoV-2, COVID-19, Asymptomatic, Mild, Feces
Abbreviations used in this paper: COVID-19, coronavirus disease 2019; Ct, cyclic threshold; GI, gastrointestinal; LTC, Living Treatment Center; RT-PCR, reverse-transcriptase polymerase-chain reaction; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2
What You Need to Know.
Background
This study investigated gastrointestinal symptoms and shedding of virus into feces of patients with asymptomatic or mild coronavirus disease 2019 (COVID-19).
Findings
An analysis of fecal and respiratory specimens from patients with COVID-19 in quarantine in Korea found that the gastrointestinal tract could be a route of transmission of severe acute respiratory syndrome–coronavirus, even in patients with asymptomatic or mild disease, with no gastrointestinal symptoms. The viral load of the respiratory specimens appears be related to shedding of the virus into feces in this group of patients.
Implications for patient care
Even patients with mild or no symptoms of COVID-19, and no gastrointestinal symptoms, still shed virus into feces.
Only a few weeks after the first case of coronavirus disease 2019 (COVID-19) was reported in Wuhan, China, the first case of COVID-19 was confirmed in the Republic of Korea on January 20, 2020.1 On February 18, when a suspicious index case was identified in a religious group, a sharp increase in the number of COVID-19 cases was observed, with most infections being reported in specific clusters and geographic regions; the highest number of COVID-19 cases was reported in Daegu, followed by Gyeongbuk.1, 2, 3 Until April 4, 2020, 10,156 cumulative cases of COVID-19 were reported in Korea. Although the number of new cases is decreasing in Korea, as of April 4, 2020, there were 3654 patients (36%) still in quarantine; therefore, the possibility of prolonged respiratory shedding of SARS-CoV-2 has become a matter of concern.
It is well established that most patients with COVID-19 have fever along with respiratory symptoms, and human-to-human transmission occurs among close contacts, mainly through respiratory droplets and direct contact.4 , 5 However, the diseases caused by novel coronaviruses are found commonly to be accompanied with gastrointestinal (GI) manifestations,6 , 7 and these viruses may be viable in environments/conditions that facilitate fecal–oral transmission.8 In the of severe acute respiratory syndrome (SARS) outbreak of 2002 to 2003, viral RNA in the feces was detectable for more than a month in some patients.9 In experimental conditions, the stability of severe acute respiratory syndrome-coronavirus (SARS-CoV-2) has been found to be similar to that of SARS-CoV-1.10 However, SARS-CoV-2 and SARS-CoV-1 have different viral kinetics at high viral loads, with prolonged shedding in the upper respiratory tract even in patients with asymptomatic and mildly symptomatic disease (unpublished data). Hence, the GI symptoms and fecal viral shedding in prolonged respiratory shedders with asymptomatic or mild COVID-19, who were in quarantine for more than a month, must be evaluated.
Methods
Study Participants
We conducted a prospective cohort study involving the patients diagnosed with asymptomatic or mild COVID-19, quarantined between April 4 and April 24 at the Youngdeok Samsung Living and Treatment Center (LTC). Patients quarantined at the beginning of Youngdeok Samsung LTC’s operation (March 4) (group 1) and patients transferred to Youngdeok Samsung LTC on April 5 (group 2) were included. Patients were defined as having asymptomatic or mild COVID-19 as per the Korea Centers for Disease Control and Prevention severity criteria (http://ncov.mohw.go.kr/en), when the following conditions were met: (1) age younger than 60 years, (2) no underlying chronic diseases, (3) alert mentality, (4) body temperature lower than 37.5°C, and (5) no radiologic evidence of pneumonia. Originally, Youngdeok Samsung LTC was a human resource training center of Samsung, but on March 2, 2020, out-of-hospital quarantine and care for patients with asymptomatic or mild COVID-19 was mandated by the Korea Centers for Disease Control and Prevention. Therefore, the accommodation facilities including Youngdeok Samsung human resource training centers were transformed to a LTC, and COVID-19 patients in Daegu and Gyeongbuk were quarantined there on March 4.11 This center has 300 individual rooms in 7 buildings, and each patient was quarantined in a single-bed room.
COVID-19 was confirmed by real-time reverse-transcription polymerase-chain reaction (RT-PCR) assay for SARS-CoV-2 in upper respiratory tract specimens using nasopharyngeal and oropharyngeal swabs and/or lower respiratory tract specimens (ie, sputum).12 Although none of the patients showed fever or respiratory symptoms with suspected pneumonia, real-time RT-PCR assays were performed because they were close contacts.
This study was approved by the Institutional Review Board of Kang Buk Samsung Medical Center (KBSMC-2020-03-065). Written informed consent was obtained from all patients.
Collection of Respiratory Specimens and Monitoring Symptoms
Starting March 4, 2020, all quarantined patients’ respiratory specimens were collected at Youngdeok Samsung LTC every Monday by medical teams tested, and respiratory specimens were tested for SARS-CoV-2 nucleic acid by real-time RT-PCR assay. Respiratory specimens included upper respiratory specimens (nasopharyngeal swab and oropharyngeal swab) and lower respiratory specimens (sputum). Patients who showed cyclic threshold (Ct) values were reported the following day. Then, the specimens showing SARS-CoV-2–positive results were retested the following Monday. The patients whose specimens showed negative or inconclusive results on Monday were retested on Wednesday, and in cases of inconclusive and negative results on Monday and Wednesday, an additional test was performed on Thursday. Since April 13, respiratory specimens were collected on Mondays and Thursdays regularly. Only patients with 2 consecutive negative results on RT-PCR assay at least 24 to 48 hours apart were discharged from the LTC.
Official reports including past medical history and symptoms at the time of diagnosis were obtained from the district public health centers. Since the beginning of the operation of Youngdeok Samsung LTC, 11 medical staff (4 doctors and 7 nurses) had been carefully monitoring the patients’ symptoms twice a day (at 9 am and 5 pm) by a mobile application. Thermometers were given to each patient, and the patients were monitored for the following symptoms every day: fever (>37.5°C), headache, myalgia, cough, sputum, rhinorrhea, nasal obstruction, chest pain, anosmia, fatigue, nausea, vomiting, diarrhea, and loss of appetite.
For this study, we collected additional data on underlying digestive diseases including reflux esophagitis, gastritis, gastric ulcer, irritable bowel syndrome, inflammatory bowel disease, fatty liver, viral hepatitis, cirrhosis, pancreatitis, cholecystitis, and cholangitis. Since the day we started collecting fecal specimens, we also collected additional data on GI manifestations, such as abdominal pain and dyspepsia, once a day by telephone call, because they were not included in the list of symptoms that were investigated using the mobile application. Data regarding abdominal pain and dyspepsia that occurred at the time of diagnosis and before the day we started collecting fecal specimens were collected by patient recall.
Collection of Fecal Specimens
To ensure proper collection and testing of fecal specimens for SARS-CoV-2, fecal specimens of patients who were admitted to KangBuk Samsung Hospital were tested in various conditions (see the Supplementary Methods and Supplementary Table 1 for further details). Considering the earlier-described results, from April 4 and 5, 2020, for groups 1 and 2, respectively, stool collection kits and refrigerators were provided in each room of the LTC and stool collection was conducted by patients until April 24 (see the Supplementary Methods section for further details).
Reverse-Transcription Polymerase Chain Reaction Assay for Severe Acute Respiratory Syndrome-Coronavirus 2
RT-PCR assays were performed in the Green Cross Laboratories for respiratory specimens, and in the KangBuk Samsung Hospital for fecal specimens (see the Supplementary Methods section for further details).
Statistical Analysis
Baseline characteristics including age, sex, and days between laboratory diagnosis and admission were summarized using descriptive statistics including proportion, means ± SD, median, and range. All statistical analyses were performed using SPSS software (SPSS 26.0 for Windows; SPSS, Chicago, IL).
Results
Clinical Characteristics of Asymptomatic and Mildly Symptomatic Patients
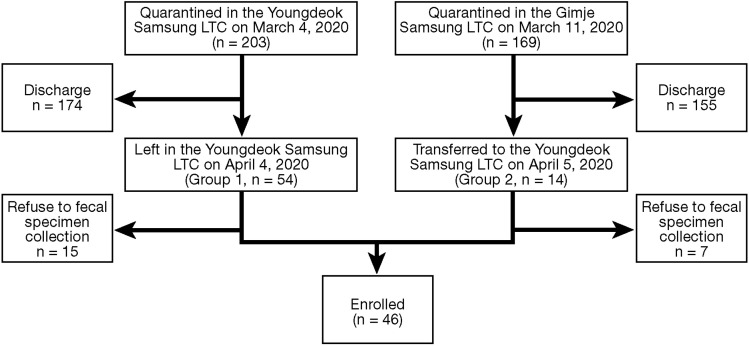
A total of 203 patients were diagnosed with COVID-19 between February 18 and March 1, 2020, and quarantined in the Youngdeok Samsung LTC on March 4, 2020. Until April 4, 2020, 147 patients were discharged on the basis of 2 consecutive negative results on RT-PCR assay and 54 patients remained, of whom 39 agreed to provide fecal specimens (group 1). Another group of 169 patients was diagnosed with COVID-19 between February 25 and March 8, 2020, and quarantined in the Gimje Samsung LTC. Until April 4, 2020, 155 patients were discharged and the remaining 14 patients were transferred to Youngdeok Samsung LTC on April 5, 2020 (group 2). Among them, 7 patients agreed to provide fecal specimens. Finally, a total of 46 patients were enrolled in this study (Figure 1 ).
Figure 1.
Flow chart of enrolled patients. LTC, Living and Treatment Center.
The clinical characteristics of study participants with asymptomatic and mildly symptomatic COVID-19 are described in Table 1 . The median age was 26 years, and 46% were men. The median number of days from diagnosis to quarantine was 6.5 days (range, 4.0–18.0 d). The median number of days from diagnosis to fecal sample examination was 37.0 (range, 29.0–41.0 d); this indicates that patients had prolonged respiratory shedding of SARS-CoV-2 for a median of 37 days at the start of fecal examination. We followed up the patients for up to 61 days (median, 52 d; range, 39–61 d).
Table 1.
Clinical Characteristics of Enrolled Patients
| N = 46 | |
|---|---|
| Age, median (range) | 26 (18–57) |
| Male, n (%) | 21 (45.6%) |
| Days from diagnosis to quarantine (March 4 or 11, 2020),a median (range) | 6.5 (4.0–18.0) |
| Days from diagnosis to fecal sample examination (April 4 or 5, 2020),b median (range) | 37.0 (29.0–41.0) |
| Days from diagnosis to last follow-up evaluation (April 24, 2020), median (range) | 52.0 (39.0–61.0) |
| Medical history, digestive, n (%) | 11 (23.9%) |
| Reflux esophagitis | 6 (13.0%) |
| Gastritis | 3 (6.5%) |
| Gastric ulcer | 0 |
| Irritable bowel disease | 3 (6.5%) |
| Inflammatory bowel disease | 0 |
| Fatty liver | 1 (2.1%) |
| Viral hepatitis | 0 |
| Cirrhosis | 0 |
| Pancreatitis | 0 |
| Cholecystitis | 0 |
| Cholangitis | 0 |
| Medical history, others,c n (%) | 3 (6.5%) |
| General symptoms from diagnosis to last follow-up evaluation, n (%) | 38 (82.6%) |
| Feverd | 1 (2.2%) |
| Headache | 12 (26.1%) |
| Myalgia | 6 (13.0%) |
| Cough | 17 (36.9%) |
| Sputum | 26 (56.5%) |
| Rhinorrhea | 15 (32.6%) |
| Nasal obstruction | 5 (10.9%) |
| Chest pain | 7 (15.2%) |
| Anosmia | 4 (8.7%) |
| Fatigue | 11 (23.9%) |
| Loss of appetite | 1 (2.2%) |
Group 1 on March 4, 2020, and group 2 on March 11, 2020.
Group 1 on April 4, 2020, and group 2 on April 5, 2020.
Other diseases included gout (n = 1), thyroid cancer (n = 1), and pneumothorax (n = 1).
Fever occurred after quarantine in 1 patient.
A medical history of digestive diseases was reported in 24% of the study population: reflux esophagitis (13%), gastritis (7%), irritable bowel disease (7%), and fatty liver (2%). A total of 38 patients (83%) complained of symptoms during the monitoring period after diagnosis, mostly sputum (57%) and coughing (37%).
Detailed digestive symptoms are shown in Table 2 . A total of 16 patients (35%) presented with digestive symptoms, and diarrhea was the most common (15%), followed by abdominal pain (11%), dyspepsia (11%), and nausea (2%). Only 1 patient (2%) presented with abdominal pain, but no respiratory symptoms at diagnosis. Most of the patients (94%; 15 of 16) complained of digestive symptoms within 1 month after diagnosis. Five patients presented with digestive symptoms after 1 month from diagnosis (Supplementary Figure 1). Eight (17%) patients did not have any symptoms (general or digestive) from the time of diagnosis to the last follow-up evaluation.
Table 2.
Digestive Symptoms of Enrolled Patients
| Total (N = 46) | Time of symptom occurrence |
|||
|---|---|---|---|---|
| At diagnosis | Within 1 month from diagnosis | After 1 month from diagnosis | ||
| Symptoms, digestive, n (%) | 16 (34.7) | 1 (2.1) | 15 (32.6) | 5 (10.8) |
| Nausea | 1 (2.1) | 0 | 1 (2.1) | 1 (2.1) |
| Vomiting | 0 | 0 | 0 | 0 |
| Abdominal pain | 5 (10.8) | 1 (2.1) | 2 (4.3) | 2 (4.3) |
| Diarrhea | 7 (15.2) | 0 | 7 (15.2) | 1 (2.1) |
| Dyspepsia | 5 (10.8) | 0 | 3 (6.5) | 2 (4.3) |
Supplementary Figure 1.
Timeline of the fecal specimen collection according to days since diagnosis. Feces collection dates are color-coded yellow and positive results are color-coded red. The numbers shown are Bristol stool scale scores. Patient 45 had dyspepsia at 37 days, patient 29 had abdominal pain at 44 days, patient 19 had nausea at 45 days, and patient 36 had dyspepsia at 42 days, abdominal pain at 42, 43, and 47 days, and diarrhea at 41, 47, and 50 days. #, indicates digestive symptoms. DC, discharge; Pt, patient; TF, transfer.
Reverse-Transcription Polymerase Chain Reaction Assay for Severe Acute Respiratory Syndrome-Coronavirus 2 in Fecal Specimens
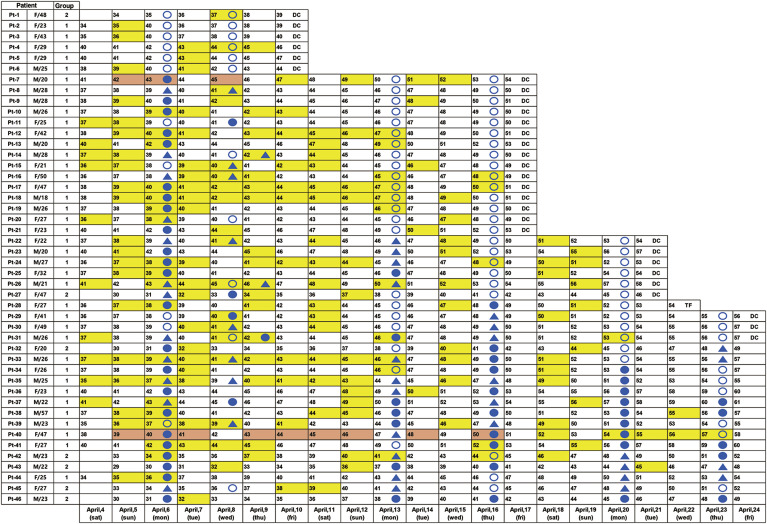
Fecal specimens were collected from April 4 to April 24, 2020 (21 days), and the median interval between COVID-19 diagnosis to the start of fecal sample collection was 37 days (range, 29–41 d) (Table 1, Figure 2 ). A total of 213 fecal specimens were collected from 46 patients (Figure 2). During the study period, 30 (65%) patients were discharged from the LTC, and 1 patient was transferred to a hospital for persistent fever.
Figure 2.
Timeline of the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) test results of the respiratory and fecal specimens from patients at the Living Treatment Center. Numbers denote days since diagnosis. Feces collection dates are color-coded in yellow, and positive results are color-coded in red. A filled blue circle represents a positive result of a respiratory specimen, a filled blue triangle represents an inconclusive result, and an open circle represents a negative result. DC, discharge; TF, transfer.
Among 46 patients, 2 patients (patient 7 [21-year-old man] and patient 40 [48-year-old woman]) were positive for SARS-CoV-2 (2 of 46; 4%) per their fecal samples (Figure 2); 12 fecal specimens were positive for SARS-CoV-2 (12 of 213; 6%) (Figure 2). The Bristol scale of positive fecal specimens was 4 in patient 40, and 3 or 2 in patient 7 (Supplementary Figure 1). None of the patients had underlying digestive disease or other diseases, and none had digestive symptoms from diagnosis to last follow-up evaluation. Patient 7 had symptoms such as fatigue, rhinorrhea, sputum, and headache on several days during quarantine in the LTC and was discharged 54 days after diagnosis. Patient 40 complained of fatigue, sputum, cough, chest pain, myalgia, and headache during quarantine and could not be discharged during the study period because of consistent detection of SARS-CoV-2 in respiratory samples (until April 24, 2020). After follow-up evaluation, she was discharged on day 62 because 2 consecutive negative results were obtained from her respiratory specimens on day 57 and day 61 (Figure 2).
Comparing Cyclic Threshold Values From Reverse-Transcription Polymerase Chain Reaction Testing of Respiratory Specimens and Fecal Specimens Between Patients With Positive and Negative Fecal Samples
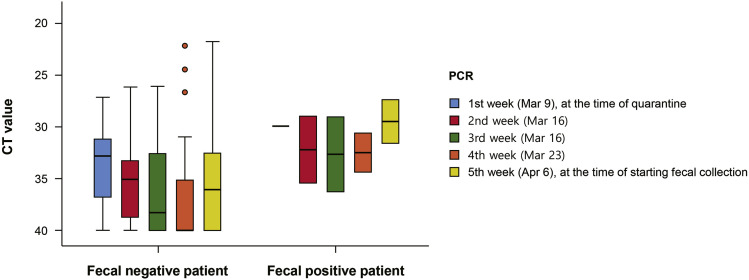
The Ct values of SARS-CoV-2 were based on the results of RdRp gene amplification. To compare the viral kinetics between patients with positive and negative fecal samples during 1 month of quarantine, we obtained weekly Ct values on RT-PCR testing of respiratory samples from March 9, 2020, the first Monday after starting operations of the LTC, until April 6, 2020, the first Monday after starting fecal collection. In the first month after quarantine, Ct values were available only for group 1 (n = 39), and the mean Ct value of respiratory specimens for group 1 was 33.46 (median, 32.79; range, 27.13–40.0) at the time of quarantine (based on data from March 9, 2020). The mean Ct of the 2 patients with positive fecal samples was 29.91 (29.94 and 29.88, respectively), and the mean Ct of patients with negative fecal samples (n = 37) was 33.67 (median, 32.8; range, 27.13–40.00) (Figure 3 ). At the time of starting fecal collection (based on data from April 6, 2020), the mean Ct of the respiratory specimens of group 1 was 35.39 (median, 36.27; range, 21.74–40.0). In the 2 patients with positive fecal samples, the mean Ct was 29.47 (31.58 and 27.36, respectively) and that of patients with negative fecal samples (n = 44) was 35.71 (median, 36.48; range, 21.7–440.00) (Figure 3).
Figure 3.
Comparison of cyclic threshold (Ct) values of respiratory specimens between patients with positive and negative fecal samples. PCR, polymerase chain reaction.
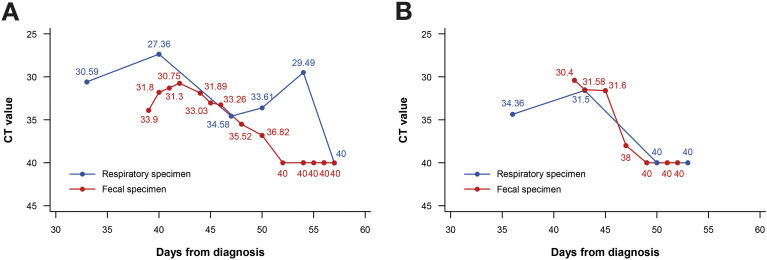
We investigated the chronologic changes in RT-PCR test results in both fecal and respiratory specimens of the 2 patients with positive fecal samples (Figure 4 ). In patient 40, RT-PCR results of both respiratory and fecal specimens were positive on day 50, but fecal specimens converted to negative on day 52, and the negative results were sustained on day 54, whereas the respiratory specimens still yielded positive results. Respiratory specimens were converted to negative on day 57 (Figures 2 and 4 A). In patient 7, the RT-PCR results of the fecal specimens converted to negative on day 47, and the respiratory specimens converted to negative on day 50 (Figures 2 and 4 B).
Figure 4.
Chronologic changes in reverse-transcription polymerase chain reaction testing results. (A) Patient 40. (B) Patient 7. Ct, cyclic threshold.
Discussion
In this prospective study of 46 patients with asymptomatic or mild COVID-19 in an out-of-hospital LTC, GI manifestations were found in 35% of the study participants during 2 months of evaluation, in 2% at the time of diagnosis, in 33% within 1 month from diagnosis, and in 11% after 1 month from diagnosis. Viral RNA was detected in the feces of 2 (4%) patients, although they did not have any GI manifestations since diagnosis to discharge. Of note, the mean Ct values from the time of quarantine to the time of fecal collection tended to be lower in patients with positive fecal samples than in patients with negative fecal samples (29.91 vs 33.67 in the first week, 29.47 vs 35.71 in the fifth week, respectively). Fecal viral shedding of SARS-CoV-2 persisted until day 50 and converted to negative before or around the time of negative conversion of the respiratory specimen results.
In a meta-analysis of 60 studies, comprising 4243 patients, the pooled prevalence rate of all GI symptoms was 17.6%, and when compared with the prevalence of GI symptoms according to the severity of COVID-19, pooled prevalence rates of GI symptoms were 11.8% and 17.1% in patients with nonsevere and severe COVID-19, respectively.13 In others studies that were not included in previous meta-analysis, the prevalence rates of GI symptoms were 11.4% (75 of 651),14 50.5% (101 of 204),15 and 61.1% (58 of 95), respectively.16 In our study, although the enrolled patients had nonsevere, mild-to-asymptomatic disease, the prevalence of GI symptoms was 34.7%. Considering the possibility of selection bias, we also investigated digestive symptoms in 22 patients who did not agree to participate in the stool test. There were no difference in digestive symptoms and diarrhea between those who participated in the study and those who did not (digestive symptoms, 34.8% vs 31.8%, P = .81; diarrhea, 15.2% vs 18.2%, P = .75) (data not shown). The reason for this high prevalence compared with that in the meta-analysis might be explained by the following reasons. First, we enrolled patients who had prolonged respiratory viral shedding of SARS-CoV-2 for at least 39 days (days since diagnosis to last follow-up evaluation; median, 52 d; range, 39–61 d), and 15 patients could not be discharged during the study period because of persistent respiratory shedding on days 49 to 61 (Figure 2). Second, we collected data prospectively from Youngdeok Samsung LTC, where medical teams carefully monitored symptoms twice per day and automatically recorded them by a mobile application, whereas because of their retrospective design, most previous studies in the meta-analysis might have underestimated the prevalence.13 After starting fecal collection, we checked the Bristol stool scale for all fecal samples, and when we defined diarrhea as a Bristol scale score of more than 6 (Supplementary Figure 1), the prevalence of diarrhea after 1 month of diagnosis increased up to 26%, and the prevalence of all GI symptoms increased up to 57% (data not shown).
The pooled prevalence rate of fecal samples that were positive for SARS-CoV-2 was 48.1% in the meta-analysis,13 and 1 study reported a viral RNA positive rate of 29% (44 of 153) according to the number of stool specimens.17 In our study, the viral RNA positive rates were low at 4% of patients and 6% of specimens. However, we collected fecal specimens after a median of 37.0 days from diagnosis and all of our patients were nonhospitalized with asymptomatic or mild conditions, as compared with the patients in the meta-analysis who accounted for 1.3% to 62.3% of severe disease cases in the studies, and feces were collected at 1 to 33 days.13 In another study, the viral RNA positive rate was 47.7%, with 20% of the patients being severe cases.16 Although our patients showed prolonged respiratory shedding over 1 month, the viral loads in the respiratory specimen (mean Ct) of group 1 (n = 39) were low at 33.46 and 35.39, respectively, at the time of quarantine in LTC and at the time of fecal collection. A remarkable finding in our study was that the respiratory specimens of 2 patients with positive fecal samples showed low Ct values from the time of quarantine to the time of fecal collection compared with patients with negative fecal samples. Thus, the Ct values of respiratory specimens may predict positive findings on fecal testing.
Regarding the relationship between digestive symptoms and the rate of positive fecal samples, 1 study with 65 patients reported positive rates of 52.4% and 39.1% for patients with and without GI symptoms, respectively, without statistical significance (P = .31).16 However, Cheung et al,13 who studied 59 patients, reported that viral RNA was more prevalent in the stool of patients with diarrhea than in the stool of patients without diarrhea (38.5% vs 8.7%; P = .019). In our study, 2 patients with positive fecal samples had no digestive symptoms from diagnosis to discharge; therefore, further large-scale studies are needed to confirm this correlation.
In our study, in the 2 patients with positive fecal samples, the positive fecal samples converted to negative earlier than the respiratory specimens in 1 patient, and fecal and respiratory specimens converted to negative at a similar time in another patient. These results are not in agreement with those of previous studies (eg, 70.3% of the fecal specimens collected after loss of virus from respiratory specimens tested positive for the virus).13 The persistence of viral RNA in the stool samples was longer than that in respiratory specimens in 34% (13 of 38) of the patients.13 However, there were 7 (53.8%, 7 of 13) pediatric patients and several studies showed that SARS-CoV-2 may exist for a longer time in children’s GI tracts than in their respiratory system.18, 19, 20 Thus, whether viral shedding is sustained for a longer time in the respiratory system of adults than in the respiratory system of children needs to be evaluated further. One feature of respiratory viral shedding in our study was that there were dynamic changes in respiratory specimens because even after reporting negative results, positive or inconclusive results were observed repeatedly. On the other hand, when fecal specimens were tested negative or converted to negative, the finding persisted (Figure 2). Thus, it remains to be determined whether the viral RNA in the respiratory system or feces is the source of the recurrent infection.
The strength of our study was that it was a prospective cohort study involving asymptomatic and mild COVID-19 patients who were quarantined in the LTC, not hospitalized. We carefully monitored them not only for diarrhea but also other GI symptoms for up to 61 days of quarantine. In addition, with systematic specimen collection protocols, we reported prolonged fecal viral shedding for up to 50 days in the patients with prolonged respiratory viral shedding, indicating a correlation between the viral loads of respiratory and fecal specimens.
However, there were several limitations. First, because we started collecting fecal specimens 1 month after diagnosis, the full extent of the positive findings on fecal testing, including the peak timing and extent of fecal shedding, was limited. Second, because there were only 2 patients with positive fecal samples, the comparison of Ct values and digestive symptoms between patients with positive and negative findings on fecal testing also was limited. Third, because we did not perform endoscopy in these patients, the understanding of viral replication in the intestinal mucosa was limited, and whether these were live virus particles or just RNA fragments released from the intestinal cells was unclear.
In conclusion, we found that GI symptoms were present in 35% of the patients with asymptomatic and mild COVID-19, who had prolonged respiratory shedding of SARS-CoV-2. Viral shedding in the feces was detected in 4% of the patients who did not show any GI manifestations and could persist for up to 50 days or more. A relatively high viral load in respiratory specimens might be related to fecal viral shedding. Future research needs to focus on this correlation to predict the fecal shedding of SARS-CoV-2 and its clinical significance. In addition, it also is important to understand whether the GI tract is a potential transmission route even in asymptomatic or mild COVID-19 cases with no GI symptoms so that appropriate pandemic prevention and control measures can be developed.
CRediT Authorship Contributions
Soo-kyung Park (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead);
Chil-Woo Lee (Data curation: Equal; Writing – review & editing: Equal);
Dong-Il Park (Conceptualization: Equal; Data curation: Equal);
Hee-Yeon Woo (Data curation: Equal; Methodology: Equal);
Hae Suk Cheong (Data curation: Equal; Methodology: Equal);
Ho Cheol Shin (Conceptualization: Equal; Data curation: Equal);
Kwangsung Ahn (Data curation: Equal; Formal analysis: Equal);
Min-Jung Kwon (Conceptualization: Lead; Writing – review & editing: Lead);
Eun-Jeong Joo, MD (Conceptualization: Lead; Data curation: Lead; Investigation: Equal; Methodology: Equal; Writing – review & editing: Lead).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.06.005.
Supplementary Methods
Collection of Fecal Specimens
To ensure proper collection and testing of fecal specimens for SARS-CoV-2, 3 consecutive fecal specimens (specimen 1-1, 1-2, and 1-3) from patient 1 (35-year-old woman) and 2 fecal specimens (specimen 2-1 and 2-1) from patient 2 (72-year-old woman) who were admitted to KangBuk Samsung Hospital were tested in various conditions (Supplementary Table 1). Both patients were admitted to the hospital for pneumonia or fever, and the intervals between the COVID-19 diagnosis and collection of fecal specimens 1-1, 1-2, 1-3, 2-1, and 2-2 were 3, 5, 7, 4, and 7 days, respectively. We performed the real-time RT-PCR assay for SARS-CoV-2 in various conditions, such as testing the original feces immediately and testing the feces in a nucleic acid preservative kit (NBgene-GUT; Noble Bioscience, Gyeonggi-do, Korea) stored at 2 different temperatures (room temperature or cold temperature [4°C]) 3 times (immediately, 3 days after, and 7 days after). All fecal specimens that were tested in their original form immediately showed negative results for SARS-CoV-2. However, specimens 1-1 and 1-2, which were placed in the nucleic acid preservative kit and tested immediately, showed positive results, and the positive finding persisted even when the nucleic acid preservative kit with the stool was stored at a cold temperature for 7 days.
Considering the earlier-described results, from April 4 and 5, 2020, for groups 1 and 2, respectively, stool collection kits and refrigerators were provided in each room of the LTC and stool collection was conducted by patients until April 24, 2020. The stool collection kit included the following: (1) 1 nucleic acid preservative kit (NBgene-GUT), (2) 1 tube without nucleic acid preservative, (3) 1 nucleic acid preservative kit (Stool Nucleic Acid Collection and Preservation Tubes, Norgen Biotek Corp, Ontario, Canada) for storage purpose, and (4) stool collection paper (Fe-Col; Alpha Laboratories, Eastleigh, United Kingdom). In addition, an image of the Bristol stool scale, which classifies human feces into 7 categories, was provided to the patients. The patients were instructed to collect a stool sample daily, once a day, and keep it in the refrigerator labeled with their name, date of collection, and Bristol stool scale. The stool samples were collected by the medical teams every Monday and Thursday, when they visited their rooms to collect respiratory specimens.
Reverse-Transcription Polymerase Chain Reaction Assay for Severe Acute Respiratory Syndrome-Coronavirus 2
The respiratory specimens were sent to Green Cross Laboratories, which was one of the designated laboratories for SARS-CoV-2 testing in Korea. The fecal specimens were sent to KangBuk Samsung Hospital for storage and testing. The RNA was extracted using the MagNA Pure 96 DNA and Viral NA Small-Volume Kit (Roche Diagnostics, Mannheim, Germany) at Green Cross Laboratories and using the QIAamp DSP Viral RNA Mini Kit (Qiagen, Hilden, Germany) at our institute according to the manufacturer’s instructions. At both Green Cross Laboratories and KangBuk Samsung Hospital, real time RT-PCR assays were performed using the Allplex 2019-nCoV Assay (Seegene, Seoul, South Korea) running on a CFX96 (Bio-Rad, Pleasanton, CA), according to the manufacturer’s instruction. A total of 8 μL RNA was used in a total of 25 μL, and thermal cycling was performed at 50°C for 20 minutes for reverse transcription, followed by 95°C for 15 minutes, and 45 cycles at 94°C for 15 seconds and 58°C for 30 seconds. A sample was considered positive when a positive reaction signal for the target genes, namely the envelope protein (E) gene, the RdRp gene, and the nucleocapsid protein (N) gene, were detected at less than 40 Ct. If 1 or 2 of the 3 target genes were detected, the result was considered inconclusive. In the case of inconclusive results, respiratory specimens were re-analyzed the following day, and fecal specimens were re-analyzed the same day with the same specimens. A sample was considered to be negative for the virus if the internal control was amplified, but not the viral E, RdRp, and N genes.
Supplementary Material
References
- 1.Kim J.Y., Choe P.G., Oh Y. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim I., Lee J., Shin E. KCDC risk assessments on the initial phase of the COVID-19 outbreak in Korea. Osong Public Health Res Perspect. 2020;11:67–73. doi: 10.24171/j.phrp.2020.11.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-Associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Amico F., Baumgart D.C., Danese S. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan K.H., Poon L.L., Cheng V.C. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35:e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong K.H., Lee S.W., Kim T.S. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung K.S., Hung I.F., Chan P.P. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 17.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T., Cui X., Zhao X. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020;92:909–914. doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing Y.H., Ni W., Wu Q. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y., Li X., Zhu B. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.