Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic has already caused more than 300,000 deaths worldwide. Several studies have elucidated the central role of cardiovascular complications in the disease course. Herein, we provide a concise review of current knowledge regarding the involvement of cardiovascular system in the pathogenesis and prognosis of COVID-19. We summarize data from 21 studies involving in total more than 21,000 patients from Asia, Europe, and the USA indicating that severe disease is associated with the presence of myocardial injury, heart failure, and arrhythmias. Additionally, we present the clinical and laboratory differences between recovered and deceased patients highlighting the importance of cardiac manifestations. For the infected patients, underlying cardiovascular comorbidities and particularly existing cardiovascular disease seem to predispose to the development of cardiovascular complications, which are in turn associated with higher mortality rates. We provide mechanistic insights into the underlying mechanisms including direct myocardial damage by the virus and the consequences of the hyperinflammatory syndrome developed later in the disease course. Finally, we summarize current knowledge on therapeutic modalities and recommendations by scientific societies and experts regarding the cardiovascular management of patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Pandemic, Cardiovascular comorbidities, Cardiovascular complications
Graphical abstract
Abbreviations
- RNA
ribonucleic acid
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- COVID-19
coronavirus disease 2019
- SARS
severe acute respiratory syndrome
- MERS
Middle East respiratory syndrome
- SARS-CoV
severe acute respiratory syndrome coronavirus
- MERS-CoV
Middle East respiratory syndrome coronavirus
- ACE
angiotensin converting enzyme
- ACEI
angiotensin converting enzyme inhibitor
- ARB
angiotensin II receptor blocker
- RAAS
renin–angiotensin–aldosterone system
- O-GlcNAc
O-linked β-N-acetylglucosamine
- IRF5
interferon regulatory factor–5
- RT-PCR
real-time reverse transcription polymerase chain reaction
- CT
computed tomography
- IL
interleukin
- CVD
cardiovascular disease
- ICU
intensive care unit
1. Introduction
In early December 2019, the first cases of a pneumonia-like disease emerged in Wuhan, Hubei Province, China.1 All cases were linked to a seafood market in the same city2 and were confirmed to be associated with a novel RNA Betacoronavirus, which was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)3,4. On February 11, 2020, the novel disease was named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), which declared a pandemic on March 11, 2020.5 COVID-19's high reproduction number has led to worldwide expansion of the disease6 and has gripped the world in a health and economic crisis.7 , 8
2. Etiology – Pathophysiology of SARS-CoV-2 infection
2.1. Structure and genome sequence of SARS-CoV-2
SARS-CoV-2 is a round or elliptic Betacoronavirus and has a diameter of approximately 60–140 nm9. It belongs to the large family of coronaviruses, which are responsible for 5-10% of all respiratory tract infections.10 Coronaviruses have also been the cause of two previous infectious disease outbreaks; severe acute respiratory syndrome (SARS)11 and Middle East respiratory syndrome (MERS).12 Comparative homology analysis has revealed the close relation of SARS-CoV-2 with bat coronavirus (RaTG13) demonstrating an overall sequence homology of 96.2%,13 whereas the association with SARS-CoV (approximately 79% sequence identity) and MERS-CoV (approximately 50% sequence identity) was less significant4.
2.2. Viral cell entry
Angiotensin-converting enzyme 2 (ACE2), a type 1 membrane protein expressed in the intestine, kidneys, heart and type II alveolar cells in the lungs14 has been recognized as a cell receptor for SARS-CoV15 , 16 and can also serve as a cell receptor for the novel SARS-CoV-2.13 , 17 The spike glycoprotein (S protein) on the virion surface of SARS-CoV is responsible for receptor recognition and membrane fusion.18 Similarly, an S protein on the surface of the novel SARS-CoV-2 binds to the peptidase domain of ACE2 at least ten times more tightly than of (SARS)–CoV19 and subsequently causes membrane fusion, releasing its RNA into the host cell.19 , 20 Transmembrane Serine Protease 2 (TMPRSS2) is essential for viral entry being involved in S protein priming and the cleavage of the site.21 Ou et al. reported that phosphoinositide 5-kinase (PIKfyve), two-pore segment channel 2 (TPC2), and cathepsin L are also critical for viral entry,22 Once endocytosis is completed, SARS-CoV-2 RNA is translated into viral polyproteins, which are assembled with genome RNA into virions and transported through exocytosis out of the host cell.23 The SARS-CoV-2 binding to the ACE2 receptors and the subsequent membrane fusion and viral invasion result in the downregulation and the loss of the catalytic effect of ACE2 receptors at the external site of the cell membrane.24
3. Epidemiology of COVID-19
On January 24, 2020, the first three cases of COVID-19 were reported in Europe, all located in France.25 At that point, Asia had already recorded 1312 COVID-19 cases and 41 deaths.26 In Greece, the first confirmed patient with COVID-19 was reported on February 26, 2020.27 As of May 18, 2020, the WHO has confirmed 4,819,372 cases, 316,961 deaths, and 1,864,269 recovered patients worldwide.28 The overall fatality rate is currently at 6,6%; however, there are wide variations depending on age, comorbidities, and country.29 In comparison, SARS-CoV had 8,098 confirmed cases and 774 deaths from November 2002 to July 2003 (mortality 9.6%)30 and MERS-CoV had 2,494 confirmed cases and 858 deaths from September 2012 to September 2019 (mortality 34.4%).31
The high sequence homology of SARS-CoV-2 with bat coronavirus13 and the vast number of coronaviruses carried by distinct bat species32 , 33 have suggested that SARS-CoV-2 has originated from bats.13 The transmission of SARS-CoV-2 occurs mainly from person to person through respiratory droplets34, 35, 36 and has an incubation period ranging from 2 to 14 days37 or in extreme cases up to 32 days.38 Li et al. analyzed the first 425 cases in Wuhan by January 22, 2020 and estimated the mean incubation period of COVID-19 at 5.2 days with the 95th percentile of the distribution at 12.5 days.36 Airborne transmission39 and transmission through the oral-fecal route40 have also been recorded. Of note, Zou et al. reported that the viral load of SARS-CoV-2 in asymptomatic patients was comparable to that in symptomatic patients,41 suggesting potential transmission by asymptomatic patients in concordance with other studies also reporting transmission from asymptomatic SARS-CoV-2 carriers.42 , 43 Despite the ability of SARS-CoV-2 to infect different pet species,44 the risk of human contamination from pets has not been elucidated so far.45
On January 23, 2020, the WHO estimated the basic reproductive number (R0) of COVID-19 at 1.4-2.5.46 Liu et al suggested that SARS-CoV-2 has a higher R0 in comparison to SARS-CoV, estimating the mean R0 of SARS-CoV-2 at 3.28 by analyzing data from 12 studies.47 However, as there has not been adequate evidence regarding how asymptomatic carriers contribute to the transmission rate of this novel infectious disease and how each treatment or preventive strategy affects it, the accurate estimation of R0 is difficult.
Gender differences have been reported in the epidemiology of COVID-19, as women have lower infection and mortality rates than men.29 , 48 A recent study in patients with heart failure found that circulating levels of ACE2 were higher in men than in women, suggesting increased ACE2 tissue expression, which could contribute to susceptibility to SARS-CoV-2 infection and disease progress.49 However, further studies are needed to elucidate the gap between sex difference and COVID-19 susceptibility and prognosis.
4. Cardiovascular complications in patients with COVID-19
4.1. Myocardial injury
Myocardial injury has been a remarkable finding, which contributes to worse prognosis (Figure 3, Figure 4 ) in most patient cohorts with COVID-19 so far34 , 48 , 50, 51, 52, 53, 54, 55, 56(Table 1 ), and being reported in >50% of deceased patients in most included studies. (Table 3 ) Case reports of probable COVID-19-induced myocarditis claim the direct myocardial injury by SARS-CoV-2.57, 58, 59, 60 According to the fourth universal definition of myocardial infarction by the European Society of Cardiology (ESC), myocardial injury is defined as being present when blood levels of cardiac troponin are increased above the 99th percentile upper reference limit.61 Most of the published reports have used the same or similar definitions (Appendix).34 , 48 , 52, 53, 54, 55, 56 The exact underlying mechanism for the COVID-19-mediated myocardial damage is not clear;62 however, the following four hypotheses are the main mechanisms considered so far (Fig. 1):
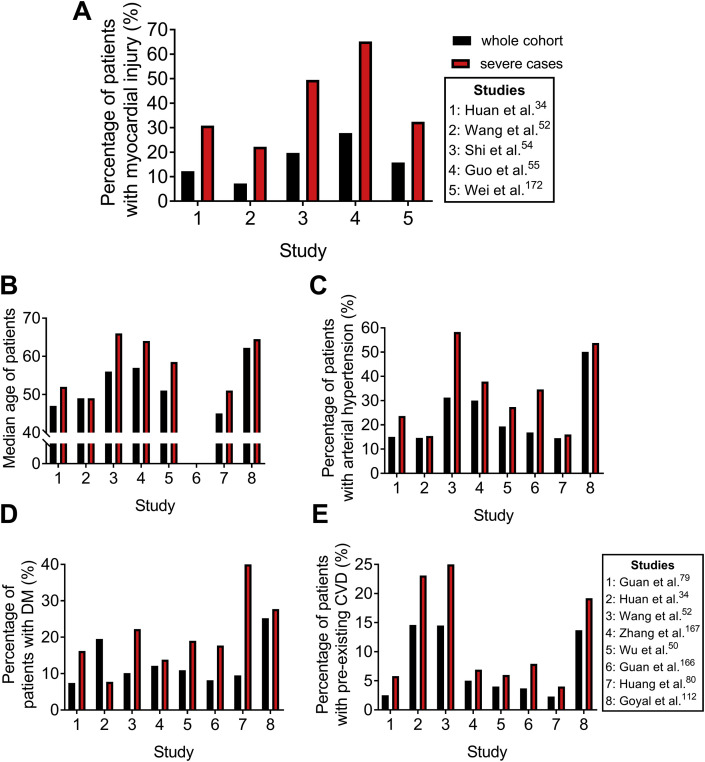
Figure 3.
Comparative analysis of myocardial injury frequency between total and severe patients with COVID-19. The number of patients included in the whole cohort vs. severe cases in the depicted cohort studies (n whole cohort/severe cases): Huan34 et al. 41/13, Wang52 et al. 138/36, Shi54 et al. 416/97, Guo55 et al. 187/46, Wei172 et al. 101/37, Guan79 et al. 1099/173, Zhang167 et al. 140/58, Wu50 et al. 201/84, Guan166 et al. 1590/254, Huang80 et al. 221/25, and Goyal112 et al. 393/130. Bar graphs represent: (A) the percentage of patients who developed myocardial injury, (B) median age of patients, (C) the percentage of patients with arterial hypertension, (D) the percentage of patients with DM and (E) the percentage of patients with preexisting CVD in the whole cohort (black) and among the severe COVID-19 cases (red) per study. DM: diabetes mellitus and CVD: cardiovascular disease.
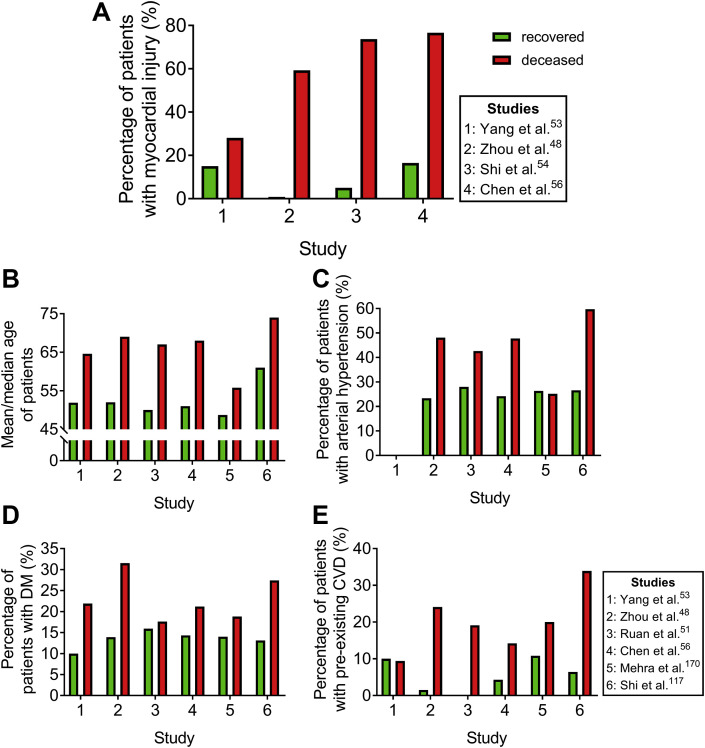
Figure 4.
Comparative analysis of myocardial injury frequency between recovered and deceased patients with COVID-19. The number of recovered patients vs. deceased patients in the depicted cohort studies (n recovered patients/deceased patients): Yang53 et al. 20/32, Zhou48 et al. 37/54, Shi54 et al. 40/57, Chen56 et al. 161/113, Ruan51 et al. 82/68, Mehra170 et al. 8395/515, and Shi117 et al. 609/62. Bar graphs represent: (A) the percentage of patients who developed myocardial injury, (B) the median (Zhou48, Ruan51, Chen56, and Shi117) or mean (Yang53 and Mehra170) age of patients, (C) the percentage of patients with arterial hypertension, (D) the percentage of patients with DM, and (E) the percentage of patients with preexisting CVD in recovered (green) and deceased patients with COVID-19 (red) per study. DM: diabetes mellitus and CVD: cardiovascular disease.
Table 1.
Frequency of cardiovascular comorbidities, preexisting cardiovascular disease, and cardiovascular complications in patients with COVID-19
| n | Median age, years | CV comorbidities |
Pre existing CVD |
CV complications |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Current smoker | HTN | DM | CVD | Myocardial injury | HF | Arrhythmia | |||
| Guan79 | 1099 | 47 | 12.6 | 15.0 | 7.4 | 2.5 | N/A | N/A | N/A |
| Huan34 | 41 | 49 | 7.3 | 14.6 | 19.5 | 14.6 | 12.2 | N/A | N/A |
| Wang52 | 138 | 56 | N/A | 31.2 | 10.1 | 14.5 | 7.2 | N/A | 16.7 |
| Zhang167 | 140 | 57 | 1.4 | 30.0 | 12.1 | 5.0D | N/A | N/A | N/A |
| Wu50 | 201 | 51 | N/A | 19.4 | 10.9 | 4.0 | 4.5 | N/A | N/A |
| Guan166 | 1590 | 48.9A | 7.0B | 16.9 | 8.2 | 3.7 | N/A | N/A | N/A |
| Huang80 | 221 | 45 | 7.7C | 14.5 | 9.5 | 2.3 | 1.7 | N/A | N/A |
| Xu168 | 90 | 50 | N/A | 18.9 | 5.6 | 3.3 | N/A | N/A | N/A |
| Zhou48 | 191 | 56 | 5.8 | 30.4 | 18.8 | 7.9D | 17.3 | 23.0 | N/A |
| Ruan51 | 150 | N/A | N/A | 34.7 | 16.7 | 8.7 | N/A | N/A | N/A |
| Shi54 | 416 | 64 | N/A | 30.5 | 14.4 | 10.6D 4.1E |
19.7 | N/A | N/A |
| Guo55 | 187 | 58.5A | 9.6 | 32.6 | 15.0 | 11.2D | 27.8 | N/A | 5.9 |
| Chen56 | 274 | 62 | 4.4 | 33.9 | 17.2 | 8.4 | 43.8 | 24.4 | N/A |
| Mehra170 | 8910 | 49A | 5.5 | 26.3 | 14.3 | 11.3D 2.1F 3.4G |
N/A | N/A | N/A |
| Goyal112 | 393 | 62.2 | 5.1 | 50.1 | 25.2 | 13.7D | N/A | N/A | 7.4 |
| Lechien171 | 1420H | 39.2A | 14.3 | 9.2 | 1.7 | 1.8 | N/A | N/A | N/A |
| Wei172 | 101 | 49 | 7.9 | 20.1 | 13.9 | 5.0D | 15.8 | N/A | N/A |
| Richardson173 | 5700 | 63 | N/A | 56.6 | 33.8 | 11.1D 6.9F |
N/A | N/A | N/A |
| Shi117 | 671 | 63 | N/A | 29.7 | 14.5 | 8.9D 3.3E |
N/A | N/A | N/A |
CV: cardiovascular, CVD: cardiovascular disease, DM: diabetes mellitus, HF: heart failure, HTN: hypertension, n: total patients, and N/A: not applicable. A Mean age, B Former and current smoker, C Smoking history, D Coronary artery disease, E Chronic heart failure, F Congestive heart failure, G Arrhythmia, and H Only patients with mild-to-moderate COVID-19 included.
Table 3.
Frequency of cardiovascular comorbidities, preexisting cardiovascular disease, and cardiovascular complications in patients who died due to COVID-19
| n | Median age, years | CV comorbidities |
Pre existing CVD |
CV complications |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Current smoker | HTN | DM | CVD | Myocardial injury | HF | Arrhythmia | |||
| Guan166 | 50 | N/A | N/A | 56.0 | 26.0 | 16.0 | N/A | N/A | N/A |
| Yang53 | 32A | 64.6B | 0 | N/A | 21.9 | 9.4 | 28.1 | N/A | N/A |
| Zhou48 | 54 | 69 | 9.3 | 48.1 | 31.5 | 24.1C | 59.3 | 51.9 | N/A |
| Ruan51 | 68 | 67 | N/A | 42.6 | 17.6 | 19.1 | N/A | N/A | N/A |
| Shi54 | 57 | N/A | N/A | N/A | N/A | N/A | 73.7 | N/A | N/A |
| Guo55 | 43 | N/A | N/A | N/A | N/A | N/A | 72.1 | N/A | N/A |
| Chen56 | 113 | 68 | 6.2 | 47.8 | 21.2 | 14.2 | 76.6 | 49.4 | N/A |
| Mehra170 | 515 | 55.8B | 8.9 | 25.2 | 18.8 | 20.0C 5.6D 6.8E |
N/A | N/A | N/A |
| Shi117 | 62 | 74 | N/A | 59.7 | 27.4 | 33.9C 21.0F |
30.6 | 19.4 | N/A |
CV: cardiovascular, CVD: cardiovascular disease, DM: diabetes mellitus, HF: heart failure, HTN: hypertension, n: total deceased patients, N/A: not applicable.
A Only critically ill patients with COVID-19 were included in the study, B Mean age, C Coronary artery disease, D Congestive heart failure, E Arrhythmia, F Chronic heart failure.
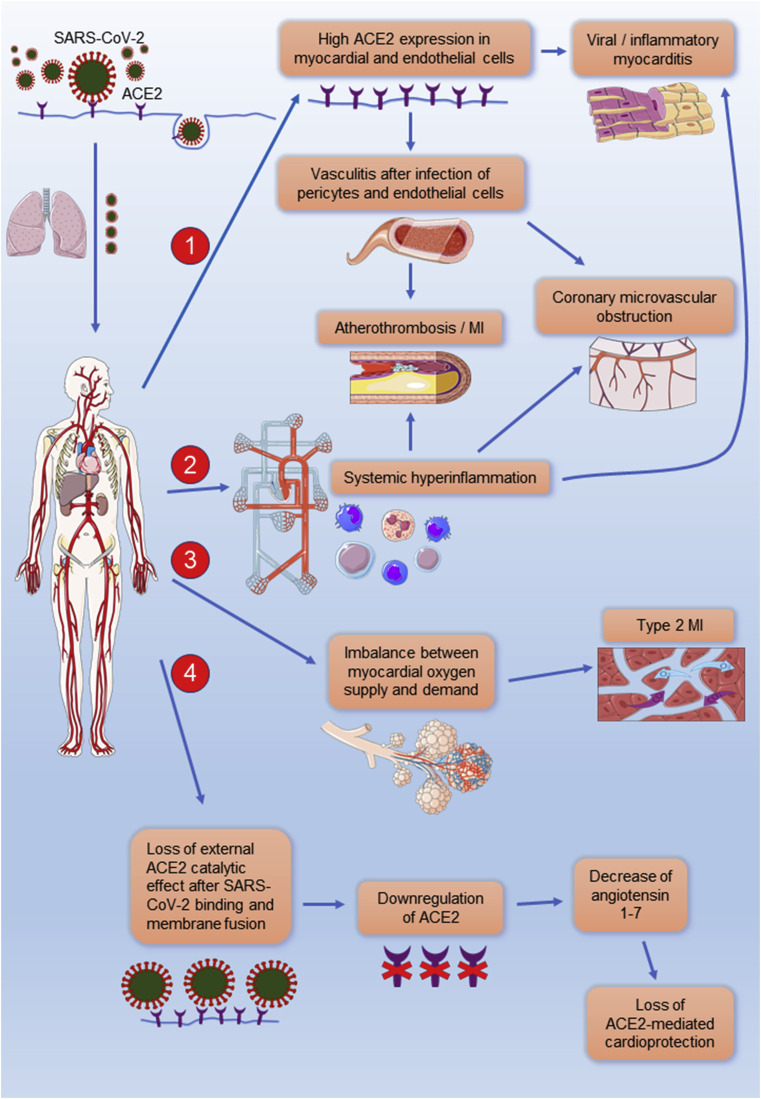
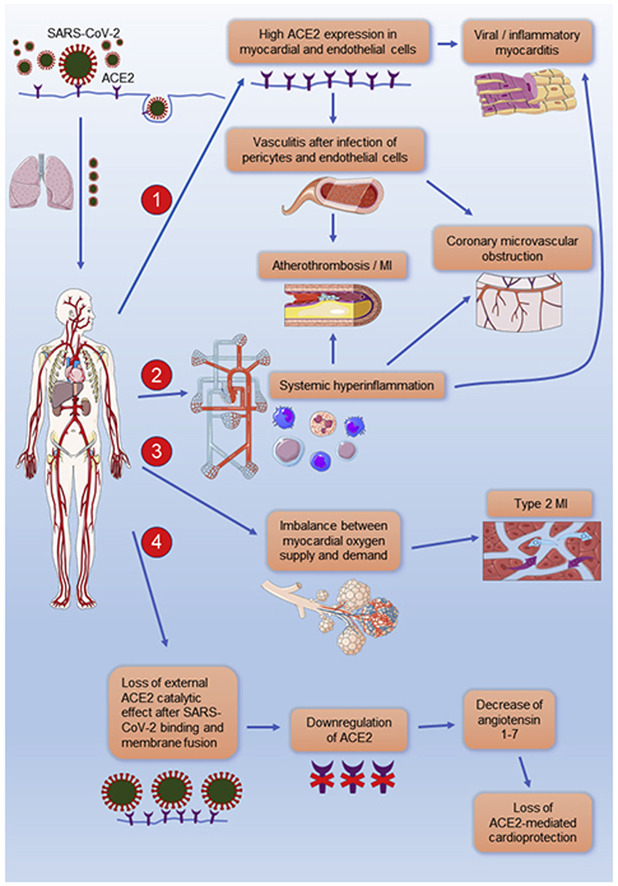
Figure 1.
Clinical manifestations of cardiovascular disease after infection with SARS-CoV-2. (1) High ACE2 expression is detected in cardiac and vascular tissue and may therefore facilitate cellular entry of SARS-CoV-2 resulting in myocardial and vascular damage. (2) An aberrant T-cell and monocyte activation has been observed in patients with COVID-19 leading to a systemic hyperinflammatory response. Increased circulating proinflammatory cytokines may result in inflammatory cardiomyopathy or atherothrombosis, causing an acute coronary syndrome. Systemic inflammatory response can also activate the microvascular endothelium, provoking the dysfunction of the coronary microvasculature, and consequently resulting in myocardial ischemia and myocardial injury. (3) Decreased myocardial oxygen supply, due to severe COVID-19 respiratory complications and hypoxia, along with increased myocardial oxygen demand, mainly due to high systemic metabolic needs, can provoke myocardial injury and type 2 myocardial infarction. (4) The binding of SARS-CoV-2 to ACE2 is expected to lead to the internalization of ACE2 and loss of the external ACE2 catalytic effect. Therefore, the possible downregulation of ACE2 and the subsequent decrease of angiotensin 1-7 in patients with COVID-19 may also compromise heart function. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com. ACE2: angiotensin-converting enzyme 2, MI: myocardial infarction, and SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
4.1.1. Direct ACE2-mediated myocardial cell invasion
As already described, high ACE2 expression is detected in cardiac tissue,63 and may therefore facilitate cellular entry of the virus resulting in endothelial dysfunction and myocardial damage (Table 6 ) In particular, ACE2 is widely expressed in cardiomyocytes, cardiac pericytes, and coronary endothelial cells.64 Therefore, SARS-CoV-2 could directly enter cardiomyocytes and provoke myocardial injury. Furthermore, pericytes, which are perivascular mural cells with high ACE2 expression, have been suggested as target host cells by SARS-CoV-2.64 Considering the essential role of cardiac pericytes in maintaining endothelial cell function in capillary vessels, their infection could lead to coronary microvascular dysfunction and cardiac injury.64 SARS-CoV-2 has also been shown to infect human blood vessel organoids in vitro.65 Recent pathology reports provided the evidence of direct endothelial cell infection and diffuse endothelial inflammation, which could suggest the induction of “endotheliitis” and endothelial dysfunction, potentially contributing to the destabilization of coronary plaques, atherothrombosis, and vascular disease.66
Table 6.
Clinical relevance of cardiovascular and circulatory cells in patients with COVID-19 and potential underlying mechanisms leading to cardiovascular disease.
| Contributing cells | Clinical relevance | Potential underlying mechanisms leading to CVD |
|---|---|---|
| Cardiovascular cells | Cardiovascular cell-related mechanisms: | |
| Cardiomyocytes1 | Wide expression of ACE2 | SARS-CoV-2 uses ACE2 as a cell receptor → direct myocardial damage |
| Cardiac pericytes2 | High ACE2 expression | SARS-CoV-2 uses ACE2 as a cell receptor → pericyte is a potential host cell targeted by SARS-CoV-2 in cardiac tissue capillary → capillary endothelial cells dysfunction → coronary microvascular dysfunction |
| Endothelial cells3, 4, 5 | Evidence of direct SARS-CoV-2 infection of the endothelial cells and diffuse endothelial inflammation | Increased ACE2 expression by endothelial cells and evidence of direct viral infection of vascular organoids in vitro → endothelial inflammation (“endotheliitis”) and increased leukocyte infiltration in heart tissue → atherosclerotic plaque destabilization → acute coronary syndrome |
| Blood cells | Leukocyte-related mechanisms: | |
| Lymphocytes6, 7, 8, 9, 10 | ↓ in all cases, especially in severe disease |
|
| CD4+ T cells11, 12, 13, 14 | ↓ in severe disease ✓Autopsy-confirmed CD4+ T infiltration of myocardium |
|
| CD8+ T cells15,16 | ↓ in severe disease |
|
| NK cells14,15 | ↓ in all cases |
|
| Neutrophils17, 18, 19 | ↑ in severe disease |
Platelet-related mechanism: Platelets become activated → platelets adhere to vascular endothelium promoting further recruitment of leukocytes to vascular wall → vascular inflammation and tissue inflammation → inflammatory cardiomyopathy, atherothrombosis, and vasculitis |
| Platelets177,178 | ↓ in severe disease | |
ACE2: angiotensin-converting enzyme 2, COVID-19: coronavirus disease 2019, CV: cardiovascular.
CVD: cardiovascular disease, NK: natural killer, and SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
4.1.2. Systemic Hyperinflammation
Viral infections are recognized as one of the most frequent causes of infectious myocarditis, which trigger the activation of the host antiviral immune response, including natural killer cells, macrophages, and virus-specific T lymphocytes.67 Similarly, an aberrant T-cell and monocyte response has been observed in patients with COVID-19 leading to a systemic hyperinflammatory response characterized by increased proinflammatory cytokine and chemokine production (tumor necrosis factor, IL-2, IL-6, IL-7, and CCL2 among others)34 , 48 , 50 , 68 , 69 (Table 5) (Fig. 2). This could lead to consequent myocardial damage as suggested by autopsy reports describing inflammatory mononuclear cell infiltration in cardiac tissues of patients with fulminant myocarditis and high SARS-CoV-2 viral load.59 , 70 Systemic inflammation could further stimulate tissue-resident macrophages and leukocyte adhesion molecule expression on the endothelial cells of preexisting atherosclerotic lesions, enhancing their propensity to be disrupted and cause an acute coronary syndrome.62 , 71 Elevated circulating cytokines can also activate the microvascular endothelium, provoking dysfunction of the coronary microvasculature, and consequent myocardial ischemia and injury.72 In line with this concept, blockade of IL-6, which is central in leukocyte transmigration into peripheral tissues, has shown promising results in severe cases of COVID-19.73 , 74 Despite the low severity and low mortality of COVID-19 in children75 there have been reports of severe Kawasaki-like disease (which is a type of vasculitis) cases across Europe.76 Macrophage activation syndrome-like manifestations, classically associated with rheumatic diseases including Kawasaki disease,77 have also been reported in patients with COVID-19 supporting the hypothesis that the increase of Kawasaki-like presentations could be a result of COVID-19-induced systemic hyperinflammation and consequent vasculitis.78
Table 5.
Clinical value of cardiovascular and inflammatory biomarkers in patients with COVID-19
| Cardiovascular biomarkers | Clinical relevance |
|---|---|
| Troponin | ↑ in severe COVID-19 as compared to nonsevere COVID-19 |
| |
| ↑ in deceased patients compared to discharged patients | |
| |
| |
| NT-proBNP | ↑ in severe COVID-19 as compared to mild COVID-19 |
| |
| ↑ in deceased patients as compared to discharged patients | |
| |
| |
| |
| D-Dimer |
↑ in severe COVID-19 as compared to nonsevere COVID-19 |
| |
| ↑ in patients with ARDS as compared to patients without ARDS | |
| |
| ↑ in deceased patients as compared to recovered patients | |
| |
|
Inflammatory biomarkers | |
| CRP | ↑ in severe COVID-19 as compared to nonsevere COVID-19 |
| |
| ↑ in deceased patients as compared to discharged patients | |
| |
| |
| IL-6 | ↑ in severe COVID-19 compared to nonsevere COVID-19 |
| |
| ↑ in deceased patients as compared to discharged patients | |
| |
| Procalcitonin | ↑ in severe COVID-19 as compared to nonsevere COVID-19 |
| |
| ↑ in patients who required ICU care as compared to no ICU care | |
| |
| Neutrophil-to-lymphocyte ratio (NLR) |
|
| |
ARDS: acute respiratory distress syndrome, COVID-19: coronavirus disease 2019, CRP: C-reactive protein, ICU: intensive care unit, IL-6: interleukin 6, NLR: neutrophil-to-lymphocyte ratio, and NT-proBNP: N-terminal (NT)- proB-type natriuretic peptide.
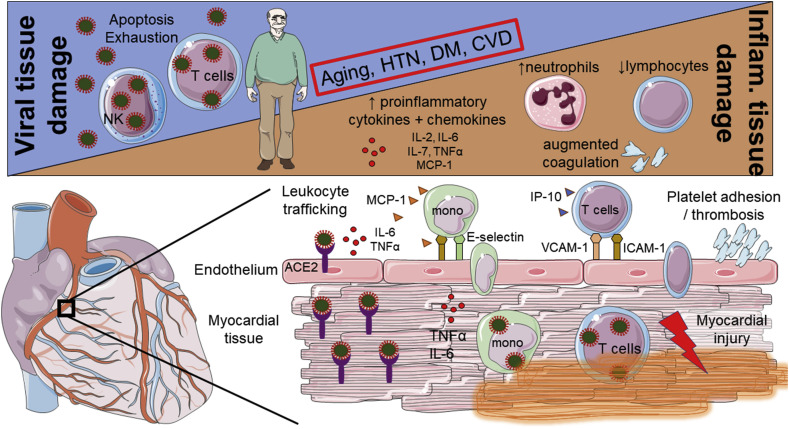
Figure 2.
Mechanistic insights into viral and inflammatory myocardial and vascular tissue damage in COVID-19. Two phases of COVID-19 have been described: a) an early phase where tissue damage is mainly induced directly by the virus and b) in some severe cases a 2nd phase, where aberrant immune response (hyperinflammation) is the cause of tissue damage (upper panel). A large number of proinflammatory cytokines (TNFα, IL-2, IL-6, and IL-7) and chemokines (MCP-1 and IP-10) have been found increased in the circulation of patients with more severe disease. Circulating cytokines can activate endothelial cells and upregulate the expression of leukocyte adhesion molecules such as E-selectin, ICAM-1, and VCAM-1. This could lead to the transmigration of leukocytes into peripheral tissues, such as the myocardium, and cause inflammatory tissue damage (lower panel). This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com. ACE2: angiotensin-converting enzyme 2, CVD: cardiovascular disease, DM: diabetes mellitus, HTN: hypertension, ICAM-1: intercellular adhesion molecule 1, IL: interleukin, IP10: interferon γ-induced protein 10, MCP1: monocyte chemoattractant protein-1, mono: monocytes, TNFα: tumor necrosis factor alpha, and VCAM-1: vascular cell adhesion molecule.
4.1.3. Imbalance between myocardial oxygen supply and demand
Myocardial injury can be the result of a mismatch between myocardial oxygen supply and demand, being classified as type 2 myocardial infarction.61 Severe respiratory complications and potential subsequent hypoxia are common findings in patients with COVID-19.48 , 53 , 79, 80, 81 In a meta-analysis of 19 studies, including a total of 2,874 patients, the most predominant chest x-ray finding was bilateral pneumonia (72.9%, 95% CI 58.6–87.1%) with ground glass opacity being reported in 68.5% (95% CI 51.8–85.2%) of patients.82 In addition, ground glass opacity was the most frequent chest CT finding (97.6%) in a Chinese cohort of 83 patients with COVID-19-related pneumonia and was associated with severe outcomes in all (100%) patients.83 Hypoxia may also contribute to the development of tissue inflammation, which in turn may lead to cardiac damage.84 Furthermore, hypotension, a frequent clinical sign in sepsis and in cytokine storm syndrome, can also reduce myocardial oxygen supply.72 On the other hand, systemic infection and fever increase the metabolic needs of peripheral tissues and end organs resulting in a rise of the metabolic demands of the myocardial cells.85 The decrease in diastolic perfusion time during tachycardia can induce inadequate subendocardial perfusion in patients with coronary artery disease, resulting in cardiac injury.86 Therefore, the viral infection caused by SARS-CoV-2 may provoke myocardial oxygen supply and demand imbalance, which is translated into myocardial ischemia and injury.
4.1.4. Loss of ACE2-mediated cardioprotection
ACE2 plays an important role in the renin–angiotensin system by catalyzing the conversion of the vasoconstrictor angiotensin II to the vasodilator angiotensin 1-7, which exerts antiarrhythmogenic and antiremodeling protective effects in the cardiovascular system.87 , 88 Angiotensin 1-7 has also antiproliferative effects on vascular smooth muscle cells89 and cardiac fibroblasts.90 Additionally, ACE2 has a counterregulatory function to ACE1, which hydrolyzes angiotensin I to the octapeptide angiotensin II and inactivates the vasodilator bradykinin.91 The activation of angiotensin II elicits heterogeneous signaling cascades in the vasculature, which can result in the expression of proinflammatory mediators and endothelial dysfunction.92 The binding of SARS-CoV-2 to ACE2 is expected to lead to the internalization of ACE2 and loss of the external ACE2 catalytic effect.24 , 93 Therefore, the possible downregulation of ACE2 and the subsequent increase of the proatherosclerotic angiotensin II together with the decrease of the cardioprotective angiotensin 1-7 in patients with COVID-19 may ultimately compromise heart function.94 , 95 Remarkably, severe COVID-19 has been associated with hypokalemia and higher blood pressure, supporting suggestions of decreased ACE2 function and augmented levels of angiotensin II after SARS-CoV-2 infection.96
4.1.5. Heart failure
Current data regarding the incidence of heart failure among patients with COVID-19 are limited (Table 1). Viral infections are the most common cause of myocarditis97. Despite the high recovery rates, nearly one out of three biopsy-proven myocarditis patients will later develop dilated cardiomyopathy.98 Recurrent viral myocarditis and persistent viral replication have also been associated with the deterioration of myocardial function.99 , 100 Similarly, fulminant myocarditis, which may be a clinical manifestation of COVID-19,57 , 58 can result in left ventricular systolic dysfunction and even cardiogenic shock.101 , 102
Viruses can also contribute to the etiology of heart failure through immune-mediated and inflammatory myocardial damage.103 Acute systemic inflammation and septic shock can result in an increase of left ventricular end-diastolic volume together with the depression of myocardial function.104 , 105 Moreover, an excessive T lymphocyte response in enterovirus-induced myocarditis has been reported to provoke left ventricular dilatation and/or dysfunction.106 Finally, high levels of circulating cytokines, such as TNF-α, IL-1β, and IL-6 have been shown to cause the deterioration of myocardial cell contraction and relaxation in vitro 107 , 108 and could suggest a potential relation between COVID-19-induced hyperinflammatory syndrome and myocardial dysfunction.
Chen et al. reported heart failure as a complication in 24.4% (n = 43) of a Chinese COVID-19 population (n = 176), using age-related amino-terminal pro-brain natriuretic peptide cutoffs, which yielded 90% sensitivity and 84% specificity for acute heart failure;56 there was a remarkable difference in the prevalence of heart failure between COVID-19 survivors and nonsurvivors (3.2% vs. 49.4%).56 Another study including 191 patients reported heart failure as a cardiovascular complication in 23.0% (n = 44) of the population, 63.6% (n = 28) of whom had a fatal outcome48 (Table 3). Lastly, in a meta-analysis of 43 studies involving 3,600 patients, the prevalence of heart failure as a complication was 17.1% (95%, CI: 1.5–42.2%) among critically ill patients as compared to 1.9% (95% CI: 0.0-26.0%) among non-critically ill patients.109
4.2. Arrhythmias
Sustained ventricular arrhythmias are significant clinical features of acute myocarditis,102 which is increasingly being reported as a clinical complication of COVID-19.57, 58, 59, 60 Guo et al. reported sustained ventricular tachycardia or ventricular fibrillation in 5.9% (n = 11) of 187 patients in a designated hospital to treat patients with COVID-19 in China.55 Arrhythmias could also be precipitated by electrolyte imbalances that have been observed in populations with COVID-19.56 The interaction of SARS-CoV-2 with the renin-angiotensin-aldosterone system (RAAS) has caused increasing concern about sodium and potassium disorders, which may increase vulnerability to various tachyarrhythmias.96 , 110 In addition, hypoxia, a common clinical manifestation of severe COVID-19,48 , 53 , 79, 80, 81 has been associated with alterations of cardiomyocyte gap-junctions, which could contribute to the development of atrial arrhythmias, particularly atrial fibrillation.111 A recent retrospective case series study characterizing the first 393 consecutive patients with COVID-19 in two hospitals in New York City found that patients who received mechanical ventilation were more likely to have atrial arrhythmias (18.5% vs. 1.9%).112
Arrhythmias can also be induced by novel medical therapies for COVID-19; despite the unclear data about the effectiveness of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of COVID-19,113 the Food and Drug Administration of the United States of America issued an emergency authorization for their use under determined circumstances in patients with COVID-19.114 Both agents may increase the risk for Torsades de Pointes or other ventricular arrhythmias through QTc prolongation115 and could also lead to advanced types of atrioventricular block.116
4.3. The role of cardiovascular comorbidities and preexisting CVD in the development of CVD complications
Preexisting cardiovascular disease and cardiovascular comorbidities, including arterial hypertension and type 2 diabetes mellitus, are predictors of myocardial injury in hospitalized patients with COVID-19117 (Table 1, Table 2, Table 3, Table 4). An association between preexisting cardiac disease and higher frequency of cardiovascular complications has been previously shown among patients with pneumonia.118 , 119 Recent results indicate multi-organ tropism of SARS-CoV-2, including heart, vascular system, and the circulation, which is speculated to influence the course of the disease as well as aggravate preexisting conditions.120 The increased myocardial expression of ACE2 in patients with cardiovascular disease and COVID-19121 , 122 has been suggested as a possible mechanism of myocardial cell invasion and injury leading to worse outcomes64 (Fig. 1).
Table 2.
Frequency of cardiovascular comorbidities, preexisting cardiovascular disease, and cardiovascular complications in patients with severe COVID-19
| n | Median age, years | CV comorbidities |
Pre existing CVD |
CV complications |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Current smoker | HTN | DM | CVD | Myocardial injury | HF | Arrhythmia | |||
| Guan79 | 173 | 52 | 16.9 | 23.7 | 16.2 | 5.8 | N/A | N/A | N/A |
| Huan34 | 13 | 49 | 0 | 15.4 | 7.7 | 23.1 | 30.8 | N/A | N/A |
| Wang52 | 36 | 66 | N/A | 58.3 | 22.2 | 25.0 | 22.2 | N/A | 44.4 |
| Zhang167 | 58 | 64 | 3.4 | 37.9 | 13.8 | 6.9A | N/A | N/A | N/A |
| Wu50 | 84 | 58.5 | N/A | 27.4 | 19.0 | 6.0 | N/A | N/A | N/A |
| Guan166 | 254 | N/A | N/A | 34.6 | 17.7 | 7.9 | N/A | N/A | N/A |
| Yang53 | 52 | 59.7 | 3.8 | N/A | 17.3 | 10.4 | 23.1 | N/A | N/A |
| Huang80 | 25 | 51 | 8.0E | 16.0 | 40.0 | 4.0 | N/A | N/A | N/A |
| Shi54 | 97 | N/A | N/A | N/A | N/A | N/A | 49.5 | N/A | N/A |
| Guo55 | 46 | N/A | N/A | N/A | N/A | N/A | 65.2 | N/A | N/A |
| Han81 | 60B 15C |
59B,D 57C,D |
N/A | N/A | N/A | N/A | 76.7B 80.0C |
25.0B 33.3C | N/A |
| Arentz169 | 21 | 70D | N/A | N/A | 33.3 | 42.9 | N/A | 33.3 | N/A |
| Goyal112 | 130 | 64.5 | 4.6 | 53.8 | 27.7 | 19.2D | N/A | N/A | 18.5 |
| Wei172 | 37 | N/A | N/A | N/A | N/A | N/A | 32.4 | N/A | N/A |
CV: cardiovascular, CVD: cardiovascular disease, DM: diabetes mellitus, HF: heart failure, HTN: hypertension, n: total severe cases, and N/A: not applicable.
ACoronary artery disease, B Severe COVID-19 C Critical COVID-19, D Mean age, and E Smoking history.
Table 4.
Frequency of cardiovascular comorbidities, preexisting cardiovascular disease and cardiovascular complications in patients with COVID-19 who recovered the disease.
| n | Median age, years | CV comorbidities |
Pre existing CVD |
CV complications |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Current smoker | HTN | DM | CVD | Myocardial injury | HF | Arrhythmia | |||
| Yang53 | 20A | 51.9C | 10.0 | N/A | 10.0 | 10.0 | 15.0 | N/A | N/A |
| Zhou48 | 137B | 52 | 4.4 | 23.4 | 13.9 | 1.5D | 0.8 | 11.7 | N/A |
| Ruan51 | 82B | 50 | N/A | 28.0 | 15.9 | 0 | 6.2E | N/A | N/A |
| Shi54 | 40B | N/A | N/A | N/A | N/A | N/A | 5.0 | N/A | N/A |
| Chen56 | 161 | 51 | 3.1 | 24.2 | 14.3 | 4.3 | 16.5 | 3.2 | N/A |
| Mehra170 | 8395B | 48.7C | 5.3 | 26.4 | 14.0 | 10.8D 1.9F 3.2G |
N/A | N/A | N/A |
| Lechien171 | 264H | 34.1C | 15.9 | 10.6 | 1.5 | 1.9 | N/A | N/A | N/A |
| Shi117 | 609 | 61 | N/A | 26.6 | 13.1 | 6.4D 1.5I |
N/A | N/A | N/A |
CV: cardiovascular, CVD: cardiovascular disease, DM: diabetes mellitus, HF: heart failure, HTN: hypertension, n: total recovered patients, N/A: not applicable.
A Only critically ill patients with COVID-19 were included in the study, B Discharged patients, C Mean age, D Coronary artery disease, E Continuous variable representing the median value of cardiac troponin, pg/mL (2.0-28.0), F Congestive heart failure, G Arrhythmia, H Only patients with mild-to-moderate COVID-19 were included in the study, I Chronic heart failure.
In a cohort of 416 patients with COVID-19, individuals with cardiac injury were more commonly affected by arterial hypertension (59.8% vs 23.4%), diabetes (24.4% vs 12.0%), coronary heart disease (29.3% vs 6.0%), and chronic heart failure (14.6% vs 1.5%) as compared to patients without cardiac injury.54 Similarly, 52 patients with COVID-19 with elevated troponin T levels had significantly higher rates of comorbidities including arterial hypertension (63.5% vs 20.7%), coronary heart disease (32.7% vs 3.0%), cardiomyopathy (15.4% vs 0%), and diabetes (30.8% vs 8.9%) in comparison to 135 patients with normal troponin T levels.55 On the other hand, rates of smoking did not differ significantly between those with normal or elevated troponin T levels (8.1% vs 13.5%).55 The data about the contribution of smoking to the severity of COVID-19 are conflicting. Although a preliminary meta-analysis of 1,399 Chinese patients with COVID-19 suggested that current smoking is not associated with increased risk of developing severe disease,123 another systematic review of five studies including a total of 1,549 patients concluded that smoking is most likely associated with worse outcomes,124 probably due to its detrimental effects in the lungs and cardiovascular system. On the other hand, decreased levels of ACE2 have been observed in smokers,125 , 126 while current smoking was reported in less than 17% of patients with severe disease in all 5 original studies included herein (Table 2).
Insufficient glycemic control in patients with diabetes has been strongly associated with the overall risk of serious infections.127 A recent study revealed the central role of deregulated glucose metabolism in influenza virus-induced cytokine storm through O-GlcNAcylation of IRF-5.128 Considering that a cytokine storm syndrome has been reported as a potential cause of COVID-19 complications.129 a similar mechanism may be present in diabetic patients with SARS-CoV-2 infection. Lastly, Liraglutide, a glucagon-like peptide 1 receptor, has been found to increase ACE2 expression in rat models,130 and could therefore facilitate viral infection in accordance with the aforementioned hypothesis of the potential ACE2 role in COVID-19 cardiovascular complications.
5. Current treatment strategies for CVD patients
There are limited data to guide the clinical treatment strategies for COVID-19 and its cardiovascular complications. The best possible approach should be reached with a multidisciplinary team, which includes specialized infectious disease advice, and should be based upon the available information provided by the World Health Organization or reputable societies (Table 7 ). Numerous clinical trials are currently testing experimental therapies or repurposing of current drugs for the treatment of COVID-19.131
Table 7.
Recommendations for the management of CVD patients with COVID-19 as suggested from Societies/Organizations/Experts.
| Society/Organization/Expert and date issued | Recommendations | Precautions |
|---|---|---|
| American College of Cardiology179 6 March 2020 |
|
|
| ESC Council on Hypertension140 13 March 2020 |
Continuation of treatment with the usual antihypertensive therapy. | No evidence about ACEIs and ARBs in humans; however, preclinical evidence suggests that these medications might be rather protective. |
| Chinese Medical Association180 27 March 2020 |
Severe emergent cardiovascular diseases for which hospitalization and conservative medical treatment is recommended:
|
|
| 1) Heart Rhythm Society COVID-19 Task Force 2) Electrophysiology Section of the American College of Cardiology 3) Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association181 01 April 2020 |
|
|
| European Association of Cardiovascular Imaging182 3 April 2020 |
|
Obligatory preventive measures during TTE and TOE:
|
ACE: angiotensin converting enzyme, ARB: angiotensin II receptor blocker, AMI: acute myocardial infarction, CrCl: creatinine clearance, CVD: cardiovascular, ECMO: extracorporeal membrane oxygenation, ESC: European Society of Cardiology, GRACE score: Global Registry of Acute Coronary Events score. HCQ: Hydroxychloroquine, HFOT: high flow oxygen therapy, NSTEMI: Non-ST-elevation myocardial infarction, PPE: personal protective equipment, STEMI: ST-elevation myocardial infarction, TOE: transesophageal echocardiogram, and TTE: transthoracic echocardiogram.
The participation of ACE2 in the pathogenesis of COVID-19, acting as a cell receptor for SARS-CoV-213 has caused increasing concern about the role of antihypertensive therapy with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) in patients with COVID-19.132 An observational analysis in a cohort of 12,594 patients who were tested for COVID-19 in New York City indicated that previous treatment with medications acting on the RAAS was not associated with a higher risk of either testing positive for COVID-19 or developing severe COVID-19.133 Of interest, another retrospective, multicenter study including 1,128 hospitalized hypertensive patients diagnosed with COVID-19 showed that inpatient use of ACEIs or ARBs was associated with lower risk of all-cause mortality compared to ACEI/ARB nonusers (adjusted HR, 0.42; 95% CI, 0.19-0.92).134 Despite initial concerns that the use of RAAS inhibitors in patients with COVID-19 could increase ACE2 expression, and therefore facilitate viral entry,135 , 136 no evident association between RAAS use and more severe COVID-19 course has been observed in large clinical cohorts to date.49 , 133 , 134 , 137 , 138 Thus, the European Society of Cardiology and Heart Failure Society of America/American College of Cardiology/American Heart Association guidelines suggest no change in treatment with RAAS antagonists.139 , 140
Chloroquine, an antimalarial agent with known anti-viral effects,141 has been proven to have beneficial effects against SARS-CoV infection,142 opening the possibility of its implementation in the prophylaxis and treatment of COVID-19. The available published data have been conflicting so far,113 , 143, 144, 145 highlighting the necessity of awaiting the results of randomized controlled clinical trials.
Another agent being tested in several clinical trials is Remdesivir131. It acts as a chain terminator during RNA replication and was initially developed for the treatment of Ebola virus disease146. Activity of Remdesivir against SARS-CoV-2 has been indicated in vitro,147 while clinical data have shown improvement in patients with severe forms of COVID-19.148
In addition, severe forms of COVID-19 have been associated with nonspecific widespread immune reactions and cytokine storm syndromes.129 In line with this, increased levels of inflammatory biomarkers have been associated with high risk of critical COVID-19 cases and death.34 , 48 , 50 Consequently, monoclonal IL-6 receptor inhibitors, such as tocilizumab and siltuximab, IL-1 receptor antagonists (Anakinra), fully human anti-interferon-gamma antibodies (Emapalumab), azithromycin, and corticosteroids are currently being investigated in clinical trials,131 as they have proven their efficacy against exaggerated immune activation.149, 150, 151, 152, 153 Finally, in view of colchicine's favorable anti-inflammatory profile,154 including its use in the treatment of pericarditis and postpericardiotomy syndrome,155 a prospective, randomized, controlled study in Greece (among others) will investigate the effects of colchicine in the prognosis of COVID-19.156
6. Future perspectives
Epidemiologists have forecasted that 40%–70% of the world's population will be infected by SARS-CoV-2 in the coming year and will present with a wide range of clinical manifestations.157 Apart from the direct effects of SARS-CoV-2 infection, patients who avoid infection may be affected by self-isolation and social distancing.158 Hospital attendance and hospital admissions for diseases other than COVID-19 have been significantly decreased since the beginning of the pandemic.159 There have been reports of significant decrease in the number of patients with acute coronary syndromes as well as large delays in presentation.160 The reduced healthcare staffing levels along with the increasing ICU demands are causing services to become overwhelmed,161 while elective procedures and outpatient clinics are being postponed or cancelled.162 The impact of these factors on the care of cardiovascular patients warrants further investigation.
Cardiologists offering front-line services during the COVID-19 crisis have a pivotal role in the composition of appropriate therapeutic schemes. Because of the scarce ICU resources, critical care triage has become increasingly challenging. Possible delay in the management of urgent cardiac conditions could lead to a remarkable rise in morbidity and mortality. It is crucial for patients with new or worsening symptoms to be encouraged to seek medical assistance. The management of acute coronary syndromes, particularly ST-elevation myocardial infarctions, requires calculated measures,163 particularly in the era of a pandemic. We believe that further research is needed toward the establishment of comprehensive guidelines, which will provide sufficient preparation and knowledge for such extraordinary conditions.
7. Conclusions
COVID-19 poses an outstanding clinical hazard to the general population and the healthcare community. Although most patients develop no or mild symptoms, approximately 20% experience severe or critical COVID-19 symptoms.164
For the infected patients, underlying cardiovascular comorbidities and particularly preexisting cardiovascular disease are linked to worse outcomes,34 , 48 , 50 , 51 , 79 , 80 , 164, 165, 166, 167 while the development of cardiovascular complications, including myocardial injury, heart failure, and arrhythmias, are associated with higher mortality rates.34 , 48 , 50, 51, 52, 53, 54, 55, 56 , 79 , 109 , 112 Continuous efforts are underway to uncover the pathogenetic mechanisms of COVID-19's cardiovascular complications and develop appropriate and targeted treatment strategies, with the repurposing of available drugs and identification of novel therapeutic targets. However, the acuteness and rapid spread of the COVID-19 pandemic has complicated the elucidation of effective, preventive, and therapeutic schemes.
On the whole, our knowledge of the pathogenesis, diagnosis, clinical course, and treatment of COVID-19 is expeditiously evolving. Nevertheless, in the wake of these unprecedented times, the scientific world has rallied united to progress in the understanding of COVID-19 and develop optimal treatment solutions.
Conflict of Interest
None.
Sources of Funding
Dr. K. Stellos is supported by grants from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 759248) and the DFG (SFB834 project number 75732319). Dr. C. Lazaridis is supported by a scholarship from the Hellenic Society of Cardiology.
Footnotes
Peer review under responsibility of Hellenic Society of Cardiology.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hjc.2020.05.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18(3) doi: 10.1038/s41579-020-0332-0. 123-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D.S., I Azhar E., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Coronavirus disease (COVID-19) outbreak. https://www.who.int
- 6.Zhao S., Lin Q., Ran J. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai C.-C., Wang C.-Y., Wang Y.-H., Hsueh S.-C., Ko W.-C., Hsueh P.-R. Global epidemiology of coronavirus disease 2019: disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55(4):105946. doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson H.C., Gossner C.M., Colzani E. Potential scenarios for the progression of a COVID-19 epidemic in the European Union and the European Economic Area, March 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiris J.S.M., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat Med. 2004;10(S12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 13.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donoghue M., Hsieh F., Baronas E. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ Res. 2000;87(5) doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 15.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang J., Ye G., Shi K. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher T.M., Buchmeier M.J. Coronavirus Spike Proteins in Viral Entry and Pathogenesis. Virology. 2001;279(2):371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. The Novel Coronavirus 2019 (2019-NCoV) Uses the SARS-Coronavirus Receptor ACE2 and the Cellular Protease TMPRSS2 for Entry into Target Cells. Mol Biol. 2020;181(2):271–280. e8. [Google Scholar]
- 21.Matsuyama S., Nao N., Shirato K. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci Unit States Am. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou X., Liu Y., Lei X. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiteri G., Fielding J., Diercke M. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolrd Health Organization Novel Coronavirus (2019-nCoV) Situation Report. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200125-sitrep-5-2019-ncov.pdf?sfvrsn=429b143d_8
- 27.Greek National Organization of Public Health . 2020. Daily Report.https://eody.gov.gr/wp-content/uploads/2020/03/covid-gr-daily-report-20200325.pdf [Google Scholar]
- 28.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. J Am Med Assoc. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . 2020. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003.www.who.int/csr/sars/country/table2003_09_23/en/ [Google Scholar]
- 31.World Health organization . 2020. Middle East respiratory syndrome coronavirus (MERS-CoV)https://www.who.int/emergencies/mers-cov/en/ [Google Scholar]
- 32.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W. Bats Are Natural Reservoirs of SARS-Like Coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 34.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To K.K.-W., Tsang O.T.-Y., Yip C.C.-Y. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q., Guan X., Wu P. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu C., Deng Z., Xiao Q. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. J Med Virol. 2020 doi: 10.1002/jmv.25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. Published online March 4, 2020. Accessed 11.04.2020. [DOI] [PMC free article] [PubMed]
- 40.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou L., Ruan F., Huang M. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan X., Chen D., Xia Y. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi J., Wen Z., Zhong G. Susceptibility of Ferrets, Cats, Dogs, and Different Domestic Animals to SARS-Coronavirus-2. Microbiology. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leroy E.M., Ar Gouilh M., Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;13:100133. doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization . 2020. Statement on the meeting of the International Health Regulations (2005). Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)https://www.who.int/news-room/detail/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov [Google Scholar]
- 47.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Trav Med. 2020;27(2) doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sama I.E., Ravera A., Santema B.T. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J. 2020;41(19):1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. Accessed 18.04.2020 [DOI] [PMC free article] [PubMed]
- 51.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi S., Qin M., Shen B. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;25 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo T., Fan Y., Chen M. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;27 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;26:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020;16:ehaa190. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng J.H., Liu Y.-X., Yuan J. First Case of COVID-19 Infection with Fulminant Myocarditis Complication: Case Report and Insights. Infection. 2020;10:1–5. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inciardi R.M., Lupi L., Zaccone G. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;9:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 62.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 63.Gembardt F., Sterner-Kock A., Imboden H. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26(7):1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;1:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monteil V., Kwon H., Prado P. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fung G., Luo H., Qiu Y., Yang D., McManus B. Myocarditis. Circ Res. 2016;118(3):496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 68.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hansson G.K., Libby P., Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278(5):483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Libby P. The Heart in COVID19: Primary Target or Secondary Bystander? JACC Basic Transl Sci. 2020;10:537–542. doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;19:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choy E.H., De Benedetti F., Takeuchi T., Hashizume M., John M.R., Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 76.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W., Gong F., Zhu W., Fu S., Zhang Q. Macrophage activation syndrome in Kawasaki Disease: More common than we thought? Semin Arthritis Rheum. 2015;44(4):405–410. doi: 10.1016/j.semarthrit.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guan W., Ni Z., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang R., Zhu L., Xue L. Clinical Findings of Patients with Coronavirus Disease 2019 in Jiangsu Province, China: A Retrospective, Multi-Center Study. PLoS Negl Trop Dis. 2020;14(5) doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han H., Xie L., Liu R. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92(7):819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Trav Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li K., Wu J., Wu F. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eltzschig H.K., Carmeliet P. Hypoxia and Inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute Infection and Myocardial Infarction. Longo D.L., editor. N Engl J Med. 2019;380(2):171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 86.Giuseppe Ferro, Carlo Duilio, Letizia Spinelli, Giovanni Antonio Liucci, Felice Mazza, Ciro Indolfi. Relation Between Diastolic Perfusion Time and Coronary Artery Stenosis During Stress-Induced Myocardial Ischemia. Circulation. 1995;92(3):342–347. doi: 10.1161/01.cir.92.3.342. [DOI] [PubMed] [Google Scholar]
- 87.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure. Circ Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang F., Yang J., Zhang Y. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol. 2014;11(7):413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freeman E.J., Chisolm G.M., Ferrario C.M., Tallant E.A. Angiotensin-(1-7) inhibits vascular smooth muscle cell growth. Hypertension. 1996;28(1):104–108. doi: 10.1161/01.hyp.28.1.104. [DOI] [PubMed] [Google Scholar]
- 90.McCollum L.T., Gallagher P.E., Ann Tallant E. Angiotensin-(1–7) abrogates mitogen-stimulated proliferation of cardiac fibroblasts. Peptides. 2012;34(2):380–388. doi: 10.1016/j.peptides.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sampaio Walkyria O., de Castro Carlos Henrique, Santos Robson A.S., Schiffrin Ernesto L., Touyz Rhian M. Angiotensin-(1-7) Counterregulates Angiotensin II Signaling in Human Endothelial Cells. Hypertension. 2007;50(6):1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 92.Touyz R.M., Schiffrin E.L. Signal Transduction Mechanisms Mediating the Physiological and Pathophysiological Actions of Angiotensin II in Vascular Smooth Muscle Cells. Pharmacol Rev. 2000;52(4):639. [PubMed] [Google Scholar]
- 93.Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo J., Huang Z., Lin L., Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020;9(7):e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen D., Li X., Song Q., Hu C., Su F., Dai J. pokalemia and Clinical Implications in Patients with Coronavirus Disease 2019 (COVID-19) medRxiv. 2020;29 https://www.medrxiv.org/content/10.1101/2020.02.27.20028530v1 [Google Scholar]
- 97.Shauer A., Gotsman I., Keren A. Acute Viral Myocarditis: Current Concepts in Diagnosis and Treatment. Isr Med Assoc J. 2013;15(3):180–185. [PubMed] [Google Scholar]
- 98.Caforio A.L.P., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 99.Nikolaou M., Lazaros G., Karavidas A., Hatzianastasiou S., Miliopoulos D., Adamopoulos S. Recurrent viral myocarditis: The emerging link toward dilated cardiomyopathy. Hellenic J Cardiol. 2018;59(1):60–63. doi: 10.1016/j.hjc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Kühl U., Pauschinger M., Seeberg B. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112(13):1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 101.Felker G.M., Boehmer J.P., Hruban R.H. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36(1):227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 102.Ammirati E., Cipriani M., Moro C. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry. Circulation. 2018;138(11):1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 103.Ponikowski P., Voors A.A., Anker S.D. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failureThe Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 104.Suffredini A.F., Fromm R.E., Parker M.M. The Cardiovascular Response of Normal Humans to the Administration of Endotoxin. N Engl J Med. 1989;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 105.Vieillard-Baron A., Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med. 2014;40(10):1560–1563. doi: 10.1007/s00134-014-3367-8. [DOI] [PubMed] [Google Scholar]
- 106.Badorff C., Knowlton K.U. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: from bench to bedside. Med Microbiol Immunol. 2004;193(2-3):121–126. doi: 10.1007/s00430-003-0189-7. [DOI] [PubMed] [Google Scholar]
- 107.Kumar A., Thota V., Dee L., Olson J., Uretz E., Parrillo J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183(3):949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atefi G., Zetoune F.S., Herron T.J. Complement dependency of cardiomyocyte release of mediators during sepsis. Faseb J. 2011;25(7):2500–2508. doi: 10.1096/fj.11-183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu L., Wang B., Yuan T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cavusoglu Y., Kaya H., Eraslan S., Yilmaz M.B. Hyponatremia is associated with occurrence of atrial fibrillation in outpatients with heart failure and reduced ejection fraction. Hellenic J Cardiol. 2019;60(2):117–121. doi: 10.1016/j.hjc.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Severino A., Narducci M.L., Pedicino D. Reversible atrial gap junction remodeling during hypoxia/reoxygenation and ischemia: a possible arrhythmogenic substrate for atrial fibrillation. Gen Physiol Biophys. 2012;31(4):439–448. doi: 10.4149/gpb_2012_047. [DOI] [PubMed] [Google Scholar]
- 112.Goyal P., Choi J.J., Pinheiro L.C. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.U.S. Food and Drug Administration . 2020. Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease.https://www.fda.gov/media/136534/download [Google Scholar]
- 115.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19) Mayo Clin Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Verny C., de Gennes C., Sébastien P. [Heart conduction disorders in long-term treatment with chloroquine. Two new cases] Presse Medicale Paris Fr. 1992;21(17):800–804. [PubMed] [Google Scholar]
- 117.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong M., Liu T., Li G. Association between acute infections and risk of acute coronary syndrome: a meta-analysis. Int J Cardiol. 2011;147(3):479–482. doi: 10.1016/j.ijcard.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 119.Corrales-Medina V.F., Musher D.M., Wells G.A., Chirinos J.A., Chen L., Fine M.J. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125(6):773–781. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 120.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2011400. https://doi.org/10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nicin L., Abplanalp W.T., Mellentin H. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41(19):1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur J Intern Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vardavas C., Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis. 2020;20(18):20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. Gallagher T. J Virol. 2020;94(7):e00127–e00220. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Critchley J.A., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care. 2018;41(10):2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 128.Wang Q., Fang P., He R. O-GlcNAc transferase promotes influenza A virus–induced cytokine storm by targeting interferon regulatory factor–5. Sci Adv. 2020;6(16) doi: 10.1126/sciadv.aaz7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology. 2015;156(10):3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 131.Clinicaltrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=&cntry=&state=&city=&dist=; 2020
- 132.Tignanelli C.J., Ingraham N.E., Sparks M.A. Antihypertensive drugs and risk of COVID-19? Lancet Respir Med. 2020;8(5):e30–e31. doi: 10.1016/S2213-2600(20)30153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reynolds H.R., Adhikari S., Pulgarin C. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang P., Zhu L., Cai J. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38(5):781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 136.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel A.B., Verma A. COVID-19 and Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: What Is the Evidence? JAMA. 2020 doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 138.Danser A.H.J., Epstein M., Batlle D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension. 2020;75(6):1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. 2020. https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHA-statement-addresses-concerns-re-using-RAAS-antagonists-in-COVID-19.jsp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.ESC Council on Hypertension . 2020. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [Google Scholar]
- 141.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Magagnoli J., Narendran S., Pereira F. 2020. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with Covid-19. Infectious Diseases (except HIV/AIDS) Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Molina J.M., Delaugerre C., Le Goff J. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mulangu S., Dodd L.E., Davey R.T. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grein J., Ohmagari N., Shin D. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Q., Zhou Y., Yang Z. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13(1):3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guo C., Li B., Ma H. Tocilizumab treatment in severe COVID-19 patients attenuates the inflammatory storm incited by monocyte centric immune interactions revealed by single-cell analysis. Preprint at bioRxiv https://doi.org/10.1101/2020.04.08.029769 (2020) 2020 [Google Scholar]
- 151.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ascierto P.A., Fox B., Urba W. Insights from immuno-oncology: the Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maude S.L., Barrett D., Teachey D.T., Grupp S.A. Managing Cytokine Release Syndrome Associated With Novel T Cell-Engaging Therapies. Canc J. 2014;20(2):119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marques-da-Silva C., Chaves M.M., Castro N.G., Coutinho-Silva R., Guimaraes M.Z.P. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br J Pharmacol. 2011;163(5):912–926. doi: 10.1111/j.1476-5381.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Adler Y., Charron P. The 2015 ESC Guidelines on the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2873–2874. doi: 10.1093/eurheartj/ehv479. [DOI] [PubMed] [Google Scholar]
- 156.Deftereos S.G., Siasos G., Giannopoulos G. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J Cardiol. 2020;S1109-9666(20)30061-0 doi: 10.1016/j.hjc.2020.03.002. doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Norman Joseph, Bar-Yam Yaneer, Nassim Nicholas Taleb . New England Complex Systems Institute; 2020. Systemic risk of pandemic via novel pathogens – Coronavirus: A note. Published online January 26. [Google Scholar]
- 158.Wang C., Pan R., Wan X. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Publ Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Owens Ben. Reduced attendances and admissions: lessons from the covid crisis. Health Service Journal. 2020 https://www.hsj.co.uk/coronavirus/reduced-attendances-and-admissions-lessons-from-the-covid-crisis/7027541.article (online) [Google Scholar]
- 160.Tam Chor-Cheung Frankie, Kent-Shek Cheung, Lam Simon. Impact of Coronavirus Disease 2019 (COVID-19) Outbreak on ST-Segment–Elevation Myocardial Infarction Care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4) doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moghadas S.M., Shoukat A., Fitzpatrick M.C. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci U S A. 2020;117(16):9122–9126. doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Iacobucci G. Covid-19: all non-urgent elective surgery is suspended for at least three months in England. BMJ. 020;368:m1106. [DOI] [PubMed]
- 163.Koutsoukis A., Kanakakis I. Challenges and unanswered questions in STEMI management. Hellenic J Cardiol. 2019;60(4):211–215. doi: 10.1016/j.hjc.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 164.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 165.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guan W., Liang W., Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang J., Dong X., Cao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 168.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imag. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arentz M., Yim E., Klaff L. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mehra M.R., Desai S.S., Kuy S., Henry T.D., PatelCardiovascular Disease A.N. Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020;18382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Lechien J.R., Chiesa-Estomba C.M., Place S. Clinical and Epidemiological Characteristics of 1,420 European Patients with mild-to-moderate Coronavirus Disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wei J.-F., Huang F.-Y., Xiong T.-Y. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. 2020 doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Richardson S., Hirsch J.S., Narasimhan M. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gao L., Jiang D., Wen X. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21(1):83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu Y., Du X., Chen J. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta Int J Clin Chem. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.American College of Cardiology A. 2020. COVID-19 Clinical Guidance for the Cardiovascular Care Team.https://www.acc.org/latest-in-cardiology/features/∼/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/2020/02/S20028-ACC-Clinical-Bulletin-Coronavirus.pdf [Google Scholar]
- 180.Han Y., Zeng H., Jiang H. CSC Expert Consensus on Principles of Clinical Management of Patients with Severe Emergent Cardiovascular Diseases during the COVID-19 Epidemic. Circulation. 2020;141(20):e810–e816. doi: 10.1161/CIRCULATIONAHA.120.047011. [DOI] [PubMed] [Google Scholar]
- 181.Lakkireddy D.R., Chung M.K., Gopinathannair R. Guidance for Cardiac Electrophysiology During the Coronavirus (COVID-19) Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.028. S1547-5271(20)30289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Skulstad H., Cosyns B., Popescu B.A. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21(6):592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.