Introduction
After a cancer diagnosis, many people engage in lifestyle behaviors that can improve their health (Demark-Wahnefried et al., 2005; Rock et al., 2012). Physical activity and a nutritious diet among cancer survivors are associated with a number of positive health-related outcomes, including decreased functional decline and impairment (Morey et al., 2009; Pinto et al., 2002), and improvements in quality of life (Blanchard et al., 2004; Patterson et al., 2003). Much of this research, however, has focused on the behavioral engagement of people with early-stage cancers or long-term survivors (Courneya & Friedenreich, 2007). There is a need for more research on the health behaviors of people with advanced cancer.
Due to the complexities of advanced cancer, including the management of symptoms and numerous associated side-effects, people with advanced cancers may be more likely than those at earlier stages to rely on support from caregivers (Given et al., 2001). A substantial body of literature has shown that caring for someone with cancer is an emotionally and psychologically taxing experience (Bevans & Sternberg, 2012; Northouse et al., 2012); less is known, however, about advanced cancer caregivers’ health behaviors. Exploratory research in this area can begin to unpack whether there is an interdependent relationship between patient and caregiver health-promoting behaviors, and factors that may positively or negatively influence their behavior engagement at this stage of disease.
Compared to those with early-stage disease, patients with advanced cancers may experience worse quality of life (Ravasco et al., 2004), more pain (van den Beuken-van Everdingen et al., 2007), higher symptom burden (Shi et al., 2011), and poorer physical functioning (Hebert et al., 2009; Payne, 2007). The challenges of advanced disease may be an impediment to patient engagement in health-promoting behaviors. For example, patient fatigue due to treatment or caregiver fatigue due to the demands of caregiving could be impediments to their engagement in exercise. However, a growing body of research supports the idea that patient engagement in health-promoting behaviors can be beneficial, even at advanced stages (Albrecht & Taylor, 2012). Physical activity interventions may help to decrease fatigue and improve quality of life in advanced cancer patients (Oldervoll et al., 2006; Rummans et al., 2006), whereas diet interventions may combat malnutrition and weight loss (Barrera, 2002; Ravasco et al., 2005; Rock et al., 2012).
Interdependence theory (Lewis et al., 2006; Van Lange & Rusbult, 2012) supports the examination of the between-persons effects that often exist within dyads, for example, how patient physical activity influences caregiver physical activity. Interdependence is especially important to consider in the advanced cancer context. The first dimension of interdependence theory, degree of interdependence, recognizes that a person’s dependence on a dyadic partner can increase or decrease over time. For example, over the course of the patients’ advanced cancer diagnosis, their dependence on their caregiver may vary depending on their medical treatments and side effects (Fiszer et al., 2014). The second dimension, mutuality of interdependence, recognizes that in a dyadic relationship, each person has the ability to influence the outcomes of the other (Holmes, 2002). Previous research has shown that patient health may also have an influence on the wellbeing of their family members (Northouse, 2005; Kershaw et al., 2015). Social support has long been recognized as an important factor influencing health and wellbeing (Uchino, 2006; Thoits, 2011). Perceived levels of social support between patients and caregivers may help to explain – or mediate – relationships that may exist between their health behaviors or health problems; however, there is a lack of research in this area.
It also important to consider that patients with cancer may have other ongoing health problems (i.e., comorbidities) and symptoms of functional impairment associated with these conditions that can further impede their ability to engage in health-promoting behaviors (Deimling et al., 2007; Ogle et al., 2000). According to a recent study, approximately one-third of older adults with breast or prostate cancer have comorbidities, with prevalence rates higher for patients with colorectal (40.7%) or lung cancers (52.9%; Edwards et al., 2014). Patient comorbidities can lead to a number of difficulties, including uncertainty in symptom attribution and increased complexity of health care (Fortin et al., 2004; Vogeli et al., 2007). Few studies, however, have explored the influence of cancer patients’ comorbid conditions or symptom experiences on their health-promoting behaviors (Kim & Given, 2008). Moreover, the physical health status of caregivers during the caregiving experience is another area research that needs more attention (Kim & Schulz, 2008). Because chronic disease prevalence increases with age (CDC, 2003; Rowland & Yancik, 2006), cancer caregivers, who tend to be older (Romito et al., 2013), may be managing health conditions of their own and/or physical and mental health symptoms that could be attributed to those conditions.
A 2008 review of literature on the quality of life of cancer caregivers found no studies of caregiver exercise and diet behaviors (Kim & Given, 2008), and to date, literature in this area remains limited. In studies enrolling primarily early-stage survivors, there are a few notable exceptions. For example, a recent study found relationships between fruit and vegetable consumption among people with colorectal cancer and their cancer caregivers in the year following diagnosis (Shaffer et al., 2016). In one of the first intervention studies of its kind, Winter-Stone and colleagues (2016) found positive outcomes for prostate cancer survivors and wives who participated in an exercise program. In their study of advanced cancer caregivers, Mazanec and colleagues (2011) found that while most caregivers reported usually eating a healthy diet, they also reported low levels of physical activity. Similarly, another study reported no change in fruit and vegetable consumption among ovarian cancer caregivers, but a decrease in caregiver physical activity since the time of diagnosis (Beesley et al., 2011). However, more research is needed that takes into account the advanced stage of disease and examines the degree of interdependence between patient and caregiver behaviors.
Purpose & Hypotheses
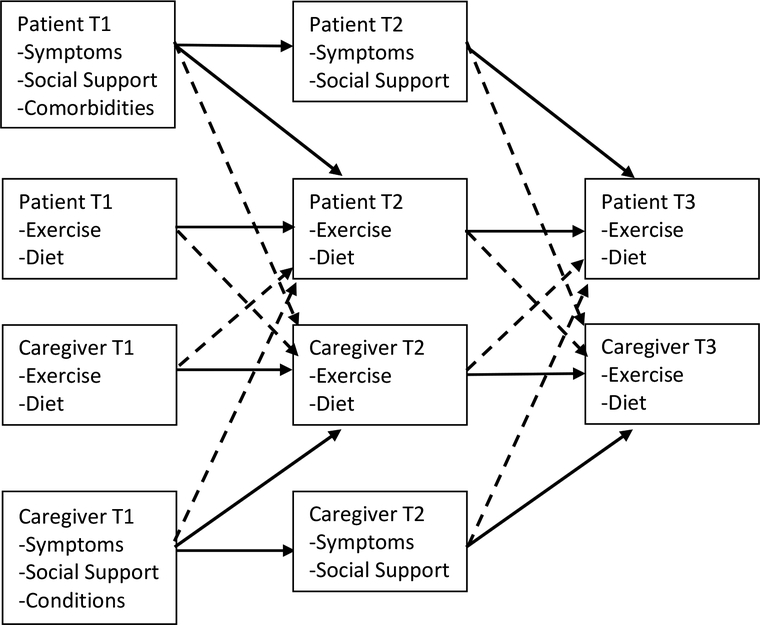
The purpose of this exploratory study is to examine the exercise and diet behaviors of patients with advanced cancer and their caregivers over three time points, with a particular focus on (1) the interdependence of their health behaviors, (2) the effect of patient and caregiver perceived social support on engagement in health behaviors, and (3) the effect of their health status (patient comorbidities/caregiver chronic conditions and patient/caregiver symptom experiences) on their engagement in health behaviors (see Figure 1). The specific study hypotheses are as follows:
H1: Patients’ and caregivers’ exercise and diet will be positively related to their own individual-level measures of those variables at three time points (actor effects).
H2: Patients’ and caregivers’ exercise and diet will be positively related to their dyadic partner’s individual-level measures of those variables at three time points (partner effects).
H3: Patients’ and caregivers’ number of comorbidities/conditions, symptom distress, and level of perceived social support assessed at Time 1 & Time 2 will be significantly related to each person’s own exercise and diet at Time 2 and Time 3 assessments (actor effects).
H4: Patients’ and caregivers’ comorbidities/conditions, symptom distress, and level of perceived social support assessed at Time 1 & Time 2 will be significantly related to the other dyad member’s exercise and diet at Time 2 and Time 3 assessments (partner effects).
H5: The relationship between comorbidities/conditions and symptom distress at Time 1 and exercise and diet behaviors at Time 3 will be mediated by symptom distress and perceived social support at Time 2.
Figure 1. Hypothesized Actor-Partner Effects on Patient and Caregiver Exercise and Diet.
Note: Independent effects (i.e. actor effects) are represented by solid lines. Interdependent effects (i.e. partner effects) are represented by dashed lines. T1 = Time 1; T2= Time 2; T3 = Time 3.
Method
Study Design
A longitudinal, prospective exploratory design was used to examine the study hypotheses. Details about the study design and procedure have been published elsewhere (Northouse et al., 2013; Kershaw et al., 2015). Briefly, data were from a randomized clinical trial (RCT) that tested the efficacy of brief and extensive forms of the evidence-based FOCUS program and control condition (usual care) on outcomes for patients with advanced cancer and their family caregivers (i.e., dyads; Northouse et al., 2013). This dyadic program provides information and support to patients and caregivers jointly. Research nurses blinded to the dyads’ group assignments collected data from patients and caregivers, separately, in their home. Assessments were conducted at three time points: Time 1 (T1) occurred at baseline prior to the intervention (N=484), Time 2 (T2) occurred following the intervention at 3 months after baseline (N=347), and Time 3 (T3) occurred at 6 months after baseline (N=307). Informed consent was obtained from all individual participants included in the study. Institutional Review Boards at the patient’s cancer center and the University of Michigan (coordinating institution) approved all study procedures and documents. The parent RCT study examined intervention outcomes for dyads who participated in the RCT. Outcomes associated with the FOCUS Program were less avoidant coping, greater engagement in health lifestyle behaviors (overall), higher self-efficacy, and higher emotional QOL for patients, caregivers, or dyads. Detailed information related to the RCT procedures, intervention content, and study outcomes have been published elsewhere (Northouse et al., 2013). This secondary analysis was conducted to analyze more closely the extent to which patients and caregivers influenced one another’s exercise and diet behaviors over time and to determine the influence of co-occurring health problems (comorbidities, chronic conditions and symptom distress) and perceived social support on these behaviors.
Study Participants
At baseline, the sample population for this study included 484 patients with advanced cancer and their family caregivers (N=484 dyads). All patients: were age 21 or older; had a confirmed diagnosis of advanced breast (32.4%), colorectal (29.1%), lung (29.1%) or prostate cancer (13%) and were within 6 months of the diagnosis, progression of their advanced cancer, or change of treatment for it. Patients were excluded from the study if they were diagnosed with multiple, primary cancer sites. A majority of patients were undergoing chemotherapy treatment (68.8%). Patients also reported that they were currently receiving hormone therapy (16.5%), radiation therapy (8.3%), watchful waiting (7.6%), surgical treatment (2.9%) or another treatment not specified (5.4%). Multiple responses for treatment options were possible.
A family caregiver was defined as the family member or significant other identified by the patient as his or her primary source of emotional or physical support during the advanced phase of cancer and confirmed by the designated person (Northouse et al., 2013). Family caregivers were age 18 or older and were excluded from the study if they had been diagnosed with cancer during the previous year and/or were in active treatment for cancer.
Instruments
The following instruments were used to measure the selected study variables.
Health behaviors.
Patients and caregivers reported engagement in health behaviors at three time points. Their responses to questions about their exercise and diet behaviors are included in the analysis. Based on recommendations by the American Cancer Society at the time of the study, participants were asked how often in a typical week they a) exercised by walking or doing moderate to vigorous physical activity or b) ate a balanced diet including fruits and vegetables (if allowed). Response options on the 5-point scale ranged from (0) “not at all” (1) … (2)… (3)…to (4) “5–7 times a week.” These items were included in a scale that measured health behaviors, with reported baseline reliabilities of α = 0.61 for patients and α = 0.67 for caregivers.
Comorbidities/conditions.
At baseline, patients and caregivers responded to the following question: “Do you have any other health problems (such as heart disease, arthritis, diabetes, etc.) at this time?” Respondents who answered “yes” to this question were then asked to name their health problems. The number of comorbidities/conditions variable was based on a count of the number of physical and mental health problems reported in response to this question. Comorbidities refer to the health problems reported by patients, with cancer as their index condition; conditions refer to the health problems reported by caregivers. There was no significant change in the number of comorbidities/conditions reported across the three time points, and the correlations between the number of comorbidities/conditions across time were high (above .920), so only T1 comorbidities/conditions were included in the analyses.
Social support.
Social support was measured with the 7-item Social Support Scale (patients: α = .84; caregivers: α = .87) and was used to assess patient and caregiver perceptions of social support within the dyad (Northouse, 1988, 2000). An example item is “My family member is willing to listen to me when I just need to talk.” Response options ranged from 1) never true to 5) always true. Higher average scores indicated more perceived social support (possible range 0–5).
Symptom distress.
The 16-item Symptom Scale of the Risk for Distress Scale (patients: α = .74; caregivers: α = .89) was used to assess patient and caregiver physical and psychological symptom distress (Mood & Bickes, 1989; Northouse et al., 2007). Patients reported on the trouble they experienced because of cancer- related and/or non-cancer symptoms during the past week; conversely, caregivers reported on the trouble they experienced because of their own symptoms during the past week. Descriptive response options were appropriate for the symptoms and generally represented: 0) no trouble, 1) some, and, 2) a lot. Higher summary scores indicated higher symptom distress (possible range 0–32).
Covariates.
Age, sex, race, income, cancer type, treatment, length of time since patient diagnosis, living arrangements, and the relationship type were used as covariates. This information was obtained from patient medical records and the self-administered Risk for Distress Scale, which was adapted from the original Omega Clinic Screening Interview (Mood & Bickes, 1989; Northouse et al., 2013; Northouse et al., 2007). Intervention group was also controlled for in the analysis in order to remove treatment effects.
Data Analysis Strategy
The hypotheses for this exploratory study were examined using the actor-partner interdependence mediational model (APIMeM; Kenny et al., 2006; Rayens & Svavarsdottir, 2003). This model consists of three pairs of variables corresponding to each dyad member: predictor variables (T1); mediator variables (T2); and, outcome variables (T3). Path analysis with structural equation modeling (SEM) was used to estimate the model parameters using MPlus version 7 (Muthén & Muthén, 2015). Actor effects refer to the influence of a patient or caregiver on his/her own outcomes (e.g., influence of patient social support on patient exercise). Partner effects - an example of interdependence - refer to the influence of patients’ or caregivers’ on the outcomes of the other member of the patient/caregiver dyad (e.g., influence of patient social support on caregiver exercise). For hypothesis 1 (H1) and hypothesis 2 (H2), the actor and partner effects of patient and caregiver diet and exercise behaviors were examined. For H3 & H4, the actor and partner influences of comorbidities/conditions, symptom distress, and social support on subsequent diet and exercise behaviors were examined. For H5, potential mediating effects of symptom distress and social support at T2 were examined.
Mediation effects were tested using the bootstrapping procedure in MPlus. The comparative fit index (CFI), the root mean squared error of approximation (RMSEA), and the standardized root-mean-square residual (SRMR) were used to determine the adequacy of model fit. The indicators of adequate model fit for these indices (i.e. the indication that the model fits the sample data well) are a CFI above .90; a RMSEA value of .08 or less; and, an SRMR value of .08 or less (Hu & Bentler, 1999; Little & Card, 2013). Full information maximum likelihood estimation was the estimation method. All models included covariances between predictor variables and error terms of the patient and caregiver behaviors at the same time point (not shown in figures).
Results
Descriptive Findings
Background demographic information obtained from patients and caregivers at baseline is presented in Table 1. The average age at baseline (T1) of patients was 60.5 years (SD: 11.5; range: 26–95) and of caregivers 56.5 years (SD: 13.4; range: 18–88). Most patients (62%) and caregivers (56.8%) were female. Patients and caregivers were predominantly White (approximately 80%). A majority of patients and caregivers (70%) were in a marital relationship with each other.
Table 1.
Patient and Caregiver Demographic Information at Baseline
| Patients (N=484) | Caregivers (N=484) | Difference Testsa | |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 60.5 (11.5) | 56.5 (13.4) | * |
| Range | 26–95 | 18–88 | |
| Sex (%) | |||
| Female | 62.0 | 56.8 | NS |
| Male | 38.0 | 43.1 | |
| Race (%) | |||
| American Indian/Alaskan Native | 0.2 | 0 | NS |
| Asian | 1.0 | 1.2 | |
| Black | 15.3 | 15.9 | |
| Pacific Islander | 0.2 | 0 | |
| White | 79.3 | 79.6 | |
| Multiracial | 3.9 | 2.5 | |
| Highest level of education in years | |||
| Mean (SD) | 14.5 (2.7) | 14.6 (2.8) | NS |
| Marital Status (%) | |||
| Married/Living as married | 75.6 | 82.9 | * |
| Divorced/Separated | 13.2 | 8.1 | |
| Widowed | 6.0 | 2.3 | |
| Never married | 5.2 | 6.8 | |
| Relationship to patient (%; caregiver only) | -- | ||
| Spouse | -- | 70.0 | |
| Daughter | -- | 12.0 | |
| Son | -- | 3.3 | |
| Sister/Brother | -- | 0.2 | |
| Other relative | -- | 5.6 | |
| Friend | -- | 4.3 | |
| Unknown/Coding error | -- | 4.5 | |
| Currently living with patient (caregiver only) | |||
| % Yes | -- | 82.6 | |
Paired sample t-tests, McNemar’s Test, or Wilcoxon Signed-Rank Test.
p<.05;
NS: not significant.
There were significant differences in the frequency of patient and caregiver exercise behaviors (p=.025) and diet behaviors (p=.006) at baseline. That is, caregivers engaged in exercise more frequently than patients, while patients more frequently ate a balanced diet. Among patients, 36% reported engaging in moderate or vigorous exercise at least 3 days a week at T1, 38.5% at T2, and 38.1% at T3. Among caregivers, 43.9% reported engaging in moderate or vigorous exercise at least 3 days a week at T1, followed by 44.6% at T2, and 40.4% at T3. Most patients (78.6%) reported eating a balanced diet at least 3 days a week at T1. At follow-up assessments, 80.3% (T2) and 77.6% (T3) of patients reported eating a balanced diet at least 3 days a week. Similarly, most caregivers (72.2%) reported eating a balanced diet at least 3 days a week at T1, followed by 75.3% at T2 and 72.7% at T3.
Tables 2 & 3 report correlations among the main study variables. Where significant, primarily small to moderate correlations were observed. The average number of comorbidities/conditions reported was 1.82 among patients (SD: 1.44; range 0–5) and 1.48 among caregivers (SD: 1.35; range 0–5). Most patients (77.5%) and caregivers (68.1%) reported at least one comorbidity/condition and almost one-third of patients (32.3%) and one-quarter of caregivers (23%) reported three or more comorbidities/conditions. There were significant differences between the number of patient and caregiver comorbidities/conditions (p<.001), with patients reporting significantly more conditions. Average symptom distress scores for patients was 10.25 (SD: 4.65, range: 0–23) at T1 and 8.86 (SD: 4.85, Range, 0 – 23) at T2. Average symptom distress scores for caregivers was 5.98 (SD: 4.31, range, 0–23) at T1 and 5.16 (SD: 4.27, range, 0–19) at T2. Significant differences were also found between patient and caregiver symptom distress at T1 and T2 (p<.001), with patients reporting more symptom distress. Lastly, average social support scores for patients at baseline was 4.28 (SD: 0.66, range: 1.57–5.00) and 4.30 (SD: 0.66, range: 1.43–5) at T2. Average social support scores for caregivers was 3.99 (SD: 0.76, range: 1.43–5) at baseline and 3.95 at T2 (SD: 0.79, range, 1.29–5). Significant differences were also found between patient and caregiver social support at T1 and T2 (p<.001), with patients reporting more social support.
Table 2.
Correlations among Exercise, Diet, Social Support, Symptom Distress and Comorbidities for Patients
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Measures | ||||||||||||
| 1. Exercise T1 | -- | |||||||||||
| 2. Exercise T2 | .493 | -- | ||||||||||
| 3. Exercise T3 | .436 | .483 | -- | |||||||||
| 4. Diet T1 | .233 | .164 | .284 | -- | ||||||||
| 5. Diet T2 | .198 | .160 | .190 | .559 | -- | |||||||
| 6. Diet T3 | .265 | .259 | .370 | .529 | .590 | -- | ||||||
| 7. Social Support T1 | .035 | −.038 | .109 | .115 | .121 | .176 | -- | |||||
| 8. Social Support T2 | .019 | −.023 | .080 | .107 | .163 | .133 | .705 | -- | ||||
| 9. Social Support T3 | .021 | −.012 | .076 | .080 | .155 | .108 | .685 | .641 | -- | |||
| 10. Symptoms Distress T1 | −.232 | −.190 | −.194 | −.279 | −.154 | −.216 | −.171 | −.176 | −.171 | -- | ||
| 11. Symptoms Distress T2 | −.101 | −.268 | −.197 | −.051 | −.182 | −.163 | −.129 | −.181 | −.146 | .571 | -- | |
| 12. Symptoms Distress T3 | −.152 | −.194 | −.275 | .033 | −.083 | −.243 | −.184 | −.151 | −.189 | .525 | .632 | -- |
| 13. Comorbidities | −.118 | .212 | .441 | .093 | .051 | .029 | −.056 | −.010 | −.055 | .147 | .092 | .109 |
Correlations that are significant at the .05 level are boldface; correlations that are significant at the .10 level are underlined. T1 = Time 1; T2 = Time 2; T3 = Time 3
Table 3.
Correlations among Exercise, Diet, Social Support, Symptom Distress, and Chronic Conditions for Caregivers
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caregiver Measures | ||||||||||||
| 1. Exercise T1 | -- | |||||||||||
| 2. Exercise T2 | .551 | -- | ||||||||||
| 3. Exercise T3 | .534 | .660 | -- | |||||||||
| 4. Diet T1 | .234 | .205 | .179 | -- | ||||||||
| 5. Diet T2 | .124 | .288 | .270 | .629 | -- | |||||||
| 6. Diet T3 | .176 | .280 | .309 | .627 | .678 | -- | ||||||
| 7. Social Support T1 | .066 | .080 | .189 | .124 | .217 | .258 | -- | |||||
| 8. Social Support T2 | .052 | .053 | .137 | .032 | .153 | .129 | .631 | -- | ||||
| 9. Social Support T3 | .087 | .108 | .197 | .071 | .137 | .170 | .698 | .746 | -- | |||
| 10. Symptom Distress T1 | −.191 | −.172 | −.138 | −.117 | −.042 | −.137 | −.243 | −.178 | −.257 | -- | ||
| 11. Symptom Distress T2 | −.189 | −.170 | −.111 | −.117 | −.135 | −.163 | −.279 | −.248 | −.266 | .681 | -- | |
| 12. Symptom Distress T3 | −.208 | −.217 | −.194 | −.185 | −.173 | −.235 | −.321 | −.220 | −.298 | .761 | .713 | -- |
| 13. Chronic Conditions | −.093 | −.009 | −.047 | .026 | .064 | .112 | −.010 | .023 | −.037 | .347 | .222 | .253 |
Correlations that are significant at the .05 level are boldface; correlations that are significant at the .10 level are underlined. T1 = Time 1; T2 = Time 2; T3 = Time 3
Exercise Behaviors
Figure 2 provides the unstandardized estimates for APIMeM models examining exercise. The model fit statistics were good across all models.
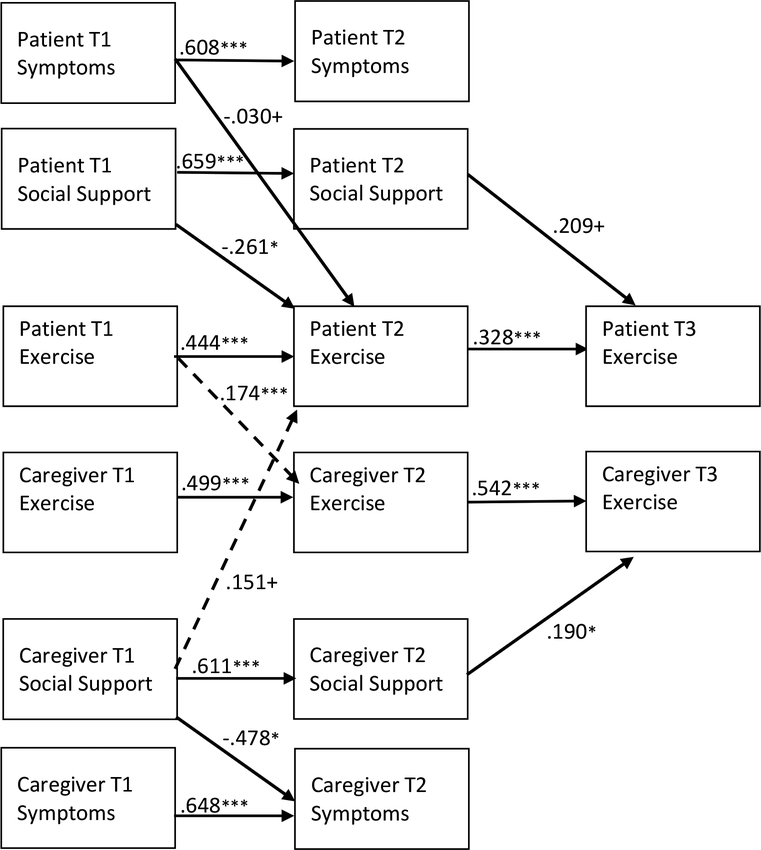
Figure 2. Estimated Actor-Partner Effects on Patient and Caregiver Exercise.
Note: Estimates are unstandardized; only significant parameter estimates are reported. Independent effects (actor effects) are represented by solid lines. Interdependent effects (partner effects) are represented by dashed lines. T1 = Time 1; T2= Time 2; T3 = Time 3. Covariances and/or error terms between patient comorbidities/conditions, social support, symptoms, and exercise were included in the analysis but are not shown in the model above. Model covariates included age, sex, race, income, cancer type, patient treatment, relationship type, length of time since patient diagnosis, living arrangement, and intervention group. Model fit: CFI=.968; RMSEA=.029; SRMR = .023. +p < .10; *p < .05; **p<.01; ***p<.001
Exercise actor effects (H1).
Patient exercise at T1 was positively associated with their own exercise at T2 (β=.444; p<.001) and patient exercise behavior at T2 was positively associated with their own exercise behavior at T3 (β=.328; p<.001). Caregiver exercise at T1 was positively associated with their own exercise at T2 (β=.499; p<.001) and caregiver exercise behavior at T2 was positively associated with their own exercise behavior at T3 (β=.542; p<.001).
Exercise partner effects (H2).
Patient exercise at T1 was positively associated with caregiver exercise at T2 (β=.174; p<.001). No other exercise partner effects were observed.
Influence of comorbidities/conditions, symptom distress and social support on exercise – actor effects (H3).
The number of patient and caregiver comorbidities/conditions at T1 did not have a statistically significant influence on their own exercise, symptom distress, or social support at T2. Patient symptom distress at T1 had a positive influence on patient symptom distress at T2 (β= −.608, p<.001) and a marginally significant negative influence on patient exercise at T2 (β= −.030, p=.061). Patient social support at T1 had positive influence on patient social support at T2 (β= .659, p<.001) and negative influence on patient exercise at T2 (−.261, p=.013). Patient social support at T2 had a marginally significant positive influence on patient exercise at T3 (β= .209, p=.066).
Caregiver symptom distress at T1 had a positive influence on caregiver symptom distress at T2 (β= .648, p<.001) but did not have a significant influence on caregiver exercise behaviors. Caregiver social support at T1 had positive influence on caregiver social support at T2 (β= .611, p<.001) and a negative influence on caregiver symptom distress at T2 (β= −.478, p=.036). Caregiver social support at T2 had a positive influence on caregiver exercise at T3 (β= .190, p=.022).
Influence of comorbidities/conditions, symptom distress and social support on exercise – partner effects (H4).
Patient and caregiver comorbidities/conditions at T1 did not have a statistically significant influence on the other dyad member’s exercise, symptom distress, or social support at T2. Neither patient symptom distress nor patient social support had an influence on caregiver exercise. Caregiver symptom distress did not have an influence on patient exercise; however, caregiver social support at T1 had a marginally significant positive influence on patient exercise at T2 (β= .151, p=.096).
Mediating effects on exercise (H5).
Caregiver social support at T1 had a positive indirect effect on caregiver exercise at T3 through caregiver social support at T2 (β=.116; 95% CI: 0.014, .223). No other mediating effects of social support or symptom distress on exercise were observed.
Diet Behaviors
Figure 3 provide the unstandardized estimates for APIMeM models examining diet. The model fit statistics were good across all models.
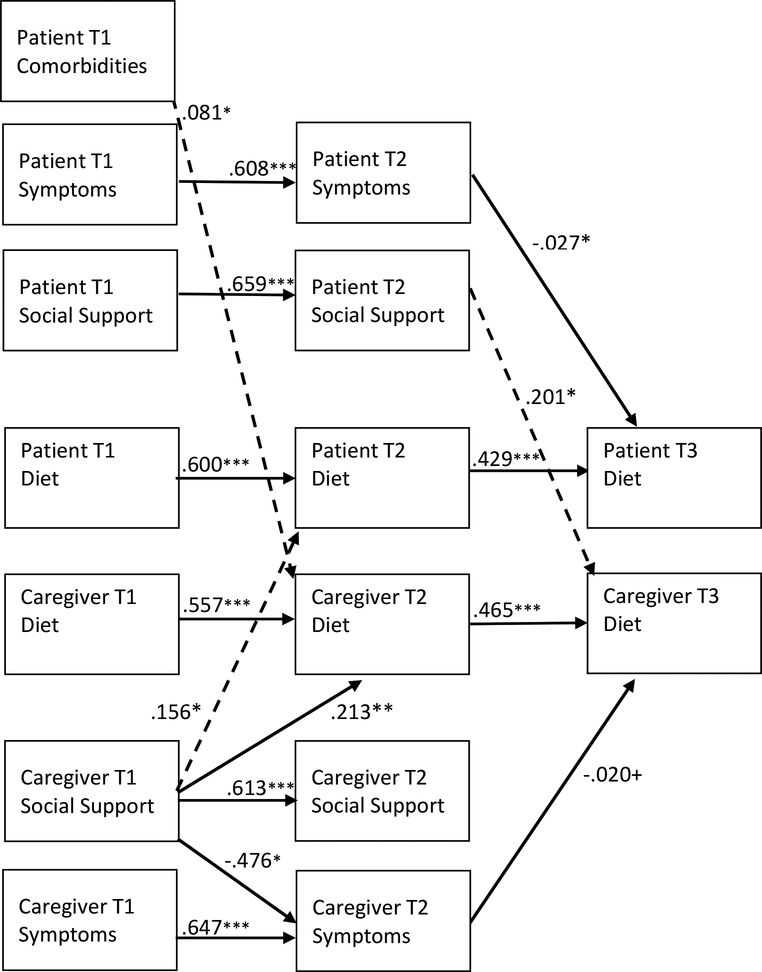
Figure 3. Estimated Actor-Partner Effects on Patient and Caregiver Diet.
Note: Estimates are unstandardized; only significant parameter estimates are reported. Independent effects (actor effects) are represented by solid lines. Interdependent effects (partner effects) are represented by dashed lines. T1 = Time 1; T2= Time 2; T3 = Time 3. Covariances and/or error terms between patient comorbidities/conditions, social support, symptoms, and diet were included in the analysis but are not shown in the model above. Model covariates included age, sex, race, income, cancer type, patient treatment, relationship type, length of time since patient diagnosis, living arrangement, and intervention group. Model fit: CFI=.958; RMSEA=.035; SRMR = .027. +p < .10; *p < .05; **p<.01; ***p<.001
Diet actor effects (H1).
Patient diet at T1 was positively associated with their own diet at T2 (β=.600; p<.001) and patient diet behavior at T2 was positively associated with their own diet behavior at T3 (β=.429; p<.001). Caregiver diet at T1 was positively associated with their own diet at T2 (β=.557; p<.001) and caregiver diet behavior at T2 was positively associated with their own diet behavior at T3 (β=.465; p<.001).
Diet partner effects (H2).
No diet partner effects were observed.
Influence of comorbidities/conditions, symptom distress and social support on diet – actor effects (H3).
Patient and caregiver comorbidities/conditions at T1 did not have a significant influence on their own exercise, symptom distress, or social support at T2. Patient symptom distress at T1 had a positive influence on patient symptom distress at T2 (β= −.608, p<.001). Patient symptom distress at T2 had a negative influence on patient diet at T3 (β= −.027, p=.017). Patient social support at T1 had positive influence on patient social support at T2 (β= .659, p<.001).
Caregiver symptom distress at T1 had a significant positive influence on caregiver symptoms at T2 (β= .647, p=<.001) and caregiver symptom distress at T2 had a marginally significant negative influence on caregiver diet at T3 (β= −.020, p=.086). Caregiver social support at T1 had positive influence on caregiver social support at T2 (β= .613, p<.001), a negative influence on caregiver symptom distress at T2 (β= −.476, p=.037), and a positive influence on caregiver diet at T2 (β= .213 p=.002).
Influence of comorbidities/conditions, symptom distress and social support on diet – partner effects (H4).
Number of patient comorbidities at T1 had a positive influence on caregiver diet at T2 (β= .081, p=.012). The number of caregiver conditions at T1 did not have a significant influence on patient exercise, symptom distress, or social support at T2. Neither patient or caregiver symptom distress had an influence on the other dyad member’s exercise behavior. Patient social support at T2 had a significant positive influence on caregiver diet at T3 (β= .201, p=.013). Caregiver social support at T1 had a significant positive influence on patient diet at T2 (β= .156, p=.026).
Mediating effects on diet (H5).
Patient symptom distress at T1 had a negative indirect effect on patient diet at T3 through patient symptoms at T2 (β=−.017; 95% CI: −.034, −.005). Patient social support at T1 had a positive indirect effect on caregiver diet at T3 through patient social support at T2 (β=.132, 95% CI: 0.023, .256). No other mediating effects of social support or symptom distress on diet were observed.
Discussion
The purpose of this exploratory study was to examine the exercise and diet behaviors of patients with advanced cancer and their caregivers, with a particular focus on the interdependence of their health behaviors, and the effect of perceived social support and chronic health problems (number of conditions and symptom distress) on their engagement in health behaviors. This secondary analysis was conducted with large sample of patients with advanced cancers (breast, colorectal, lung and prostate) and their primary family caregivers. There is a need for more research on the experiences of patients and caregivers at this disease stage given the specific challenges that accompany advanced disease (Hopkinson et al., 2012).
Health Behaviors of Advanced Cancer Patients and their Caregivers
For much of our sample of advanced cancer patients and their caregivers, their exercise behavior was much lower than recommended while their dietary behavior was near recommended guidelines. At baseline, approximately one-third reported engaging in moderate or vigorous exercise at least 3 days a week while over two-thirds reported eating a balanced diet. Much of the previous research evaluating the health behaviors of patients with cancer has focused on people with earlier stage disease or did not analyze data by stage of disease. Furthermore, few studies of cancer patients examined the behavior patterns of their family caregivers (Bellizzi et al., 2005; Demark-Wahnefried et al., 2005; Patterson et al., 2003; Shaffer et al., 2016). Data in the present study appear similar to other studies of cancer survivors of early stage disease or all-stages combined. In those studies only 30% to 58% met recommended exercise guidelines or engaged in routine exercise (Bellizzi et al., 2005; Demark-Wahnefried et al., 2005); about half (45%) ate at least five servings of fruits and vegetables per day, and most (69%) followed a low fat diet (Demark-Wahnefried et al., 2005). In a dyadic study by Shaffer and colleagues (2016), less than 10% of cancer patients nor their caregivers consumed the recommended guideline of five servings of fruits and vegetables per day, indicating that this in important area for intervention.
Influence of Patient and Caregivers on Their Own Health Behaviors (Actor Effects)
In this study, an individual’s previous behavior was a strong predictor of their future behavior (i.e. actor effects) for both exercise and dietary health-promoting behaviors. This highlights the importance of assessing health behavior engagement and intervening early with patients and caregivers in order to increase health-promoting behaviors and support the maintenance of these behaviors. The adjustment to chronic and advanced disease is a dynamic and non-linear process (Clark, 2003; Stanton et al., 2007) that requires ongoing assessment because patient and caregiver needs will vary overtime. Schulman-Green and colleagues (2011; 2012) identified the transitional period from curable to non-curable cancers as a key phase for understanding and supporting patient self-management of their illness, which includes participation in health-promoting behaviors. Future research should examine the effects of transitions on patient and caregiver health behaviors.
Influence on One Another’s Health Behaviors (Partner Effects)
When patients engaged in more exercise at baseline, their caregivers engaged in more exercise at the subsequent time point three months later. It is possible that patients who were able to exercise also felt better and required less care, freeing up their caregivers to engage in exercise in the following few months. However, there was no significant relationship between patients’ and caregivers exercise behavior at the final assessment, possibly due to the patient’s diminishing health status over time. We found no associations between advanced cancer patients’ and caregivers’ diet behaviors at any time point, although Shaffer and colleagues (2016) found that patient and caregiver intake of fruits and vegetables tended to change in tandem. Health behaviors are highly influenced by individual, familial and environmental factors (Gordon-Larsen, 2006; Lewis et al., 2006; Umberson et al., 2010) and may also be influenced by stage of disease. In the present study all patients had advanced cancer, while in the study by Shaffer and colleagues (2016) study the majority (82%) had localized or regional disease. Research indicates that patients face significant challenges such as fatigue and changes to their appetite and diet as their cancer progresses (Brown et al., 2001); as a result, it is possible that these results are highly influenced by cancer stage and/or progression.
Comorbidities/Chronic Conditions, Symptom Distress, Social Support and Behaviors
A fairly high prevalence of patient comorbidities and caregiver chronic conditions were observed in this sample. In addition, patients and caregiver also reported experiencing mental and physical health symptoms (i.e., symptom distress) that could be attributed to the cancer and caregiving experience or their health problems. In our analysis, these conditions and symptoms had a significant influence on diet behaviors, but not exercise behaviors. First, results indicated that more patient comorbidities at baseline was associated with better caregiver diet three months later. The management recommendations for many chronic conditions include engagement in healthy diet behaviors (Barlow et al., 2002). It is possible that patients with a greater number of health problems may receive more encouragement to engage in health-promoting behaviors from health professionals due to the additional clinic appointments focused on managing their other chronic conditions. In turn, this could have a positive influence on caregivers as well. Results also indicated, however, that more patient symptom distress was associated with poorer patient diet (via direct and indirect effects). Weight loss and appetite changes are a common problem faced by individuals with advanced cancer (Barrera, 2002; Brown et al., 2001). Thus, while this finding might be expected, it points towards the need for continued palliative care and support to help patients manage their symptoms and the effect that cancer and its treatment are having on their diet (Ravasco et al., 2005; Rock et al., 2012).
We also examined the influence of social support on health behaviors, and the role of perceptions of social support within the dyad (i.e., how well dyad members felt supported by each other) as a possible mediator of the relationship between health problems and exercise behavior. While these mediating effects were not supported by our data, we did observe a number of direct and indirect effects of social support on health behaviors. As expected, the more social support caregivers perceived from patients, the better their exercise and diet behaviors. These findings are aligned with broader literature supporting the role of social support for health behavior (Uchino, 2006; Thoits, 2011), particularly as it relates to dyadic relationships (Lewis et al., 2006). Caregiver concern and attention to their own health is an ongoing issue of concern for researchers and practitioners. This finding suggests that positive support received from the patient (i.e., care recipient) could be beneficial to patient health and an important target for intervention efforts.
Conversely, our findings also indicated that better patient perceptions of social support received from the caregiver was associated with less patient exercise. Given that our measure of social support was general, and not related to health behavior engagement specifically, it is difficult to tease apart this finding. As noted previously, most patients and caregivers in our sample did not meet recommended physical activity levels. Thus, the provision of general social support (i.e., willingness to listen, helping me cope with illness) could have led to caregiver support of a patient’s desire to decrease physical activity levels in response to illness burden or treatment demands. Furthermore, among patients with advanced cancer, lack of clear guidelines for physical activity has been noted as a possible barrier to physician-initiated interventions and recommendations (Denlinger & Engstrom, 2011; Rock et al., 2012), suggesting patients and caregivers may be unaware of the level of physical activity patients might strive for. Moreover, Lewis and colleagues (2006) argue that when examining dyadic interdependence and behavior change, it is important to understand the degree to which dyad members preferences for outcomes correspond (i.e., agreement on acceptable levels of physical activity). Unfortunately, this information was not captured in our study, but would be a useful area of future research.
We also observed a number of interesting partner effects of social support on health behavior. Better caregiver perceptions of social support received from the patient also had a positive influence on patient diet; that is, the more positively a caregiver viewed the support received by the patient, the better the patient’s diet. Similarly, better patient perceptions of social support received from the caregiver had a positive influence of caregiver diet. Providing social support (as evidenced here by patient and caregiver perceptions of support received) may fulfill patients’ and caregivers’ desire to maintain valued familial roles, give meaning to their lives (Patterson, 2002), and as our results suggest, may also have a positive influence on their diet behaviors. This finding points to the importance of supporting the quality of the caregiver-patient relationship for both of their health outcomes, and diet behaviors in particular.
Limitations
There are a number of limitations that should be noted. First, the measures of physical activity and diet asked patients and caregivers to report on their engagement in behaviors in the week prior to data collection. Other more reliable measures of these activities could provide more accurate assessments of behavior engagement. The choice of method(s), however, should not be overly burdensome to dyads, especially those facing advanced cancer. Wearable fitness trackers that record physical activity and other technology for capturing health behavior in real-time, could be a good option. These technologies would also be useful for capturing more data points, which could be used to analyze dyadic behavior change over time and factors that might influence observed patterns. Second, the measure of patient and caregiver conditions was based upon their responses to an open-ended question; it is possible that providing a checklist of conditions for patients and caregivers to choose from and/or using medical records could provide more accurate assessment of chronic disease diagnoses. Another limitation of this study is the use of the “number of chronic conditions” rather than a measure of chronic disease severity or management burden, which may be a stronger predictor of health behaviors than number of conditions. While our measure of symptom distress may have captured some aspects of disease burden, a measure of chronic disease burden tailored to the reported diseases could yield different results. As mentioned previously, our measure of social support was general in nature and did not ask specifically about social support related to physical activity or diet behaviors. A social support measure more clearly associated with these target behaviors could identify more associations between dyadic social support and behavior engagement.
Lastly, these findings may not generalize to all advanced cancer patient/caregiver dyads. All dyads in this study included a patient and a caregiver who were motivated to participate; the level of motivation and support in these dyads could be reflected in the frequency of engagement in healthy exercise and diet behaviors. A focus on different cancer types and the possible effects on patient and caregiver behavior, and examining relationships between cancer, gender and role (patients vs. caregiver) are also worthwhile areas of future study. Future studies should also examine these findings in a large, multiethnic population of cancer patients and caregivers. Differences in factors such as familial and cultural orientations toward illness and caregiving (Baider, 2012; Dilworth-Anderson et al., 2002), varying quality and availability of health care, and disparities in the accessibility and availability of resources (Cohen et al., 2000; Jackson & Knight, 2006; Sanders et al., 2013), could influence the associations between chronic conditions and health-promoting behaviors observed in this study.
Conclusion
Our exploratory study examined the exercise and diet behaviors of patients with advanced cancer and their caregivers, with a particular focus on the interdependence of their health behaviors and factors that might influence behavior engagement. Engagement in health-promoting behaviors among patients with advanced cancer and their family caregivers may be difficult due to the consequences of advanced disease associated caregiving. As such, health professionals need to assess the health needs and behaviors of patients and caregivers early in the course of illness to identify ways to intervene and support their health behaviors. An important contribution of this study is the examination of how caregivers’ own health behaviors, health conditions and symptoms may influence patient behaviors (and vice-versa). Caregiver assessments could help to identify caregivers who may need additional support managing their own health problems in addition to caring for the patients.
Moreover, interventions need to be directed to patients and caregivers as a unit of care. While patient and caregiver health problems may present challenges to behavior engagement, it may provide opportunities as well, due to increased contact with providers to manage the conditions and possibility of reciprocal social support and reinforcement for positive behaviors between patients and caregivers. For those patients and caregivers for whom behavioral interventions are appropriate and desired, it will be useful to tailor those interventions to and identify resources that meet the dyad’s specific needs. The value that patients and caregivers place on health-promoting behaviors, especially as patients near the end of life, should not be assumed. While a number of studies point towards the benefits of these behaviors with advanced disease, it is important to take individual preferences and priorities into account.
Funding:
The preparation of this manuscript by the first author was supported by Rackham Graduate School at the University of Michigan, and the Cancer Health Disparities Training Program (2T32CA128582-06) and Center for Health Equity Research at the University of North Carolina. Data come from a study funded by a grant from the National Cancer Institute (RO1CA107383, L. Northouse).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
References
- Albrecht TA, & Taylor AG (2012). Physical activity in patients with advanced-stage cancer: A systematic review of the literature. Clinical Journal of Oncology Nursing, 16(3), 293–300. doi: 10.1188/12.cjon.293-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baider L (2012). Cultural diversity: Family path through terminal illness. Annals of Oncology, 23(Suppl. 3), 62–65. doi: 10.1093/annonc/mds090 [DOI] [PubMed] [Google Scholar]
- Barlow J, Wright C, Sheasby J, Turner A, & Hainsworth J (2002). Self-management approaches for people with chronic conditions: A review. Patient Education and Counseling, 48(2), 177–187. [DOI] [PubMed] [Google Scholar]
- Barrera R (2002). Nutritional support in cancer patients. Journal of Parenteral and Enteral Nutrition, 26(Suppl. 5), S63–S71. doi: 10.1177/014860710202600516 [DOI] [PubMed] [Google Scholar]
- Beesley VL, Price MA, Webb PM, AOCS Group, AOCS-QOL Investigators (2011). Loss of lifestyle: health behaviour and weight changes after becoming a caregiver of a family member diagnosed with ovarian cancer. Supportive Care in Cancer, 19(12), 1949–1956. [DOI] [PubMed] [Google Scholar]
- Bellizzi KM, Rowland JH, Jeffery DD, & McNeel T (2005). Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. Journal of Clinical Oncology, 23(34), 8884–8893. doi: 10.1200/jco.2005.02.2343 [DOI] [PubMed] [Google Scholar]
- Bevans M, & Sternberg EM (2012). Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA, 307(4), 398–403. doi: 10.1001/jama.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard CM, Stein KD, Baker F, Dent MF, Denniston MM, Courneya KS, & Nehl E (2004). Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychology & Health, 19(1), 1–13. doi: 10.1080/08870440310001606507 [DOI] [Google Scholar]
- Brown J, Byers T, Thompson K, Eldridge B, Doyle C, & Williams AM (2001). Nutrition during and after cancer treatment: A guide for informed choices by cancer survivors. CA: A Cancer Journal for Clinicians, 51(3), 153–187. [DOI] [PubMed] [Google Scholar]
- Clark NM (2003). Management of chronic disease by patients. Annual Review of Public Health, 24(1), 289–313. doi: doi: 10.1146/annurev.publhealth.24.100901.141021 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2003). Trends in aging--United States and worldwide. MMWR. Morbidity and Mortality Weekly Report, 52(6), 101. [PubMed] [Google Scholar]
- Cohen DA, Scribner RA, & Farley TA (2000). A structural model of health behavior: A pragmatic approach to explain and influence health behaviors at the population level. Preventive Medicine, 30(2), 146–154. doi: 10.1006/pmed.1999.0609 [DOI] [PubMed] [Google Scholar]
- Courneya KS, & Friedenreich CM (2007). Physical activity and cancer control. Seminars in Oncology Nursing, 23(4), 242–252. doi: 10.1016/j.soncn.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Deimling GT, Bowman KF, & Wagner LJ (2007). Cancer survivorship and identity among long-term survivors. Cancer Investigation, 25(8), 758. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Aziz NM, Rowland JH, & Pinto BM (2005). Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology, 23(24), 5814–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger CS, & Engstrom PF (2011). Colorectal cancer survivorship: Movement matters. Cancer Prevention Research, 4(4), 502–511. doi: 10.1158/1940-6207.capr-11-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth-Anderson P, Williams IC, & Gibson BE (2002). Issues of race, ethnicity, and culture in caregiving research: A 20-year review (1980–2000). The Gerontologist, 42(2), 237–272. doi: 10.1093/geront/42.2.237 [DOI] [PubMed] [Google Scholar]
- Edwards BK, Noone A-M, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, … Ward EM (2014). Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer, 120(9), 1290–1314. doi: 10.1002/cncr.28509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszer C, Dolbeault S, Sultan S, & Brédart A (2014). Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: a systematic review. Psycho-Oncology, 23(4), 361–374. doi: 10.1002/pon.3432 [DOI] [PubMed] [Google Scholar]
- Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, & Maltais D (2004). Multimorbidity and quality of life in primary care: A systematic review. Health and Quality of Life Outcomes, 2(51). doi: 10.1186/1477-7525-2-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given BA, Given CW, & Kozachik S (2001). Family support in advanced cancer. CA: A Cancer Journal for Clinicians, 51(4), 213–231. doi: 10.3322/canjclin.51.4.213 [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P (2006). Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics, 117(2), 417. [DOI] [PubMed] [Google Scholar]
- Hebert R, Zdaniuk B, Schulz R, & Scheier M (2009). Positive and negative religious coping and well-being in women with breast cancer. Journal of Palliative Medicine, 12(6), 537–545. doi: 10.1089/jpm.2008.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JG (2002). Interpersonal Expectations as the Building Blocks of Social Cognition: An Interdependence Theory Perspective. Personal Relationships, 9(1), 1–26. doi: 10.1111/1475-6811.00001 [DOI] [Google Scholar]
- Hopkinson JB, Brown JC, Okamoto I, & Addington-Hall JM (2012). The effectiveness of patient-family carer (couple) intervention for the management of symptoms and other health-related problems in people affected by cancer: A systematic literature search and narrative review. Journal of Pain and Symptom Management, 43(1), 111–142. [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- Jackson JS, & Knight KM (2006). Race and self-regulatory health behaviors: The role of the stress response and the HPA axis in physical and mental health disparities In Schaie KW & Carstensen LL (Eds.), Social structures, aging, and self-regulation in the elderly (pp. 189–239). New York: Springer Publishing. [Google Scholar]
- Kenny DA, Kashy DA, & Cook WL (2006). Dyadic data analysis. New York: Guilford Press. [Google Scholar]
- Kershaw T, Ellis K, Yoon H, Schafenacker A, Katapodi M, & Northouse L (2015). The Interdependence of Advanced Cancer Patients’ and Their Family Caregivers’ Mental Health, Physical Health, and Self-Efficacy over Time. Annals of Behavioral Medicine, 49(6), 901–911. doi: 10.1007/s12160-015-9743-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, & Given BA (2008). Quality of life of family caregivers of cancer survivors: Across the trajectory of the illness. Cancer, 112(Suppl. 11), 2556–2568. [DOI] [PubMed] [Google Scholar]
- Kim Y, & Schulz R (2008). Family caregivers’ strains: Comparative analysis of cancer caregiving with dementia, diabetes, and frail elderly caregiving. Journal of Aging and Health, 20(5), 483–503. doi: 10.1177/0898264308317533 [DOI] [PubMed] [Google Scholar]
- Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, & Emmons KM (2006). Understanding health behavior change among couples: An interdependence and communal coping approach. Social Science & Medicine, 62(6), 1369–1380. doi: 10.1016/j.socscimed.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Little TD, & Card NA (2013). Longitudinal structural equation modeling. New York: The Guilford Press. [Google Scholar]
- Mazanec SR, Daly BJ, Douglas SL, & Lipson AR (2011). Work productivity and health of informal caregivers of persons with advanced cancer. Research in Nursing & Health, 34(6), 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mood D, & Bickes J (1989). Strategies to enhance self-care in radiation therapy. Oncology Nursing Forum, 16(Suppl), 143. [Google Scholar]
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, … Demark-Wahnefried W (2009). Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: A randomized controlled trial. JAMA, 301(18), 1883–1891. doi: 10.1001/jama.2009.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2015). Mplus User’s Guide. Sixth Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Northouse LL (1988). Social support in patients’ and husbands’ adjustment to breast cancer. Nursing research, 37(2), 91–97. [PubMed] [Google Scholar]
- Northouse LL (2005). Helping families of patients with cancer. Oncology Nursing Forum, 32(4), 743–750. doi: 10.1188/04.onf.743-750 [DOI] [PubMed] [Google Scholar]
- Northouse LL, Mood DW, Montie JE, Sandler HM, Forman JD, Hussain M, Pienta KJ, Smith DC, Sanda MG, Kershaw T (2007) Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. Journal of Clinical Oncology 25 (27):4171–4177. doi: 10.1200/jco.2006.09.6503 [DOI] [PubMed] [Google Scholar]
- Northouse LL, Mood DW, Schafenacker A, Kalemkerian G, Zalupski M, LoRusso P, … Kershaw T (2013). Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family caregivers. Psycho-Oncology, 22(3), 555–563. doi: 10.1002/pon.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northouse LL, Mood DW, Schafenacker A, Montie JE, Sandler HM, Forman JD, … Kershaw T (2007). Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer, 110(12), 2809–2818. doi: 10.1002/cncr.23114 [DOI] [PubMed] [Google Scholar]
- Northouse L, Mood D, Templin T, Mellon S, & George T (2000). Couples’ patterns of adjustment to colon cancer. Social Science & Medicine, 50(2), 271–284. [DOI] [PubMed] [Google Scholar]
- Northouse LL, Williams AL, Given B, & McCorkle R (2012). Psychosocial care for family caregivers of patients with cancer. Journal of Clinical Oncology, 30(11), 1227–1234. doi: 10.1200/jco.2011.39.5798 [DOI] [PubMed] [Google Scholar]
- Ogle KS, Swanson GM, Woods N, & Azzouz F (2000). Cancer and comorbidity: Redefining chronic diseases. Cancer, 88(3), 653–663. [DOI] [PubMed] [Google Scholar]
- Oldervoll LM, Loge JH, Paltiel H, Asp MB, Vidvei U, Wiken AN, … Kaasa S (2006). The effect of a physical exercise program in palliative care: A phase II study. Journal of Pain and Symptom Management, 31(5), 421–430. doi: 10.1016/j.jpainsymman.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, & Bowen DJ (2003). Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. Journal of the American Dietetic Association, 103(3), 323–328. doi: 10.1053/jada.2003.50045 [DOI] [PubMed] [Google Scholar]
- Payne S (2007). Living with advanced cancer In Feuerstein M (Ed.), Handbook of Cancer Survivorship (pp. 429–446): Springer US. [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, & Dallman MF (2004). Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology, 145(8), 3754–3762. doi: 10.1210/en.2004-0305 [DOI] [PubMed] [Google Scholar]
- Pinto BM, Trunzo JJ, Reiss P, & Shiu S-Y (2002). Exercise participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psycho-Oncology, 11(5), 389–400. doi: 10.1002/pon.594 [DOI] [PubMed] [Google Scholar]
- Ravasco P, Monteiro-Grillo I, Vidal PM, & Camilo ME (2004). Cancer: Disease and nutrition are key determinants of patients’ quality of life. Supportive Care in Cancer, 12(4), 246–252. doi: 10.1007/s00520-003-0568-z [DOI] [PubMed] [Google Scholar]
- Ravasco P, Monteiro-Grillo I, Vidal PM, & Camilo ME (2005). Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. Journal of Clinical Oncology, 23(7), 1431–1438. doi: 10.1200/jco.2005.02.054 [DOI] [PubMed] [Google Scholar]
- Rayens MK, & Svavarsdottir EK (2003). A new methodological approach in nursing research: An actor, partner, and interaction effect model for family outcomes. Research in Nursing & Health, 26(5), 409–419. doi: 10.1002/nur.10100 [DOI] [PubMed] [Google Scholar]
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, … Gansler T (2012). Nutrition and physical activity guidelines for cancer survivors. CA Cancer Journal for Clinicians, 62(4), 242–274. [DOI] [PubMed] [Google Scholar]
- Romito F, Goldzweig G, Cormio C, Hagedoorn M, & Andersen BL (2013). Informal caregiving for cancer patients. Cancer, 119(0 11), 10.1002/cncr.28057. doi: 10.1002/cncr.28057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JH, & Yancik R (2006). Cancer survivorship: The interface of aging, comorbidity, and quality care. Journal of the National Cancer Institute, 98(8), 504–505. [DOI] [PubMed] [Google Scholar]
- Rummans TA, Clark MM, Sloan JA, Frost MH, Bostwick JM, Atherton PJ, … Hanson J, (2006). Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: A randomized controlled trial. Journal of Clincal Oncology, 24(4), 635–642. doi: 10.1200/jco.2006.06.209 [DOI] [PubMed] [Google Scholar]
- Sanders J, Solberg B, & Gauger M (2013). Breaking barriers to care: A community of solution for chronic disease management. The Journal of the American Board of Family Medicine, 26(3), 311–315. doi: 10.3122/jabfm.2013.03.120236 [DOI] [PubMed] [Google Scholar]
- Schulman-Green D, Bradley EH, Knobf MT, Prigerson H, DiGiovanna MP, & McCorkle R (2011). Self-management and transitions in women with advanced breast cancer. Journal of Pain and Symptom Management, 42(4), 517–525. doi: 10.1016/j.jpainsymman.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman-Green D, Bradley EH, Nicholson NR, George E, Indeck A, & McCorkle R (2012). One step at a time: Self-management and transitions among women with ovarian cancer. Oncology Nursing Forum, 39(4), 354–360. doi: 10.1188/12.onf.354-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer K, Kim Y, Llabre M, & Carver C (2016). Dyadic associations between cancer-related stress and fruit and vegetable consumption among colorectal cancer patients and their family caregivers. Journal of Behavioral Medicine, 39(1), 75–84. doi: 10.1007/s10865-015-9665-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, & Cleeland CS (2011). Symptom burden in cancer survivors 1 year after diagnosis. Cancer, 117(12), 2779–2790. doi: 10.1002/cncr.26146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Revenson TA, & Tennen H (2007). Health psychology: Psychological adjustment to chronic disease. Annual Review of Psychology, 58(1), 565–592. doi: doi: 10.1146/annurev.psych.58.110405.085615 [DOI] [PubMed] [Google Scholar]
- Thoits PA (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52(2), 145–161. doi: 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Uchino B (2006). Social Support and Health: A Review of Physiological Processes Potentially Underlying Links to Disease Outcomes. J Behav Med, 29(4), 377–387. doi: 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, & Reczek C (2010). Social relationships and health behavior across the life course. Annual Review of Sociology, 36(1), 139–157. doi: doi: 10.1146/annurev-soc-070308-120011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, & Patijn J (2007). Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Annals of Oncology, 18(9), 1437–1449. [DOI] [PubMed] [Google Scholar]
- Van Lange PAM, & Rusbult CE (2012). Interdependence Theory In Van Lange PAM, Kruglanski AW, & Higgins ET (Eds.), Handbook of theories in social psychology. (pp. 251–272). Los Angeles: SAGE. [Google Scholar]
- Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, & Blumenthal D (2007). Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. Journal of General Internal Medicine, 22, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters-Stone KM, Lyons KS, Dobek J, Dieckmann NF, Bennett JA, Nail L, & Beer TM (2015). Benefits of partnered strength training for prostate cancer survivors and spouses: results from a randomized controlled trial of the Exercising Together project. Journal of Cancer Survivorship, 1–12. [DOI] [PubMed] [Google Scholar]