Abstract
Background:
Binge drinking is associated with increased risk for cardiovascular (CV) disease. MicroRNA-21 (miR21) is upregulated in the setting of excessive alcohol consumption and CV disease. Therefore, the goal of this study was to examine the vasodilatory responses to flow and acetylcholine in the absence and presence of an anti-miR21 inhibitor in the microcirculation of young adult repeated binge drinkers.
Methods:
Gluteal subcutaneous adipose tissue biopsies were obtained from young adults (18–30 yrs, n = 35 vessels from binge drinkers [BDs] and n = 28 vessels from abstainers). Resistance arteries (RAs) were isolated, incubated with anti-miR21 or a negative control to miR-21 (12 hours; 5 nM), and lumen diameters measured with video microscopy. MiRNA-21 of adipose tissues was determined by quantitative PCR (qPCR).
Results:
Flow-induced dilation (FID) and acetylcholine (ACh)-induced dilation (AChID) were reduced in BDs as compared to abstainers. The miR-21 inhibitor but not the negative control abrogated these effects in BDs, but did not affect vasodilation in abstainers. Nitric oxide synthase inhibition with L-NAME reduced vasodilation in abstainers but not in BDs. In BDs, vasodilation was reduced by L-NAME in the presence of anti-miR-21 but not the negative control. Scavenging the reactive oxygen species hydrogen peroxide with polyethyleneglyco-catalase reduced dilation in BDs but did not affect the restored dilation by miR-21 inhibitor. Maximum dilation to papaverine (endothelium-independent) was similar between groups and unaffected by pharmacological inhibition. Finally, vascular endogenous miR-21 was increased in BDs compared to abstainers.
Conclusions:
Endogenous microRNA-21 is increased in RAs of young BDs, leading to reduced flow and acetylcholine-induced vasodilation in the microcirculation.
Keywords: Binge drinking, young adults, microcirculation, micro-RNA21, endothelium
Introduction
Compared to previous generations, today’s youth binge drink with a prevalence, intensity, and frequency that may place them at greater risk for more profound rates of alcohol-induced harm (Kanny et al., 2013, Piano et al., 2017). This alcohol-related harm may include premature cardiovascular (CV) disease. We previously reported that young adults (18–25 years) with a history of binge drinking (mean of four years) had reduced flow-mediated vasodilation and endothelium-independent (nitroglycerin) dilations compared to age-matched abstainers(Goslawski et al., 2013). Others have also reported that, in young adults, a binge pattern of drinking was associated with higher blood pressure (Wellman et al., 2016), increased carotid intimal thickness (Pletcher et al., 2005), and increased coronary calcification (van Trijp et al., 2005). These findings suggest that binge drinking may be associated with premature CV risk in young adults.
The biologic links between premature CV risk and binge drinking are not known; however, evidence is accumulating that micro-ribonucleic acids (microRNA) which regulate gene expression, may play a significant role in mediating the adverse effects of ethanol (Miranda et al., 2010). MicroRNAs are non-coding RNAs that regulate gene expression at the post-translational level by modulating the stability and/or translational efficiency of target messenger RNAs (Schulte et al., 2017). In subjects with coronary artery disease, Fichtlscherer et al. found circulating vessel wall- and inflammatory-derived miRNAs (in particular those, expressed in endothelial cells) were downregulated whereas other miRNAs are increased (Fichtlscherer et al., 2010). Others have reported that, in an animal model of vascular balloon injury, microRNA-21 (MiR21) is significantly upregulated in coronary artery atherosclerotic plaques (Ji et al., 2007). Also, in a laboratory model of stress-induced drinking, Beech et al. found increases in circulating miR21 levels in subjects with a history of heavy drinking (defined as regular alcohol use over the past year of at least 8 standard drinks/week for women and at least 15 standard drinks/week for men) and that miR21 levels correlated with the amount of alcohol consumed by the heavy drinkers (Beech et al., 2014). Others have also found that, in the setting of alcoholic liver injury, miR-21 modulated cell proliferation and survival (apoptosis) in a way that contributed to the progression of alcoholic liver disease (Francis et al., 2014). MiR21 is involved in inflammation, apoptosis, and nitric oxide signaling (Vickers et al., 2014). Given the critical role of miR-21 in the pathophysiology of the vasculature, miR-21appears to be an attractive target for treatment of cardiovascular and cardiometabolic disease.
Therefore, the purpose of this study was to examine vasodilatory responses to flow and acetylcholine in the absence and presence of an anti-miR21 inhibitor in microvessels isolated from young adult repeated binge drinkers. We focused on the microcirculation because changes in the microcirculation precede the development of overt CV disease and other adverse CV sequelae (Gutterman et al., 2016). Importantly, there is a need to understand the pathways leading from alcohol consumption to the early origins of CV disease.
Material and Methods
Study Design and Subjects
Subjects were part of a larger ongoing study designed to examine the CV effects of binge drinking. Young adult (18–30 years) abstainers (n = 7) and binge drinkers (BDs; n = 7) were recruited from a large Midwestern university campus. Exclusion criteria were obesity (body mass index ≥ 30), current or history of cigarette smoking, inflammatory disease (e.g., rheumatoid), diagnosed CV or renal disease, active infection (2 months prior), or pregnancy. Women were recruited during the early follicular phase of the reproductive cycle(Goslawski et al., 2013). As previously described, alcohol consumption levels and drinking patterns were determined by an alcohol intake questionnaire (AIQ) and Alcohol Use Disorders Identification Test (AUDIT) scores. (Goslawski et al., 2013, Piano et al., 2015). The AIQ is a 20-item tool and includes a modified version of the National Institute of Alcoholism and Alcohol Abuse (NIAAA) 6-item set of questions on binge drinking (Task Force on Recommended Alcohol Questions, 2003). The AUDIT is a 10-item questionnaire and has been validated in young adult populations and a total score of > 6 has high sensitivity (91%) and specificity (60.0%) for detecting high-risk drinking among young adults (Kokotailo et al., 2004).
Alcohol abstainers were defined as those that consumed no more than 1 standard drink per month in the last 2–3 years (and abstention could not be due to a medical illness or prior alcohol abuse). According to this definition abstainers may have not been alcohol free over the previous month. Binge drinkers were defined as those consuming 5 or more standard drinks if male, 4 or more standard drinks if female, either on one occasion or within a two-hour period in the last 30 days. In addition, BDs had to have a history of more than two years of repeated binge drinking.
Prior to the study visit, subjects fasted overnight; all visits were scheduled between 8 a.m. and 12 p.m. After questionnaire completion, venous blood was collected into either serum separator tubes or tubes containing sodium citrate for measurement of total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglycerides, insulin, glucose, complete blood count (CBC) differential, and high sensitivity C-reactive protein (CRP; Alverno Clinical Laboratories, Hammond, IN, USA). The study was approved by the Office of Protection of Research Subjects and Institutional Review Board, and written consent was obtained from all subjects.
Gluteal Adipose Biopsy and Resistance Artery Function
As previously described, we used resistance arteries (RAs) in subcutaneous fat to investigate microvascular function in humans (Robinson et al., 2016, Durand et al., 2014). Approximately 2 ml of fat tissue was removed and transferred to (4°C) HEPES solution. RAs (n = 28 from 7 abstainer biopsies; n = 35 from 7 BD biopsies) were isolated and cannulated in an organ chamber with glass micropipettes filled with KREBS solution (pH = 7.40; (Phillips et al., 2007). Both ends of the vessel were secured, and the vessel was maintained at an intraluminal pressure of 20 mmHg for 30 minutes, after which intraluminal pressure was gradually increased to 60 mmHg for an additional 30 minutes. Mean internal diameter of RAs was 160 ± 21 μm, and, using endothelin-1 (100–200 pM), RAs were pre-constricted to ~50% of maximum diameter. Following the 30-minute equilibration period, RA diameters were measured before and during flow-induced dilation (FID) at different pressure gradients (Δ10 to Δ100 cmH2O). In addition, RA diameters were measured before and during incremental doses of acetylcholine (ACh; 10−9-10−4 M). FID and ACh-induced dilations (AChID) were determined in the absence and presence of the nitric oxide synthase (NOS) inhibitor N-nitro-L-arginine methyl ester (L-NAME; 10−4 M), or scavenger of H2O2, poly ethyleneglycol catalase (PEG-CAT, 500 Units/ml). Maximal responses to papaverine (10−4 M) were measured at the end of each experiment.
Anti-MicroRNA 21 Experiments
Lastly, RAs were incubated with microRNA-21 inhibitor (anti-miR-21, final concentration of 50 nM; Invitrogen) or negative control (NC) to anti-miR-21 (50 nM) overnight at 37°C with 200 μl KREBS buffer in a 96-well plate, and the FID or ACh experiments were repeated as described above. The doses of anti-miR21 were determined according to our previous results showing effective knockdown of smooth muscle cell miR21 expression by 50 nM anti-miR21 (data not shown). RAs were monitored and visualized continuously with video cameras, and internal diameters were measured at the maximum diameter after each pressure gradient and drug dose (Ach; Boeckeler model VIA-100, Tucson, AZ, USA).
Determination of miRNA-21 by Quantitative Real-time Reversely Transcribed PCR (qRT-PCR)
Arterioles from each biopsy were pooled and total RNA was extracted from each subject individually using the RNeasy mini kit (Qiagen, Germantown, MD). RNA quantities and qualities were determined by measuring absorbance at 260 nm and 280 nm by spectrophotometry. RNA was reversely transcribed into cDNA using SuperScript RT III (Invitrogen). The normalized expression ratio of miR21 was determined by real-time RT-PCR using the SYBR Green Assay standard protocol and the Agilent Mx3005P qPCR System (Agilent Technologies, Santa Clara, CA). Specific primers for miR21 were designed using the online Prime3 program. The forward miR21 primer sequence is 5’-CAGACTGATGTTGACTGTTGAATC-3’, and the reverse miR21 primer sequence is 5’-GTCAGACAGCCCATCGAC-3’. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for normalization of expression data. Threshold cycle numbers (Ct) generated by the real-time RT-PCR were used to calculate the normalized expression ratio of the target genes using the 2−∆∆Ct (Livak) method.
Materials
L-NAME, ACh, ET-1, papaverine, and other chemical reagents for buffer solutions were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA) and Fisher Scientific (San Jose, CA, USA). Anti-miR21 inhibitor was obtained from Invitrogen (Carlsbad, CA, USA).
Statistics
Analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and SigmaPlot Version 12.0 (San Jose, CA, USA). All data are reported as mean ± SE, with p < .05 as significant unless otherwise noted. Normality was tested using the Shapiro-Wilk test. The abstainer and BD group differences were determined using independent t tests. Mann-Whitney U tests were used for continuous variables (e.g., demographic, CRP, lipid levels) and chi-squared tests for categorical variables. In gradient or concentration response experiments, data are expressed as a percentage for dilation of flow or ACh, with 100% representing the change from constricted diameter (ET1, 50%) to the maximal diameter at 60 cmH2O intraluminal pressure. Vasodilator responses to ACh and flow (FID; −/+ L-NAME, −/+ miRNA inhibitor and −/+ PEG-catalase) were compared using a two-way repeated-measures analysis of variance. t test was used with two-tailed distribution and two-sample equal variance.
Results
Demographic Characteristics
Total cholesterol, LDL-c, HDL-c, and other laboratory values were not different between groups (Table 1). Total AUDIT scores were significantly greater in BDs compared to abstainers (p < 0.03). The average duration of binge drinking was 2.4 years.
Table 1.
Demographic and Metabolic Characteristics
| Abstainers | Binge Drinkers | p | |
|---|---|---|---|
| (n = 7) | (n = 7) | ||
| Age (yrs) | 23.8 ± 1.2 | 23.7± 0.64 | 0.92 |
| Female | 70% | 30% | 0.13 |
| White | 85% | 71% | 0.71 |
| Body mass index (kg/m2) | 23.4 ± 1.4 | 24.2 ± 0.84 | 0.63 |
| Total cholesterol (mg/dL) | 155.4 ± 10.9 | 170.6 ± 10.7 | 0.34 |
| LDL cholesterol (mg/dL) | 91.0 ± 8.5 | 95.8 ± 6.9 | 0.66 |
| HDL cholesterol (mg/dL) | 52.7 ± 3.3 | 51.6 ± 2.7 | 0.81 |
| Triglycerides (mg/dL) | 72.5 ± 10.1 | 95.8 ± 19.3 | 0.29 |
| Glucose (mg/dL) | 92.0± 2.2 | 92.0 ± 2.6 | 0.75 |
| Insulin (μIU/L) | 5.1 ± 0.84 | 4.1 ± 1.1 | 0.39 |
| AST (IU/L) | 21.8 ± 3.3 | 22.2 ± 2.7 | 0.69 |
| ALT (IU/L) | 17.0 ± 1.4 | 15.8 ± 2.8 | 0.71 |
| AUDIT score | 0.57 ± 0.29 | 10.4 ± 2.4 | <0.001* |
Data, MEAN ± SEM,
< 0.05, Binge Drinkers vs. Abstainers. Except for AST (n = 5) and ALT (n = 5) values all levels were measured in n = 7.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; HDL = high-density lipoprotein; LDL = low-density lipoprotein
Reduction of Vasodilation of Arterioles in Binge Drinkers
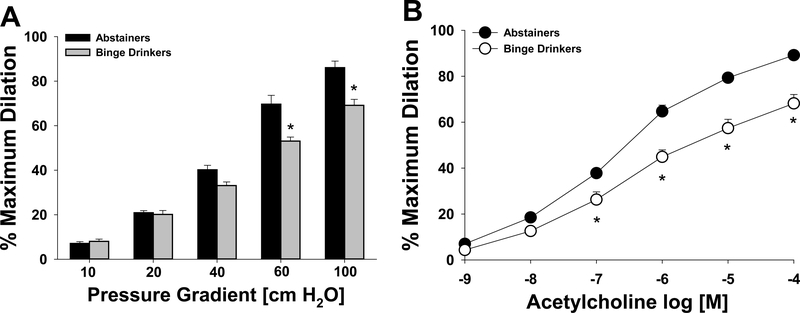
The mean internal luminal diameter of RAs was 228 ± 15 μm and 202 ± 26 μm for BD and abstainers respectively (p = 0.3), respectively. FID was significantly reduced in RAs from BDs compared to abstainers at pressure gradients greater than 60 (cm H2O; Figure 1A). Similarly, AChIDs of RAs to increasing doses of ACh (> 10−6 M) were significantly reduced in BDs compared to abstainers, as was the maximum AChID in BDs (68% ± 4%) compared to abstainers (89% ± 1%; Figure 1B). Papaverine (10−4 M) dilations were similar in the groups following either FID or AChID (data not shown).
Figure 1.
Flow-induced dilation (A) and acetylcholine (B) -induced dilation of arterioles from binge drinkers were reduced compared to abstainers.*p < 0.05 Binge drinker vs. abstainer.
Inhibition of NOS with L-NAME did not reduce FID (Figure 2A) or AChID (Figure 2C) of RAs from BDs. In contrast, PEG-catalase significantly reduced FID and AChID in BDs; whereas, in abstainer, RAs, L-NAME, and PEG-catalase significantly reduced FIDs (Figure 2B) and AChIDs (Figure 2D).
Figure 2.
Effect of L-NAME and PEG-catalase on flow-induced dilation (FID) and Ach-induced dilation (AChID) in binge drinkers (BD) and abstainers. In the presence of PEG-catalase, FID (A) and AChID (C) were significantly reduced in BDs (p < 0.05). There was no effect of L-NAME on FID and AChID in BDs. However, both L-NAME and PEG-catalase, reduced FID (B) and AChID (D) in abstainers (*p < 0.05 for PEG-catalase vs. baseline; †p < 0.05 for L-NAME vs. baseline).
Anti-miR21 Inhibitor Restored Impaired Microvascular Vasodilation of BDs
There was no difference in arterial diameters between arterioles with and without miR21 in abstainers (202 ± 26 μm control vs. 192 ± 21 μm anti-miR21) and binge drinkers (227 ± 15 um control vs. 231 ± 22 anti-miR21). Treatment with the anti-miR21 inhibitor (50 nM) reversed impaired FID and AChID in BD RAs (Figures 3A and 3C), whereas the negative control sequence of anti-miR21 (50 nM) had no effect. Exposure with either anti-miR21 or the negative control peptide to anti-miR21 had no effect on FID or AChIDs in abstainer RAs (Figures 3B and 3D).
Figure 3.
Anti-microRNA21 restores flow-induced dilation (FID) and acetylcholine-induced dilation (AChID) in arterioles of binge drinkers (BD). There was a significant increase in FID (A) and AChID (C) in arterioles from BD after exposure to anti-miR21 (*p < 0.01 vs. baseline of BD). There was no effect of anti-miR21 or the negative control miR21 sequence on FID (B) or AChID (D).
The restored FID and ACh following anti-miR21 inhibition in RA of BDs was eliminated by L-NAME (Figures 4A and 4C). In contrast, PEG-catalase had no effect on this restored FID or AChIDs (Figures 4A and 4C). These results suggest that these restored effects of anti-miR21 are nitric oxide (NO)-dependent. In abstainers, the inhibitory effects of L-NAME or PEG-catalase on dilations to flow and ACh were not altered by anti-miR21 inhibitor (Figures 4B and 4D). There was no effect of the anti-miR21 negative control peptide on the inhibitory effect of L-NAME or PEG-catalase on vasodilation (data not shown).
Figure 4.
Effect of L-NAME and PEG-catalase on flow-induced dilation (FID) and Ach-induced dilation (AChID) in arterioles of binge drinkers (BD) and abstainers following anti-microRNA21 (miR21) treatment. L-NAME completely inhibited FID (A) and AChID (C) in anti-miR21 treated arterioles from BD (*p < 0.001, vs. baseline). There was no effect of PEG-catalase on either FID (A) or AChID (C) in BD following anti-miR21. Both L-NAME and PEG-catalase significantly reduced FID (B) and AChID (D) in arterioles from abstainers exposed to anti-miR21 (*p < 0.05 L-NAME vs. baseline; †p < 0.05 PEG-catalase vs. baseline).
MiR21 Levels are Increased in Binge Drinkers
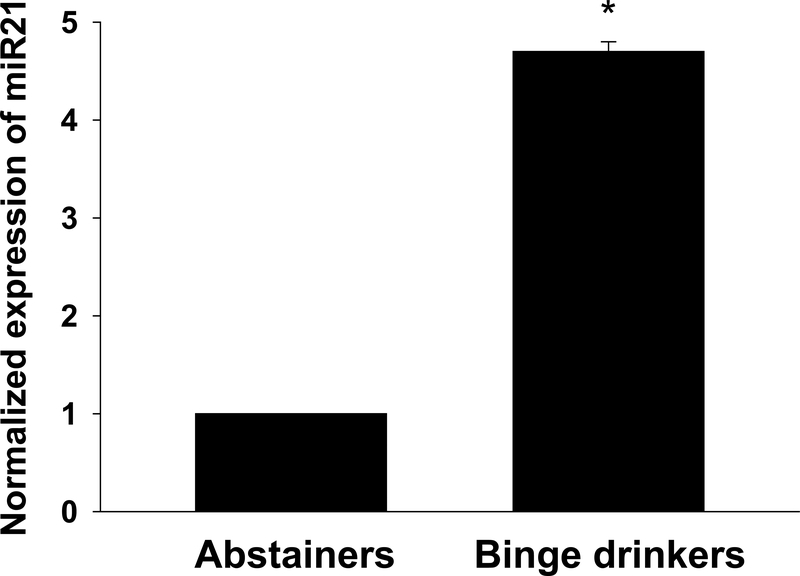
There was a 4.7-fold increase in miR21 expression in microvessels/tissue of BDs compared to abstainers (Figure 5).
Figure 5.
MicroRNA21 expression was increased in adipose tissues of binge drinkers compared to abstainers (*p < 0.001 vs. abstainer; n=3 per group).
Discussion
Our findings suggest that microvascular vasodilation to flow and ACh are reduced in young adult BDs compared to abstainers. We found no effect of NOS inhibition (via L-NAME) on RA dilations to flow and ACh in BDs, suggesting reduced NO bioavailability in the microcirculation of BDs compared to abstainers. Secondly, miR21 inhibition restored flow and ACh dilation in RAs of BDs; however, NOS inhibition eliminated the dilation of RAs to flow and ACh following the treatment of RAs with anti-miR21. This is the first investigation to examine potential mechanisms of microvascular dysfunction in young adults with a history of repeated binge drinking.
The attenuation of FID and AChID responses suggests impairments in the endothelial-nitric oxide-generating/signaling system. Interestingly, we found no effect of NOS inhibition (via L-NAME) on RA dilations to flow and ACh in BDs, whereas (as expected) in abstainers, L-NAME markedly attenuated FIDs and AChIDs. Within the microcirculation, NO generated by NOS plays an important role in mediating both flow and ACh-induced vasodilation. Our findings suggest reduced NO bioavailability in the microcirculation of BDs compared to abstainers. Various pathological stimuli have been reported to decrease NOS expression (mainly through decreasing NOS mRNA stability), and these pathological stimuli include cytokines, tumor necrosis-alpha, and oxidative stress (Valerio et al., 2006, Venugopal et al., 2002, Zhou et al., 2013). Though we did not measure any of the latter stimuli, using an animal model of long-term binge (daily gavage [9g/kg/d] x 16 weeks in rats), Zhou et al. found that oxidative stress markers and proinflammatory mediators (tumor necrosis factor-α) were significantly increased following long-term binge drinking (Zhou et al., 2013). It is possible that binge-induced elevations in oxidative stress molecules such as superoxide create an environment where NO bioavailability is reduced. In support of this supposition, we found that PEG-catalase, a hydrogen peroxide scavenger, reduced FID and AChIDs.
Flow-induced vasodilation is an important physiological regulator of tissue perfusion. The mediator of FID in human adipose is typically NO under normal physiological conditions (Phillips et al., 2007). Consistent with the results of our previous studies (Phillips et al., 2007, Robinson et al., 2016), we found that NO is the primary mediator of dilation to flow in subcutaneous adipose RAs. Previous studies have found that, in the setting of CV disease or presence of CV risk factors such as hypertension, NO is scavenged by elevated levels of reactive oxygen species (Erdei et al., 2006, Guzik et al., 2002). This is likely to be the case during binge drinking because ROS have been found to be elevated during alcohol intake (Rocha et al., 2012, Yogi et al., 2012). Further, increases in ROS such as H2O2 have been shown to compensate for reduced NO bioavailability in the human microvasculature during disease (Phillips et al., 2007, Liu et al., 2003). In the case of binge drinking in young adults, it is possible that ROS and H2O2 may contribute to FID and AChID dilations because PEG-catalase reduced dilations in BD in the absence of an L-NAME effect (Figure 2).
H2O2 is associated with inflammation and pro-inflammatory profiles in the vasculature (Cai, 2005a). It is possible that H2O2 may serve as a feed-forward mechanism or “viscous cycle” for inducing more vascular dysfunction in the context of young binge drinkers because it was recently found that ROS may induce more ROS release in the peripheral microcirculation (Cai, 2005b, Zinkevich et al., 2017). The physiological cost of maintaining vasodilation with H2O2 at the expense of further long-term vascular risk during periods of binge drinking in young adults and in the transition to mid-adulthood is not known. While alcohol may increase the propensity for inflammatory phenotype, we have previously found that there was no difference in circulating markers of c-reactive protein in young adult BD compare to abstainers (Goslawski et al., 2013). Further research is needed to examine the potential contribution of oxidative stress and the sources of ROS to these abnormal responses to physiologic and pharmacologic stimuli in the setting of binge drinking. This is especially important because the physiological cost of maintaining vasodilation with H2O2 may result in long-term vascular injury and increased cardiovascular risk during periods of binge drinking in young adults.
miR21 is one of the most upregulated miRNAs in cardiac disease and vascular injury (Small et al., 2010, Ji et al., 2007). We found a 4.7-fold increase in miR21 expression in the arterioles/tissue from BDs and that RAs from BDs treated with an anti-miR21 peptide had restored vasodilation responsiveness to flow and ACh. Our data suggest a role for miR21 in mediating the adverse effects of binge drinking on the microvasculature in young adults. Interestingly, the effect of anti-miR21 restoration appears to be entirely dependent on NOS because L-NAME eliminated the dilations to flow and ACh following anti-miR21 treatment. It is possible that miRNAs can modulate NO generation and/or alter the expression of NOS. For example, miR-155 expression was found to decrease NOS in human internal mammary arteries (Sun et al., 2012). In other studies, miR21 inhibited the conversion of superoxide to H2O2, which increased superoxide in epithelial cells (Zhang et al., 2012). On the other hand, miR21 specifically may increase the pro-inflammatory (NF-kB) pathway in vascular cells (Thulasingam et al., 2011), thereby contributing to the pro-inflammatory phenotype that scavenges NO during binge drinking. The mechanism whereby miR21 might activate this pro-inflammatory pathway and/or reduce NOS function and expression during binge drinking is unknown. However, the impact of anti-miR21 treatment of the microvascular dysfunction (reduced vasodilation) during binge drinking appears to be an important mechanistic area of future investigation.
Limitations
The small sample size and cross-sectional design disallow any conclusions about causality and the potential reversibility of the binge-induced microvascular effects. However, we commonly procure multiple vessels from each subject, which minimizes burden and enhances the robust nature of the experiments. While the current cohort consisted of more women in the abstainer group, we do not have evidence for sex differences in the vascular response to binge drinking in young adults (Goslawski et al., 2013). Future studies should incorporate the measurement of enzymes (i.e., NOS and catalase) or direct measurement of NO and ROS. However, we used PEG-catalase, which is a specific inhibitor of H2O2, and L-NAME is a specific inhibitor of NOS. In this context, the data support that the catalase-inhibited component of dilation is due to H2O2 in binge drinkers and the L-NAME-inhibited component of dilation following anti-miR21 treatment is due to NO. In addition, using immunohistochemical approaches, it may be possible to measure NOS and superoxide dismutase expression in the microvascular wall of binge drinkers. Further, while miR21 was significantly increased in the tissue of BDs compared to abstainers, future studies should include the measurement of other microRNAs.
Collectively, these study findings provide preliminary evidence for reduced microvascular flow-induced vasodilation and acetylcholine-induced vasodilation in young adult BDs compared to abstainers. We found that inhibition of the miR21 signaling response in the vascular wall restores microvascular vasodilation (FID and AChID) and that this restored vasodilation is almost entirely dependent on NO signaling. The mechanism by which miR21 is increased during binge drinking and by which anti-miR21 restores NO-dependent vasodilation warrants further investigation.
Acknowledgments
National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- BEECH RD, LEFFERT JJ, LIN A, HONG KA, HANSEN J, UMLAUF S, MANE S, ZHAO H. & SINHA R. (2014) Stress-related alcohol consumption in heavy drinkers correlates with expression of miR-10a, miR-21, and components of the TAR-RNA-binding protein-associated complex. Alcohol Clin Exp Res, 38, 2743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAI H. (2005a) Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res, 68, 26–36. [DOI] [PubMed] [Google Scholar]
- CAI H. 2005b. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res, 96, 818–22. [DOI] [PubMed] [Google Scholar]
- DURAND MJ, DHARMASHANKAR K, BIAN JT, DAS E, VIDOVICH M, GUTTERMAN DD & PHILLIPS SA (2014) Acute Exertion Elicits a H2O2-Dependent Vasodilator Mechanism in the Microvasculature of Exercise-Trained but not Sedentary Adults. Hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERDEI N, TOTH A, PASZTOR ET, PAPP Z, EDES I, KOLLER A. & BAGI Z. (2006) High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol, 291, H2107–15. [DOI] [PubMed] [Google Scholar]
- FICHTLSCHERER S, DE ROSA S, FOX H, SCHWIETZ T, FISCHER A, LIEBETRAU C, WEBER M, HAMM CW, ROXE T, MULLER-ARDOGAN M, BONAUER A, ZEIHER AM & DIMMELER S. (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res, 107, 677–84. [DOI] [PubMed] [Google Scholar]
- FRANCIS H, MCDANIEL K, HAN Y, LIU X, KENNEDY L, YANG F, MCCARRA J, ZHOU T, GLASER S, VENTER J, HUANG L, LEVINE P, LAI JM, LIU CG, ALPINI G. & MENG F. (2014) Regulation of the extrinsic apoptotic pathway by microRNA-21 in alcoholic liver injury. J Biol Chem, 289, 27526–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSLAWSKI M, PIANO MR, BIAN JT, CHURCH EC, SZCZUREK M. & PHILLIPS SA (2013) Binge drinking impairs vascular function in young adults. J Am Coll Cardiol, 62, 201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTTERMAN DD, CHABOWSKI DS, KADLEC AO, DURAND MJ, FREED JK, AIT-AISSA K. & BEYER AM (2016) The Human Microcirculation: Regulation of Flow and Beyond. Circ Res, 118, 157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZIK TJ, MUSSA S, GASTALDI D, SADOWSKI J, RATNATUNGA C, PILLAI R. & CHANNON KM (2002) Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation, 105, 1656–62. [DOI] [PubMed] [Google Scholar]
- JI R, CHENG Y, YUE J, YANG J, LIU X, CHEN H, DEAN DB & ZHANG C. (2007) MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res, 100, 1579–88. [DOI] [PubMed] [Google Scholar]
- KANNY D, LIU Y, BREWER RD & LU H. 2013. Binge drinking - United States, 2011. MMWR Surveill Summ, 62 Suppl 3, 77–80. [PubMed] [Google Scholar]
- KOKOTAILO PK, EGAN J, GANGNON R, BROWN D, MUNDT M. & FLEMING M. 2004. Validity of the alcohol use disorders identification test in college students. Alcohol Clin Exp Res, 28, 914–20. [DOI] [PubMed] [Google Scholar]
- LIU Y, ZHAO H, LI H, KALYANARAMAN B, NICOLOSI AC & GUTTERMAN DD (2003) Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res, 93, 573–80. [DOI] [PubMed] [Google Scholar]
- MIRANDA RC, PIETRZYKOWSKI AZ, TANG Y, SATHYAN P, MAYFIELD D, KESHAVARZIAN A, SAMPSON W. & HERELD D. (2010) MicroRNAs: master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res, 34, 575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS SA, HATOUM OA & GUTTERMAN DD (2007) The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol, 292, H93–100. [DOI] [PubMed] [Google Scholar]
- PIANO MR, MAZZUCO A, KANG M. & PHILLIPS SA (2017) Binge Drinking Episodes in Young Adults: How Should We Measure Them in a Research Setting? J Stud Alcohol Drugs, 78, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIANO MR, TIWARI S, NEVORAL L. & PHILLIPS SA (2015) Phosphatidylethanol levels are elevated and correlate strongly with AUDIT scores in young adult binge drinkers. Alcohol Alcohol, 50, 519–25. [DOI] [PubMed] [Google Scholar]
- PLETCHER MJ, VAROSY P, KIEFE CI, LEWIS CE, SIDNEY S. & HULLEY SB (2005) Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol, 161, 423–33. [DOI] [PubMed] [Google Scholar]
- ROBINSON AT, FRANKLIN NC, NORKEVICIUTE E, BIAN JT, BABANA JC, SZCZUREK MR & PHILLIPS SA (2016) Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J Hypertens, 34, 1309–16. [DOI] [PubMed] [Google Scholar]
- ROCHA JT, HIPOLITO UV, CALLERA GE, YOGI A, NETO FILHO MDOS A, BENDHACK LM, TOUYZ RM & TIRAPELLI CR (2012) Ethanol induces vascular relaxation via redox-sensitive and nitric oxide-dependent pathways. Vascul Pharmacol, 56, 74–83. [DOI] [PubMed] [Google Scholar]
- SCHULTE C, KARAKAS M. & ZELLER T. (2017) microRNAs in cardiovascular disease - clinical application. Clin Chem Lab Med, 55, 687–704. [DOI] [PubMed] [Google Scholar]
- SMALL EM, FROST RJ & OLSON EN (2010) MicroRNAs add a new dimension to cardiovascular disease. Circulation, 121, 1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN HX, ZENG DY, LI RT, PANG RP, YANG H, HU YL, ZHANG Q, JIANG Y, HUANG LY, TANG YB, YAN GJ & ZHOU JG (2012) Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension, 60, 1407–14. [DOI] [PubMed] [Google Scholar]
- TASK FORCE ON RECOMMENDED ALCOHOL QUESTIONS. (2003) Recommended alcohol questions [Online]. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Available: http://www.niaaa.nih.gov/research/guidelines-and-resources/recommended-alcohol-questions [Accessed September 23 2013]. [Google Scholar]
- THULASINGAM S, MASSILAMANY C, GANGAPLARA A, DAI H, YARBAEVA S, SUBRAMANIAM S, RIETHOVEN JJ, EUDY J, LOU M. & REDDY J. (2011) miR-27b*, an oxidative stress-responsive microRNA modulates nuclear factor-kB pathway in RAW 264.7 cells. Mol Cell Biochem, 352, 181–8. [DOI] [PubMed] [Google Scholar]
- VALERIO A, CARDILE A, COZZI V, BRACALE R, TEDESCO L, PISCONTI A, PALOMBA L, CANTONI O, CLEMENTI E, MONCADA S, CARRUBA MO & NISOLI E. (2006) TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest, 116, 2791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TRIJP MJ, BEULENS JW, BOS WJ, UITERWAAL CS, GROBBEE DE, HENDRIKS HF & BOTS ML (2005) Alcohol consumption and augmentation index in healthy young men: the ARYA study. Am J Hypertens, 18, 792–6. [DOI] [PubMed] [Google Scholar]
- VENUGOPAL SK, DEVARAJ S, YUHANNA I, SHAUL P. & JIALAL I. (2002) Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation, 106, 1439–41. [DOI] [PubMed] [Google Scholar]
- VICKERS KC, RYE KA & TABET F. (2014) MicroRNAs in the onset and development of cardiovascular disease. Clin Sci (Lond), 126, 183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLMAN RJ, VAUGHN JA, SYLVESTRE MP, O’LOUGHLIN EK, DUGAS EN & O’LOUGHLIN JL (2016) Relationships between current and past binge drinking and systolic blood pressure in young adults. J Adolesc Health, 58, 352–7. [DOI] [PubMed] [Google Scholar]
- YOGI A, CALLERA GE, MECAWI AS, BATALHAO ME, CARNIO EC, ANTUNES-RODRIGUES J, QUEIROZ RH, TOUYZ RM & TIRAPELLI CR (2012) Acute ethanol intake induces superoxide anion generation and mitogen-activated protein kinase phosphorylation in rat aorta: a role for angiotensin type 1 receptor. Toxicol Appl Pharmacol, 264, 470–8. [DOI] [PubMed] [Google Scholar]
- ZHANG X, NG WL, WANG P, TIAN L, WERNER E, WANG H, DOETSCH P. & WANG Y. (2012) MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNFalpha. Cancer Res, 72, 4707–13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- ZHOU JY, JIANG ZA, ZHAO CY, ZHEN Z, WANG W. & NANJI AA 2013. Long-term binge and escalating ethanol exposure causes necroinflammation and fibrosis in rat liver. Alcohol Clin Exp Res, 37, 213–22. [DOI] [PubMed] [Google Scholar]
- ZINKEVICH NS, FANCHER IS, GUTTERMAN DD & PHILLIPS SA (2017) Roles of NADPH oxidase and mitochondria in flow-induced vasodilation of human adipose arterioles: ROS induced ROS release in coronary artery disease. Microcirculation. [DOI] [PMC free article] [PubMed] [Google Scholar]