Abstract
IMPORTANCE
Human papillomavirus–related oropharyngeal carcinoma (HPV-OPC) is increasing in incidence in the United States. Although HPV-OPC has favorable prognosis, 10% to 25% of HPV-OPCs recur. Detection of human papillomavirus (HPV) DNA in oral rinses is associated with HPV-OPC, but its potential as a prognostic biomarker is unclear.
OBJECTIVE
To determine whether HPV DNA detection in oral rinses after treatment for HPV-OPC is associated with recurrence and survival.
DESIGN, SETTING, AND PARTICIPANTS
Prospective cohort study of patients with incident HPV-OPC diagnosed from 2009 to 2013 at 4 academic tertiary referral cancer centers in the United States. Oral rinse samples were collected at diagnosis and after treatment (9, 12, 18, and 24 months after diagnosis), and evaluated for HPV DNA. Among an initial cohort of 157 participants with incident HPV-OPC treated with curative intent, 124 had 1 or more posttreatment oral rinses available and were included in this study.
MAIN OUTCOMES AND MEASURES
Disease-free survival (DFS) and overall survival (OS) were estimated by the Kaplan-Meier method, and the association of HPV DNA detection in oral rinses with survival was evaluated using Cox regression analysis.
RESULTS
Oral HPV type 16 (HPV16) DNA was common at diagnosis (67 of 124 participants [54%]). In contrast, oral HPV16 DNA was detected in only 6 participants after treatment (5%), including 5 with HPV16 DNA also detected at diagnosis (persistent oral HPV16 DNA). Two-year DFS and OS were 92% (95% CI, 94%-100%) and 98% (95% CI, 93%-99%). Persistent oral HPV16 DNA was associated with worse DFS (hazard ratio, 29.7 [95% CI, 9.0-98.2]) and OS (hazard ratio, 23.5 [95% CI, 4.7-116.9]). All 5 participants with persistent oral HPV16 DNA developed recurrent disease, 3 with local disease involvement. In contrast, just 9 of 119 participants (8%) without persistent oral HPV16 DNA developed recurrent disease, only 1 (11%) with local disease involvement. Median (range) time from earliest posttreatment oral HPV16 DNA detection to recurrence was 7.0 (3.7-10.9) months.
CONCLUSIONS AND RELEVANCE
Human papillomavirus type 16 DNA in oral rinses is common at diagnosis but rare after treatment for HPV-OPC. Our data suggest that, although infrequent, persistent HPV16 DNA in posttreatment oral rinses is associated with poor prognosis and is a potential tool for long-term tumor surveillance, perhaps more so for local recurrence.
Human papillomavirus (HPV) infection is responsible for the majority of oropharyngeal carcinomas (OPCs) in the United States.1 Human papillomavirus–positive tumor status confers significantly improved prognosis; however, approximately 10% to 25% of patients experience disease progression after treatment, most within the first 2 years.2-6 Importantly, even after progression, HPV-related OPC (HPV-OPC) responds favorably to salvage treatment. Surgical salvage in particular is associated with improved outcomes for both HPV-positive and HPV-negative OPC, so surgically treatable HPV-positive disease is the most favorable scenario for patients with OPC progression.2,7-9 Currently, 35% to 45% of progressive HPV-OPCs have already spread to distant sites at the time of diagnosis, decreasing the feasibility of surgical salvage.2,7 Earlier diagnosis of progressive or recurrent disease than is currently possible, and especially of local or locoregional disease amenable to surgical treatment, would hasten initiation of salvage therapy and may improve outcomes.
To that end, the viral etiology of HPV-OPC, and the resultant association of HPV-specific biomarkers with HPV-OPC, presents an opportunity to potentially enhance posttreatment disease surveillance strategies and facilitate earlier diagnosis of progressive or recurrent disease. One such biomarker is human papillomavirus type 16 (HPV16) DNA in oral exfoliated cells, which is detected in up to two-thirds of HPV-OPC cases before treatment, and persists in a small subset of cases after treatment.10-14 Initial single-center studies suggest that HPV16 DNA detection in posttreatment oral rinses may also be associated with disease recurrence.11,15 Therefore, in this study we evaluated the prognostic and diagnostic implications of HPV16 DNA detection in serially collected posttreatment oral rinses within a prospective multicenter cohort of patients with HPV-OPC.
Methods
Study Population
The study population was a prospective multi-institutional cohort of participants with incident OPC diagnosed from 2009 through 2013 as previously described.13 Briefly, participants were enrolled at time of diagnosis, before treatment initiation (“diagnosis”). Oral rinse samples were prospectively collected with behavioral and clinical data. Computer-assisted self-interview surveys were administered at diagnosis and then at 12 and 24 months after diagnosis to collect behavioral and demographic data, including self-designated race because HPV-OPC incidence and behavioral risk factors vary by race.16 Medical record abstraction was performed annually, with abstraction last updated in October 2014 for all participants. Tumor HPV status was determined by in situ hybridization for HPV DNA and immunohistochemical analysis for p16, as previously described.13 Clinical care, including treatment regimen and posttreatment disease surveillance, was carried out consistent with established national guidelines17 at the 4 participating academic head and neck oncology centers.
Participants eligible for this analysis had HPV-OPC treated with curative intent, clinical follow-up for at least 12 months after diagnosis, and at least 1 posttreatment oral rinse. This study was approved by the institutional review board at all study sites, and all participants gave written informed consent.
HPV Detection in Oral Rinses
Oral rinse and gargle samples were collected using 10 mL of Scope mouthwash at diagnosis and again after treatment, at 9, 12, 18, and 24 months after diagnosis. Oral rinse samples were tested for 36 types of HPV DNA using PGMY 09/11 primers and line-blot hybridization as described previously.13,18 Types of HPV considered high-risk were types 16, 18, 31, 33, 35, 39, 45, 51, 56, 58, 59, 68, and 73. Persistent oral HPV DNA detection was defined as detection of the same high-risk HPV (HR-HPV) type at diagnosis and in at least 1 posttreatment oral rinse. Clearance of HR-HPV DNA was defined as detection of the HR-HPV type at diagnosis but at no time during follow-up. A new oral HPV “infection” was defined as detecting DNA from an HR-HPV type in 1 or more posttreatment rinses that was not detected at diagnosis. Oral rinses at diagnosis were also evaluated for HPV16 viral load using TaqMan quantitative polymerase chain reaction as previously described.13,19 Oral HPV16 viral load was considered either undetectable or detectable, and detectable viral load was further categorized by number of copies in 2 μL of oral rinse sample as either low (<160 [median detectable viral load]) or high (≥160).
Analytic Methods
Frequency and percent, or median and interquartile range (IQR), of participant characteristics were described overall and by eligibility for this analysis. Viral load was reported as both a categorical and a continuous variable by characteristics of interest. Categorical variables were compared using χ2 or Fisher exact tests, and continuous variables, using the Wilcoxon non-parametric rank-sum test.
Survival rates were estimated using the Kaplan-Meier method.20 Overall survival (OS) was defined as time from diagnosis to death from any cause, with censoring at last follow-up. Disease-free survival (DFS) was defined as time from diagnosis to disease recurrence, with censoring at last follow-up or death. Survival curves were compared using the log-rank test. Risk factors for mortality and recurrence were explored using Cox regression models.
Specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) of HPV16 DNA detection in posttreatment rinses as a predictor of recurrence (considered as binary) were also explored. Oral rinse results at the 9- and 12-month visits and the 18- and 24-month visits were combined for some analyses. There were no discordant oral HPV16 DNA detection results at these pairs of visits. P < .05 was considered statistically significant. Data analysis was performed using STATA 11.2.
Results
Study Population
Of the 157 participants with HPV-OPC, 124 (79%) had at least 1 posttreatment oral rinse sample and were therefore eligible for this analysis. The characteristics of the study population are summarized in Table 1. The majority of participants were male (90%), white (98%), married or living as married (85%), and never-smokers (58%). Most participants had small tumors (65%) and advanced nodal (70%) and overall stage (82%). Compared with ineligible participants, eligible participants were more likely to be married (P = .03), had higher income (P = .009), and had fewer pack-years of smoking (P = .02) (eTable 1 in the Supplement).
Table 1.
Participant Characteristics and Association With Disease-Free and Overall Survival
| Disease-Free Survival | Overall Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic (n = 124)a |
No. (%) | Univariate, HR (95%CI) |
P Value | Multivariate, aHR (95% CI)b |
P Value | Univariate, HR (95% CI) |
P Value | Multivariate, aHR (95% CI)b |
P Value |
| Age, y | |||||||||
| <50 | 32 (26) | 1 [Reference] | 1 [Reference] | ||||||
| 51-55 | 27 (22) | 1.1 (0.2-5.6) | .88 | … | 1.2 (0.2-8.3) | .88 | … | ||
| 56-60 | 33 (27) | 1.5 (0.3-6.5) | .63 | … | 0.5 (0.1-5.6) | .58 | … | ||
| >60 | 32 (26) | 1.3 (0.3-5.9) | .71 | … | 0.4 (0.04-5.0) | .52 | … | ||
| Sex | |||||||||
| Female | 12 (10) | 1 [Reference] | 1 [Reference] | ||||||
| Male | 112 (90) | 0.6 (0.1-2.8) | .55 | … | 0.5 (0.1-4.4) | .55 | … | ||
| Annual income, $ | |||||||||
| <50 000 | 16 (15) | 1 [Reference] | 1 [Reference] | ||||||
| 50 000-99 999 | 36 (33) | 1.3 (0.3-6.9) | .74 | … | 0.5 (0.03-7.5) | .59 | … | ||
| ≥100 000 | 58 (53) | 0.4 (0.1-2.6) | .36 | … | 0.3 (0.02-4.1) | .34 | … | ||
| Education | |||||||||
| High school or less | 52 (44) | 1 [Reference] | .11 | 1 [Reference] | .17 | ||||
| College or advanced degree | 67 (56) | 0.5 (0.2-1.6) | .26 | … | 0.7 (0.1-3.3) | .63 | … | ||
| Smoking, per 10 pack-years | 1.4 (1.1-1.8) | .003 | 1.2 (1.0-1.5) | 1.5 (1.1-2.1) | .01 | 1.3 (0.9-1.8) | |||
| Smoking status | |||||||||
| Current | 13 (11) | 1 [Reference] | 1 [Reference] | ||||||
| Former | 38 (31) | 1.0 (0.2-3.7) | .95 | … | 0.4 (0.06-3.1) | .40 | … | ||
| Never | 70 (58) | 0.3 (0.06-1.2) | .09 | … | 0.2 (0.03-1.5) | .11 | … | ||
| Alcohol use | |||||||||
| Current | 13 (11) | 1 [Reference] | 1 [Reference] | ||||||
| Former | 38 (31) | 3.3 (0.9-11.6) | .07 | … | 2.1 (0.3-15.2) | .45 | … | ||
| Never | 70 (58) | 3.3 (0.9-13.4) | .09 | … | 3.4 (0.5-24.2) | .22 | … | ||
| T stage | |||||||||
| T1-T2 | 75 (65) | 1 [Reference] | .16 | 1 [Reference] | .07 | 1 [Reference] | .38 | 1 [Reference] | .26 |
| T3-T4 | 40 (35) | 2.1 (0.8-6.2) | 3.1 (0.9-10.6) | 2.1 (0.4-10.2) | 2.7 (0.5-15.1) | ||||
| N stage | |||||||||
| N0-N2a | 37 (30) | 1 [Reference] | .44 | ||||||
| N2b-N3 | 85 (70) | 1.7 (0.5-5.9) | … | NAc | … | ||||
| Overall stage | |||||||||
| <IV | 22 (18) | 1 [Reference] | .33 | ||||||
| IV | 99 (82) | 2.8 (0.4-21.1) | … | NAc | … | ||||
| Oropharynx subsite | |||||||||
| Tonsil | 60 (48) | 1 [Reference] | .99 | 1 [Reference] | .92 | ||||
| Base of tongue or other oropharynx | 64 (52) | 1.0 (0.4-2.9) | … | 0.9 (0.2-4.6) | … | ||||
| Treatmentd | |||||||||
| Radiation based | 78 (64) | 1 [Reference] | .46 | 1 [Reference] | .11 | ||||
| Surgery based | 45 (37) | 1.5 (0.5-4.3) | … | 4.0 (0.7-21.8) | … | ||||
| HPV16 DNA detection in oral rinses at diagnosis | |||||||||
| No | 57 (46) | 1 [Reference] | .17 | 1 [Reference] | .18 | ||||
| Yes | 67 (54) | 2.3 (0.7-7.3) | … | 4.4 (0.5-37.4) | … | ||||
| HPV16 viral load in oral rinses at diagnosis | |||||||||
| Undetectable | 64 (53) | 1 [Reference] | .06e | 1 [Reference]b | .11e | 1 [Reference] | .01e | 1 [Reference]b | .05e |
| Low (<160 copies/2 μL) | 28 (23) | 3.9 (1.0-15.1) | 3.3 (0.7-14.2) | 2.4 (0.2-38.9) | 2.9 (0.2-50.3) | ||||
| High (≥160 copies/2 μL) | 29 (24) | 3.2 (0.9-12.0) | 2.9 (0.8-11.1) | 10.1 (1.1-90.7) | 8.3 (0.9-76.4) | ||||
| Persistent HPV16 DNA detection in oral rinses after treatment | |||||||||
| No | 119 (96) | 1 [Reference] | <.001 | 1 [Reference]b | <.001 | 1 [Reference] | <.001 | 1 [Reference]b | |
| Yes | 5 (4) | 29.7 (9.0-98.2) | 35.8 (8.6-149.1) | 23.5 (4.7-116.9) | 16.1 (2.8-92.7) | .002 | |||
Abbreviations: aHR, adjusted hazard ratio; HPV, human papillomavirus; HR, hazard ratio.
In cases in which total across categories is not equal to 124, data regarding that characteristic were missing for at least 1 participant.
The aHRs for HPV16 DNA viral load at diagnosis, and persistent HPV16 DNA detection were calculated in separate models, each adjusted for T stage and pack- years smoking. The aHRs for T stage and pack-years smoking are shown for model with persistent HPV16 DNA detection.
No deaths occurred in the early N stage group or early overall stage group, so HR was unobtainable.
Treatment was categorized according to the primary modality selected by the clinical care team. Of 78 participants who received radiation therapy, 75 (96%) also received chemotherapy. Of 45 participants who received surgery-based treatment, 32 (71%) also received radiation therapy and chemotherapy, and an additional 11 (24%) also received radiation therapy but no chemotherapy.
P value for trend across categories of increasing viral load.
HPV Detection in Oral Rinses at Diagnosis and After Treatment
Detection of HPV16 DNA in oral rinses was common in participants at diagnosis (67 of 124 [54%]) (Table 2). A median (range) of 3 (1-4) posttreatment oral rinses was available per participant. Nearly all (113 [91%]) participants had an oral rinse at 9 to 12 months after diagnosis, and 89 (72%) had an oral rinse at 18 to 24 months. Detection of HPV16 DNA in oral rinses was rare after treatment. Only 6 of 124 participants (5%) had detectable HPV16 DNA in any posttreatment oral rinse, including 4% prevalence (4 of 113) at 9 to 12 months and 3% (3 of 89) at 18 to 24 months after diagnosis. Most oral HPV16 DNA detected at diagnosis cleared after treatment (62 of 67 participants [93%]).
Table 2.
Number and Prevalence of Type-Specific High-Risk Human Papillomavirus (HR-HPV) Infections Detected in Oral Rinses at Diagnosis and After Treatment
| Timeframe of Oral Rinse Collection |
Total No. of Participants With Oral Rinses |
DNA Detected | |||||
|---|---|---|---|---|---|---|---|
| HPV16 | Other HR-HPVa | Any HR-HPVb | |||||
| Infections, No. |
Participants, No. (%) |
Infections, No. |
Participants, No. (%) |
Infections, No. |
Participants, No. (%) |
||
| Diagnosis | 124 | 67 | 67 (54) | 28 | 27 (22) | 95 | 80 (65) |
| Posttreatment | |||||||
| Postdiagnosis, mo | |||||||
| 9-12c | 113 | 4 | 4 (4) | 20 | 16 (14) | 24 | 20 (18) |
| 18-24d | 89 | 3 | 3 (3) | 9 | 9 (10) | 12 | 12 (14) |
| Any time | 124 | 6 | 6 (5) | 21 | 17 (14) | 27 | 22 (18) |
| Persistently detected | 5 | 5 (4) | 7 | 7 (6) | 12 | 12 (10) | |
| Newly detected | 1 | 1 (1) | 14 | 12 (10) | 15 | 13 (11) | |
| Cleared after treatmente | 124 | 62 | 62 (93) | 21 | 20 (74) | 83 | 68 (85) |
Abbreviation: HPV16, human papillomavirus type 16.
HR-HPV not including HPV16.
Any HR-HPV, including HPV16.
Participants with an oral rinse from either the 9-month and/or the 12-month (after diagnosis) study visit were included (n = 113): 58 had both 9- and 12-month oral rinses (all concordant), 18 had only 9-month and 37 participants had only 12-month oral rinses.
Participants with an oral rinse from either the 18-month and/or the 24-month (after diagnosis) study visit were included (n = 89): 45 had both 18- and 24-month oral rinses (all concordant), 17 had only 18-month and 27 had only 24-month oral rinses.
Defined as detection of HR-HPV DNA in oral rinse at diagnosis but not in any posttreatment oral rinse.
Detailed characteristics of the 6 participants with HPV16 DNA detected in any posttreatment oral rinse are displayed in Table 3. The most common pattern observed among these 6 participants was persistent detection of oral HPV16 DNA at diagnosis and in all posttreatment rinses (4 participants [patients 1-4]) (timeline shown in eFigure 1 in the Supplement). In contrast, 1 participant (patient 5) had oral HPV16 DNA detected at diagnosis that cleared temporarily but was again detected at 18 and 24 months. Finally, there was 1 participant (patient 6) in whom oral HPV16 DNA was not detected at diagnosis, then was newly detected at 12 months, and subsequently cleared.
Table 3.
Characteristics of Participants With Human Papillomavirus Type 16 (HPV16) DNA Detected in Any Posttreatment Oral Rinse
| ID | HPV16 DNA Detection in Oral Rinses |
Smoking Status |
T Stage |
N Stage |
Primary Treatment |
Clinical Follow-up After Diagnosis, mo |
Site of Recurrence |
Salvage Treatment |
Time From First HPV16-Positive Posttreatment Oral Rinse to Recurrence, mo |
Vital Status at Last Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Persistent | Never | T2 | N2b | CRT | 24.2 | Local, regional, distant | CT | 10.9 | AWD |
| 2 | Persistent | Former | T4 | N2c | CRT | 20.0 | Regional, distant | CT | NAa | DOD |
| 3 | Persistent | Never | T2 | N2c | CRT | 36.8 | Local | Surgery + RT | 6.9 | NED |
| 4 | Persistent | Never | T3 | N2c | CRT | 34.6 | Local, regional | Surgery, then CT, then palliative RT | 3.7 | DOD |
| 5 | Persistent | Current | T2 | N2b | Surgery + RT | 36.2 | Distant | CT | 7.0 | DOD |
| 6 | New after treatment | Never | T3 | N2c | CRT | 23.0 | NAb | NAb | NAb | NED |
Abbreviations: AWD, alive with disease; CT, chemotherapy; CRT, chemoradiotherapy; DOD, died of disease; NA, not applicable; NED, no evidence of disease; RT, radiation therapy.
First posttreatment oral rinse obtained after diagnosis of recurrence.
Patient 6 did not have disease recurrence during the study period.
Oral rinses were also evaluated for DNA from 12 other HR-HPV types besides HPV16 (called HR-HPV hereafter) (Table 2). High-risk–HPV DNA was less common than HPV16 DNA in oral rinses at diagnosis, observed in 27 (22%) compared with 67 (54%) of participants (P < .001). Most type-specific HR-HPV infections present at diagnosis cleared after treatment (21 of 28 [75%] infections corresponding to 20 of 27 [74%] participants), whereas 7 (25%) infections persisted (corresponding to 7 [26%] participants) (Table 2). Interestingly, there were also 14 new type-specific HR-HPV infections detected after treatment in 12 participants. Thus, the majority of times that non-HPV16 HR-HPV DNA was detected after treatment appeared to represent newly detectable, as opposed to persistent, HR-HPV infections (14 of 21 type-specific infections [67%]). Participants with vs without newly detected type-specific HR-HPV infections did not significantly differ with regard to sexual behaviors, smoking status, or other characteristics (eTable 2 in the Supplement).
Recurrence and Survival
Median (IQR) follow-up time was 33 (24-41) months. There were 14 recurrences and 6 deaths observed during follow-up among the 124 participants. All 6 deaths were from recurrent disease. Two years after diagnosis, disease-free survival (DFS) was 92% (95% CI, 94%-100%) and overall survival (OS) was 98% (95% CI, 93%-99%).
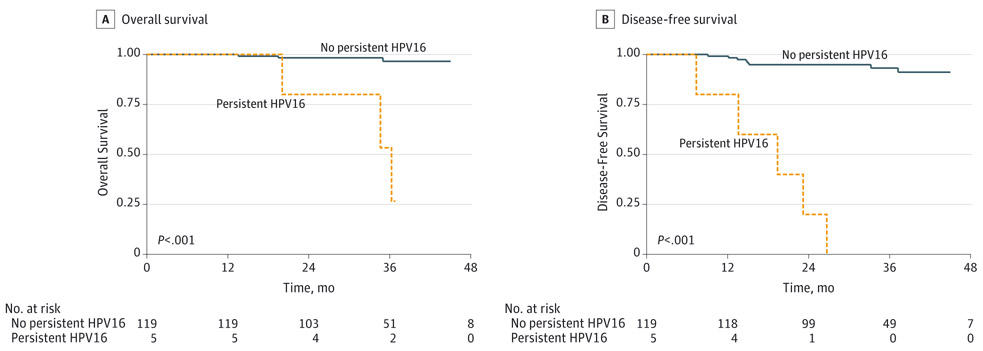
Detection of HPV16 DNA in oral rinses at diagnosis was not associated with DFS (P = .15) or OS (P = .14). In contrast, persistent HPV16 DNA detection in oral rinses (eg, both at diagnosis and any time after treatment) was associated with a greater than 20-fold increased risk of recurrence (hazard ratio [HR], 29.7 [95% CI, 9.0-98.2]) and death (HR, 23.5 [95% CI, 4.7-116.9]) in univariate analysis (Table 1, Figure). After adjustment for pack-years of smoking and tumor stage, persistent detection of oral HPV16 DNA remained associated with both DFS (adjusted HR [aHR], 35.8 [95% CI, 8.6-149.1]) and OS (aHR, 16.1 [95% CI, 2.8-92.7]). Number of pack-years of cigarette smoking was also associated with worse DFS (HR, 1.4 [95% CI, 1.1-1.8] per 10 pack-years) and OS (HR, 1.5 [95% CI, 1.1-2.1]) in univariate analysis, consistent with previous research,21 although these associations were no longer significant after adjustment for persistent oral HPV16 DNA and tumor stage (aHR for DFS, 1.2 [95% CI, 1.0-1.5] and aHR for OS, 1.3 [95% CI, 0.9-1.8]). Sex, alcohol use, tumor subsite, age, and study site were not significantly associated with DFS or OS.
Figure.
Survival by Detection of Persistent Human Papillomavirus Type 16 (HPV16) DNA in Pretreatment and Posttreatment Oral Rinse Samples
Persistent HPV16 indicates detection of HPV16 DNA in oral rinses both at diagnosis and at any posttreatment visit.
All 5 participants with persistent oral HPV16 DNA detected developed recurrent disease and 3 died of disease, whereas the 1 participant with newly detected oral HPV16 DNA after treatment that subsequently cleared (patient 6) was alive without disease at last follow-up, 23 months after diagnosis (Table 3, eFigure 1 in the Supplement). The median (range) time from first posttreatment detection of oral HPV16 DNA to recurrence was 7.0 (3.7-10.9) months for the 4 participants with study visits prior to recurrence (patient 2 did not have any posttreatment study visits until after recurrence).
Four of the 5 participants with persistent oral HPV16 were originally treated with chemoradiation therapy and the fifth with surgery and adjuvant radiation therapy, which did not differ from the overall study population (P = .65, Fisher exact test) or from the 9 participants who experienced disease recurrence but did not have persistent oral HPV16 DNA (P = .30).
Three of the recurrences among the 5 participants with persistent oral HPV16 DNA included local disease (Table 4). In comparison, nearly all recurrences among participants without persistent oral HPV16 DNA were regional and/or distant, without local disease (8 of 9 [89%]; P = .10, Fisher exact test) (Table 4). Overall, only 3 participants developed isolated local or locoregional recurrence without distant disease; 2 of these had persistent oral HPV16 DNA (patients 3 and 4) and 1 did not.
Table 4.
Patterns of Recurrence for Participants With and Without Persistent Human Papillomavirus Type 16 (HPV16) DNA Detected in Oral Rinses
| No. (%) | |||||||
|---|---|---|---|---|---|---|---|
| Persistent Oral HPV16 DNA Detected |
No. | Participants With Recurrent Disease |
Isolated Local or Local+Regional Recurrences |
Recurrences Including Distant Disease | |||
| Local Only | Local + Regional | Locoregional + Distant | Regional + Distant | Distant Only | |||
| Yes | 5 | 5 (100) | 1 (20) | 1 (20) | 1 (20) | 1 (20) | 1 (20) |
| No | 119 | 9 (8) | 1 (11) | 0 | 0 | 3 (33) | 5 (56) |
| Total | 124 | 14 | 4 (28) | 1 (7) | 1 (7) | 4 (29) | 6 (43) |
We estimated the ability of persistent HPV16 DNA in oral rinses to predict future recurrence by considering only oral rinses collected within 9 to 12 months after diagnosis and evaluating recurrence risk only within 12 to 24 months after diagnosis, eg, recurrences within approximately 1 year subsequent to the oral rinse sampling. There were 3 participants with persistent oral HPV16 DNA detected at 9 to 12 months, of whom all 3 experienced disease recurrence between 12 and 24 months after diagnosis. In contrast, there were 108 participants without persistent oral HPV16 DNA at 9 to 12 months, of whom 4 (4%) experienced recurrence. Therefore, although there were few events, detection of HPV16 DNA in oral rinses at 9 to 12 months after diagnosis in our study population had high specificity (100% [95% CI, 96%-100%]), PPV (100% [95% CI, 29%-100%]), and NPV (96% [95% CI, 91%-99%]), but low sensitivity (43% [95% CI, 12%-80%]), in predicting any subsequent recurrence within the following year. Interestingly, all 3 recurrences predicted by the presence of persistent oral HPV16 DNA involved local disease (1 local, 1 locoregional, and 1 locoregional and distant), compared with none of the 4 recurrences in participants without persistent oral HPV16 DNA (2 regional and distant, 2 distant only; P = .03, Fisher exact test). When only recurrences involving local disease were considered in this analysis, specificity and PPV remained at 100% (95% CI, 97%-100% and 29%-100%, respectively), whereas sensitivity increased to 100% (95% CI, 29%-100%), as did NPV (100% [95% CI, 97%-100%]).
In contrast to the observed association of persistent oral HPV16 DNA detection with survival, persistent detection of DNA from HR-HPV types other than HPV16 was not associated with survival (P = .40 and .65 for DFS and OS, respectively). Among the 7 participants with persistently detected HR-HPV other than HPV16 (including HPV 33, 39, 45, 51, and 52 and 2 participants with HPV59), there was only 1 recurrence, in a participant with persistent oral HPV52 DNA who developed pulmonary metastases.
HPV16 Viral Load in Oral Rinses at Diagnosis
There were 57 participants with detectable HPV16 viral load in oral rinses at diagnosis, 56 (98%) of whom also had HPV16 DNA detected by line-blot hybridization (eTable 3 in the Supplement). Median (IQR) detectable HPV16 viral load in oral rinses at diagnosis was 161 (21-846) copies/2 μL. Participants with larger tumors more commonly had a detectable oral HPV16 viral load than those with smaller tumors (62% vs 43%; P = .05), and current smokers were more likely than never or former smokers to have high (>160 copies/2 μL) viral load (46% vs 21%; P = .04). High viral load was also more common in participants with tonsil compared with nontonsil tumors (39% vs 11%; P < .001).
Higher HPV16 viral load in oral rinses at diagnosis was associated with significantly worse OS (P for trend = .01) but was not significantly associated with worse DFS (P for trend = .06) in univariate analysis (Table 1, eFigure 2 in the Supplement). After adjustment for number of smoking pack-years and tumor stage, higher HPV16 viral load in oral rinses at diagnosis continued to be associated with OS (P for trend = .05) but not DFS (P for trend = .11).
Discussion
Although most HPV-OPCs respond favorably to treatment, a subset of patients experience disease progression.2-6 There is a need for clinically relevant biomarkers of disease recurrence to facilitate timely initiation of aggressive diagnostic investigation and subsequent salvage treatment to potentially improve outcomes for the growing population of HPV-OPC survivors. Detection of recurrent local or locoregional disease prior to distant spread is particularly desirable given the favorable response of HPV-OPC to surgical salvage. We have demonstrated that persistent HPV16 DNA detection in posttreatment oral rinses, although infrequent, is predictive of HPV-OPC recurrence, perhaps even more so for local recurrence, and is associated with survival. Furthermore, we observed a clinically meaningful lead time from first positive posttreatment oral rinse result to diagnosis of recurrence (median, 7 months). Therefore, HPV16 DNA detection in oral rinses may prove a valuable tool for long-term posttreatment surveillance of HPV-OPC for local recurrence.
Our study corroborates in a multicenter, prospective cohort setting the findings of 2 previous smaller cohort studies that described an association of HPV16 DNA detection in posttreatment oral rinses with survival. Chuang et al15 detected HPV16 DNA in oral rinses from 2 of 20 patients with HPV-OPC or HPV-positive unknown primary tumors from 1999 through 2005, both of whose disease recurred 3.5 months after the oral rinse sample was collected. Ahn et al11 reported that of 83 patients with OPC and unknown primary tumors from 1999 to 2010 (72 HPV positive), 4 had HPV16 DNA detected in posttreatment oral rinses, and 3 subsequently had recurrent cancers (aHR for recurrence-free survival, 10.7 [95% CI, 2.4-48.5]). The higher HR that we report (aHR, 35.8 [95% CI, 8.6-149.1]) is likely explained by our restriction to patients with HPV-OPC and use of persistent, rather than any, posttreatment oral HPV16 DNA for our analysis. Risk estimates may also be influenced by different laboratory methods for HPV detection in each study. Of note, 1 additional study by Koslabova et al14 did not observe prognostic significance of posttreatment oral HPV16 DNA detection. Reasons for this discrepancy are unclear but may relate to their use of a single posttreatment oral rinse, heterogeneous patient population, and/or limited clinical follow-up.
The consistent finding that HPV16 DNA detection in posttreatment oral rinses is predictive of recurrence in our and several previous studies supports its potential utility as a clinical test. Our study has several strengths. First, the multisite, prospective cohort design and recent calendar period (2009-2013) lend generalizability and current relevance to our findings. We included only participants with HPV-OPC, decreasing heterogeneity. Finally, the serial collection of posttreatment oral rinse samples at predefined intervals allowed us to preliminarily describe a time lag from first HPV16 DNA–positive oral rinse to recurrence.
These findings highlight the question of what HPV16 DNA detected in posttreatment rinses represents pathophysiologically. We hypothesize that the persistent presence of oral HPV16 DNA represents in most cases integrated or episomal viral DNA in persistent or recurrent microscopic disease that may not yet be detectable by conventional methods. Of note, our laboratory methods cannot distinguish between HPV DNA in infectious viral particles and integrated or episomal HPV DNA in tumor cells (for this reason, we have largely referred to “oral HPV DNA detection” rather than “oral HPV infection”). However, it has been shown that most HPV16 strains detected in oral rinses of patients with HPV-OPC are genetically identical to the HPV16 found in corresponding tumors.12 Furthermore, we previously reported that partners of patients with HPV-OPC do not exhibit a higher than expected prevalence of HPV16 DNA detection in oral rinses.13 Taken together, this is more consistent with noninfectious fragments of HPV16 DNA in exfoliated tumor cells, rather than an active infection, as the primary source of the HPV16 DNA detected in oral rinses from patients with HPV-OPC.
Although based on few events, our analysis of the predictive value of persistent oral HPV16 DNA at 9 to 12 months after diagnosis demonstrated high specificity, sensitivity, PPV, and NPV for subsequent recurrence involving local disease in the following year. This is clinically significant, in that isolated local or locoregional tumor is more amenable to salvage treatment, especially surgical salvage. Although only 2 of the 5 participants with persistent oral HPV16 DNA had such isolated local or locoregional recurrence, it is conceivable that earlier and more aggressive diagnostic workup for the remaining 3 participants, precipitated by an HPV16-positive oral rinse, may have facilitated detection of disease at a more limited, and therefore treatable, stage.
The low sensitivity of our assay for any recurrence, including regional and distant disease, is not surprising because regional or distant metastastic disease would not, in the absence of a direct connection to the primary site, be expected to shed tumor cells into an oral rinse. A potential strategy to increase sensitivity for recurrence overall is to combine oral rinses with plasma-based testing for HPV16 biomarkers, such as HPV16 DNA or antibodies to HPV16 antigens, which has also been shown to be sensitive for HPV-OPC and is less anatomically constrained.11,14,22
The prognostic value of persistent oral HR-HPV DNA detection in posttreatment oral rinses was limited in our study to HPV16, whereas persistence of other HR-HPV types was not associated with recurrence or survival. Because HPV16 is responsible for more than 90% of HPV-OPCs,23,24 DNA from other HR-HPV types is less likely to be etiologically related to tumor recurrence. In addition, the demographic characteristics of our study population are such that a relatively high baseline prevalence of oral HR-HPV DNA detection is expected: the prevalence of oral HR-HPV infection in the general population is 3.7% but is greater among men in their mid-50s with high numbers of lifetime oral sex partners,25 all characteristics typical of patients with HPV-OPC. This may also explain the high rate of newly detected non-HPV16 HR-HPV DNA after treatment, which was also noted in previous studies.12,14
Our finding that HPV16 viral load in oral rinses at diagnosis was associated with worse survival has not to our knowledge been previously reported, and the clinical relevance is unclear because there were few events in our study. It is possible that higher viral copy number may correlate to some measure of disease activity, for example, tumor size, and portend increased resistance to treatment. Additional studies are necessary to replicate and further characterize this association. The higher viral load among smokers is consistent with previously reported data from the general population,26 and the association of detectable viral load with tumor size is not surprising because larger tumors would be expected to shed more tumor cells; however, the significantly increased viral load in participants with tonsillar compared with other oropharyngeal tumors was unexpected. This may reflect a differential capacity of an oral rinse to sample tumor tissue depending on primary site of disease.
The conclusions of this study are limited by the infrequency of persistent oral HPV16 DNA detection and small number of deaths and recurrences. In addition, the lack of posttreatment disease surveillance standardization restricted analysis of the timing of oral rinse results relative to clinical and imaging findings. Finally, eligible participants were significantly more likely than ineligible participants to have several characteristics associated with improved prognosis after head and neck cancer treatment, in that they were more likely to be married, had higher income, and had fewer pack-years of smoking, which may have biased our results.
Conclusions
Detection of HPV16 DNA in oral rinse samples is common at diagnosis but rare after treatment for HPV-OPC. Our data suggest that persistent HPV16 DNA detection in posttreatment oral rinses, although uncommon, is associated with poor prognosis and may be predictive of disease recurrence, in particular local recurrence. Therefore, HPV16 DNA detection in oral rinses is a potentially useful tool for long-term tumor surveillance for the growing population of HPV-OPC survivors.
Supplementary Material
At a Glance.
Among patients with human papillomavirus–positive oropharyngeal cancer (HPV-OPC), 10% to 25% experience disease progression after treatment. Earlier diagnosis of progressive or recurrent disease may allow for earlier treatment and improved outcomes.
Human papillomavirus type 16 (HPV16) DNA was detected in oral rinses from 54% of 124 patients with HPV-OPC at diagnosis but was persistently detected after treatment in 4% of patients with HPV-OPC.
All participants with persistent oral HPV16 DNA detected after treatment experienced disease recurrence, with a median of 7.0 months between the first HPV16-positive posttreatment oral rinse and recurrence.
Although infrequent, persistent HPV16 DNA detection in posttreatment oral rinses was associated with disease-free (hazard ratio, 29.7 [95% CI, 9.0-98.2]) and overall survival (hazard ratio, 23.5 [95% CI, 4.7-116.9]).
Detection of HPV16 DNA in oral rinses after treatment for HPV-OPC may be a useful adjunct to current posttreatment tumor surveillance strategies, potentially facilitating earlier diagnosis of progressive or recurrent HPV-OPC.
Acknowledgments
Funding/Support: This research was supported financially by the Johns Hopkins Richard Gelb Cancer Prevention Award (Dr D’Souza), the Oral Cancer Foundation (Dr D’Souza), and the National Institute of Dental and Craniofacial Research and National Institutes of Health (NIH) Research Training in Otolaryngology grant 2T32DC000027-26 (Dr Rettig).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Gross has received travel expense reimbursement from Intuitive Surgical, Inc, and Medrobotics, Inc. Dr Haddad has been compensated as a consultant for Celgene, Merck, and Eisai and has received institutional research funding from Merck, Bristol-Myers Squibb, VentiRx, and Boehringer-Ingelheim. Dr Sikora has received research funding from Advaxis, LLC. Dr Lorch has been compensated as a consultant for Eisai and has received institutional research funding from Novartis. Dr Miles has received institutional research funding from Advaxis. Dr Anderson has received honoraria from and been paid to participate in a speakers’ bureau for Ethicon and travel expense reimbursement from Medrobotics. Dr Chung has been compensated as a consultant for Novartis, has received research funding from Immunogen and Boehringer-Ingelheim, and has received travel expense reimbursement from Merck. Dr D’Souza has received prior institutional research funding from Merck. No other disclosures are reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Contributor Information
Eleni M. Rettig, Otolaryngology–Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Alicia Wentz, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Marshall R. Posner, Tisch Cancer Institute, Head and Neck Oncology Center, Icahn School of Medicine at Mount Sinai Medical Center, New York, New York.
Neil D. Gross, Division of Surgery, Department of Head and Neck Surgery, University of Texas MD Anderson Cancer Center, Houston.
Robert I. Haddad, Department of Adult Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts.
Maura L. Gillison, Viral Oncology Program, Ohio State University Comprehensive Cancer Center, Columbus.
Carole Fakhry, Otolaryngology–Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Harry Quon, Department of Radiation Oncology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Andrew G. Sikora, Department of Otolaryngology–Head and Neck Surgery, Baylor College of Medicine, Houston, Texas.
William J. Stott, Department of Otolaryngology–Head and Neck Surgery, Oregon Health and Science University, Portland, Oregon.
Jochen H. Lorch, Department of Adult Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts.
Christine G. Gourin, Otolaryngology–Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Yingshi Guo, Viral Oncology Program, Ohio State University Comprehensive Cancer Center, Columbus.
Weihong Xiao, Viral Oncology Program, Ohio State University Comprehensive Cancer Center, Columbus.
Brett A. Miles, Department of Otolaryngology, Icahn School of Medicine at Mount Sinai Medical Center, New York, New York.
Jeremy D. Richmon, Otolaryngology–Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Peter E. Andersen, Department of Otolaryngology–Head and Neck Surgery, Oregon Health and Science University, Portland, Oregon.
Krzysztof J. Misiukiewicz, Tisch Cancer Institute, Head and Neck Oncology Center, Icahn School of Medicine at Mount Sinai Medical Center, New York, New York.
Christine H. Chung, Otolaryngology–Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland; Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Jennifer E. Gerber, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Shirani D. Rajan, Louisiana State University School of Medicine, New Orleans.
Gypsyamber D’Souza, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
REFERENCES
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1): 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–550. [DOI] [PubMed] [Google Scholar]
- 5.Lin BM, Wang H, D’Souza G, et al. Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer. 2013;119(19):3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. [DOI] [PubMed] [Google Scholar]
- 7.Guo T, Qualliotine JR, Ha PK, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer. 2015;121(12):1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. ; SPECTRUM investigators. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14(8):697–710. [DOI] [PubMed] [Google Scholar]
- 9.Argiris A, Li S, Ghebremichael M, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol. 2014;25(7):1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith EM, Ritchie JM, Summersgill KF, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108(5):766–772. [DOI] [PubMed] [Google Scholar]
- 11.Ahn SM, Chan JY, Zhang Z, et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2014; 140(9):846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal Y, Koch WM, Xiao W, et al. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14 (21):7143–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32(23):2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koslabova E, Hamsikova E, Salakova M, et al. Markers of HPV infection and survival in patients with head and neck tumors. Int J Cancer. 2013;133 (8):1832–1839. [DOI] [PubMed] [Google Scholar]
- 15.Chuang AY, Chuang TC, Chang S, et al. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44(10): 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One. 2014;9(1):e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head and Neck Cancers. NCCN Clinical Practice Guidelines in Oncology 2015; Version 1.2015. http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp Accessed May 27, 2015.
- 18.Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. J Clin Virol. 2011;50(4):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshiol J, Rotunno M, Gillison ML, et al. Assessment of human papillomavirus in lung tumor tissue. J Natl Cancer Inst. 2011;103(6):501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 21.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KS, Gerber J, D’Souza G, et al. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: the HOTSPOT study [published online June 18, 2015]. Oral Oncol. doi: 10.1016/j.oraloncology.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrero R, Castellsagué X, Pawlita M, et al. ; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. [DOI] [PubMed] [Google Scholar]
- 24.Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(suppl 5):F34–F54. [DOI] [PubMed] [Google Scholar]
- 25.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaturvedi AK, Graubard BI, Pickard RK, Xiao W, Gillison ML. High-risk oral human papillomavirus viral load in the US population, National Health and Nutrition Examination Survey 2009-2010. J Infect Dis. 2014;210(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.