Abstract
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been declared a pandemic. This study analysed 95 SARS-CoV-2-infected patients, including 62 moderate COVID-19 patients, 21 severe COVID-19 patients and 12 critical COVID-19 patients (6 patients died, all critical). The results showed that the mean serum procalcitonin (PCT) levels were over four times higher in severe patients than in moderate patients and were over eight times higher in critical patients than in moderate patients. For discharged patients, both high-normal PCT levels and abnormal PCT levels decreased during recovery. However, in death cases, serum levels of PCT increased as the disease worsened. We demonstrate that PCT may be an indicator of disease severity in COVID-19 and may contribute to determining the severity of patients infected with SARS-CoV-2. Moreover, serial PCT measurements may be useful in predicting the prognosis.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, Procalcitonin, Severity, Prognosis
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly throughout more than 200 countries, areas or territories [1]. SARS-CoV-2 is a novel positive-sense RNA virus with 79% homology with severe acute respiratory syndrome coronavirus (SARS-CoV) and 50% homology with Middle East respiratory syndrome coronavirus (MERS-CoV) [2], however it is far more infectious than either. As of 24 May 2020, a total of 5 103 006 people were confirmed to have had COVID-19 and ~333 401 deaths have occurred [1]. COVID-19 has become a pandemic.
Procalcitonin (PCT) is the 116-amino acid precursor of the hormone calcitonin. Recently, several studies reported that elevated PCT levels are positively associated with the severity of COVID-19 [3], [2], [5], [6]. A meta-analysis also demonstrated that increased PCT values are related to an ~5-fold higher risk of severe SARS-CoV-2 infection [7]. In order to improve the diagnosis to distinguish between severe/critical patients and moderate patients with COVID-19 and to better predict the prognosis, the aim of this study was to investigate the role of changes in PCT values.
2. Materials and methods
2.1. Study design and participants
This retrospective cohort study initially enrolled 95 patients meeting the inclusion criteria from 30 January 2020 to 17 March 2020 from the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The inclusion criteria were as follows: adult patients (males and non-pregnant females) with laboratory-confirmed COVID-19. The diagnostic criteria of COVID-19 were based on the New Coronavirus Pneumonia Prevention and Control Program (6th edition) published by the National Health Commission of the People's Republic of China [8]. Written informed consent was waived by the Ethics Commission of the designated hospital due to the emergency situation of COVID-19.
2.2. Definitions
According to the New Coronavirus Pneumonia Prevention and Control Program (6th edition) [8], COVID-19 is classified into four types, namely mild, moderate, severe, and critical pneumonia. Mild pneumonia represents asymptomatic infection or mild clinical symptoms without abnormal chest imaging findings. Moderate pneumonia is defined as having both clinical symptoms and abnormal chest imaging findings. Patients are diagnosed as having severe pneumonia when the disease progresses to meet any of the following criteria: (i) significantly increased respiration rate of ≥30 breaths/min; (ii) oxygen saturation ≤93% in resting state; or (iii) PaO2/FiO2 (partial pressure of oxygen/fraction of inspired oxygen) ≤300 mmHg (1 mmHg = 0.133 kPa). Critical pneumonia occurs when the disease progresses rapidly with any of the following conditions: (i) respiratory failure requiring mechanical ventilation; (ii) shock; or (iii) other organ failure requiring intensive care unit admission for monitoring and treatment.
Fever was defined when the axillary temperature was ≥37.5 °C. The length of hospital stay was recorded. Bacterial infection was defined according to clinical symptoms, increased white blood cell (WBC) count, PCT or C-reactive protein (CRP) levels, and positive results of bacterial culture.
2.3. Data collection
Demographic, clinical and laboratory data were obtained from electronic medical records using a data collection form. Chemiluminescence was used to measure the PCT concentration.
2.4. Statistical analysis
Continuous variables with a normal distribution are expressed as the mean ± standard deviation and were compared by analysis of variance (ANOVA). Continuous data with a skewed distribution were compared with the Wilcoxon rank-sum test. Categorical variables are summarised as counts (percentages). All analyses were performed with IBM SPSS Statistics v.23 (IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
3. Results
A total of 95 COVID-19 patients (39 male and 56 female) were included in the study. According to their severity of pneumonia on admission to hospital, they were classified into three groups as follows: moderate, n = 62; severe, n = 21; and critical, n = 12. The overall mean age was 57.6 ± 14.7 years. The mean age was 54.2 ± 15.0 years in the moderate group, 65.0 ± 10.3 years in the severe group and 62.2 ± 14.7 years in the critical group. Approximately one-third of the patients had underlying diseases (36; 37.9%), including hypertension (27; 28.4%), diabetes (13; 13.7%), cardiovascular disease (8; 8.4%), hyperlipidaemia (1; 1.1%), cerebrovascular disease (1; 1.1%), chronic obstructive pulmonary disease (COPD) (1; 1.1%) and hepatitis B virus infection (1; 1.1%). There was no history of other diseases in these patients.
Clinical symptoms of the patients on admission in the three groups are compared in Supplementary Table S1. The most common symptoms at illness onset were fever (68/95; 71.6%), dry cough (54/95; 56.8%) and dyspnoea (48/95; 50.5%); less common symptoms were fatigue (32/95; 33.7%), expectoration (26/95; 27.4%), nausea (14/95; 14.7%), myalgia (14/95; 14.7%), vomiting (11/95; 11.6%), diarrhoea (9/95; 9.5%), dizziness (7/95; 7.4%), sore throat (5/95; 5.3%), headache (3/95; 3.2%) and abdominal pain (2/95; 2.1%). The mean length of hospital stay was 16.8 ± 8.8 days.
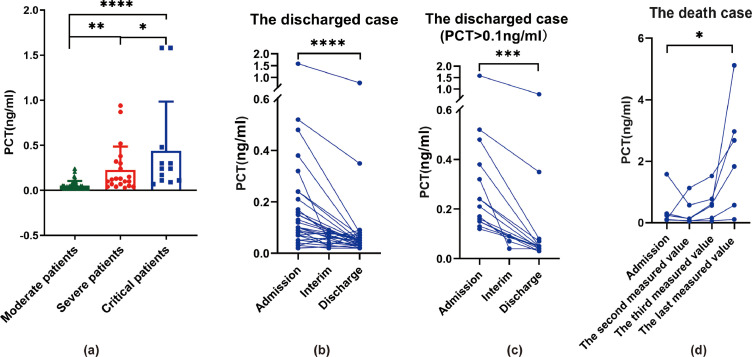
Laboratory data within 3 days of admission demonstrated that the mean serum PCT levels were 0.05 ± 0.05 ng/mL in the moderate group (n = 62), 0.23 ± 0.26 ng/mL in the severe group (n = 21) and 0.44 ± 0.55 ng/mL in the critical group (n = 12) (Fig. 1 A). Total serum levels of PCT increased as the disease worsened (P < 0.05). A total of 38 patients (32 discharged cases and 6 deaths) had serial PCT values measured (within 3 days of admission, during the hospital stay and within 7 days before hospital discharge) (Fig. 1B,D). Results for the 32 discharged patients showed that the serum PCT levels decreased during recovery and there was a significant difference between levels within 3 days of admission and within 7 days before hospital discharge (Fig. 1B). Among them, the PCT levels of 14 patients were >0.1 ng/mL within 3 days of admission, which decreased significantly during recovery (Fig. 1C). Interestingly, most (5/6; 83.3%) of the second PCT values measured in the death cases decreased slightly after the patients were admitted and received treatment. However, the third PCT value measured all increased significantly again (6/6; 100%) (Fig. 1D). It then continuously increased as the disease worsened in the death cases (P < 0.05) (Fig. 1D). The WBC count was normal in 66/93 cases (71.0%), decreased in 16/93 cases (17.2%) and increased in 11/93 cases (11.8%). The neutrophil count was decreased in 10/93 cases (10.8%) and increased in 19/93 cases (20.4%). Other laboratory findings are shown in Supplementary Table S2.
Fig. 1.
Procalcitonin (PCT) levels in COVID-19 patients. (A) PCT values of patients (n = 95) with differing severity of COVID-19. Data are the mean ± standard deviation. * P < 0.5; ** P < 0.01; **** P < 0.0001 [compared by analysis of variance (ANOVA)]. (B) Serial PCT values for COVID-19 patients who were discharged (n = 32) (high-normal and abnormal PCT values). **** P < 0.0001 (tested by Wilcoxon rank-sum test). (C) Serial PCT values for discharged COVID-19 patients with PCT >0.1 ng/mL within 3 days of admission (n = 14). *** P < 0.001 (tested by Wilcoxon rank-sum test). (D) Serial PCT values of COVID-19 patients who died (n = 6). * P < 0.05 (tested by Wilcoxon rank-sum test).
In addition, most patients were administered antiviral therapy, glucocorticoid therapy and oxygen therapy (Supplementary Table S1).
4. Discussion
Here we report on 95 patients with laboratory-confirmed SARS-CoV-2 infection in Union Hospital of Tongji Medical College. A total of 62 moderate COVID-19 patients, 21 severe COVID-19 patients and 12 critical COVID-19 patients were included in the study. Of the 95 patients in this cohort, 6 (6.3%) died (all critical patients).
PCT, which is the 116-amino acid precursor of the hormone calcitonin, is normally synthesised and released by thyroid parafollicular C cells. However, it can also be synthesised in many extrathyroid tissues during bacterial infection, which is mediated by increased concentrations of tumour necrosis factor-alpha (TNFα) and interleukin 6 [9]. Recently, several studies reported that elevated PCT is positively associated with the severity of COVID-19 [3], [2], [5], [6]. A meta-analysis also demonstrated that increased PCT values are related to an ~5-fold higher risk of severe COVID-19 [7].
This study further classified severe infections into severe patients and critical patients. The results showed that mean serum PCT levels were approximately four times higher in severe patients than in moderate patients and approximately eight times higher in critical patients than in moderate patients. PCT levels appeared to be disease severity-dependent and may be associated with bacterial co-infection, as the co-infection rate was close to the rate of elevated PCT levels in patients with moderate disease severity (~10%). Notably, the co-infection rates were only 20% and 50% in severe and critical patients, respectively, whereas the rates of elevated PCT were 50% and 80%, respectively.
In addition, a recent study hypothesised that a progressive increase in PCT levels may predict a worse prognosis [10]. A total of 38 patients who had serially measured PCT values were included in the current study, of whom 32 were discharged from hospital and 6 died. For the 32 discharged patients, both high-normal and abnormal PCT levels decreased during recovery. However, for the 6 death cases, serum PCT levels increased as the disease worsened. These results demonstrate that serial PCT measurements can predict the prognosis of COVID-19 patients.
5. Conclusion
This study demonstrates that PCT may be an indicator of disease severity and may contribute to determining the severity of patients with COVID-19. In addition, serial PCT measurements may be useful in predicting the prognosis. Additional investigation is needed to further illustrate the mechanisms by which increased PCT is synthesised and released in patients infected with SARS-CoV-2.
Acknowledgments
The authors would like to thank the doctors, nurses and medical students who helped with data collection.
Funding: This work was supported by grants from the General Program the National Natural Science Foundation of China [81874138 and 62041208].
Competing interests: None declared.
Ethical approval: Not required.
Editor: Jean-Marc Rolain
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106051.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). Coronavirus disease 2019 (COVID-19) Situation report – 124.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200523-covid-19-sitrep-124.pdf?sfvrsn=9626d639_2[accessed 24 May 2020].
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 Feb 19 doi: 10.1111/all.14238. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.State Council of The People's Republic of China . 2020. The notice of launching guideline on diagnosis and treatment of the novel coronavirus pneumonia (6th edition) [in Chinese]http://www.gov.cn/zhengce/zhengceku/2020-02/19/content_5480948.htm [accessed 2 April 2020]. [Google Scholar]
- 9.Lippi G, Cervellin G. Procalcitonin for diagnosing and monitoring bacterial infections: for or against? Clin Chem Lab Med. 2018;56:1193–1195. doi: 10.1515/cclm-2018-0312. [DOI] [PubMed] [Google Scholar]
- 10.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020 Mar 3 doi: 10.1515/cclm-2020-0198. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.