Abstract
Purpose
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) and Adipose Insulin Resistance index (ADIPO-IR) values are often concordant. In this study we evaluated whether there are groups discordant for HOMA-IR and ADIPOpalmitate-IR and, if so, what are their defining characteristics.
Methods
The body composition, basal metabolic rate (BMR), fasting plasma lipids, insulin, glucose, and free fatty acid (FFA) palmitate concentrations data of 466 volunteers from previous research studies were abstracted and analyzed. The middle 2 population quartiles for HOMA-IR and Adipose Insulin Resistance index palmitate concentration (ADIPOpalmitate-IR) defined medium HOMA-IR and ADIPOpalmitate-IR (MH and MA), the top and bottom quartiles were defined as high/low HOMA (HH/LH), and high/low ADIPOpalmitate as HA/LA. Because ADIPOpalmitate-IR was significantly greater in women than in men, we established sex-specific quartiles for each index. We identified groups discordant for HOMA-IR and ADIPO-IR (HHMA, LHMA, MHHA, and MHLA).
Results
Body fat and fasting triglycerides (TGs) were significantly greater with higher indices in the concordant groups (HHHA > MHMA > LHLA). MHHA differed from MHLA by visceral fat (P < .01) and fasting TGs (P < .05), whereas HHMA differed (P < .01) from LHMA by BMR. By multivariate regression, the group factor contributed to BMR (P < .01) and visceral fat (P < .05).
Conclusions
Adults discordant for HOMA-IR and ADIPO-IR have unique features including differences in visceral fat, TGs, and BMR. This suggests different forms of insulin resistance are present, which should be considered when studying insulin resistance in the future.
Keywords: Free fatty acids, obesity, body composition, metabolic rate, triglycerides
Insulin resistance is associated with the pathogenesis of many disorders, including type 2 diabetes mellitus, hypertension, dyslipidemia, cardiovascular disease, and metabolic syndrome (1, 2). Among many indices to present the insulin resistance status, the most widely used (3, 4) is the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), a parameter that focuses on glucose metabolism, which encompasses hepatic and muscle tissues. The Adipose tissue Insulin Resistance index (ADIPO-IR) has been proposed to uniquely identify insulin resistance with regards to adipose tissue lipolysis, and has been shown to be abnormal in patients with nonalcoholic fatty liver disease, type 2 diabetes mellitus, and metabolic syndrome (5–7).
A number of studies have shown that most people with type 2 diabetes mellitus, polycystic ovary syndrome, nonalcoholic fatty liver disease, and metabolic syndrome are concordant for elevated values of HOMA-IR and ADIPO-IR when compared to a healthy population (6-10). However, we found no previous studies addressing the question of whether there are groups of people discordant for HOMA-IR and ADIPO-IR. The aim of this study was to determine whether there are groups that are discordant for HOMA-IR and ADIPO-IR and, if so, to identify the phenotypes of these populations.
Materials and Methods
Participants
The following data were abstracted from the results of 19 previous institutional review board–approved research studies conducted between November 1995 and July 2010 that included 466 unique participants. All participants were healthy, nonsmoking adults when recruited to the studies without diabetes mellitus or thyroid disorders. We excluded volunteers with diseases or taking medications that alter free fatty acid (FFA) metabolism or metabolic rate. Participants were also were required to be weight stable for at least 3 months before the study (weight stable to within ± 2%) and consumed an isoenergetic diet of consistent macronutrient distribution for 3 to 7 days prior to the study day. The data regarding sex, age, race, body mass index (BMI), measured and predicted basal metabolic rate (BMR) (11), body composition, fasting plasma glucose (FPG), insulin, and palmitate concentrations were collected. Residual BMR was calculated by subtracting the measured BMR from the predicted BMR (11). FPG, palmitate, and insulin concentrations were the average of 3 to 4 samples collected over 20 to 30 minutes. Fasting plasma triglycerides (TGs), total cholesterol, and high-density lipoprotein cholesterol (HDL-C) concentrations were measured during the screening examination for each study. For all our studies of FFA metabolism the blood samples are immediately placed on ice, spun in a refrigerated centrifuge, and frozen prior to analysis. We have documented that the contribution of very low-density lipoprotein triglyceride fatty acids to plasma FFA is only 3 ± 1% (12). We have also documented that between-individual differences in FFA concentrations are due to differences in FFA release, not FFA clearance (13).
To investigate the intraindividual variability of HOMA-IR and Adipose Insulin Resistance index palmitate concentration (ADIPOpalmitate-IR), we reanalyzed data from a group of volunteers who were studied for 4 consecutive days with measures of fasting plasma insulin, glucose, and palmitate concentrations (13).
Assays
Plasma palmitate concentrations were measured using a liquid chromatography–ultraviolet detection method (14) and/or liquid chromatography–mass spectrometry (15). To ensure the 2 methods provided the same results, we performed crossover comparisons using the same standard curves and same quality control samples for 12 months—there were no differences in the concentrations measured between the 2 methods. A Beckman Instrument was used to measure plasma glucose. Plasma insulin concentrations were measured with a chemiluminescent immunoassay (Sanofi Diagnostics Pasteur).
Body composition was assessed using a whole-body, dual-energy, x-ray absorptiometry scan and a single-slice abdominal computed tomography scan at the L2-3 interspace to calculate fat-free mass, total body fat, visceral fat, and lower-body and upper-body subcutaneous fat masses (16).
Calculations
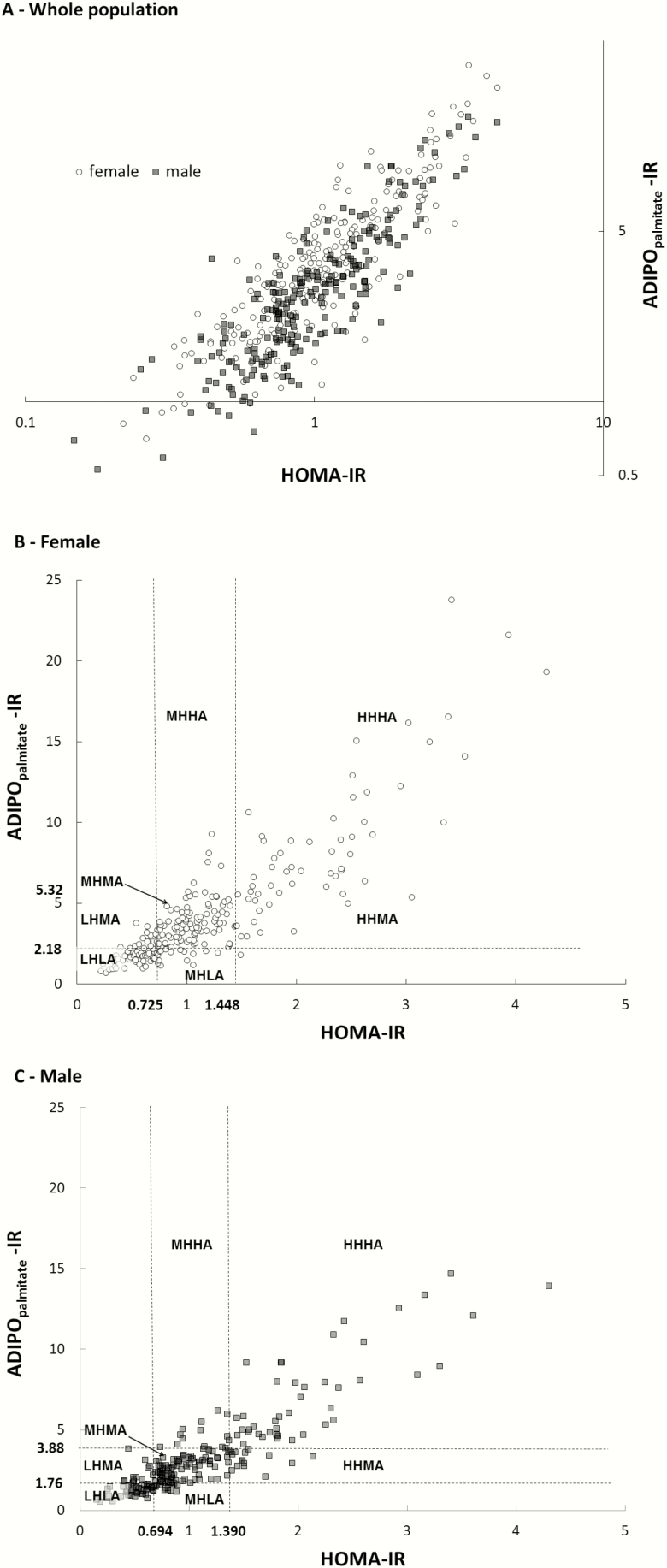
HOMA-IR and ADIPOpalmitate-IR were calculated using a published formula (3, 17), except that we used plasma palmitate. Plasma palmitate concentration is minimally different in our study population, comprising between 22% and 25% of total FFA (18-20). To compare our results with published studies using total FFA concentrations, we multiplied ADIPOpalmitate-IR by 4 (ADIPOpalmitate-IR = fasting palmitate concentration (µmol/L) × fasting insulin concentration (pmol/L) ADIPO-IR = ADIPOpalmitate-IR × 4). Although we measured palmitate flux in virtually all these studies, ADIPO-IR is calculated using concentrations; therefore we report ADIPOpalmitate-IR to allow broader utility of these data. We found sex-specific differences in fasting plasma palmitate, glucose concentrations, and ADIPOpalmitate-IR (see “Results”), which prompted us to develop sex-specific quartiles for HOMA-IR and ADIPOpalmitate-IR. Fig. 1A depicts the log-transformed HOMA-IR and ADIPOpalmitate-IR among all 466 participants. Fig. 1B and 1C depict the cutoffs for the top and bottom quartiles for HOMA-IR and ADIPOpalmitate-IR for women and men, respectively. The middle 2 quartiles of HOMA-IR and ADIPOpalmitate-IR were combined to create groups that were “medium” for both parameters. The group LHLA (low HOMA-IR and low ADIPOpalmitate-IR) was defined as those with both values below the cutoffs for lowest quartiles, and the group HHHA (high HOMA-IR and high ADIPOpalmitate-IR) included participants with both indices in the top quartiles. Participants with both indices in the middle 2 quartiles constituted the group MHMA (medium HOMA-IR medium ADIPOpalmitate-IR). We also identified volunteers who were discordant for HOMA-IR and ADIPOpalmitate-IR as follows: groups LHMA (low HOMA-IR and medium ADIPOpalmitate-IR) and HHMA (high HOMA-IR and medium ADIPOpalmitate-IR) included those with ADIPOpalmitate-IR in the middle 2 quartiles, and with HOMA-IR in the bottom or top quartiles, respectively; groups MHLA (medium HOMA-IR low ADIPOpalmitate-IR) and MHHA (medium HOMA-IR high ADIPOpalmitate-IR) consisted of participants with HOMA-IR in the middle 2 quartiles and ADIPOpalmitate-IR in the bottom or top quartiles, respectively. Because only one participant had an ADIPOpalmitate-IR less than the bottom quartile and HOMA-IR in the top quartile, this participant was included in the MHLA group. The primary, a priori comparisons for this study were to test for differences in the characteristics of the participants in the LHMA, MHHA, HHMA, and MHLA groups. The LHLA, MHMA, and HHHA groups were assessed primarily to provide context for our volunteer characteristics as to how the groups progressively differ.
Figure 1.
Adipose Insulin Resistance index palmitate concentration (ADIPOpalmitate-IR) as a function of Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). A, HOMA-IR vs ADIPOpalmitate-IR for the entire population (both axes are log-transformed). B, HOMA-IR vs ADIPOpalmitate-IR for women with quartiles marked to identify the 7 groups. C, HOMA-IR vs ADIPOpalmitate-IR for men with quartiles marked to identify the 7 groups.
Statistical analysis
To analyze the differences of age, BMI, BMR, plasma lipid concentrations, and body composition between groups, we used nonpaired t tests if the data were normally distributed. If not, data were log-transformed before analyzed by t test. If data could not be log-transformed to normally distributed, the Wilcoxon rank-sum test was performed to analyze the data. We used a P value of less than .05 as significantly different. To test for differences in the distribution of men and women between groups, we used chi-square tests with a P value of .05 as statistically significant. To test for changes in ADIPOpalmitate-IR and HOMA-IR categories in the 50 volunteers who complete 4 consecutive days of studies (13), we used repeated-measures analysis of variance in the general linear models. The Mauchly sphericity test would not be considered violated when the P value was greater than or equal to .05, which means that variances of data in each day were equal. If the P was less than .05 in the Mauchly sphericity test, then it would be corrected by Greenhouse-Geisser test to read the final P value. To test for between-group differences in body composition, plasma lipid concentrations, and residual BMR, we used analysis of variance. To test for the effect of group, we used multivariate regression analysis, with the variables of sex, BMI, group, and either fat mass or percentage body fat as independent variables to predict body composition, plasma lipid concentrations, and residual BMR for the entire study population. We repeated this analysis for the 4 discordant groups (LHMA, HHMA, MHLA, and MHHA) using the data only from that subset of the entire population. We accepted independent variables as significant contributors to the model at a P value of less than .05. The effect of each significant, independent variable to predict the dependent variables was further analyzed using partial correlation analysis; again, a P value less than .05 was considered statistically significant.
Results
Participant characteristics
The population included 238 female and 228 male volunteers. Age (41 ± 18 vs 41 ± 19 years) and BMI (26.6 ± 5.3 vs 26.5 ± 3.9 kg/m2) were not different between women and men, respectively.
Stability of Homeostatic Model Assessment for Insulin Resistance and Adipose Insulin Resistance index palmitate concentrations
Because the fasting plasma concentrations of glucose, insulin, and FFA may vary from day to day, we tested the extent to which HOMA-IR and ADIPOpalmitate-IR are stable. We analyzed HOMA-IR and ADIPOpalmitate-IR data from volunteers who underwent measures of FPG, insulin, and palmitate consecutive days. All these data were available for 4 days for 42 participants, and data were available for 3 consecutive days for 3 additional participants. FPG concentrations were 90 ± 9, 90 ± 9, 90 ± 8, and 89 ± 7 mg/dL from day 1 to day 4; insulin concentrations were 50 ± 22, 47 ± 18, 50 ± 20, and 47 ± 18 pmol/L from day 1 to day 4; palmitate concentrations were 93 ± 28, 87 ± 23, 86 ± 22, and 84 ± 27 μmol/L from day 1 to day 4 (P = not significant for all 3). Likewise, HOMA-IR and ADIPOpalmitate-IR did not change significantly.
We examined the likelihood that an individual would move between the bottom and top quartiles of HOMA-IR and ADIPOpalmitate-IR over 3 to 4 days; none of the volunteers whose values were in the bottom or top HOMA-IR quartiles changed to the opposite quartile, whereas one participant changed from the top to the bottom quartile of ADIPOpalmitate-IR. We also tested whether any of the individuals in the 7 groups based on the combination of HOMA-IR and ADIPOpalmitate-IR on day 1 would change to different groups on subsequent days. No one changed from LHLA to HHHA, from MHLA to MHHA, or from LHMA to HHMA. On the basis of these observations, we concluded that a single day’s measurement of HOMA-IR and ADIPOpalmitate-IR is sufficiently representative to allow us to evaluate between-group differences.
To explore the intra-individual and interindividual relationships between FPG concentrations and fasting insulin concentrations, we plotted the values for each day for each volunteer for this study. A representative set of data from 6 volunteers is provided in Supplementary Fig. 1 (21). For the same individual, on mornings where plasma glucose concentrations were higher, fasting plasma insulin concentrations were higher. Small percentage differences in fasting glucose are associated with much larger percentage differences in fasting plasma insulin concentrations. For the set of values in Supplementary Fig. 1 (21), the highest FPG was 31% greater than the lowest concentration, whereas the highest fasting plasma insulin concentration is 287% greater than the lowest fasting plasma insulin concentration. Each individual appears to have a unique relationship between fasting glucose and fasting insulin. This is consistent with the concept that HOMA-IR defines this relationship of insulin resistance with respect to glucose metabolism as a single number even if the FPG concentration varies from day to day.
We also investigated the relationship between insulin and FFA for this group of volunteers to examine the theoretical construct that the glucose-induced changes in fasting plasma insulin concentrations results in reciprocal changes in FFA-palmitate concentrations because insulin suppresses lipolysis as a “healthy” adipose tissue response. The intraindividual palmitate vs insulin concentrations for these same 6 individuals is provided in Supplementary Fig. 2 (21). The relationship is not as clear, likely indicating that the adipose tissue responses are not as predictable as the glucose/insulin responses; perhaps the effects of adipose insulin resistance. There also appear to be even more interindividual differences in the relationships between insulin and palmitate, with some individuals having palmitate concentrations double those of others with the same insulin concentrations and some individuals having triple the insulin concentrations at the same palmitate concentrations.
Sex differences in Homeostatic Model Assessment for Insulin Resistance and Adipose Insulin Resistance index palmitate concentrations
We initially created 7 groups based on HOMA-IR and ADIPOpalmitate-IR quartiles. The HOMA-IR and ADIPOpalmitate-IR (mean ± SD) were 0.50 ± 1.40 and 1.39 ± 0.37, respectively for the LHLA group, 1.00 ± 1.20 and 3.11 ± 0.80, respectively for the MHMA group and 2.34 ± 0.97 and 8.84 ± 4.75, respectively for the HHHA group. Thus, the mean value for HOMA-IR in the HHHA group was 468% greater than the mean value for the LHLA group, and the mean ADIPOpalmitate-IR value was 636% greater in the HHHA group than the mean value for the LHLA group.
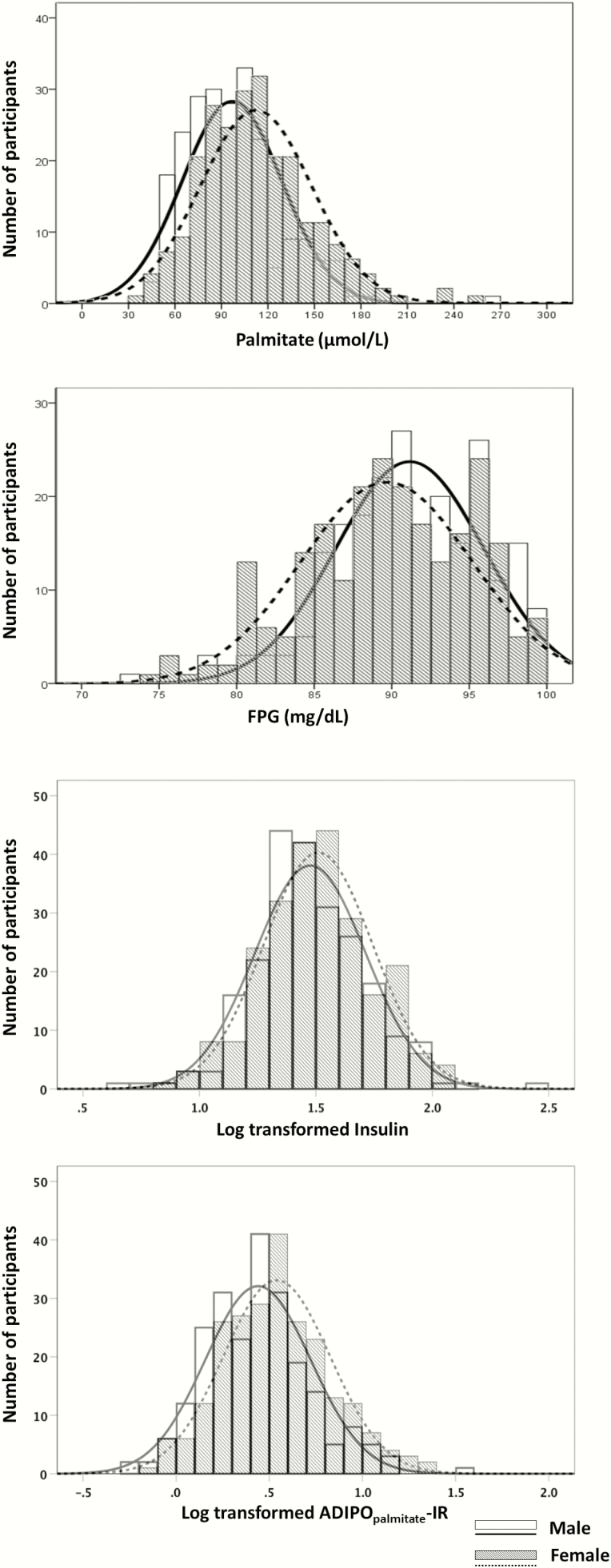
Fig. 2 depicts the population distributions of FPG and palmitate concentrations. Although the distribution for fasting plasma insulin was not different between sexes, fasting plasma palmitate concentrations were 16% greater in women (P < .001, Fig. 2), whereas fasting glucose concentrations were slightly but significantly greater in men (P < .01, Fig. 2). ADIPOpalmitate-IR, but not HOMA-IR, was significantly different between women and men (P < .001, Fig. 2). Because of these sex differences, some of the initial 7 groups based on HOMA-IR and ADIPOpalmitate-IR were imbalanced with regards to men and women. The number of women was greater in the LHMA (21 women/10 men), MHHA (22 women/5 men), and HHHA groups (37 women/21 men), whereas there were more men in the HHMA (9 women/17 men) and MHLA (8 women/22 men) groups. Fig. 1A plots the distribution of HOMA-IR by ADIPOpalmitate-IR for the entire population by sex; as a function of HOMA-IR, ADIPOpalmitate-IR is shifted up in the women compared to the men. To avoid the confounding effect of sex difference when assessing clinical and metabolic characteristics of the discordant groups, we created sex-specific cutoffs for the top and bottom quartiles of ADIPOpalmitate-IR for women (Fig. 1B) and men (Fig. 1C).
Figure 2.
Distribution of palmitate, glucose, insulin concentrations and Adipose Insulin Resistance index palmitate concentration (ADIPOpalmitate-IR) between men and women. The x-axis of insulin concentration is log-transformed because of the skewed distribution of insulin concentration in the population. The x-axis of ADIPOpalmitate-IR is also log-transformed because it was calculated using insulin and palmitate concentrations.
Characteristics of groups concordant for Homeostatic Model Assessment for Insulin Resistance and Adipose Insulin Resistance index palmitate concentrations
As expected, BMI, indices of body fat, and fasting plasma lipid concentrations (TGs, total cholesterol, and HDL cholesterol) progressively worsened from the LHLA to MHMA to HHHA groups (Table 1). The ratio of leg fat to total body fat was the lowest in the HHHA group.
Table 1.
Characteristics of groups concordant for Homeostatic Model Assessment for Insulin Resistance and Adipose Insulin Resistance index palmitate concentrations
| LHLA group | MHMA group | HHHA group | ||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| Age, y | 87 | 42 ± 19 | 177 | 42 ± 20 | 91 | 38 ± 15 |
| Sex, female/male | 87 | 44/43 | 177 | 90/87 | 91 | 47/44 |
| BMI, kg/m2 | 87 | 23.5 ± 2.7c,d | 177 | 26.2 ± 4.0c | 91 | 31.4 ± 4.2 |
| TG, mg/dL | 84 | 82 ± 33c,e | 167 | 108 ± 59c | 79 | 161 ± 97 |
| TCHO, mg/dL | 82 | 163 ± 37c | 169 | 169 ± 31b | 86 | 185 ± 42 |
| HDL-C, mg/dL | 82 | 54 ± 15c | 169 | 53 ± 22c | 86 | 41 ± 12 |
| FFM, kg | 87 | 53.3 ± 12.3a | 177 | 53.0 ± 12.1b | 91 | 57.4 ± 12.2 |
| FM, kg | 87 | 16.5 ± 6.5c,e | 177 | 23.5 ± 9.8c | 91 | 35.6 ± 10.2 |
| % FM | 87 | 23.9 ± 9.1c,e | 177 | 30.4 ± 10.2c | 91 | 38.0 ± 9.3 |
| CT visceral FM, cm2 | 83 | 57 ± 58c,e | 175 | 97 ± 84c | 89 | 162 ± 87 |
| CT subcutaneous FM, cm2 | 83 | 107 ± 61c,e | 175 | 158 ± 84c | 89 | 277 ± 111 |
| UB subcutaneous FM, kg | 79 | 8.5 ± 3.8c,e | 167 | 12.3 ± 5.4c | 80 | 18.7 ± 5.9 |
| Leg fat, kg | 82 | 6.4 ± 2.9c,e | 167 | 8.5 ± 4.0c | 83 | 11.8 ± 4.2 |
| Leg fat/Whole-body fat | 82 | 0.38 ± 0.05c | 167 | 0.37 ± 0.07c | 83 | 0.33 ± 0.07 |
Data presented as mean value and SD.
Abbreviations: % FM, percentage of fat mass; ADIPOpalmitate-IR, Adipose Insulin Resistance index palmitate concentration; BMI, body mass index; CT, computed tomography; FFM, fat-free mass; FM, fat mass; HDL-C, high-density lipoprotein cholesterol; HHHA, high HOMA-IR and high ADIPOpalmitate-IR; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LHLA, low HOMA-IR and low ADIPOpalmitate-IR; MHMA, medium HOMA-IR medium ADIPOpalmitate-IR; TCHO, total cholesterol; TG, triglyceride; UB subcutaneous FM, upper body subcutaneous fat mass.
a P less than .05 vs HHHA.
b P less than .01 vs HHHA.
c P less than .001 vs HHHA.
d P less than .05 vs MHMA.
e P less than .001 vs MHMA.
The HHHA group had significantly greater amounts of fat-free mass and higher measured BMR. With these results as an anchor for the phenotypes of concordant participants, we proceeded to test for differences between groups discordant for HOMA-IR and ADIPOpalmitate-IR.
Characteristics of groups discordant for Homeostatic Model Assessment for Insulin Resistance and Adipose Insulin Resistance index palmitate concentrations
The HOMA-IR and ADIPOpalmitate-IR (mean ± SD) were 0.58 ± 0.10 and 2.54 ± 0.58, respectively, for the LHMA group and 1.64 ± 0.25 and 3.57 ± 0.81, respectively, for the HHMA group; the 41% difference in ADIPOpalmitate-IR between these 2 groups was statistically significant (P < .001); the 283% difference in HOMA-IR between the 2 groups is not subject to statistical testing because the groups were selected to be different. The HOMA-IR and ADIPOpalmitate-IR were 0.89 ± 0.18 and 1.64 ± 0.32, respectively, for the MHLA group and 1.13 ± 0.16 and 5.55 ± 1.32, respectively, for the MHHA group. Likewise, the 27% difference in HOMA-IR between these 2 groups was statistically significant (P < .001) and the 338% difference in ADIPOpalmitate-IR between the 2 groups was not subjected to statistical testing.
In considering the 2 groups with HOMA-IR in the medium range and ADIPOpalmitate-IR in either the top quartile or bottom quartile, BMI (P = .057), body fat mass, visceral fat, abdominal subcutaneous fat, and fasting plasma TG concentrations were all significantly greater in the MHHA than the MHLA group (Table 2).
Table 2.
Characteristics of groups discordant for Homeostatic Model Assessment for Insulin Resistance and Adipose Insulin Resistance index palmitate concentrations
| LHMA group | HHMA group | MHLA group | MHHA group | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| Age, y | 30 | 41 ± 20 | 25 | 37 ± 19 | 30 | 37 ± 16 | 26 | 46 ± 21 |
| Sex, female/male | 30 | 16/14 | 25 | 12/13 | 30 | 16/14 | 26 | 13/13 |
| BMI, kg/m2 | 30 | 23.4 ± 3.1a,c | 25 | 27.2 ± 3.8 | 30 | 24.7 ± 4.7e | 26 | 27.1 ± 4.9 |
| TGs, mg/dL | 27 | 107 ± 48 | 25 | 112 ± 63 | 27 | 87 ± 26b | 24 | 118 ± 48 |
| TCHO, mg/dL | 27 | 169 ± 31 | 25 | 165 ± 28 | 26 | 168 ± 35 | 24 | 172 ± 40 |
| HDL-C, mg/dL | 26 | 51 ± 14 | 25 | 48 ± 15 | 27 | 56 ± 21 | 26 | 47 ± 18 |
| FFM, kg | 30 | 52.2 ± 11.4 | 25 | 53.7 ± 12.8 | 30 | 53.4 ± 13.8 | 26 | 51.8 ± 8.9 |
| FM, kg | 30 | 17.8 ± 7.4b,d | 25 | 26.2 ± 10.2 | 30 | 19.5 ± 11.2b,e | 26 | 26.1 ± 12.8 |
| % FM | 30 | 25.4 ± 9.7b,e | 25 | 32.4 ± 10.8 | 30 | 26.2 ± 10.2e | 26 | 32.4 ± 12.6 |
| CT visceral FM, cm2 | 28 | 69 ± 65b | 25 | 90 ± 60 | 30 | 68 ± 78a,e | 26 | 129 ± 93 |
| CT subcutaneous FM, cm2 | 28 | 108 ± 62b,c | 25 | 191 ± 95 | 30 | 116 ± 106b,d | 26 | 189 ± 119 |
| UB subcutaneous FM, kg | 25 | 9.3 ± 3.8e | 24 | 13.2 ± 5.3 | 28 | 10.1 ± 6.2e | 22 | 11.9 ± 5.8 |
| Leg fat, kg | 27 | 6.8 ± 2.9e | 24 | 10.0 ± 4.7 | 28 | 7.4 ± 4.3e | 23 | 9.1 ± 4.9 |
| Leg fat/Whole-body fat | 28 | 0.36 ± 0.09 | 24 | 0.38 ± 0.06 | 28 | 0.38 ± 0.05 | 23 | 0.36 ± 0.06 |
Data presented as mean value and SD.
Abbreviations: % FM, percentage of fat mass; ADIPOpalmitate-IR, Adipose Insulin Resistance index palmitate concentration; BMI, body mass index; CT, computed tomography; FFM, fat-free mass; FM, fat mass; HDL-C, high-density lipoprotein cholesterol; HHMA, high HOMA-IR and medium ADIPOpalmitate-IR; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LHMA, low HOMA-IR and medium ADIPOpalmitate-IR; MHHA, medium HOMA-IR high ADIPOpalmitate-IR; MHLA, medium HOMA-IR and low ADIPOpalmitate-IR; TCHO, total cholesterol; TG, plasma triglycerides; UB subcutaneous FM, upper body subcutaneous fat mass.
a P less than .01 vs MHHA.
b P less than .05 vs MHHA.
c P less than .001 vs HHMA.
d P less than .01 vs HHMA.
e P less than .05 vs HHMA.
When contrasting the groups discordant for HOMA-IR but with ADIPOpalmitate-IR in the middle quartiles (HHMA vs LHMA), the LHMA group had a lower mean BMI, lower fat mass, fat percentage, subcutaneous abdominal fat, and leg fat than HHMA. Of note, fasting plasma TG concentrations and visceral fat were not different between these 2 groups (Table 2).
The concentrations of total and HDL cholesterol of the LHMA, HHMA, MHLA, and MHHA groups were similar to those in the MHMA group, and there were no significant differences in total or HDL cholesterol between the 4 discordant groups (Table 2).
Multivariate regression analysis of predictors of visceral fat, residual basal metabolic rate, and plasma triglycerides
To further characterize the differences between the concordant and discordant groups, we used multivariate logistic regression analysis to test how the factors of group, sex, BMI, fat mass (or percentage body fat) related to indices of body composition, plasma lipid concentrations, and residual BMR (Table 3). For the 4 discordant groups, the only factor that “group” contributed a significant portion of the variance was visceral fat, which is consistent with the significant differences in visceral fat presented in Table 2.
Table 3.
Multivariate regression analysis of clinical characteristics, body composition, and lipid concentration
| Visceral fat mass, cm2 | Residual BMR, Kcal/d | TGs, mg/dL | |||||
|---|---|---|---|---|---|---|---|
| Entire population (n = 466) | Discordant groups (n = 111) | Entire population (n = 466) | Discordant groups (n = 111) | Entire population (n = 466) | Discordant groups (n = 111) | ||
| Model R2 | 0.474 | 0.419 | 0.021 | 0.108 | 0.138 | 0.047 | |
| Model sig | < 0.001 | < 0.001 | 0.040 | 0.016 | < 0.001 | 0.310 | |
| Partial correlation, sig | Sex | < 0.001 | < 0.001 | 0.434 | 0.832 | 0.047 | 0.871 |
| BMI | 0.032 | 0.141 | 0.798 | 0.395 | 0.001 | 0.938 | |
| Fat mass, kg | < 0.001 | 0.068 | 0.686 | 0.750 | 0.880 | 0.447 | |
| Group | 0.330 | 0.047 | 0.007 | 0.064 | 0.640 | 0.163 | |
| Model R2 | 0.527 | 0.469 | 0.025 | 0.107 | 0.153 | 0.078 | |
| Model sig | < 0.001 | < 0.001 | 0.022 | 0.016 | < 0.001 | 0.087 | |
| Partial correlation, sig | Sex | < 0.001 | < 0.001 | 0.152 | 0.992 | 0.003 | 0.175 |
| BMI | 0.003 | 0.047 | 0.893 | 0.087 | 0.001 | 0.521 | |
| Percentage of fat mass, % | < 0.001 | < 0.001 | 0.204 | 0.986 | 0.056 | 0.049 | |
| Group | 0.295 | 0.043 | 0.007 | 0.066 | 0.610 | 0.169 | |
Data presented as the significance values from the multivariate linear regression analysis and the partial correlation analysis.
Abbreviations: BMI, body mass index; BMR, basal metabolic rate; sig, significance; TGs, plasma triglycerides.
For the entire population, group predicted a significant portion of the variance of residual BMR. The measured and residual BMR data are presented in Table 4. The mean residual BMR values were close to zero for the 3 groups concordant for HOMA-IR and ADIPOpalmitate-IR, whereas the residual BMRs in the discordant groups were either lower than (HHMA, MHLA, and MHHA) or greater than (LHMA) zero. The comparisons between the 3 concordant groups and the 4 discordant groups are provided in Table 4. The residual BMR of the HHMA group was significantly less than the 3 concordant groups (P < .01 compared to MHMA and HHHA, P < .05 compared to LHLA, respectively), whereas the differences between the 3 concordant groups and the other 3 discordant groups did not reach statistical significance (P = .06 MHHA vs HHHA; P = .07 MHHA vs MHMA; P values in other comparisons were > .1).
Table 4.
Measured, predicted, and residual basal metabolic rate of concordant and discordant groups
| n | Measured BMR, Kcal/d | Predicted BMR, Kcal/d | Residual BMR, Kcal/d | ||
|---|---|---|---|---|---|
| Concordant groups | LHLA group | 87 | 1534 ± 304a,d | 1551 ± 261a,d,e | –17 ± 165f |
| MHMA group | 177 | 1630 ± 309a | 1618 ± 238a | 13 ± 189e | |
| HHHA group | 91 | 1819 ± 296 | 1818 ± 250 | 1 ± 159e | |
| Discordant groups | LHMA group | 30 | 1594 ± 323b | 1547 ± 254a | 48 ± 199e,g |
| HHMA group | 25 | 1602 ± 319b | 1679 ± 280c | –77 ± 160 | |
| MHLA group | 30 | 1536 ± 275a | 1601 ± 275a | –66 ± 150 | |
| MHHA group | 26 | 1540 ± 226a | 1607 ± 190a | –67 ± 155 |
Data presented as mean value and SD.
Abbreviations: ADIPOpalmitate-IR, Adipose Insulin Resistance index palmitate concentration; BMR, basal metabolism rate; HHHA, high HOMA-IR and high ADIPOpalmitate-IR; HHMA, high HOMA-IR and medium ADIPOpalmitate-IR; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LHLA, low HOMA-IR and low ADIPOpalmitate-IR; LHMA, low HOMA-IR and medium ADIPOpalmitate-IR; MHHA, medium HOMA-IR high ADIPOpalmitate-IR; MHLA, medium HOMA-IR low ADIPOpalmitate-IR; MHMA, medium HOMA-IR medium ADIPOpalmitate-IR.
a P less than .001 vs HHHA.
b P less than .01 vs HHHA.
c P less than .05 vs HHHA.
d P less than .05 vs MHMA.
e P less than .01 vs HHMA.
f P less than .05 vs HHMA.
g P less than .05 vs MHHA.
Discussion
This study was designed to investigate whether there are groups of adults that are discordant for HOMA-IR and ADIPOpalmitate-IR and, if so, to define their metabolic characteristics. Because most studies sample blood on one day to measure insulin, glucose, and FFA, we first evaluated the intraindividual variability of HOMA-IR and ADIPOpalmitate-IR over 4 days using data from one of our previous studies (13). We found that these indices were sufficiently stable that we could test for discordance in our larger population with less concern that the members of the discordant groups are there by random chance. We also found that women on average have greater ADIPOpalmitate-IR than men because of higher fasting plasma FFA (palmitate) concentrations. Although men in our studies had statistically significantly greater plasma glucose concentrations, the difference was not clinically meaningful. To avoid overrepresenting men or women in the discordant groups, we developed sex-specific quartiles both for ADIPOpalmitate-IR and HOMA-IR. Using this approach we found that visceral fat was uniquely greater in the group with high vs low ADIPOpalmitate-IR when matched for HOMA-IR. Consistent with this, fasting plasma TG concentrations were also greater in the MHHA than in the MHLA group, whereas they were not different in the HHMA and LHMA groups. Finally, groups discordant for ADIPOpalmitate-IR and HOMA-IR appear to differ significantly with respect to BMR. These findings suggest that adult humans with selective differences in adipose insulin resistance vs insulin resistance with regard to glucose metabolism have recognizable metabolic profiles.
Obesity, and especially upper body obesity, is associated with adipose tissue insulin resistance (22). The excess FFA release as a result of adipose insulin resistance (measured by ADIPO-IR) is thought to contribute substantially to insulin resistance in liver and/or muscle (measured by HOMA-IR) (17). If this is the only pathway for hepatic and muscle insulin resistance, we would expect that HOMA-IR and ADIPO-IR would vary concordantly, which was the case for the majority of participants and has been previously reported (6-10). The possibility of discordance between adipose tissue insulin resistance, liver, and muscle insulin resistance has been raised in the past (23), but the lack of a straightforward quantification method to address this issue prevented investigators from defining the problem. Our results indicate that this phenomenon can occur and that relatively simple methods can be used to find those who are discordant. We found some leaner, low–HOMA-IR adults with moderate adipose tissue insulin resistance as measured by ADIPOpalmitate-IR, indicating a subpopulation that is somewhat insensitive to excess FFA. We also found a subpopulation of overweight individuals with high HOMA-IR, but similar middle-range ADIPOpalmitate-IR, suggesting that the muscle/liver in this group may be more sensitive to moderately elevated FFA, and another group with moderate muscle/liver insulin resistance and high ADIPOpalmitate-IR, again suggesting some degree of resistance to excess FFA. Finally, there appeared to be a subset of our population with moderate muscle/liver insulin resistance as measured by HOMA-IR with low ADIPOpalmitate-IR, suggesting an FFA-independent mechanism. The reasons for this heterogeneity are not clear, but these observations have implications for the study of how FFA relate to muscle/liver insulin action depending on the phenotypes we describe.
Although the 27% difference in HOMA-IR between the MHLA vs MHHA and the 41% difference in ADIPOpalmitate-IR between LHMA vs HHMA were statistically significant, the degree of difference is dwarfed by the between-group differences in the concordant groups. We attempted to further rule out the confounding impact of these small differences for the medium range by matching participants for HOMA-IR in the MHLA vs MHHA and ADIPOpalmitate-IR in the LHMA vs HHMA groups. Unfortunately, we could find only 6 pairs in the MHLA vs MHHA groups with the same HOMA-IR and 7 pairs in the LHMA vs HHMA groups with the same ADIPOpalmitate-IR. This sample size was too small to detect meaningful differences in the clinical characteristics of these pairs.
Because the participants in the studies we included for these analyses were recruited for a variety of research protocols, we provide detailed information on the groups concordant for HOMA-IR and ADIPOpalmitate-IR to allow other investigators to assess whether our population has characteristics comparable to those they might study. Two previous studies reported HOMA-IR quartiles similar to those we developed (24, 25), whereas other studies (26-28) have reported quartiles of HOMA-IR higher than ours. This may reflect differences in the populations, including the study of older adults and those with hypertension (26), as well as difference in the ethnicity of the study population (27, 28). We also compared ADIPO-IR with 2 previous studies with ADIPO-IR quartiles. The quartiles of ADIPO-IR in our study were similar to those reported by some investigators (10), but greater than those in a population of younger women with polycystic ovary syndrome and lower BMI (8). Given that HOMA-IR and ADIPO-IR values appear to vary considerably between studies, it is essential to provide as much information as possible regarding the characteristics of each quartile to allow better comparisons between studies.
We and others have previously reported that fasting plasma FFA concentrations are slightly higher in women than men (18, 29), whereas fasting plasma insulin concentrations are not. The age and BMI of the men and women in our studies were not different, and therefore the plasma FFA differences are not due to having more obese female volunteers in our studies. Thus, it may not be surprising that, for any level of HOMA-IR, ADIPO-IR values would be greater in women than men. To the best of our knowledge, however, the implications of this have not been previously described. Investigators have considered whether HOMA-IR is different between women and men; some report findings regarding sex-differences (or lack thereof) of HOMA-IR similar to ours (30, 31), whereas others had different results (27, 32). The sex-differences in HOMA-IR in the latter 2 studies may be caused by the greater BMI of women in those studies.
Although most adults are concordant for HOMA-IR and ADIPO-IR, our observations regarding the phenotypes of the discordant groups suggest these indices are sufficiently distinct as to have important physiological implications. Elevated ADIPO-IR (with medium HOMA-IR) is more closely linked with visceral adiposity and hypertriglyceridemia, whereas elevated HOMA-IR (with medium ADIPO-IR) is associated with lower than predicted BMR. It is interesting that the average BMI in the LHMA and MHLA groups was in the normal range, which is consistent with the observation that a not-insignificant proportion of normal-weight adults have abnormal metabolic risk profiles (33). Some investigators have reported a correlation between visceral fat mass and HOMA-IR (34, 35). Fasting plasma TG concentrations have been reported to correlate with HOMA-IR (36, 37), but this may be related to the greater visceral fat mass of those participants, which may indicate an underlying ADIPO-IR abnormality (38, 39).
The average BMR was significantly greater in the LHMA group than in the HHMA and MHHA groups. The predicted BMR for our cohort was developed specifically for our population as previously described (11), and thus we anticipated that the residual BMR values would average close to zero in each group. Although this was what we observed for the concordant groups, the discordant groups were sufficiently different that it seems likely there is something metabolically different about these discordant phenotypes. Volunteers with untreated or undertreated thyroid disorders were excluded from our studies, indicating that thyroid disease is not an explanation for this observation. Likewise, all our volunteers were required to be weight stable for at least 3 months before the study and are required to consume an isoenergetic diet for 3 to 7 days before the measurements, which would seem to exclude acute nutritional challenges as the cause of differences in BMR. A few previous reports have suggested differences in BMR in those with insulin resistance. Measured BMR was significantly decreased in insulin-resistant Greek women with polycystic ovary syndrome vs noninsulin-resistant Greek women with polycystic ovary syndrome and healthy controls (40). Another group reported that resting metabolic rate was less in obese individuals with metabolic syndrome than obese individuals without metabolic syndrome (41). Assuming our observations of differences in BMR in the discordant groups can be reproduced in other populations, it may be that the participants in these studies (40, 41) were coincidentally discordant for HOMA-IR and ADIPO-IR. We found no publications that would explain why BMR might be shifted in those discordant for HOMA-IR and ADIPO-IR.
There are limitations to our study. Almost all the participants were white, and thus we cannot extrapolate these findings to other races. In addition, our data regarding the reproducibility of HOMA-IR and ADIPOpalmitate-IR are likely best-case scenarios because the participants in that study received an isocaloric diet that was fixed in macronutrient proportions for 7 days prior to and during the replicate measurements. The greater day-to-day variability in fasting plasma insulin and FFA-palmitate concentrations might also be a concern when it comes to defining these groups with respect to discordancy. We suggest that the greater variability in fasting plasma insulin concentrations is an inherent biological relationship between glucose and insulin in healthy humans (Supplementary Fig. 1, [21]), whereas the adipose tissue responses are not as consistent (Supplementary Fig. 2, [21]). Had we not had a very large cohort, we might not have been able to identify these phenotypes. That said, our results do support the concept that populations exist that are truly discordant for HOMA-IR and ADIPO-IR. In addition, the other 18 studies we included also required a prestudy diet control period of 3 to 7 days.
Conclusions
In summary, we found evidence that there are adults who are discordant for insulin resistance with regards to glucose and adipose tissue lipolysis. Disproportionate elevation of ADIPOpalmitate-IR is associated with visceral adiposity and hypertriglyceridemia. We also found that BMR is shifted in groups that are discordant for HOMA-IR and ADIPO-IR. Because of higher fasting plasma FFA concentrations in women and higher glucose in me, we needed to develop sex-specific quartiles of ADIPOpalmitate-IR and HOMA-IR. These findings support the use of both indices in future studies of metabolic health.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (Grants DK-45343, DK-40484, and NCRR 1UL1 RR024150). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Data curation, formal analysis, investigation, writing of original draft, writing, review, and editing: Y.S.; conceptualization, investigation, methodology, writing, review, and editing: E.S.; conceptualization, data curation,; formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing, review, and editing: M.D.J.
Glossary
Abbreviations
- ADIPO-IR
Adipose tissue Insulin Resistance index
- BMI
body mass index
- BMR
basal metabolic rate
- FFA
free fatty acid
- FPG
fasting plasma glucose
- HDL-C
high-density lipoprotein cholesterol
- HHHA
high HOMA-IR and high ADIPOpalmitate-IR
- HHMA
high HOMA-IR and medium ADIPOpalmitate-IR
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- LHLA
low HOMA-IR and low ADIPOpalmitate-IR
- LHMA
low HOMA-IR and medium ADIPOpalmitate-IR
- MHHA
medium HOMA-IR high ADIPOpalmitate-IR
- MHLA
medium HOMA-IR low ADIPOpalmitate-IR
- MHMA
medium HOMA-IR medium ADIPOpalmitate-IR
- TGs
plasma triglycerides
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Moller DE, Flier JS. Insulin resistance—mechanisms, syndromes, and implications. N Engl J Med. 1991;325(13):938-948. [DOI] [PubMed] [Google Scholar]
- 2. Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3Suppl 1):S12-S18. [DOI] [PubMed] [Google Scholar]
- 3. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 4. Antuna-Puente B, Disse E, Rabasa-Lhoret R, Laville M, Capeau J, Bastard JP. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011;37(3):179-188. [DOI] [PubMed] [Google Scholar]
- 5. Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barchetta I, Angelico F, Del Ben M, et al. Phenotypical heterogeneity linked to adipose tissue dysfunction in patients with type 2 diabetes. Clin Sci (Lond). 2016;130(19):1753-1762. [DOI] [PubMed] [Google Scholar]
- 7. Adams-Huet B, Devaraj S, Siegel D, Jialal I. Increased adipose tissue insulin resistance in metabolic syndrome: relationship to circulating adipokines. Metab Syndr Relat Disord. 2014;12(10):503-507. [DOI] [PubMed] [Google Scholar]
- 8. Mu L, Li R, Lai Y, Zhao Y, Qiao J. Adipose insulin resistance is associated with cardiovascular risk factors in polycystic ovary syndrome. J Endocrinol Invest. 2019;42(5):541-548. [DOI] [PubMed] [Google Scholar]
- 9. Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1389-1397. [DOI] [PubMed] [Google Scholar]
- 10. Jorge-Galarza E, Posadas-Romero C, Torres-Tamayo M, et al. Insulin resistance in adipose tissue but not in liver is associated with aortic valve calcification. Dis Markers. 2016;2016:9085474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anthanont P, Jensen MD. Does basal metabolic rate predict weight gain? Am J Clin Nutr. 2016;104(4):959-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bush NC, Triay JM, Gathaiya NW, Hames KC, Jensen MD. Contribution of very low-density lipoprotein triglyceride fatty acids to postabsorptive free fatty acid flux in obese humans. Metabolism. 2014;63(1):137-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111(7):981-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen MD, Heiling V, Miles JM. Measurement of non-steady-state free fatty acid turnover. Am J Physiol. 1990;258(1 Pt 1):E103-E108. [DOI] [PubMed] [Google Scholar]
- 15. Persson XM, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51(9):2761-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61(2):274-278. [DOI] [PubMed] [Google Scholar]
- 17. Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102(4):1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shadid S, Kanaley JA, Sheehan MT, Jensen MD. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab. 2007;292(6):E1770-E1774. [DOI] [PubMed] [Google Scholar]
- 19. Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738-1748. [DOI] [PubMed] [Google Scholar]
- 20. Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79(1):207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yilin S, Esben S, Jensen MD. Unique metabolic features of adults discordant for indices of insulin resistance. Figshare 2020. Deposited April 29, 2020. 10.6084/m9.figshare.12217667.v1. [DOI] [PMC free article] [PubMed]
- 22. Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94(2):206-218. [DOI] [PubMed] [Google Scholar]
- 23. Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14(12):1132-1143. [DOI] [PubMed] [Google Scholar]
- 24. Do HD, Lohsoonthorn V, Jiamjarasrangsi W, Lertmaharit S, Williams MA. Prevalence of insulin resistance and its relationship with cardiovascular disease risk factors among Thai adults over 35 years old. Diabetes Res Clin Pract. 2010;89(3):303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakai Y, Nakaishi S, Kishimoto H, et al. The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med. 2002;19(4):346-347. [DOI] [PubMed] [Google Scholar]
- 26. Laws A, Hoen HM, Selby JV, Saad MF, Haffner SM, Howard BV. Differences in insulin suppression of free fatty acid levels by gender and glucose tolerance status. Relation to plasma triglyceride and apolipoprotein B concentrations. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Arterioscler Thromb Vasc Biol. 1997;17(1):64-71. [DOI] [PubMed] [Google Scholar]
- 27. Tohidi M, Ghasemi A, Hadaegh F, Derakhshan A, Chary A, Azizi F. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy Iranian adults: Tehran Lipid and Glucose Study. Clin Biochem. 2014;47(6):432-438. [DOI] [PubMed] [Google Scholar]
- 28. Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696-703. [DOI] [PubMed] [Google Scholar]
- 29. Soeters MR, Sauerwein HP, Groener JE, et al. Gender-related differences in the metabolic response to fasting. J Clin Endocrinol Metab. 2007;92(9):3646-3652. [DOI] [PubMed] [Google Scholar]
- 30. Salazar MR, Carbajal HA, Espeche WG, et al. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am J Cardiol. 2012;109(12):1749-1753. [DOI] [PubMed] [Google Scholar]
- 31. Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motamed N, Miresmail SJ, Rabiee B, et al. Optimal cutoff points for HOMA-IR and QUICKI in the diagnosis of metabolic syndrome and non-alcoholic fatty liver disease: a population based study. J Diabetes Complications. 2016;30(2):269-274. [DOI] [PubMed] [Google Scholar]
- 33. Stefan N, Schick F, Häring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292-300. [DOI] [PubMed] [Google Scholar]
- 34. Bi X, Seabolt L, Shibao C, et al. DXA-measured visceral adipose tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African-American women. Eur J Clin Nutr. 2015;69(3):329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SK, Kim HJ, Hur KY, et al. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004;79(4):593-599. [DOI] [PubMed] [Google Scholar]
- 36. Taniguchi A, Fukushima M, Sakai M, et al. The role of the body mass index and triglyceride levels in identifying insulin-sensitive and insulin-resistant variants in Japanese non-insulin-dependent diabetic patients. Metabolism. 2000;49(8):1001-1005. [DOI] [PubMed] [Google Scholar]
- 37. Kim JS, Kang HT, Shim JY, Lee HR. The association between the triglyceride to high-density lipoprotein cholesterol ratio with insulin resistance (HOMA-IR) in the general Korean population: based on the National Health and Nutrition Examination Survey in 2007-2009. Diabetes Res Clin Pract. 2012;97(1):132-138. [DOI] [PubMed] [Google Scholar]
- 38. Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26(8):2341-2344. [DOI] [PubMed] [Google Scholar]
- 39. Tremblay AJ, Després JP, Piché ME, et al. Associations between the fatty acid content of triglyceride, visceral adipose tissue accumulation, and components of the insulin resistance syndrome. Metabolism. 2004;53(3):310-317. [DOI] [PubMed] [Google Scholar]
- 40. Georgopoulos NA, Saltamavros AD, Vervita V, et al. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertil Steril. 2009;92(1):250-255. [DOI] [PubMed] [Google Scholar]
- 41. Buscemi S, Verga S, Caimi G, Cerasola G. A low resting metabolic rate is associated with metabolic syndrome. Clin Nutr. 2007;26(6):806-809. [DOI] [PubMed] [Google Scholar]